Abstract
Objective:
Calcium sulfate beads (CSBs) are biocompatible hydrophilic crystals that are used to deliver local antibiotics in periprosthetic joint infections. Hypercalcemia after placement of CSBs is uncommon and poorly understood.
Methods:
We present the case of a woman who presented with symptomatic hypercalcemia after placement of antibiotic-eluting CSBs.
Results:
A 58-year-old, Caucasian woman presented with altered mental status, respiratory failure, and septic shock 2 days after placement of antibiotic-eluting CSBs for a left prosthetic hip infection. Laboratory analysis revealed severe hypercalcemia at presentation. She had no known history of fractures, kidney stones, parathyroid, or calcium disorders. She was not on any medications that could induce hypercalcemia. She was treated with aggressive intravenous hydration and 8 doses of calcitonin. Due to impaired renal function, bisphosphonate was contraindicated. She subsequently became anuric with worsening renal failure and volume overload and the decision was made to initiate dialysis. She received 8 days of continuous renal replacement therapy followed by 2 sessions of hemodialysis which improved her serum calcium levels, mental status, and renal failure with no long-term complications.
Conclusion:
Hypercalcemia secondary to the placement of antibiotic-eluting CSBs is rare. Larger volumes of CSBs may contribute to hypercalcemia. In some cases, hypercalcemia can be severe and symptomatic as in the case of our patient. Serum calcium levels should be monitored frequently after placement of CSBs and managed as appropriate.
INTRODUCTION
Calcium sulfate beads (CSBs) are biocompatible hydrophilic crystals that are used to deliver local antibiotics in joint and bone infections. Transient hypercalcemia following placement of these beads is uncommon. We present a case of a woman who presented with septic shock, respiratory failure, and altered mental status with findings of severe hypercalcemia 2 days after placement of CSBs for a left prosthetic hip infection.
CASE REPORT
We present the case of a 58-year-old, Caucasian woman with no history of parathyroid, calcium, or kidney disorders who presented to our hospital with septic shock, respiratory failure, and altered mental status. A review of her medical history revealed placement of antibiotic-eluting CSBs in her left hip for an infected hip prosthesis that had not responded to multiple surgical debridements nor a course of intravenous antibiotics. Placement of the beads was 2 days before presentation to our endocrinology consult service. There was no known history of fractures or kidney stones. She was on no medications that could cause hypercalcemia. Her serum calcium level and kidney function had previously been in normal range.
On presentation, total serum calcium (corrected for hypoalbuminemia) was elevated to 12.2 mg/dL (reference range is 8.4 to 10.2 mg/dL) with peak levels 2 days later at 15.7 mg/dL. Her intact parathyroid hormone (PTH) level was <10.0 pg/mL (reference range is 15.0 to 65.0 pg/mL), PTH-related peptide was 0.4 pmol/L (reference range is <2 pmol/L). 1,25-dihydroxyvitamin D was 8.9 pg/mL (reference range is 18.0 to 78.0 pg/mL) and 25-hydroxyvitamin D was 13 pg/mL (reference range is 30 to 80 pg/mL) thus ruling out primary hyperparathyroidism, paraneoplastic production of PTH-related peptide, granulomatous disease, and vitamin D toxicity as the causes for her hypercalcemia. Testing for multiple myeloma including serum protein electrophoresis was negative (Table 1).
Table 1.
Laboratory Values on Presentation
| Value | Reference range | |
|---|---|---|
| Total corrected serum calcium (mg/dL) | 12.2 | 8.4–10.2 |
| Creatinine (mg/dL) | 1.79 | 0.51–0.95 |
| Parathyroid hormone (pg/mL) | <10 | 15–65 |
| 1,25-dihydroxyvitamin D (pg/mL) | 8.9 | 18.0–78.0 |
| 25-hydroxyvitamin D (pg/mL) | 13 | 30–80 |
| Parathyroid hormone-related peptide (pmol/L) | 0.4 | <2 |
| Serum protein electrophoresis | Negative | – |
She received 2 doses of calcitonin (4 U/kg) but due to her worsening kidney function and low creatinine clearance (<30 mL/min), she could not receive a bisphosphonate. She became volume overloaded with pulmonary edema and anuric with intraveous fluids subsequently needing dialysis to correct her hypercalcemia and renal function. Her mental status improved with improvement of her calcium levels. She received continuous renal replacement therapy for 8 days followed by 2 sessions of hemodialysis. Dialysis was stopped 22 days after antibiotic-eluting beads were placed and subsequent calcium levels and renal function remained in normal range (Fig. 1).
Fig. 1.
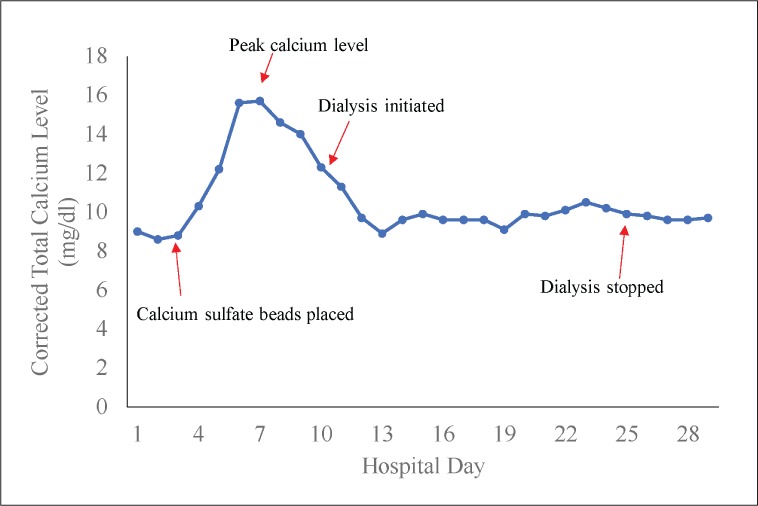
Calcium levels during hospitalization.
As no other secondary cause of hypercalcemia was discovered during the workup, we attributed her elevated calcium levels to the antibiotic-eluting CSBs made worse by immobilization from prolonged hospitalization and critical illness.
DISCUSSION
We present a case of prolonged severe hypercalcemia from antibiotic-eluting CSBs. CSBs are used for periprosthetic joint infections, osteomyelitis, open fractures, and combat fractures (1–3). Antibiotic beads can be used for new bone formation at sites of bone defects and can be used as a local antibiotic delivery system. In contrast to traditional polymethyl methacrylate spacers, absorbable CSBs allow for more local release of antibiotics along with higher sustained concentrations of antibiotics. CSBs have the additional advantage of not needing subsequent removal as they are completely resorbed (2,4).
Hypercalcemia from CSBs was first described in a canine model but elevated serum calcium levels were not sustained and no symptoms were noted (5). Kallala et al (6) reported the outcomes and complications in a cohort of 15 patients undergoing placement of CSBs for a peri-prosthetic joint infection in a revision arthroplasty. Three of these patients developed hypercalcemia with 1 patient developing symptoms of altered mental status and lethargy with a peak calcium level of 14.2 mg/dL. This patient had received 40 mL of CSBs and subsequently received treatment for his hypercalcemia with intravenous fluids and bisphosphonate. He had improvement of his symptoms and correction of his calcium levels within 10 days.
The largest study evaluating complications of CSBs involved a cohort of 755 patients (7). A mean of 23.39 mL (with a range of 5 to 80 mL) of CSBs were used per patient. In all, 41 patients developed hypercalcemia of which 2 developed symptoms and were treated with intravenous fluids and 1 dose of bisphosphonate. Mean serum calcium level was 11.7 mg/dL (ranging from 10.8 to 14.9 mg/dL). Hypercalcemia corrected in all patients within a maximum of 5 days after the operation. The volume of CSBs placed in this study was not related to hypercalcemia. The U.S. Food and Drug Administration has an adverse reaction report about transient hypercalcemia following the placement of vancomycin-infused CSBs in a patient undergoing hip arthroplasty (8).
Our case demonstrates an uncommon etiology of hypercalcemia. Unlike the cases reported in the literature, our patient developed severe hypercalcemia and renal failure. Patients who have had placement of CSBs should be monitored with serum calcium and creatinine levels within 24 to 48 hours of this procedure. Patients who are at risk of developing hypercalcemia including those with renal impairment, critical illness, prolonged immobilization, and history of calcium or parathyroid disorders should be approached with additional caution and alternative treatment for infection should be considered. In addition, a large volume of CSBs may increase risk of transient hypercalcemia.
CONCLUSION
We present a case of severe, prolonged hypercalcemia secondary to antibiotic-eluting CSBs. We recommend considering alternate treatments for infection in patients at high risk of developing hypercalcemia and to monitor calcium levels and kidney function frequently after placement of these beads.
Abbreviations
- CSB
calcium sulfate bead
- PTH
parathyroid hormone
Footnotes
DISCLOSURE
The authors have no multiplicity of interest to disclose.
REFERENCES
- 1.Beuerlein MJ, McKee MD. Calcium sulfates: what is the evidence? J Orthop Trauma. 2010;24(suppl 1):S46–S51. doi: 10.1097/BOT.0b013e3181cec48e. [DOI] [PubMed] [Google Scholar]
- 2.McKee MD, Li-Bland EA, Wild LM, Schemitsch EH. A prospective, randomized clinical trial comparing an antibiotic-impregnated bioabsorbable bone substitute with standard antibiotic-impregnated cement beads in the treatment of chronic osteomyelitis and infected nonunion. J Orthop Trauma. 2010;24:483–490. doi: 10.1097/BOT.0b013e3181df91d9. [DOI] [PubMed] [Google Scholar]
- 3.Helgeson MD, Potter BK, Tucker CJ, Frisch HM, Shawen SB. Antibiotic-impregnated calcium sulfate use in combat-related open fractures. Orthopedics. 2009;32:323. doi: 10.3928/01477447-20090501-03. [DOI] [PubMed] [Google Scholar]
- 4.Sanicola SM, Albert SF. The in vitro elution characteristics of vancomycin and tobramycin from calcium sulfate beads. J Foot Ankle Surg. 2005;44:121–124. doi: 10.1053/j.jfas.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 5.Peltier LF. The use of plaster of paris to fill large defects in bone. Am J Surg. 1959;97:311–315. doi: 10.1016/0002-9610(59)90305-8. [DOI] [PubMed] [Google Scholar]
- 6.Kallala R, Haddad FS. Hypercalcaemia following the use of antibiotic-eluting absorbable calcium sulphate beads in revision arthroplasty for infection. Bone Joint J. 2015;97-B:1237–1241. doi: 10.1302/0301-620X.97B9.34532. [DOI] [PubMed] [Google Scholar]
- 7.Kallala R, Harris WE, Ibrahim M, Dipane M, McPherson E. Use of Stimulan absorbable calcium sulphate beads in revision lower limb arthroplasty: safety profile and complication rates. Bone Joint Res. 2018;7:570–579. doi: 10.1302/2046-3758.710.BJR-2017-0319.R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.U.S. Food and Drug Administration Calcium Osteoset Beads, April 29, 2005. MAUDE (medical device) Adverse Event Cases. Available at: http://fdable.com/basic_query/maude/fc8db54924f17b3cd7d8051d392d3892 Accessed September 1, 2019.


