Abstract
Telomeres are essential for chromosomal integrity. Telomere shortening during cell division restricts cellular proliferative capacity and leads to cellular senescence when critically shortened telomere lengths are reached. Similar to hematopoietic stem cells, T cells can upregulate telomerase activity to compensate for telomere loss incurred during activation-induced proliferation that occurs following engagement of the T cell antigen receptor (TCR) or exposure to homeostatic cytokines. However, this compensation for telomere loss by telomerase in T cells is imperfect or limited, as shortening of T cell telomeres is observed in human aging. In this review, we summarize the current state of knowledge regarding the expression and regulation of telomerase in human T cells and changes of telomerase expression during development, activation, differentiation, aging and disease conditions. In conclusion, we discuss how controlled enhancement of telomerase activity could be a potential strategy to improve T cell function in the elderly and in immunotherapy.
I. Introduction
Telomerase is a ribonucleoprotein enzyme that synthesizes the 3’ ends of telomeres, which in turn compensates for telomere loss during cell division [1]. Human telomerase is composed of a catalytic subunit encoded by telomerase reverse transcriptase (hTERT) and an RNA component (hTERC) that serves as a template for the synthesis of telomeric DNA. While hTERC is present in all cells and tissues [2], hTERT is expressed during fetal tissue development and in germline cells but not in most somatic cells [3]. Regulation of hTERT expression is complex involving multiple levels such as epigenetic, transcriptional, alternative splicing, and post-translational mechanisms [4–6]. This complex regulation ensures a tightly controlled telomerase activity at the right time, under the right conditions, and in a specific cell type.
T cells are key players of the adaptive immune response against both exogenous pathogens including bacteria, viruses, fungi, and parasites and internal insults such as cancer cells. During an immune response, extensive cell divisions are essential to generate large numbers of effector cells for containing and eliminating the infected or cancerous cells. This extensive cell division occurs not only during the primary (naïve cells) immune response but also during subsequent (memory cells) immune responses throughout the lifespan of the host. Although it is currently unknown the precise number of cell divisions that an individual T cell undergoes in a lifetime, the estimated average number of T cell divisions during one immune response in mouse is 6-7 divisions [7]. How T cells handle telomere loss with this magnitude of cell division is a topic of intense interest. It has long been known that human T and B cells are capable of expressing telomerase in a regulated manner during development and activation, and also that telomere attrition is observed with aging [8–10]. Although the precise dynamic relationship between telomerase expression and telomere attrition in human T cells in vivo is not fully understood, the impact of T cell differentiation and aging on telomerase expression and activity was recently examined. In this review, we will summarize what is known about the regulation of telomerase activity in T cells over the trajectory of their maturation from thymus to periphery and take into account the roles of differentiation, activation, aging, and disease.
II. Telomerase activity and hTERT mRNA expression during T cell development
a. Regulation of telomerase activity in T cell development
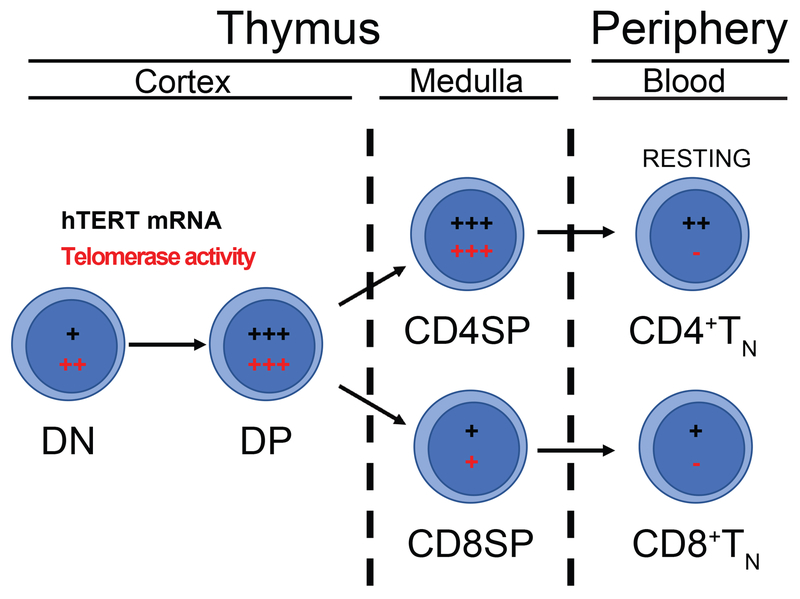
In the thymus, T cell precursors undergo stepwise development before emigration to the blood as naïve T cells. Defined by cell surface expression of CD4 and CD8 coreceptor molecules, the least mature CD4−CD8− double negative (DN) thymocytes progress to CD4+CD8+ double positive (DP) cells that undergo selection on thymic epithelial cells presenting self-peptides via MHCII or MHCI to become CD4+CD8− or CD4−CD8+ single positive (SP) thymocytes (Figure 1). In unseparated primary human thymocytes, telomerase activity is detected at high levels comparable to tumor cells. Analysis of sorted thymocyte subsets showed that expression was similar in the DN, DP, and CD4SP populations and lower in CD8SP [11–13]. The telomerase activity levels in thymocytes are nearly 30 times greater than those in resting peripheral blood T cells suggesting that maturation of T lineage cells is associated with decreased telomerase activity, similar to other somatic cells.
Figure 1. hTERT/Telomerase expression during T cell development.
T cell precursors develop in the thymus through a stepwise process. CD4−CD8− double negative (DN) thymocytes become CD4+CD8+ double positive (DP) cells that are selected on thymic epithelial cells to generate lineage-committed CD4+ or CD8+ (SP) T cells. These cells exit the thymus and enter the blood as TN cells. There is high expression of hTERT mRNA (depicted in black) and telomerase activity (depicted in red) in unsorted thymocytes, while there are slight variations in expression in individually sorted subsets. Resting peripheral CD4+ and CD8+ T cells lack telomerase activity but express hTERT mRNA.
b. Regulation of hTERT expression in T cell development
Telomerase activity is partially regulated by its two core components: telomerase RNA template (hTERC) and telomerase reverse transcriptase (hTERT) [11, 13]. hTERC is present in all subsets of thymocytes examined and fluctuates similarly to telomerase activity during T cell maturation. Furthermore, hTERT mRNA is also present in these thymocyte subsets and the levels of hTERT mRNA are correlated with levels of telomerase activity [13]. Although post-transcriptional and post-translational modification of hTERT have been reported, no data are available for human thymocytes.
III. Regulation of hTERT/telomerase expression during T cell activation and differentiation
a. Multi-level regulation of hTERT/telomerase expression during T cell activation
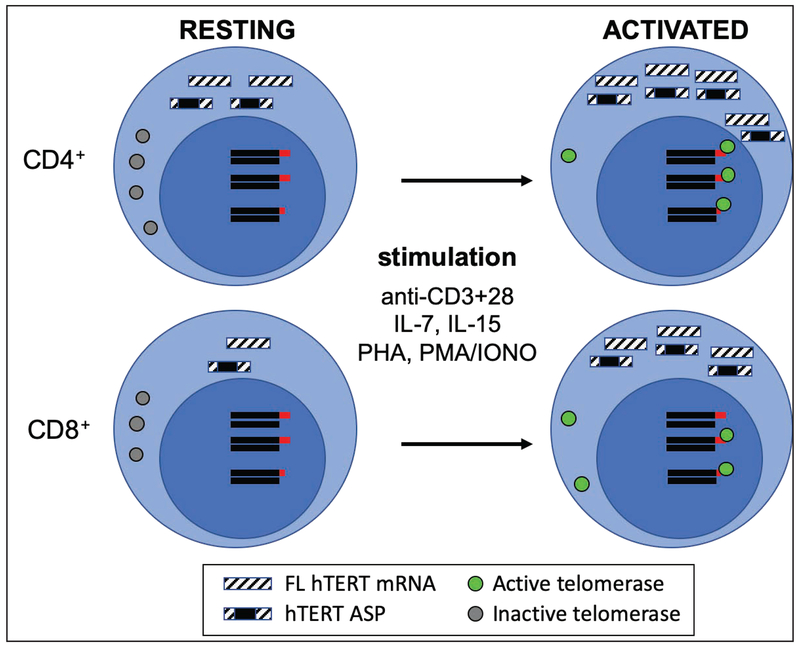
In the blood, resting T cells have detectable levels of hTERT mRNA but do not have detectable telomerase activity [13–15]. Following antigenic engagement of TCR and co-stimulatory receptors [12, 13] or exposure to homeostatic cytokines such as IL-7 and IL-15 [16–18] (Figure 2), T cells divide and are capable of upregulating hTERT mRNA expression and telomerase activity. When T cells are stimulated by anti-CD3 and anti-CD28 antibody in vitro, hTERT is upregulated within 24 hours, along with detectable telomerase activity, and the peak level of hTERT mRNA and telomerase activity arrive around 3 days post stimulation, after which both gradually decline [13, 14]. In contrast, exposure to IL-7 or IL-15 leads to a slow upregulation of telomerase activity in T cells with long-lasting low level upregulation of both telomerase and hTERT mRNA [16–18]. Such differences in the degree of upregulation of hTERT mRNA and telomerase activity may reflect the different proportions of active cycling T cells in the cell population or the different signal strengths of these two different pathways. Telomerase expression during T cell activation in vivo has been examined. CD4+ and CD8+ T cells isolated from tonsils of patients with chronic infections show an elevated level of telomerase activity which is higher than resting T cells of peripheral blood but lower than that of thymocytes [13, 19].
Figure 2. hTERT/Telomerase expression during T cell activation.
T cell activation through the engagement of T cell receptor and co-stimulatory receptor or exposure to homeostatic cytokines (IL-7 or IL-15) or other mitogens induces upregulation of hTERT mRNA and telomerase activity. hTERT mRNA, both full-length (FL) and alternatively spliced products (ASPs), are more abundant in resting CD4+ compared to resting CD8+ T cells. In the resting state, T cells have detectable hTERT mRNA and protein (in the cytosol) without detectable telomerase activity. After activation, T cells increase expression of hTERT mRNA and protein which is translocated into nucleus and have detectable telomerase activity.
Regulation of telomerase activity presents an example of a complex control mechanism as it is regulated at multiple levels from transcription to RNA splicing to posttranslational modification and translocation from cytosol to nucleus. Transcription factor regulation of hTERT expression in human cells has been reviewed extensively [4, 20, 21]. Although most of the studies are conducted in non-lymphoid cell lines, several transcription factors known to regulate hTERT expression have also been reported to bind to the promoter of hTERT and regulate its transcription in human T cells and T cell lines.
Myc binds to the hTERT promoter in proliferating cells when telomerase is active but is replaced by Mad1/Max in differentiated cells that have lost hTERT expression. Myc is known to recruit histone acetyltransferases as the transcriptional activation of hTERT by Myc is inhibited by the HDAC inhibitor trichostatin A [22]. In line with these findings, HDAC inhibitor treatment of normal resting human T cells, which have very low levels of hTERT expression, led to increased hTERT expression levels comparable to anti-CD3/CD28 stimulated cells [22].
Nuclear factor of activated T cells (NFAT) has been implicated in the regulation of hTERT expression, as treatment with the calcineurin inhibitor FK506 inhibits hTERT upregulation in activated peripheral blood lymphocytes [23]. Among five identified NFAT binding sites, a region −40 bp from the transcriptional start site is shown to be the most critical for hTERT transactivation by NFAT and acts in synergy with SP1. In the Jurkat T cell line, hTERT expression is also responsive to NFAT levels and correlated with NFAT levels in either NFAT knocked down or overexpressed conditions [23].
Kruppel-like factor 2 (KLF2) regulates T cell quiescence and trafficking to lymphoid organs [24], and is also responsible for the active repression of hTERT expression in resting human T cells. KLF2 is shown to bind to a region just downstream of the hTERT transcriptional start site and repress its expression whereas this binding is lost after T cell activation [25]. In addition, the expression of KLF2 itself is shown to be inversely correlated with hTERT expression as ectopic expression of KLF2 in activated T cells reduced hTERT expression and knockdown of KLF2 in resting cells increased hTERT mRNA [25].
Post-transcriptional regulation by alternative splicing produces several forms of hTERT alternatively spliced products (ASPs) during development [26] and activation [15]. Among them, α-, β-, and α+β-deletion have been observed in human T cells [15, 23, 27–31]. Briefly, α-deletion loses 36 bp within exon 6 resulting in deletion of part of the hTERT reverse transcriptase domain; β-deletion loses 182 bp resulting in a frame shift mutation; and the α+β-deletion loses 218 bp resulting in a frame shift mutation. Only the full-length mRNA can encode an hTERT subunit with telomerase activity [32, 33]. Although the precise functions of these three hTERT ASPs in T cells have not been determined, the α-deletion of hTERT functions as a dominant negative regulator that results in shortened lifespan of telomerase positive tumor cells [33] and overexpression of the β-deletion confers resistance to cisplatin-induced apoptosis in breast cancer cells [34]. Despite the presence of a premature termination codon in the β-deletion containing ASPs, they have been shown to be associated with polyribosomes [34, 35] indicating their potential for translation to proteins. Jalink et al., reported that the β-deletion ASP, but not the full-length, is present in resting T cells from analysis of a limited number of human subjects and concluded that hTERT ASPs regulate telomerase activity in resting T cells [27]. However, a recent study of 98 human subjects showed that both full-length hTERT and the β-deletion are present in resting CD4+ and CD8+ T cells [15]. This suggests that the lack of telomerase activity in resting T cells is not due to alternative splicing of hTERT mRNA, but rather other mechanisms.
Posttranslational phosphorylation of hTERT protein is detected in both resting and activated T cells by western blot and the amount of hTERT protein does not correlate well with telomerase activity in these cells, particularly because resting cells lack telomerase activity [36]. Early studies in cancer cell lines showed phosphorylation of hTERT protein [37, 38] via protein kinase C and Akt, respectively, is required for telomerase activity. In human T cells, phosphorylation of hTERT occurs after activation, i.e., there is no detectable phosphorylation of hTERT in resting cells but phosphorylation is detected in activated T cells [36], and correlates with telomerase activity. The importance of phosphorylation of hTERT by the kinase Akt is further demonstrated by the observation that terminally differentiated (CD27−CD28−) CD8+ T cells lose the ability to upregulate telomerase activity as a result of defective Akt phosphorylation [39]. Thus, post-translational phosphorylation of hTERT protein is another key regulatory step that controls telomerase activity in resting and activated T cells. Lastly, the phosphorylation of hTERT in T cells is associated with the translocation of cytoplasmic hTERT protein into the nucleus [36]. Non-phosphorylated hTERT protein locates to the cytosol in resting T cells, but, following activation, phosphorylated hTERT protein is located inside the nucleus.
b. Regulation of hTERT/telomerase expression during T cell differentiation
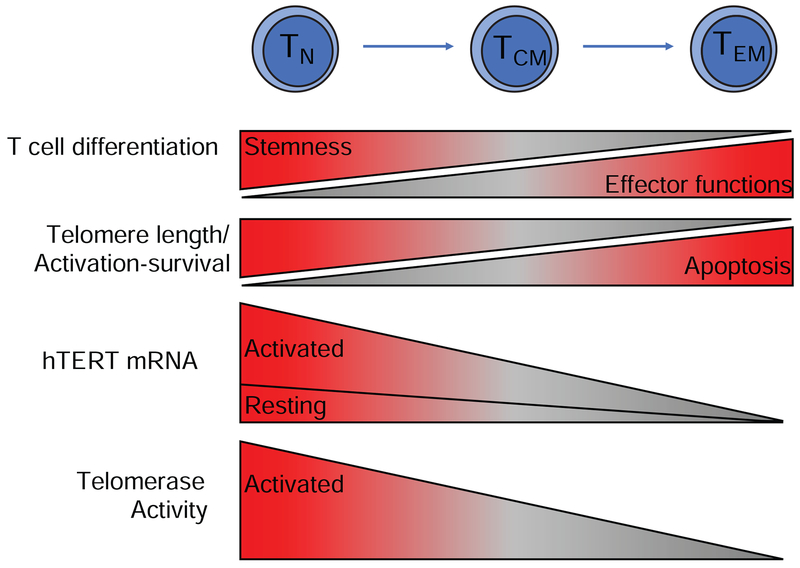
Although it has been known for decades that telomerase expression is tightly regulated in T cell development and activation, its role in T cell differentiation has been discovered recently [15]. We observe a gradual reduction of hTERT mRNA levels in freshly isolated naïve (TN), central (TCM) and effector memory (TEM) CD4+ and CD8+ T cells (Figure 3). The analysis of six T cell subsets from 98 human subjects showed that TN cells express the highest levels of hTERT mRNA, followed by TCM and TEM cells, and CD4+ T cell subsets express higher hTERT mRNA than their corresponding CD8+ T cell subsets. CD8+ TEM cells express the lowest levels of hTERT mRNA, and CD8+ TEM cells from more than 75% of healthy donors do not have detectable hTERT mRNA by a sensitive real time quantitative RT-PCR assay. Collectively, these findings demonstrate that hTERT mRNA levels decline with advancing T cell differentiation from naïve to memory T cells and that hTERT is expressed higher in CD4+ than in CD8+ T cells.
Figure 3. hTERT/Telomerase expression during T cell differentiation.
T cell differentiation from TN to TCM and TEM cells results in loss of telomere length, reduced expression of hTERT mRNA and telomerase but gain of effector functions. TN cells have longer telomeres than TCM and TEM cells due to fewer past cell divisions. Correspondingly, there is a progressive decrease in hTERT mRNA and telomerase activity with differentiation that correlates with decreased proliferative capacity, and increased propensity for apoptosis.
Further analysis of hTERT ASPs shows that both FL hTERT and ASPs are present in T cell subsets that express hTERT [15]. The β-deletion is the most frequently detected ASP in T cells. Interestingly, the amounts of ASPs correlate with the total amount of hTERT mRNA. This suggests that the alternative splicing machinery processes a fixed portion of the hTERT mRNA throughout T cell differentiation. In vitro activation of T cell subsets increases the amount of hTERT mRNA [15], but the ratio of FL and ASPs remain roughly the same as prior to stimulation in naïve and memory T cell subsets. Furthermore, the levels of telomerase activity in these T cell subsets after in vitro stimulation is closely correlated with their hTERT mRNA levels, i.e., CD4+ TN cells express the highest levels of telomerase activity followed by TCM and TEM cells. These findings indicate T cell differentiation results in reduced hTERT/telomerase expression in these T cells regardless of the cell cycle status. It is currently unknown how differentiation precisely controls hTERT expression.
The functional significance of this regulated hTERT/telomerase expression during T cell differentiation has been further assessed. CD4+ and CD8+ TN, TCM, and TEM cells are cultured in vitro for 15 days following activation by anti-CD3 and anti-CD28 antibodies. Activation-induced levels of both hTERT mRNA and telomerase activity were positively correlated with the degree of cell expansion, e.g., CD4+ TN cells have the highest expansion while CD8+ TEM have the lowest expansion. In both CD4+ and CD8+ subsets, telomerase activity and expansion are positively correlated over the course of the 15-day culture. Furthermore, the highest levels of hTERT mRNA in CD4+ TN cells are correlated with the highest percentage of viable cells. Although it has been reported that ectopic expression of hTERT in T cells increases telomerase activity and prolongs cell proliferation in vitro [40–43], the impact of reduced telomerase expression on primary T cells has only recently been examined . In two reports, knockdown of hTERT mRNA by siRNA or antisense oligonucleotide in CD4+ or CD4+ TN cells demonstrates that reduced telomerase activity results in reduced cell expansion and increased apoptosis [15, 44]. Surprisingly, Gazzaniga et. al., found that shRNA-mediated knockdown of hTERT in primary human CD4+ T cells enhanced T cell resistance to dexamethasone-induced apoptosis [45]. Yet, the increased propensity for T cell apoptosis related to hTERT insufficiency is corroborated by observations in T cells from patients with rheumatoid arthritis [44], myelodysplastic syndrome [46], and short telomere syndromes [47]. These findings establish a causal and quantifiable relationship between hTERT/telomerase expression and T cell activation-induced proliferation and survival.
IV. Reduction of hTERT/telomerase expression in T cells with aging
Decline of immune function with age is well documented [48]. Thymic involution contributes to diminished naïve T cell generation, while lifelong exposure to external and internal insults, in particular with chronic viral infections (e.g., CMV), lead to the accumulation of virus-specific (e.g., CMV) memory T cells and terminally differentiated CD8+CD28− T cells [49, 50]. An early study on the effects of aging on activation-induced telomerase activity in isolated T cells (CD4+ and CD8+, n=90) [10] shows highly variable levels of induced telomerase activity (by anti-CD3 and anti-CD28 antibodies and PHA) among the study subjects and no statistically significant age-associated reduction of induced telomerase activity. In a subsequent study of a larger cohort, it was shown that telomerase activity declines with age in resting T cells (age range 20s-90s, n=366, p<0.0001) as well as in activated T cells (stimulation by anti-CD3 and anti-CD28 antibodies) (n=195, p<0.01) [51]. A more recent report supports that activation-induced telomerase activity in T cells is significantly decreased with aging from early 20s to early 80s (n=114) [52]. However, the induced telomerase activity in centenarians appears similar to younger individuals (under 66 years of age) [52]. Collectively, these findings suggest that induced telomerase activity in T cells decreases with age in a general population. However, those exceptionally healthy old individuals may have a superior maintenance of telomerase expression and T cell function. Further studies of these centenarians may reveal new insights into telomerase regulation in healthy aging.
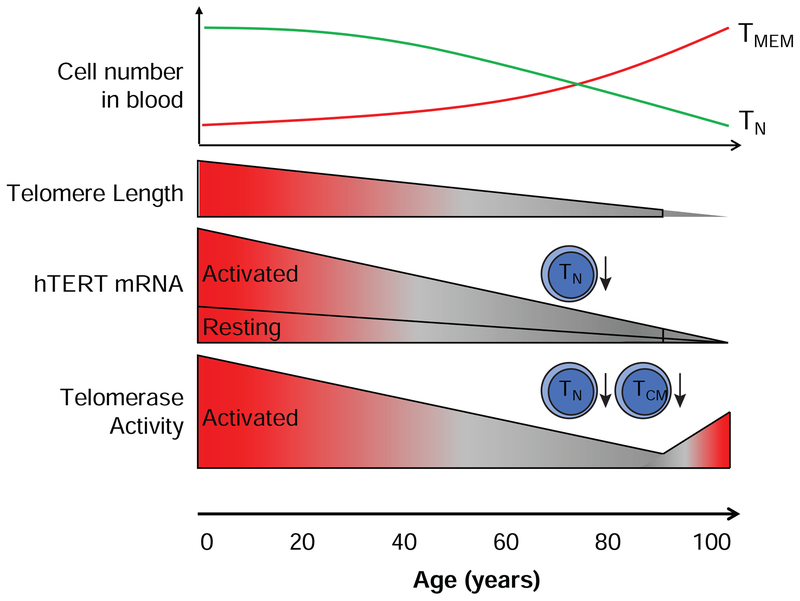
Because the proportions of TN cells decrease and TCM/TEM cells increase within total T cells with age [53] and induced telomerase activity decreases with T cell differentiation, these studies did not reveal whether the reduction of induced telomerase activity with age occurs in TN cells or TCM/TEM cells or both cells. Our recent study addresses this issue by comparison of induced hTERT expression and telomerase activity in isolated T cell subsets between young (under 40 years old) and old (over 68 years old) [15]. We observe that the level of hTERT mRNA is significantly decreased in aged CD4+ TN cells (Figure 4). In parallel, induced telomerase activity correlated with hTERT mRNA expression and also significantly decreased in CD4+ TN cells of the old age group. Intriguingly, although the reduction of hTERT mRNA does not reach statistical significance in CD4+ TCM cells, the induced telomerase activity is significantly reduced in the old age group compared to the young group. Thus, an age-associated decrease in induced telomerase activity in T cells is influenced by both fewer numbers of TN cells and qualitative changes that lessen induced telomerase expression in CD4+ TN and TCM cells. It is also worth noting that the age-associated reduction of induced telomerase activity occurs more profoundly in CD4+ TN and TCM cells than in their CD8+ T cell counterparts. Considering the role of CD4+ T cells, the impact of such changes may have broad consequences in adaptive immunity affecting the functions of both B cells and CD8+ T cells in the elderly.
Figure 4. hTERT/Telomerase expression during T cell aging.
With advance of age, there is a decrease in the number of circulating TN cells and increase in memory T cells (TCM and TEM) cells. In parallel, T cells have progressive telomere shortening and reduced induction of telomerase activity. Within T cell subsets, telomerase activity is significantly reduced in CD4+ TN and TCM cells from old individuals (70-90 years old) compared to young (20-40 years old). However, it has been shown that healthily aged centenarians have telomerase activity levels similar to much younger individuals.
V. Altered hTERT/telomerase expression in T cells in disease
a. hTERT mutations affect T cell function
Disorders of telomere maintenance often manifest pathology in the immune system via bone marrow failure. In patients with dyskeratosis congenita, mutations in hTERT affecting the reverse transcriptase domain result in telomerase haploinsufficiency and significant attrition of T cell telomeres [54]. Patients who are heterozygous for mutations in hTERT or hTERC have defective telomere maintenance resulting in very short telomeres in leukocytes and TN cells are the most severely affected [55]. These studies indicate the importance of telomerase dose in telomere maintenance and T cell functions. Even in patients without bone marrow failure, mutations in hTERT, hTERC, or DKC1 can lead to abnormally short telomeres and eventually to T cell immunodeficiency. It is worth noting that the immune systems of these patients have some shared characteristics of older individuals such as reductions in CD4+ and CD8+ TN cells and an accumulation of terminally differentiated CD8+ TEM cells [47].
b. T cell diseases with dysfunctional telomerase regulation
Myelodysplastic syndromes (MDS) are a group of age-related bone marrow disorders with the potential for progression to acute myeloid leukemia (AML) [46]. In MDS, T cell telomeres are shorter than healthy controls, and there is a loss of TN cells and a reduced diversity of the T cell repertoire. Although T cells from MDS patients have shorter telomere lengths, they surprisingly have higher basal levels of hTERT mRNA and telomerase activity compared to healthy controls [28]. But this is not true in TN cells from MDS patients which have lower hTERT mRNA and telomerase activity [46]. These findings suggest there is activation of telomerase in non-TN T cells in MDS patients. Intriguingly, despite an elevated telomerase activity, T cells of MDS patients exhibit abnormally rapid telomere shortening, reduced proliferative capacity, and increased cell death.
A range of other diseases without detected mutations in hTERT or hTERC [56, 57] also display altered telomerase expression or shortened telomeres in T cells. Lucas et al., report that a PI3K activating mutation leads to senescent CD8+ T cells with short telomeres [58]. Aubert et al., find that patients with cartilage-hair hypoplasia (CHH) have shorter than normal telomeres in peripheral blood lymphocytes as T cells and NK cells are the most affected [59]. These patients have a homozygous point mutation in the lncRNA component of the RMRP gene that does not affect hTERT mRNA levels, but activation-induced telomerase activity is reduced resulting in delayed or failed proliferation. Zhang et al., find reduced hTERT mRNA expression in peripheral blood CD4+ T cells in patients with oral lichen planus, a T cell mediated inflammatory disease [60]. Collectively, these findings show that alteration of telomerase expression in T cells, via either direct mutation of genes encoding telomerase components or indirectly by affecting pathways leading to telomerase activation, have profound effects on the function of T cells and contribute to the severity of disease.
VI. Conclusions and future directions
We have discussed the current understanding and recent progress of telomerase expression and regulation during human T cell development, activation, differentiation, aging and diseases. It is evident that telomerase is critically important in T cell division and functions as shown by the impact of reduced telomerase expression in T cell differentiation, aging, and diseases. Furthermore, identification of age-related reductions in induced telomerase expression in CD4+ TN and TCM cells offers hope to improve the age-related decline of T cell function by elevating telomerase expression in these T cells. Improvement of telomerase expression in T cells can also improve T cell-based immunotherapy in old patients. The less differentiated T cells (e.g., TN) exhibit superior performance against tumors [61, 62] and have relatively longer telomere lengths [63] and higher induced telomerase activity [15] to support greater T cell expansion. In conclusion, regulation of hTERT and telomerase expression in human T cells occurs at multiple levels and many of the mechanistic details remain to be elucidated. The numerous transcription factor binding sites within the hTERT promoter imply that hTERT is linked to an array of cellular pathways. Manipulating these pathways, through small molecules or genetic methods, to achieve a controlled augmentation of telomerase activity could be an avenue to improve T cell function. During production of autologous T cells for immunotherapy, inclusion of exogenous hTERT/telomerase enhancers may be a simple test of telomerase’s beneficial effects that could be realized in the near future.
Highlights:
Telomerase is critical for prolonged T cell proliferation and viability
hTERT/telomerase activity is regulated at multiple levels in T cells
T cell development, activation, differentiation, and aging affect telomerase
Enhancing telomerase may benefit T cell function in aging and immunotherapy
Acknowledgments
This research was supported entirely by the Intramural Research Program of the NIH, National Institute on Aging.
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
Reference:
- [1].Cech TR, Beginning to understand the end of the chromosome, Cell, 116 (2004) 273–279. [DOI] [PubMed] [Google Scholar]
- [2].Feng J, Funk WD, Wang SS, Weinrich SL, Avilion AA, Chiu CP, Adams RR, Chang E, Allsopp RC, Yu J, The RNA component of human telomerase, Science, 269 (1995) 1236–1241. [DOI] [PubMed] [Google Scholar]
- [3].Nakamura TM, Morin GB, Chapman KB, Weinrich SL, Andrews WH, Lingner J, Harley CB, Cech TR, Telomerase catalytic subunit homologs from fission yeast and human, Science, 277 (1997) 955–959. [DOI] [PubMed] [Google Scholar]
- [4].Ramlee MK, Wang J, Toh WX, Li S, Transcription Regulation of the Human Telomerase Reverse Transcriptase (hTERT) Gene, Genes (Basel), 7 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Avin BA, Umbricht CB, Zeiger MA, Human telomerase reverse transcriptase regulation by DNA methylation, transcription factor binding and alternative splicing (Review), Int J Oncol, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Saretzki G, Telomeres, Telomerase and Ageing, Subcell Biochem, 90 (2018) 221–308. [DOI] [PubMed] [Google Scholar]
- [7].Oehen S, Brduscha-Riem K, Differentiation of naive CTL to effector and memory CTL: correlation of effector function with phenotype and cell division, J Immunol, 161 (1998) 5338–5346. [PubMed] [Google Scholar]
- [8].Weng N, Levine BL, June CH, Hodes RJ, Regulated expression of telomerase activity in human T lymphocyte development and activation, J. Exp. Med, 183 (1996) 2471–2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Weng NP, Levine BL, June CH, Hodes RJ, Human naive and memory T lymphocytes differ in telomeric length and replicative potential, Proc Natl Acad Sci U S A, 92 (1995) 11091–11094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Son NH, Murray S, Yanovski J, Hodes RJ, Weng N, Lineage-specific telomere shortening and unaltered capacity for telomerase expression in human T and B lymphocytes with Age, J. Immunol, 165 (2000) 1191–1196. [DOI] [PubMed] [Google Scholar]
- [11].Weng N, Levine BL, June CH, Hodes RJ, Regulation of telomerase RNA template expression in human T lymphocyte development and activation, J Immunol, 158 (1997) 3215–3220. [PubMed] [Google Scholar]
- [12].Weng NP, Levine BL, June CH, Hodes RJ, Regulated expression of telomerase activity in human T lymphocyte development and activation, J Exp Med, 183 (1996) 2471–2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Liu K, Schoonmaker MM, Levine BL, June CH, Hodes RJ, Weng NP, Constitutive and regulated expression of telomerase reverse transcriptase (hTERT) in human lymphocytes, Proc Natl Acad Sci U S A, 96 (1999) 5147–5152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Huang EE, Tedone E, O’Hara R, Cornelius C, Lai TP, Ludlow A, Wright WE, Shay JW, The Maintenance of Telomere Length in CD28+ T Cells During T Lymphocyte Stimulation, Sci Rep, 7 (2017) 6785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Patrick M, Cheng N, Kim J, An J, Dong F, Yang Q, Zou I, Weng N, Human T cell differentiation negatively regulates telomerase expression resulting in reduced activation-induced proliferation and survival, Front Immunol, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Li Y, Zhi W, Wareski P, Weng NP, IL-15 activates telomerase and minimizes telomere loss and may preserve the replicative life span of memory CD8+ T cells in vitro, J Immunol, 174 (2005) 4019–4024. [DOI] [PubMed] [Google Scholar]
- [17].Yang Y, An J, N.p. Weng, Telomerase Is Involved in IL-7-Mediated Differential Survival of Naive and Memory CD4+ T Cells, The Journal of Immunology, 180 (2008) 3775–3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Wallace DL, Berard M, Soares MV, Oldham J, Cook JE, Akbar AN, Tough DF, Beverley PC, Prolonged exposure of naive CD8+ T cells to interleukin-7 or interleukin-15 stimulates proliferation without differentiation or loss of telomere length, Immunology, 119 (2006) 243–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Soares MV, Plunkett FJ, Verbeke CS, Cook JE, Faint JM, Belaramani LL, Fletcher JM, Hammerschmitt N, Rustin M, Bergler W, Beverley PC, Salmon M, Akbar AN, Integration of apoptosis and telomere erosion in virus-specific CD8+ T cells from blood and tonsils during primary infection, Blood, 103 (2004) 162–167. [DOI] [PubMed] [Google Scholar]
- [20].Avin BA, Umbricht CB, Zeiger MA, Human telomerase reverse transcriptase regulation by DNA methylation, transcription factor binding and alternative splicing (Review), Int J Oncol, 49 (2016) 2199–2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Chebel A, Ffrench M, Transcriptional regulation of the human telomerase reverse transcriptase: new insights, Transcription, 1 (2010) 27–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Xu D, Popov N, Hou M, Wang Q, Bjorkholm M, Gruber A, Menkel AR, Henriksson M, Switch from Myc/Max to Mad1/Max binding and decrease in histone acetylation at the telomerase reverse transcriptase promoter during differentiation of HL60 cells, Proc Natl Acad Sci U S A, 98 (2001) 3826–3831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Chebel A, Rouault JP, Urbanowicz I, Baseggio L, Chien WW, Salles G, Ffrench M, Transcriptional activation of hTERT, the human telomerase reverse transcriptase, by nuclear factor of activated T cells, J Biol Chem, 284 (2009) 35725–35734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Carlson CM, Endrizzi BT, Wu J, Ding X, Weinreich MA, Walsh ER, Wani MA, Lingrel JB, Hogquist KA, Jameson SC, Kruppel-like factor 2 regulates thymocyte and T-cell migration, Nature, 442 (2006) 299–302. [DOI] [PubMed] [Google Scholar]
- [25].Hara T, Mizuguchi M, Fujii M, Nakamura M, Kruppel-like factor 2 represses transcription of the telomerase catalytic subunit human telomerase reverse transcriptase (hTERT) in human T cells, J Biol Chem, 290 (2015) 8758–8763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Ulaner GA, Hu JF, Vu TH, Giudice LC, Hoffman AR, Telomerase activity in human development is regulated by human telomerase reverse transcriptase (hTERT) transcription and by alternate splicing of hTERT transcripts, Cancer Res, 58 (1998) 4168–4172. [PubMed] [Google Scholar]
- [27].Jalink M, Ge Z, Liu C, Bjorkholm M, Gruber A, Xu D, Human normal T lymphocytes and lymphoid cell lines do express alternative splicing variants of human telomerase reverse transcriptase (hTERT) mRNA, Biochem Biophys Res Commun, 353 (2007) 999–1003. [DOI] [PubMed] [Google Scholar]
- [28].Dong W, Wu L, Sun H, Ren X, Epling-Burnette PK, Yang L, MDS shows a higher expression of hTERT and alternative splice variants in unactivated T-cells, Oncotarget, 7 (2016) 71904–71914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Zhdanov DD, Vasina DA, Grachev VA, Orlova EV, Orlova VS, Pokrovskaya MV, Alexandrova SS, Sokolov NN, Alternative splicing of telomerase catalytic subunit hTERT generated by apoptotic endonuclease EndoG induces human CD4(+) T cell death, Eur J Cell Biol, 96 (2017) 653–664. [DOI] [PubMed] [Google Scholar]
- [30].Zhdanov DD, Gladilina YA, Pokrovskaya MV, Aleksandrova SS, Grishin DV, Podobed OV, Sokolov NN, Induction of Alternative Splicing and Inhibition of Activity of Telomerase Catalytic Subunit by Apoptotic Endonuclease EndoG in Human T, B, and NK Cells, Bull Exp Biol Med, 164 (2018) 478–482. [DOI] [PubMed] [Google Scholar]
- [31].Zhdanov DD, Plyasova AA, Gladilina YA, Pokrovsky VS, Grishin DV, Grachev VA, Orlova VS, Pokrovskaya MV, Alexandrova SS, Lobaeva TA, Sokolov NN, Inhibition of telomerase activity by splice-switching oligonucleotides targeting the mRNA of the telomerase catalytic subunit affects proliferation of human CD4(+) T lymphocytes, Biochem Biophys Res Commun, 509 (2019) 790–796. [DOI] [PubMed] [Google Scholar]
- [32].Wong MS, Chen L, Foster C, Kainthla R, Shay JW, Wright WE, Regulation of telomerase alternative splicing: a target for chemotherapy, Cell Rep, 3 (2013) 1028–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Yi X, White DM, Aisner DL, Baur JA, Wright WE, Shay JW, An alternate splicing variant of the human telomerase catalytic subunit inhibits telomerase activity, Neoplasia, 2 (2000) 433–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Listerman I, Sun J, Gazzaniga FS, Lukas JL, Blackburn EH, The major reverse transcriptase-incompetent splice variant of the human telomerase protein inhibits telomerase activity but protects from apoptosis, Cancer Res, 73 (2013) 2817–2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Radan L, Hughes CS, Teichroeb JH, Vieira Zamora FM, Jewer M, Postovit LM, Betts DH, Microenvironmental regulation of telomerase isoforms in human embryonic stem cells, Stem Cells Dev, 23 (2014) 2046–2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Liu K, Hodes RJ, Weng N, Cutting edge: telomerase activation in human T lymphocytes does not require increase in telomerase reverse transcriptase (hTERT) protein but is associated with hTERT phosphorylation and nuclear translocation, J Immunol, 166 (2001) 4826–4830. [DOI] [PubMed] [Google Scholar]
- [37].Li H, Zhao L, Yang Z, Funder JW, Liu JP, Telomerase is controlled by protein kinase Calpha in human breast cancer cells, J Biol Chem, 273 (1998) 33436–33442. [DOI] [PubMed] [Google Scholar]
- [38].Kang SS, Kwon T, Kwon DY, Do SI, Akt protein kinase enhances human telomerase activity through phosphorylation of telomerase reverse transcriptase subunit, J Biol Chem, 274 (1999) 13085–13090. [DOI] [PubMed] [Google Scholar]
- [39].Plunkett FJ, Franzese O, Finney HM, Fletcher JM, Belaramani LL, Salmon M, Dokal I, Webster D, Lawson ADG, Akbar AN, The Loss of Telomerase Activity in Highly Differentiated CD8+CD28-CD27- T Cells Is Associated with Decreased Akt (Ser473) Phosphorylation, The Journal of Immunology, 178 (2007) 7710–7719. [DOI] [PubMed] [Google Scholar]
- [40].Migliaccio M, Amacker M, Just T, Reichenbach P, Valmori D, Cerottini JC, Romero P, Nabholz M, Ectopic human telomerase catalytic subunit expression maintains telomere length but is not sufficient for CD8+ T lymphocyte immortalization, J Immunol, 165 (2000) 4978–4984. [DOI] [PubMed] [Google Scholar]
- [41].Rufer N, Migliaccio M, Antonchuk J, Humphries RK, Roosnek E, Lansdorp PM, Transfer of the human telomerase reverse transcriptase (TERT) gene into T lymphocytes results in extension of replicative potential, Blood, 98 (2001) 597–603. [DOI] [PubMed] [Google Scholar]
- [42].Roth A, Yssel H, Pene J, Chavez EA, Schertzer M, Lansdorp PM, Spits H, Luiten RM, Telomerase levels control the lifespan of human T lymphocytes, Blood, 102 (2003) 849–857. [DOI] [PubMed] [Google Scholar]
- [43].Luiten RM, Pene J, Yssel H, Spits H, Ectopic hTERT expression extends the life span of human CD4+ helper and regulatory T-cell clones and confers resistance to oxidative stress-induced apoptosis, Blood, 101 (2003) 4512–4519. [DOI] [PubMed] [Google Scholar]
- [44].Fujii H, Shao L, Colmegna I, Goronzy JJ, Weyand CM, Telomerase insufficiency in rheumatoid arthritis, Proc Natl Acad Sci U S A, 106 (2009) 4360–4365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Gazzaniga FS, Blackburn EH, An antiapoptotic role for telomerase RNA in human immune cells independent of telomere integrity or telomerase enzymatic activity, Blood, 124 (2014) 3675–3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Yang L, Mailloux A, Rollison DE, Painter JS, Maciejewski J, Paquette RL, Loughran TP, McGraw K, Makishima H, Radhakrishnan R, Wei S, Ren X, Komrokji R, List AF, Epling-Burnette PK, Naive T-cells in myelodysplastic syndrome display intrinsic human telomerase reverse transcriptase (hTERT) deficiency, Leukemia, 27 (2013) 897–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Wagner CL, Hanumanthu VS, Talbot CC Jr., Abraham RS, Hamm D, Gable DL, Kanakry CG, Applegate CD, Siliciano J, Jackson JB, Desiderio S, Alder JK, Luznik L, Armanios M, Short telomere syndromes cause a primary T cell immunodeficiency, J Clin Invest, 128 (2018) 5222–5234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Weng NP, Aging of the immune system: how much can the adaptive immune system adapt?, Immunity, 24 (2006) 495–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Weng NP, Akbar AN, Goronzy J, CD28(−) T cells: their role in the age-associated decline of immune function, Trends Immunol, 30 (2009) 306–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Goronzy JJ, Weyand CM, Successful and Maladaptive T Cell Aging, Immunity, 46 (2017) 364–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Lin Y, Damjanovic A, Metter EJ, Nguyen H, Truong T, Najarro K, Morris C, Longo DL, Zhan M, Ferrucci L, Hodes RJ, Weng NP, Age-associated telomere attrition of lymphocytes in vivo is co-ordinated with changes in telomerase activity, composition of lymphocyte subsets and health conditions, Clin Sci (Lond), 128 (2015) 367–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Tedone E, Huang E, O’Hara R, Batten K, Ludlow AT, Lai TP, Arosio B, Mari D, Wright WE, Shay JW, Telomere length and telomerase activity in T cells are biomarkers of high-performing centenarians, Aging Cell, 18 (2019) e12859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Lin Y, Kim J, Metter EJ, Nguyen H, Truong T, Lustig A, Ferrucci L, Weng NP, Changes in blood lymphocyte numbers with age in vivo and their association with the levels of cytokines/cytokine receptors, Immun Ageing, 13 (2016) 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Armanios M, Chen JL, Chang YP, Brodsky RA, Hawkins A, Griffin CA, Eshleman JR, Cohen AR, Chakravarti A, Hamosh A, Greider CW, Haploinsufficiency of telomerase reverse transcriptase leads to anticipation in autosomal dominant dyskeratosis congenita, Proc Natl Acad Sci U S A, 102 (2005) 15960–15964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Aubert G, Baerlocher GM, Vulto I, Poon SS, Lansdorp PM, Collapse of telomere homeostasis in hematopoietic cells caused by heterozygous mutations in telomerase genes, PLoS Genet, 8 (2012) e1002696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Opresko PL, Shay JW, Telomere-associated aging disorders, Ageing Res Rev, 33 (2017) 52–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Shay JW, Wright WE, Telomeres and telomerase: three decades of progress, Nat Rev Genet, (2019). [DOI] [PubMed] [Google Scholar]
- [58].Lucas CL, Zhang Y, Venida A, Wang Y, Hughes J, McElwee J, Butrick M, Matthews H, Price S, Biancalana M, Wang X, Richards M, Pozos T, Barlan I, Ozen A, Rao VK, Su HC, Lenardo MJ, Heterozygous splice mutation in PIK3R1 causes human immunodeficiency with lymphoproliferation due to dominant activation of PI3K, J Exp Med, 211(2014)2537–2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Aubert G, Strauss KA, Lansdorp PM, Rider NL, Defects in lymphocyte telomere homeostasis contribute to cellular immune phenotype in patients with cartilage-hair hypoplasia, J Allergy Clin Immunol, 140 (2017) 1120–1129 e1121. [DOI] [PubMed] [Google Scholar]
- [60].Zhang J, Wei MH, Lu R, Du GF, Zhou G, Declined hTERT expression of peripheral blood CD4(+) T cells in oral lichen planus correlated with clinical parameter, J Oral Pathol Med, 45(2016)516–522. [DOI] [PubMed] [Google Scholar]
- [61].Hinrichs CS, Borman ZA, Gattinoni L, Yu Z, Burns WR, Huang J, Klebanoff CA, Johnson LA, Kerkar SP, Yang S, Muranski P, Palmer DC, Scott CD, Morgan RA, Robbins PF, Rosenberg SA, Restifo NP, Human effector CD8+ T cells derived from naive rather than memory subsets possess superior traits for adoptive immunotherapy, Blood, 117 (2011) 808–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Klebanoff CA, Gattinoni L, Torabi-Parizi P, Kerstann K, Cardones AR, Finkelstein SE, Palmer DC, Antony PA, Hwang ST, Rosenberg SA, Waldmann TA, Restifo NP, Central memory self/tumor-reactive CD8+ T cells confer superior antitumor immunity compared with effector memory T cells, Proc Natl Acad Sci U S A, 102 (2005) 9571–9576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Shen X, Zhou J, Hathcock KS, Robbins P, Powell DJ Jr., Rosenberg SA, Hodes RJ, Persistence of tumor infiltrating lymphocytes in adoptive immunotherapy correlates with telomere length, J Immunother, 30 (2007) 123–129. [DOI] [PMC free article] [PubMed] [Google Scholar]