Abstract
Adverse environment in early life can modulate the adult phenotype, including blood pressure. Lipopolysaccharide (LPS) exposure in utero results in increased blood pressure in the offspring, but the exact mechanisms are not clear. Studies have shown that the renal dopamine D1 receptor (D1R) plays an important role in maintaining sodium homeostasis and normal blood pressure; dysfunction of D1R is associated with oxidative stress and hypertension. In this study, we determined if dysfunction of the renal D1R is involved in fetal-programmed hypertension, and if oxidative stress contributes to this process. Pregnant Sprague-Dawley (SD) rats were intraperitoneally injected with LPS (0.79 mg/kg) or saline at gestation days 8, 10, and 12. As compared with saline-injected (control) dams, offspring of LPS-treated dams had increased blood pressure, decreased renal sodium excretion, and increased markers of oxidative stress. In addition, offspring of LPS-treated dams had decreased renal D1R expression, increased D1R phosphorylation and G protein-coupled receptor kinase type 2 (GRK2) and type 4 (GRK4) protein expression and impaired D1R-mediated natriuresis and diuresis. All of the findings in the offspring of LPS-treated dams were normalized after treatment with TEMPOL, an oxygen free radical scavenger. In conclusion, prenatal LPS exposure, via an increase in oxidative stress, impairs renal D1R function and leads to hypertension in the offspring. Normalization of renal D1R function by amelioration of oxidative stress may be a therapeutic target of fetal programming of hypertension.
Keywords: fetal programming, hypertension, lipopolysaccharide, D1 dopamine receptor, G protein-coupled receptor kinase, TEMPOL, oxidative stress
Introduction
Hypertension, an important cardiovascular risk factor and a major contributor to end-stage renal disease, is a heterogeneous disorder that is probably caused by an interaction of genetic and environmental factors [1]. In recent years, a large number of experiments have revealed that fetal programming (also known as developmental origins of adult diseases), is another important factor contributing to the development of hypertension [2, 3]. Barker and colleagues have proposed that adverse environmental stimuli, experienced during a critical period of development in utero, could induce long-term structural and functional effects in the developing organism [4, 5].
Maternal infection and inflammation are common events during pregnancy, and a large number of studies support the relationship between infection and adverse pregnancy outcomes [6–9]. Intrauterine infection is considered as one of the major maternal insults during pregnancy [10]. Lipopolysaccharide (LPS) is a toxic component of cell walls of gram-negative bacteria that is present in the digestive tracts of humans and animals [11]. Peripheral administration of LPS from Escherichia coli is a well-characterized model of sepsis in rodents [12]. Maternal exposure to LPS alters proinflammatory cytokine levels, including IL-1, IL6, and TNF-α, in the placenta, amniotic fluid, and fetal brain, that may have a significant impact on fetal development [13]. Studies have shown that prenatal exposure to LPS results in increased blood pressure in the offspring that is associated with alterations in the renin-angiotensin system in the kidney and increased circulating leptin which can increase sympathetic nerve activity [14, 15]. However, hypertension is caused not only by increased activity of pro-hypertensive factors, such as the renin-angiotensin and sympathetic nervous systems, but also by decreased activity of anti-hypertensive mechanisms, such as the renal dopaminergic system [16].
Dopamine, produced in the kidney independent of renal nerves, is now recognized to serve an important role in the regulation of sodium balance and blood pressure. Dopamine receptors are classified into D1- (D1R, D5R) and D2-like (D2R, D3R and D4R) subtypes based on their structure and pharmacology [16–18]. Under euvolemic conditions and moderate volume expansion, dopamine, especially via D1-like receptors, acts to increase sodium excretion and keep the blood pressure in the normal range. Abnormal responses to dopamine and D1-like receptor function have been implicated in hypertensive patients and rodent models of genetic and salt-sensitive hypertension [16–21]. Previous studies have found that dysfunction of D1R can be the result of its hyper-phosphorylation caused by G protein-coupled receptor kinase type 2 (GRK2) and GRK4 [16, 19, 22], that can be linked to increased oxidative stress [23–28]. Antioxidant supplementation with TEMPOL, a water-soluble, paramagnetic nitroxyl radical with superoxide dismutase (SOD) mimetic activity decreases oxidative stress, restores D1R signaling, and lowers blood pressure [26–28]. Thus, we investigated whether or not dysfunction of renal D1R signaling is involved in the fetal programming of hypertension and whether or not inhibition of reactive oxygen species (ROS) production would ameliorate the renal D1R dysfunction and normalize blood pressure.
Materials and Methods
1. Animals and treatment
The experimental protocols were approved by The Third Military Medical University Animal Care and Use Committee. Virgin Sprague-Dawley (SD) rats (250-260g) were purchased from the Animal Centre of The Third Military Medical University (Chongqing, China). All rats were intraperitoneally administered with LPS (0.79mg/kg in 0.5 ml saline, Escherichia coli, Sigma, St Louis, MO, USA, L8274,) or saline (0.5ml) on gestation days 8, 10, and 12 (n=10 in each group), and were housed individually throughout pregnancy until delivery. The pups were weaned at 4 weeks of age to regular rat chow.
The blood pressure was measured in conscious rats at 5 weeks, and 3, 6, 9, and 12 months of age using the tail-cuff method (ML125, PowerLab, AD Instruments, Castle Hill, Australia). Urine was collected in metabolic cages, and 24-hour urine volume and sodium excretion were measured. At 12 weeks of age, male offspring of LPS- and saline-treated dams were randomly assigned into two groups: one group that drank tap water served as control, and the other group drank tap water with 1.0 mmol/L TEMPOL, that was changed two times a day for 3-4 weeks.
Renal Histology
Histological analysis was performed on kidney samples taken at 12 months of age from the offspring of vehicle- (control) and LPS-treated dams. The tissues were fixed in 4% paraformaldehyde, embedded in paraffin, and cut into 4-μm-thick sagittal sections. The sections were stained with hematoxylin and eosin (H&E) and Masson’s trichrome and examined for glomerulosclerosis, interstitial fibrosis, and tubular atrophy.
Surgical procedures and experimental protocol for renal function studies
The surgical procedures and surgical interventions were performed as reported [29]. The rats were anesthetized with pentobarbital sodium (50 mg/kg body wt), placed on a heated table to maintain rectal temperature at ~37°C, and tracheotomized (PE-240). Anesthesia was maintained by the infusion of pentobarbital at 0.8 mg/100g body wt per hour. The left carotid artery was catheterized with PE-50 tubing, which was connected to a pressure transducer (Cardiomax II; Columbus Instruments, Columbus, OH) for blood pressure measurement. The left external jugular vein was catheterized for fluid administration. A midline incision was performed; the left and right ureters were catheterized with PE-10 tubing for urine collection. The right suprarenal artery was catheterized (PE-10, heat stretched to 180μm) for vehicle (saline) or fenoldopam infusion. The duration of the surgical procedures was about 60 min. During the experiment, vehicle (saline) or fenoldopam was infused at a rate of 40 μl/h. Fluid losses throughout the experiment were replaced intravenously with 5% albumin at 1% body wt over 30 min.
After a 60-min stabilization period following the surgery, seven consecutive 40-min urine samples were collected: C1 and C2 (averaged as basal period), D1, D2, and D3 (averaged as fenoldopam period), and R1 and R2 (averaged as recovery period). During the basal and recovery periods, only saline alone was infused. During the fenoldopam period, fenoldopam (1.0 μg/kg/min in saline, Tocris Bioscience, 1659), a D1-like receptor agonist, was infused. Urine was collected every 40 min. Blood samples (300μl, replaced intravenously by equal amount of 5% albumin) were collected at the end of basal, fenoldopam, and recovery periods. Plasma was separated from blood particulate matter by centrifuging the blood samples at 2,500 g for 15 min at 4°C. Urine and plasma samples were stored at −80°C until use.
Blood and urine analysis
The urine sodium concentration was analyzed by a flame photometer 480 (Ciba Corning Diagnostics, Norwood, MA). Creatinine and urea nitrogen levels were measured with commercially available kits (Nanjing Jianchen Bioengineering Institute, Nanjing, China). Random blood glucose was measured with a glucose analyzer (Roche, Indianapolis, IN).
Biochemical markers of oxidative stress
To assess oxidative stress in the whole body, the lipid peroxidation product, malondialdehyde (MDA) in the urine was quantified using a commercially available kit (Nanjing Jianchen Bioengineering Institute, Nanjing, China). To asses antioxidants, plasma and renal samples from rats were used to measure superoxide dismutase activity using an SOD Assay Kit (Dojindo Laboratories, Kumamoto, Japan), and glutathione (GSH) levels, using a commercially available kit (Nanjing Jianchen Bioengineering Institute, Nanjing, China) following the manufacturer’s instructions.
Preparation of proximal tubular membranes
Proximal tubular membranes were prepared as described previously [28]. Briefly, proximal tubular suspensions were homogenized in buffer (10 mM Tris HCl, 250 mM sucrose, 2 mM PMSF, protease inhibitor cocktail; pH 7.4), and centrifuged at 24,000 g for 25 min at 4°C. The upper fluffy layer of the pellet was resuspended in the homogenization buffer and was considered as the membrane fraction; the supernatant was considered as the cytosolic fraction. Finally, the samples were quickly frozen and stored at −80°C until use.
Immunoblotting of D1R, GRK2, and GRK4
Equal amounts of proteins (60μg) were resolved in 10% SDS-PAGE and transferred onto nitrocellulose membranes. The membranes were blocked with 5% nonfat dry milk, in tris-buffered saline (TBS) with 0.05% tween-20, for 90 min at room temperature. Then the membranes were incubated with rabbit polyclonal D1R (1:500, Millipore, Billerica, MA, AB1765P), rabbit polyclonal GRK4 (1:400, Santa Cruz Biotechnology, CA, SC-13079), rabbit polyclonal GRK2 (1:400, Santa Cruz Biotechnology, SC-562), or rabbit polyclonal GAPDH (1:500, Santa Cruz Biotechnology, SC-25778) antibodies overnight at 4°C. The membrane-bound antibodies were visualized using horseradish peroxidase-conjugated secondary antibodies (1:12,000, Li-Cor Bioscience, 926-32213) and the Odyssey Infrared Imaging System (Li-Cor Bioscience, Bad Homburg, Germany). The densities of the bands were quantified by densitometry using Quantity-One software (Bio-Rad, Hercules, CA), and normalized with GAPDH.
Detection of serine-phosphorylated D1R
The proximal tubular membranes were incubated with D1R antibody (2μg, Millipore, Billerica, MA, AB1765P) for 2h followed by protein A/G agarose overnight with rocking at 4°C. The immunoprecipitates were pelleted and washed 3 times with phosphate-buffered saline. Then the pellets were suspended in 2× Laemmeli buffer at 95°C for 10 min, and subjected to immunoblotting with phosphoserine antibody (1:400, Immunechem, Burnaby, BC, Canada, ICP9806). Band density of the serine-phosphorylated D1R was normalized by band density of D1R. All the bands were quantified by densitometry.
Statistical Analysis
The data are expressed as mean ± SEM. Comparison within groups was made by repeated measures ANOVA (or paired t-test when only 2 groups were compared), and comparison among groups (or t-test when only 2 groups were compared) was made by factorial ANOVA with Holm-Sidak test. A value of P<0.05 was considered significant.
Results
1. Effects of prenatal LPS exposure on blood pressure and renal function in offspring
As shown in Table 1, there were no obvious differences among the four groups, regarding general characteristics, including heart rate, random plasma glucose level, and kidney/weight ratio. However, the offspring of LPS-treated dams were heavier than vehicle-treated (control) dams, consistent with previous reports [15]. Systolic blood pressure measured by tail-cuff method was higher in LPS-treated offspring than vehicle-treated control offspring starting at 5 week of age (Fig. 1A). The difference in systolic blood pressure between the offspring of control- and LPS-treated dams progressively increased with age (Fig. 1B).
Table 1.
General characteristics
| Control | Control+TEMPOL | LPS | LPS+TEMPOL | |
|---|---|---|---|---|
| Body weight(g) | 280±11 | 276±12 | 295±10* | 283±15 |
| Kidney/weight (g/kg wt) | 3.40±0.07 | 3.38±0.12 | 3.44±0.10 | 3.41±0.09 |
| Heart rate (beats/min) | 365±16 | 372±16 | 367±12 | 366±17 |
| Random plasma glucose (mmol/L) | 7.35±0.9 | 7.25±0.69 | 7.57±0.48 | 7.47±0.46 |
| Plasma creatinine (mmol/L) | 31.13±1.79 | 28.75±4.69 | 30.38±6.57 | 32.38±2.25 |
| Plasma urea nitrogen (mmol/L) | 6.10±1.56 | 6.21±1.25 | 6.03±0.69 | 6.20±2.03 |
| 24h Urine volume(ml/kg wt) | 38.8+2.69 | 41.58+3.18 | 25.58+2.09* | 55.42+4.08# |
| 24h Urine sodium (mmol/kg wt) | 0.85+0.05 | 1.06+0.10 | 0.52+0.01* | 1.25+0.07# |
These data were collected in 15 week-old offspring of vehicle (control)- and LPS-treated dams after treatment with vehicle or TEMPOL (1.0 mmol/L in drinking water) for 3 weeks. Results are mean±SE, n=6-8.
P<0.05 versus Control
P<0.05 versus LPS.
Fig. 1. Effect of prenatal LPS exposure on blood pressure in offspring.
Systolic blood pressure (A, SBP) and difference in SBP (B) between the offspring of LPS- and vehicle-treated (control) dams. SBP was measured by tail-cuff method in 5 week- and 3-, 6-, 9-, and 12-month old offspring of vehicle- (control) and LPS-treated dams. *P<0.05 versus offspring of vehicle-treated (control) dams, #P<0.05 versus 5 weeks (n=6-10).
There was no obvious difference in the gross structure of the kidneys from 12-month-old offspring of control and LPS-treated dams (Fig. 2). Moreover, the plasma urea nitrogen and creatinine concentrations were not different between the two groups (Table 1). However, 24h urine volume and sodium excretion were lower in 15-week old offspring of LPS-treated dams than the offspring of control dams (Fig. 3).
Fig. 2. Effect of prenatal LPS exposure on renal histopathology in offspring.
Renal histopathology was examined by H&E and Masson’s trichrome staining in 12-month-old offspring of vehicle (control)- and LPS-treated dams. Original magnification ×200.
Fig. 3. Effect of TEMPOL on 24h urine volume, 24h urine sodium excretion, and blood pressure in offspring of vehicle (control)- and LPS-treated dams.
24h urine volume (A), 24h urine sodium excretion (B), systolic blood pressure (SBP, C), and mean blood pressure (MBP, D) were measured in 15-week-old offspring of vehicle (control)- and LPS-treated dams after treatment with vehicle or TEMPOL (1.0 mmol/L in drinking water) for 3 weeks. *P<0.05 versus offspring of control dams, #P<0.05 versus offspring of LPS-treated dams (n=8).
2. Role of ROS in the fetal programming of hypertension
In vitro and in vivo studies have shown that LPS increases inflammation and ROS production [11, 30]. Therefore, we first measured ROS production in the offspring of LPS- and vehicle-treated (control) dams. We found that urine MDA levels were higher while renal and plasma SOD, and renal GSH levels were lower in the offspring of LPS- than vehicle-treated dams (Fig. 4). To determine directly the role of ROS in the fetal programming of hypertension, 12-week-old offspring of LPS-treated dams were treated with TEMPOL, a superoxide dismutase (SOD) mimetic, for 3 weeks. We found that the increase in sodium excretion and normalization of blood pressure by TEMPOL (Fig. 3) were accompanied by a decrease in urine MDA and an increase in plasma and renal SOD, and renal GSH levels (Fig. 4) in the offspring of LPS-treated dams. TEMPOL did not affect these variables in the offspring of vehicle-treated dams.
Fig. 4. Effect of prenatal LPS exposure on oxidative stress in offspring.
Urine MDA (A), plasma SOD (B), renal SOD (C), and GSH (D) were quantified in 15 week-old offspring of vehicle (control)- and LPS-treated dams after treatment with vehicle or TEMPOL (1.0 mmol/L in drinking water) for 3 weeks. *P<0.05 versus offspring of control dams, #P<0.01 versus offspring of LPS-treated dams (n =6).
3. Role of D1R in the fetal programming of hypertension
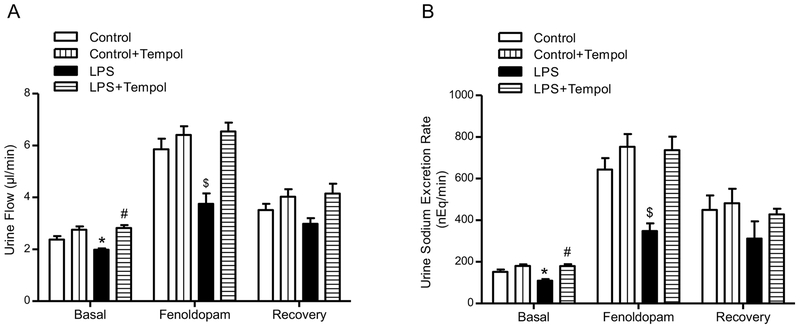
As shown in previous reports [31], D1-like receptor stimulation induced diuresis and natriuresis in Wistar-Kyoto and SD rats. To determine whether or not there is impaired D1-like receptor function in the offspring of LPS-treated dams, we studied the diuretic and natriuretic effects of fenoldopam, a D1-like receptor agonist, infused directly into the right renal artery, via the right suprarenal artery. Consistent with previous reports [20, 32], fenoldopam induced diuresis and natriuresis in the offspring of vehicle-treated dams, while the fenoldopam-mediated diuresis and natriuresis were markedly attenuated in the offspring of LPS-treated dams (Fig. 5).
Fig. 5. Effect of prenatal LPS exposure on renal D1R function in offspring.
Urine flow (A), and urine sodium excretion rates (B) were measured in 15 week-old offspring of vehicle (control)- and LPS-treated dams after treatment with vehicle or TEMPOL (1.0 mmol/L in drinking water) for 3 weeks. The D1-like receptor agonist, fenoldopam (1.0 μg/kg/min) was infused into the right renal artery via the right suprarenal artery of anesthetized rats. Basal: values before fenoldopam administration; Fenoldopam: values during fenoldopam administration; Recovery: values after fenoldopam infusion. *P<0.05 versus basal control-offspring of control dams, #P<0.05 versus basal-offspring of LPS-treated dams, $P<0.05 versus other groups in Fenoldopam period (n=8).
In the current study, we also found that renal D1R expression (Fig. 6) was lower and renal D1R serine-phosphorylation (Fig. 7) was greater in the offspring of LPS-treated dams than the offspring of vehicle-treated (control) dams. Moreover, renal GRK2 and GRK4 expressions were also greater in the offspring of LPS- than vehicle-treated dams (Fig. 6), implicating both GRK2 and GRK4 in the dysfunction of renal D1R in the fetal-programmed hypertension.
Fig. 6. Effect of prenatal LPS exposure on renal GRK2, GRK4, and D1R protein expressions in offspring.
Renal cortical membrane GRK2 (A), GRK4 (B), and D1R (C) expressions were quantified by immunoblotting in 15 week-old offspring of vehicle (control)- and LPS-treated dams after treatment with vehicle or TEMPOL (1.0 mmol/L in drinking water) for 3 weeks, *P<0.05 versus offspring of control dams, #P<0.05 versus offspring of LPS-treated dams (n =6).
Fig.7. Effect of prenatal LPS exposure on D1R serine-phosphorylation in renal cortical membranes in offspring.
Renal cortical membrane D1R serine-phosphorylation was quantified in 15 week-old offspring of vehicle (control)- and LPS-treated dams after treatment with vehicle or TEMPOL (1.0 mmol/L in drinking water) for 3 weeks. Immunoprecipitated D1R from renal cortical membranes were immunoblotted for serine-phosphorylated D1R (top band) and total D1R expressions (bottom band). The band densities of serine-phosphorylated D1R were normalized by band densities of total D1R. *P<0.05 versus others, #P<0.05 versus offspring of LPS-treated dams (n =6).
As shown in Figs. 3 and 4, TEMPOL treatment normalized the urine flow, urine sodium excretion, blood pressure, and redox status in the offspring of LPS-treated dams. To determine whether or not oxidative stress participated in the decreased renal D1R expression and increased renal D1R phosphorylation, and GRK2 and GRK4 expressions observed in the offspring of LPS-treated dams, we studied the effect of TEMPOL on these variables. After TEMPOL treatment for 3 weeks, the decreased renal D1R expression, and increased renal D1R phosphorylation and GRK2 and GRK4 expressions were reversed to normal levels in the offspring of LPS-treated dams (Figs. 6 and 7). The impaired diuretic and natriuretic effects of the D1-like receptor agonist fenoldopam in the offspring of LPS-treated dams were also ameliorated by TEMPOL (Fig 5). There were no effects of TEMPOL in the vehicle-treated (control) dams. These results could be taken to indicate that the high levels of ROS induced by LPS in the offspring of LPS-treated dams is the major factor that led to the increased renal GRK2 and GRK4 expression and impaired D1R function.
Discussion
Many epidemiological and experimental studies have shown that several adult diseases, including cardiovascular disease, have fetal origins, emphasizing the importance of fetal programming on adult diseases [4, 5, 33–35]. As summarized (Fig. 8), our current study showed that offspring of dams exposed to LPS, to model infection during pregnancy, develop hypertension, accompanied by abnormalities of the renal dopaminergic system.
Fig. 8. Schematic illustration of the effect of maternal infection in utero, mimicked by maternal LPS-injection, on renal D1R function and blood pressure in offspring.
Prenatal LPS exposure, as a model maternal infection, increases renal GRK2 and GRK4 expressions caused by oxidative stress that lead to renal hyperphosphorylation of D1R, diminished D1R-mediated diuresis and natriuresis, and ultimately hypertension.
Hypertension can be programmed by adverse environment in utero [4, 5, 33–35]. LPS, an endotoxin from gram-negative bacteria, acts as a potent trigger of oxidative stress and also a disruptor of antioxidant defenses [36]. During infection, people are constantly exposed to low levels of LPS [37]. The placenta is known to generate cytokines in response to infection; human amniochorionic membranes produce IL-1β, IL-6, and TNF-α in response to LPS in vitro [38], and these cytokines generated by the placenta are also likely to gain entry into the fetal circulation [13]. Maternal exposure to the bacterial endotoxin LPS has been used to study the effects of systemic inflammation during pregnancy [37, 39]. We chose to inject LPS in dams on gestational days 8, 10, and 12 because these periods correspond to the first-to-second trimesters of human pregnancy, one of the main periods of vulnerability of the immune system to environmental insults [40]. The dose of LPS (0.79mg/kg) used in the current study has been reported to induce systemic inflammation with low percentage of fetal anomalies and no or low rate of abortions [41] . Our present study also showed prenatal LPS exposure causes a low percentage of abortion about 10% at this dosage.
A previous study showed that exposure of dams to LPS increased blood pressure that was accompanied by decreased glomerular number and creatinine clearance and increased urinary protein in offspring [14]. However, in our current study, we did not find a significant increase in plasma creatinine in 15-week old offspring of LPS-treated dams. The reason(s) for the differences in renal function between our study and the studies of Hao et al is not clear. Age could be a factor, because creatinine clearance was decreased in 6-month-old offspring of diabetic dams [33]. In our study, we did not evaluate the renal function of the offspring at 6 months of age or beyond. The creatinine clearances were also not consistently decreased in the offspring of LPS-treated dams reported by Hao et al. In one report [14], they showed that maternal exposure to LPS caused a decrease in creatinine clearance rate and glomerular number in the offspring while in another study, the offspring of LPS-treated dams had normal plasma urea and creatinine concentrations [42].
Hypertension is characterized by impaired sodium handling and sodium retention by kidney [43], which appears to be susceptible to the effects of programming in utero [44]. The kidney and its regulation of sodium balance are of primary importance in the regulation of blood pressure, regardless of the initiating factor [45] . Our current study showed that the hypertension and decreased sodium excretion of offspring of LPS-treated dams were accompanied by abnormalities of the renal dopaminergic system. Renal dopamine, independent of renal nerves, plays an important role in maintaining sodium homeostasis and blood pressure regulation, especially during moderately increased sodium intake [16, 19, 46]. Under conditions of moderate sodium excess, the renal dopaminergic system, mainly the D1R, is responsible for more than 50% of renal sodium excretion [16]. Abnormalities in renal dopamine and D1R response to an increased sodium load have been implicated in the diminished natriuretic response and increase in blood pressure in hypertensive patients and rodents[16, 18, 19, 24, 29, 31, 46] . Our current study found that the renal D1R expression is decreased and D1R phosphorylation is increased, conditions that should lead to impaired D1R-mediated natriuresis and diuresis in the offspring of LPS-treated dams. We have reported that in spontaneously hypertensive rats, the renal D1R is hyper-phosphorylated [47] . Studies have shown that increased GRK expression and GRK4 gene variants are involved in the hyperphosphorylation of D1R, resulting in its desensitization and uncoupling from G protein partners; both GRK2 and GRK4 activities and expression are increased in some hypertensive humans and animal models of hypertension [16, 18, 19, 22–27, 31, 46–54]. Obese Zucker rats are hypertensive and also have decreased renal D1R expression, increased renal D1R phosphorylation, defective D1R and G protein subunit coupling, impaired dopamine inhibition of sodium transport and natriuretic response to D1-like receptor stimulation and increased expression of renal proximal tubule membrane GRK2 and GRK4 [52]. Consistent with these reports, we also found that GRK2 and GRK4 expressions are increased in renal cortical membranes while renal cortical membrane D1R expression and D1-like receptor function are decreased in offspring of LPS-treated dams. Thus, the current studies support the notion that both GRK2 and GRK4 are involved in the impaired function of renal D1R in hypertension.
Studies indicate that both hypertensive patients and animals have decreased antioxidant capacity and produce excessive amounts of ROS [24–27, 54–57]. Antioxidant treatment could mitigate the production of ROS, and further increase antioxidant capacity, such as SOD and GSH in plasma and tissue. LPS is known to increase maternal serum TNF-α levels [58] and stimulate macrophages to generate ROS [11, 59, 60], and enhance oxidative stress in the placenta and fetus [59]. Therefore, we hypothesized that there may be increased ROS and decreased antioxidant capacity in offspring of LPS-treated dams. Large amount of studies have shown that the impaired intrarenal D1R signaling in hypertension can cause or be caused by oxidative stress [24–28, 54, 61–64] . Treatment with the superoxide dismutase mimetic TEMPOL has been shown to reduce GRK2 and GRK4 levels and restore D1R expression and D1R coupling and function and normalize blood pressure by decreasing oxidative stress in old and obese rats [23, 27, 28, 64]. The long-term (3 weeks) intravenous infusion of TEMPOL also decreases arterial pressure of Dahl salt-sensitive rats on a high sodium intake, reducing their salt-sensitivity, renal production of O2.− and renal damage [56]. Our current study showed high ROS levels and decreased antioxidant capacity (SOD and GSH) in the offspring of LPS-treated dams. The administration of TEMPOL restored ROS to normal state, increased D1R-mediated natriuresis and diuresis, and lowered the blood pressure of the offspring of LPS-treated dams; TEMPOL also normalized the renal GRK2, GRK4, and D1R expressions and D1R phosphorylation.
In summary, our study shows direct evidence supporting the hypothesis of fetal-programmed hypertension, and, for first time, links the dysfunction of renal D1R to the fetal-programmed hypertension secondary to maternal LPS exposure. Increased oxidative stress, associated with increased GRK2 and GRK4 levels, impairs renal D1R function, and leads to hypertension. Restoration of D1R function by antioxidants may be a therapeutic target for fetal-programmed hypertension.
Prenatal LPS exposure leads to increased blood pressure and ROS in offspring.
The diuretic and natriuretic effects induced by renal D1R is decreased in LPS rats.
The level of renal GRK2 and GRK4 and serine-phosphorylation of D1R are increased.
Antioxidant TEMPOL could reverse the function of renal D1R and GRK4 level.
Dysfunction of renal D1R could be fetal origins.
Acknowledgments
Sources of Funding
These studies were supported, in part, by grants from National Natural Science Foundation of China (31130029, 81070559), Program for Changjiang Scholars and Innovative Research Team in University of Ministry of Education of China (IRT1216), and National Institutes of Health, USA (R37HL023081, and P01HL074940).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure
There are no conflicts of interest, financial or otherwise.
References
- [1].McMillen IC; Robinson JS Developmental origins of the metabolic syndrome: prediction, plasticity, and programming. Physiol Rev 85:571–633; 2005. [DOI] [PubMed] [Google Scholar]
- [2].Ritz E; Amann K; Koleganova N; Benz K Prenatal programming-effects on blood pressure and renal function. Nat Rev Nephrol 7:137–144; 2011. [DOI] [PubMed] [Google Scholar]
- [3].Zandi-Nejad K; Luyckx VA; Brenner BM Adult hypertension and kidney disease: the role of fetal programming. Hypertension 47:502–508; 2006. [DOI] [PubMed] [Google Scholar]
- [4].Barker DJ The fetal and infant origins of adult disease. BMJ 301:1111; 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Barker DJ The intrauterine environment and adult cardiovascular disease. Ciba Foundation symposium 156:3–10; discussion 10–16; 1991. [DOI] [PubMed] [Google Scholar]
- [6].Gibbs RS The relationship between infections and adverse pregnancy outcomes: an overview. Ann Periodontol 6:153–163; 2001. [DOI] [PubMed] [Google Scholar]
- [7].Patterson PH Maternal infection and immune involvement in autism. Trends Mol Med 17:389–394; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Khandaker GM; Zimbron J; Lewis G; Jones PB Prenatal maternal infection, neurodevelopment and adult schizophrenia: a systematic review of population-based studies. Psychol Med 43:239–257; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Brown AS; Derkits EJ Prenatal infection and schizophrenia: a review of epidemiologic and translational studies. Am J Psychiatry 167:261–280; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Huleihel M; Golan H; Hallak M Intrauterine infection/inflammation during pregnancy and offspring brain damages: possible mechanisms involved. Reprod Biol Endocrinol 2:17; 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Xu DX; Chen YH; Wang H; Zhao L; Wang JP; Wei W Effect of N-acetylcysteine on lipopolysaccharide-induced intra-uterine fetal death and intra-uterine growth retardation in mice. Toxicol Sci 88:525–533; 2005. [DOI] [PubMed] [Google Scholar]
- [12].Quan N; Stern EL; Whiteside MB; Herkenham M Induction of pro-inflammatory cytokine mRNAs in the brain after peripheral injection of subseptic doses of lipopolysaccharide in the rat. J Neuroimmunol 93:72–80; 1999. [DOI] [PubMed] [Google Scholar]
- [13].Urakubo A; Jarskog LF; Lieberman JA; Gilmore JH Prenatal exposure to maternal infection alters cytokine expression in the placenta, amniotic fluid, and fetal brain. Schizophr Res 47:27–36; 2001. [DOI] [PubMed] [Google Scholar]
- [14].Hao XQ; Zhang HG; Yuan ZB; Yang DL; Hao LY; Li XH Prenatal exposure to lipopolysaccharide alters the intrarenal renin-angiotensin system and renal damage in offspring rats. Hypertens Res 33:76–82; 2010. [DOI] [PubMed] [Google Scholar]
- [15].Wei YL; Li XH; Zhou JZ Prenatal exposure to lipopolysaccharide results in increases in blood pressure and body weight in rats. Acta Pharmacol Sin 28:651–656; 2007. [DOI] [PubMed] [Google Scholar]
- [16].Zeng C; Jose PA Dopamine receptors: important antihypertensive counterbalance against hypertensive factors. Hypertension 57:11–17; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Pinto V; Pinho MJ; Soares-da-Silva P Renal amino acid transport systems and essential hypertension. FASEB J 27:2927–2938; 2013. [DOI] [PubMed] [Google Scholar]
- [18].Carey RM Theodore Cooper Lecture: Renal dopamine system: paracrine regulator of sodium homeostasis and blood pressure. Hypertension 38:297–302; 2001. [DOI] [PubMed] [Google Scholar]
- [19].Harris RC; Zhang MZ Dopamine, the kidney, and hypertension. Curr Hypertens Rep 14:138–143; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Salomone LJ; Howell NL; McGrath HE; Kemp BA; Keller SR; Gildea JJ; Felder RA; Carey RM Intrarenal dopamine D1-like receptor stimulation induces natriuresis via an angiotensin type-2 receptor mechanism. Hypertension 49:155–161; 2007. [DOI] [PubMed] [Google Scholar]
- [21].Nishi A; Eklof AC; Bertorello AM; Aperia A Dopamine regulation of renal Na+,K(+)-ATPase activity is lacking in Dahl salt-sensitive rats. Hypertension 21:767–771; 1993. [DOI] [PubMed] [Google Scholar]
- [22].Watanabe H; Xu J; Bengra C; Jose PA; Felder RA Desensitization of human renal D1 dopamine receptors by G protein-coupled receptor kinase 4. Kidney Int 62:790–798; 2002. [DOI] [PubMed] [Google Scholar]
- [23].Chugh G; Lokhandwala MF; Asghar M Altered functioning of both renal dopamine D1 and angiotensin II type 1 receptors causes hypertension in old rats. Hypertension 59:1029–1036; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Asghar M; Banday AA; Fardoun RZ; Lokhandwala MF Hydrogen peroxide causes uncoupling of dopamine D1-like receptors from G proteins via a mechanism involving protein kinase C and G-protein-coupled receptor kinase 2. Free radical biology & medicine 40:13–20; 2006. [DOI] [PubMed] [Google Scholar]
- [25].Fardoun RZ; Asghar M; Lokhandwala M Role of nuclear factor kappa B (NF-kappaB) in oxidative stress-induced defective dopamine D1 receptor signaling in the renal proximal tubules of Sprague-Dawley rats. Free radical biology & medicine 42:756–764; 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Fardoun RZ; Asghar M; Lokhandwala M Role of oxidative stress in defective renal dopamine D1 receptor-G protein coupling and function in old Fischer 344 rats. Am J Physiol Renal Physiol 291:F945–951; 2006. [DOI] [PubMed] [Google Scholar]
- [27].Banday AA; Marwaha A; Tallam LS; Lokhandwala MF Tempol reduces oxidative stress, improves insulin sensitivity, decreases renal dopamine D1 receptor hyperphosphorylation, and restores D1 receptor-G-protein coupling and function in obese Zucker rats. Diabetes 54:2219–2226; 2005. [DOI] [PubMed] [Google Scholar]
- [28].Marwaha A; Lokhandwala MF Tempol reduces oxidative stress and restores renal dopamine D1-like receptor- G protein coupling and function in hyperglycemic rats. Am J Physiol Renal Physiol 291:F58–66; 2006. [DOI] [PubMed] [Google Scholar]
- [29].Ladines CA; Zeng C; Asico LD; Sun X; Pocchiari F; Semeraro C; Pisegna J; Wank S; Yamaguchi I; Eisner GM; Jose PA Impaired renal D(1)-like and D(2)-like dopamine receptor interaction in the spontaneously hypertensive rat. Am J Physiol. Regul, Integr Comp Physiol 281:R1071–1078; 2001. [DOI] [PubMed] [Google Scholar]
- [30].Yamada H; Arai T; Endo N; Yamashita K; Fukuda K; Sasada M; Uchiyama T LPS-induced ROS generation and changes in glutathione level and their relation to the maturation of human monocyte-derived dendritic cells. Life Sci 78:926–933; 2006. [DOI] [PubMed] [Google Scholar]
- [31].Felder RA; Seikaly MG; Cody P; Eisner GM; Jose PA Attenuated renal response to dopaminergic drugs in spontaneously hypertensive rats. Hypertension 15:560–569; 1990. [DOI] [PubMed] [Google Scholar]
- [32].Padia SH; Kemp BA; Howell NL; Keller SR; Gildea JJ; Carey RM Mechanisms of dopamine D(1) and angiotensin type 2 receptor interaction in natriuresis. Hypertension 59:437–445; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Nehiri T; Duong VHJ; Viltard M; Fassot C; Heudes D; Freund N; Deschenes G; Houillier P; Bruneval P; Lelievre-Pegorier M Exposure to maternal diabetes induces salt-sensitive hypertension and impairs renal function in adult rat offspring. Diabetes 57:2167–2175; 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Vehaskari VM; Aviles DH; Manning J Prenatal programming of adult hypertension in the rat. Kidney Int 59:238–245; 2001. [DOI] [PubMed] [Google Scholar]
- [35].Vehaskari VM; Woods LL Prenatal programming of hypertension: lessons from experimental models. J Am Soc Nephrol 16:2545–2556; 2005. [DOI] [PubMed] [Google Scholar]
- [36].Xu M; Sulkowski ZL; Parekh P; Khan A; Chen T; Midha S; Iwasaki T; Shimokawa N; Koibuchi N; Zavacki AM; Sajdel-Sulkowska EM Effects of perinatal lipopolysaccharide (LPS) exposure on the developing rat brain; modeling the effect of maternal infection on the developing human CNS. Cerebellum 12:572–586; 2013. [DOI] [PubMed] [Google Scholar]
- [37].Fukui H; Brauner B; Bode JC; Bode C Plasma endotoxin concentrations in patients with alcoholic and non-alcoholic liver disease: reevaluation with an improved chromogenic assay. J Hepatol 12:162–169; 1991. [DOI] [PubMed] [Google Scholar]
- [38].Fortunato SJ; Menon RP; Swan KF; Menon R Inflammatory cytokine (interleukins 1, 6 and 8 and tumor necrosis factor-alpha) release from cultured human fetal membranes in response to endotoxic lipopolysaccharide mirrors amniotic fluid concentrations. Am J Obstet Gynecol 174:1855–1861; discussion 1861–1852; 1996. [DOI] [PubMed] [Google Scholar]
- [39].Baharnoori M; Bhardwaj SK; Srivastava LK Effect of maternal lipopolysaccharide administration on the development of dopaminergic receptors and transporter in the rat offspring. PLoS One 8:e54439; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Kaufman MH The Atlas of Mouse Development. UK: Academic Press, Edinburgh University; 1992:6–26. [Google Scholar]
- [41].Ornoy A; Altshuler G Maternal endotoxemia, fetal anomalies, and central nervous system damage: a rat model of a human problem. Am J Obstet Gynecol 124:196–204; 1976. [DOI] [PubMed] [Google Scholar]
- [42].Hao XQ; Du JX; Li Y; Li M; Zhang SY Prenatal exposure to lipopolysaccharide combined with pre- and postnatal high-fat diet result in lowered blood pressure and insulin resistance in offspring rats. PLoS One 9:e88127; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Blaustein MP; Hamlyn JM Signaling mechanisms that link salt retention to hypertension: endogenous ouabain, the Na(+) pump, the Na(+)/Ca(2+) exchanger and TRPC proteins. Biochim Biophys Acta 1802:1219–1229; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Alwasel SH; Kaleem I; Sahajpal V; Ashton N Maternal protein restriction reduces angiotensin II AT(1) and AT(2) receptor expression in the fetal rat kidney. Kidney Blood Press Res 33:251–259; 2010. [DOI] [PubMed] [Google Scholar]
- [45].Hall JE; Granger JP; do Carmo JM; da Silva AA; Dubinion J; George E; Hamza S; Speed J; Hall ME Hypertension: physiology and pathophysiology. Comprehensive Physiology 2:2393–2442; 2012. [DOI] [PubMed] [Google Scholar]
- [46].Banday AA; Lokhandwala MF Dopamine receptors and hypertension. Curr Hypertens Rep 10:268–275; 2008. [DOI] [PubMed] [Google Scholar]
- [47].Sanada H; Yatabe J; Midorikawa S; Katoh T; Hashimoto S; Watanabe T; Xu J; Luo Y; Wang X; Zeng C; Armando I; Felder RA; Jose PA Amelioration of genetic hypertension by suppression of renal G protein-coupled receptor kinase type 4 expression. Hypertension 47:1131–1139; 2006. [DOI] [PubMed] [Google Scholar]
- [48].Gros R; Benovic JL; Tan CM; Feldman RD G-protein-coupled receptor kinase activity is increased in hypertension. J Clin Invest 99:2087–2093; 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Felder RA; Sanada H; Xu J; Yu PY; Wang Z; Watanabe H; Asico LD; Wang W; Zheng S; Yamaguchi I; Williams SM; Gainer J; Brown NJ; Hazen-Martin D; Wong LJ; Robillard JE; Carey RM; Eisner GM; Jose PA G protein-coupled receptor kinase 4 gene variants in human essential hypertension. Proc Natl Acad Sci U S A 99:3872–3877; 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Zeng C; Villar VA; Eisner GM; Williams SM; Felder RA; Jose PA G protein-coupled receptor kinase 4: role in blood pressure regulation. Hypertension 51:1449–1455; 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Felder RA; Jose PA Mechanisms of disease: the role of GRK4 in the etiology of essential hypertension and salt sensitivity. Nat Clin Pract Nephrol 2:637–650; 2006. [DOI] [PubMed] [Google Scholar]
- [52].Trivedi M; Lokhandwala MF Rosiglitazone restores renal D1A receptor-Gs protein coupling by reducing receptor hyperphosphorylation in obese rats. Am J Physiol Renal Physiol 289:F298–304; 2005. [DOI] [PubMed] [Google Scholar]
- [53].Rankin ML; Marinec PS; Cabrera DM; Wang Z; Jose PA; Sibley DR The D1 dopamine receptor is constitutively phosphorylated by G protein-coupled receptor kinase 4. Mol Pharmacol 69:759–769; 2006. [DOI] [PubMed] [Google Scholar]
- [54].Jose PA; Soares-da-Silva P; Eisner GM; Felder RA Dopamine and G protein-coupled receptor kinase 4 in the kidney: role in blood pressure regulation. Biochim Biophys Acta 1802:1259–1267; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Taylor NE; Glocka P; Liang M; Cowley AW Jr. NADPH oxidase in the renal medulla causes oxidative stress and contributes to salt-sensitive hypertension in Dahl S rats. Hypertension 47:692–698; 2006. [DOI] [PubMed] [Google Scholar]
- [56].Meng S; Cason GW; Gannon AW; Racusen LC; Manning RD Jr. Oxidative stress in Dahl salt-sensitive hypertension. Hypertension 41:1346–1352; 2003. [DOI] [PubMed] [Google Scholar]
- [57].Montezano AC; Touyz RM Oxidative stress, Noxs, and hypertension: experimental evidence and clinical controversies. Annals of medicine 44 Suppl 1:S2–16; 2012. [DOI] [PubMed] [Google Scholar]
- [58].Bell MJ; Hallenbeck JM; Gallo V Determining the fetal inflammatory response in an experimental model of intrauterine inflammation in rats. Pediatr Res 56:541–546; 2004. [DOI] [PubMed] [Google Scholar]
- [59].Ejima K; Koji T; Nanri H; Kashimura M; Ikeda M Expression of thioredoxin and thioredoxin reductase in placentae of pregnant mice exposed to lipopolysaccharide. Placenta 20:561–566; 1999. [DOI] [PubMed] [Google Scholar]
- [60].Bautista AP; Meszaros K; Bojta J; Spitzer JJ Superoxide anion generation in the liver during the early stage of endotoxemia in rats. J Leukoc Biol 48:123–128; 1990. [DOI] [PubMed] [Google Scholar]
- [61].Yu P; Han W; Villar VA; Yang Y; Lu Q; Lee H; Li F; Quinn MT; Gildea JJ; Felder RA; Jose PA Unique role of NADPH oxidase 5 in oxidative stress in human renal proximal tubule cells. Redox biology 2:570–579; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Cuevas S; Villar VA; Jose PA; Armando I Renal dopamine receptors, oxidative stress, and hypertension. Int J Mol Sci. 2013;14(9):17553–17572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Chugh G; Lokhandwala MF; Asghar M Oxidative stress alters renal D1 and AT1 receptor functions and increases blood pressure in old rats. Am J Physiol Renal Physiol 300:F133–138; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Banday AA; Fazili FR; Lokhandwala MF Oxidative stress causes renal dopamine D1 receptor dysfunction and hypertension via mechanisms that involve nuclear factor-kappaB and protein kinase C. J Am Soc Nephrol 18:1446–1457; 2007. [DOI] [PubMed] [Google Scholar]