Abstract
Infection induced diaphragm weakness is a major contributor to death and prolonged mechanical ventilation in critically ill patients. Infection induced muscle dysfunction is associated with activation of muscle proteolytic enzymes, and taurine is known to suppress proteolysis. We therefore postulated that taurine administration may prevent infection induced diaphragm dysfunction. The purpose of this study was to test this hypothesis using a clinically relevant animal model of infection, i.e. cecal ligation puncture induced sepsis (CLP). Studies were performed on (n=5-7 mice/group): (a) sham operated controls, (b) animals with sepsis induced by CLP, (c) sham operated animals given taurine (75 mg/kg/d, intraperitoneally), and (d) CLP animals given taurine. At intervals after surgery animals were euthanized, diaphragm force generation measured in vitro, and diaphragm calpain, caspase and proteasomal activity determined. CLP elicited a large reduction in diaphragm specific force generation at 24 hours (1-150 Hz, p<0.001) and taurine significantly attenuated CLP induced diaphragm weakness at all stimulation frequencies (p<0.001). CLP induced significant increases in diaphragm calpain, caspase and proteasomal activity; taurine administration prevented increases in the activity of all three pathways. In additional time course experiments, diaphragm force generation remained at control levels over 72 hours in CLP animals treated with daily taurine administration, while CLP animals demonstrated severe, sustained reductions in diaphragm strength (p<0.01 for all time points). Our results indicate that taurine administration prevents infection induced diaphragm weakness and reduces activation of three major proteolytic pathways. Because this agent is has been shown to be safe, non-toxic when administered to humans, taurine may have a role in treating infection induced diaphragm weakness. Future clinical studies will be needed to assess this possibility.
Keywords: sepsis, diaphragm force generation, diaphragm weakness, taurine, proteolysis
Introduction
Recent work indicates that respiratory muscle strength is a major determinant of clinical outcomes in critically ill patients (Demoule et al., 2013; Supinski and Callahan, 2013; Supinski et al., 2016b). These studies show that diaphragm weakness is associated with increased mortality and a markedly increased duration of mechanical ventilation during critical illness (Demoule et al., 2013; Supinski and Callahan, 2013; Supinski et al., 2016b). A major risk factor for the development of diaphragm weakness is infection, with diaphragm strength of infected patients averaging less than 50% of non-infected patients and less than 20% of healthy subjects (Demoule et al., 2013; Supinski and Callahan, 2013; Supinski et al., 2016b). Unfortunately, there are currently no pharmacological therapies to prevent or reverse infection induced respiratory muscle weakness in critically ill patients. In theory, such treatments could have a major impact on outcomes, with the potential to reduce duration of mechanical ventilation, reduce mortality, and improve long term outcomes after critical illness.
Taurine (2 aminoethanesulfonic acid), is a naturally occurring, ubiquitous amino acid that is present in multiple cells types, with high concentrations in cardiac and skeletal muscle. Moreover, taurine is thought to be critical for maintenance of normal skeletal muscle function. Animal studies have shown that taurine supplementation provides beneficial effects in models of neurologic disease, retinal disease, renal disease, cardiovascular disease (including hypertension, abdominal aortic aneurysm, cardiomyopathy), liver disease, pancreatic disease, diabetes, allergic induced inflammation and radiation induced pulmonary fibrosis (Froger et al., 2012; Jang et al., 2017; Schaffer and Kim, 2018; Schaffer et al., 2014; Schaffer et al., 2010; Zhao et al., 2018), #0}. In addition, taurine administration improves muscle regeneration in muscular dystrophy, enhances glucose homeostasis, protects from overuse–induced muscle damage, attenuates myotonia, and alleviates aging induced sarcopenia (Borck et al., 2018; Khalil et al., 2018; Scicchitano and Sica, 2018; Terrill et al., 2016; Thirupathi et al., 2018). Moreover, skeletal muscle depletion of taurine in rodents has been shown to accelerate muscle senescence, alter muscle metabolism, increase muscle atrophy, disrupt myofibril integrity, result in dysregulation of calcium homeostasis and shorten lifespan (Ito et al., 2010; Ito et al., 2014a; Ito et al., 2014b). Although the mechanisms by which taurine improves muscle function have not been fully elucidated, putative actions include maintenance of intracellular calcium homeostasis, inactivation of proteolytic pathways, preservation of intracellular redox status, and prevention of mitochondrial free radical generation (De Carvalho et al., 2017; Schaffer et al., 2010; Seidel et al., 2018).
We recently performed a number of studies using experimental animal models to elucidate the biological mechanisms by which infections induce muscle weakness (Supinski et al., 2015, 2016a; Supinski et al., 2014). As part of this work, we found that sepsis alters the distribution of calcium stores in muscle, augments mitochondrial free radical generation and increases activation of the calpain proteolytic systems (Supinski et al., 2015, 2016a; Supinski et al., 2014). Based on this work, we postulated that taurine, an agent that facilitates sarcoplasmic reticulum calcium uptake, inhibits mitochondrial free radical generation, and reduces calpain activation, may be capable of preventing sepsis induced skeletal muscle dysfunction (Henry and MacCormack, 2018; Schaffer et al., 2010; Seidel et al., 2018; Wu et al., 1999).
The purpose of the present study, therefore, was to test the hypothesis that taurine administration would reduce infection induced diaphragm weakness. We employed the cecal ligation puncture (CLP) model of peritonitis to induce sepsis in mice (Supinski et al., 2015) and compared diaphragm specific force generation in sham operated animals, CLP animals, sham animals given taurine and CLP animals treated with taurine. The dose of taurine used was based on previous studies in animals and in humans (Ahmadian et al., 2017a; Birdsall, 1998; Schaffer et al., 2014; Waldron et al., 2018). As a result, this dose of taurine has the potential for rapid translation into clinical trials if found to be effective in preventing infection induced muscle weakness in animal models.
Methods
Experimental Protocols
All experiments were approved by the University of Kentucky Institutional Animal Care and Use Committee. In initial experiments, we studied four groups of mice (n=5-7/group): (a) sham operated, control animals treated with vehicle (0.3 ml saline, intraperitoneally, IP); (b) cecal ligation puncture (CLP) animals treated with vehicle; (c) sham operated animals given taurine (75 mg/kg in 0.3 ml saline, IP); and (d) CLP operated animals given taurine. Vehicle or taurine was administered at the time of sham or CLP surgery. At 24 hours post-operatively, animals were euthanized, diaphragm muscle strips were dissected from a portion of the costal diaphragm and the diaphragm force-frequency relationship was assessed in vitro. The remaining diaphragm was frozen, stored in a −80°C freezer and subsequently used to determine diaphragm protein levels, proteolytic enzyme activities, and indices of reactive oxygen species mediated protein modifications.
In a subsequent group of studies, we examined the effect of repeated doses of taurine to attenuate diaphragm weakness at later time points after the induction of sepsis. For these studies, we euthanized animals at 48 hours and 72 hours after CLP surgery. Animals were given either vehicle or taurine daily until sacrificed. At the time of sacrifice, in vitro diaphragm force was determined.
Cecal Ligation Puncture (CLP) Induced Sepsis
For this procedure, animals were anesthetized with isoflurane (2–4%) delivered via a nose cone attached an anesthetic gas vaporizer. Once a steady plane of anesthesia was achieved (assessed by no response to tail and toe pinch), the abdomen was prepped with betadine and alcohol, and an incision made (1 cm in length). The cecum was then identified and a portion ligated (1.0 cm). An 18-gauge sterile needle was used to puncture the ligated cecum through-and-through and a small amount of feces was expressed into the peritoneal cavity through the needle hole. The abdominal musculature was then approximated and sutured, followed by closure of the skin with surgical staples. For sham surgery, the abdomen was opened and closed without cecal ligation or puncture. All animals were resuscitated with 60 ml/kg of saline administered subcutaneously following the surgical procedure, and received buprenorphine (0.05 mg/kg) immediately and every 12 hours following surgery. Animals were euthanized at the specified time points after surgery using an overdose of pentobarbital (150 mg/kg, IP).
Diaphragm Force–Frequency Curves and Muscle Mass
To assess diaphragm specific force generation, muscle strips were dissected from the left mid-costal portion of the diaphragm and mounted in water jacketed glass organ baths filled with Krebs-Henselheit solution as previously described (Supinski et al., 2015, 2016a; Supinski and Callahan, 2014; Supinski et al., 2014). One end of each strip was tied to the base of the organ bath and the other to a force transducer (Scientific Instruments, Heidelberg, Germany). Supramaximal currents from a biphasic constant current amplifier connected to a Grass S48 stimulator (Grass, West Warwick, Rl, USA) were delivered using platinum mesh field electrodes. After a 15 minute equilibration period, muscle length was adjusted to Lo, and strips were sequentially stimulated with trains of 1, 10, 20, 50, 100 and 150 Hz. stimuli (train duration 800 msec, 30 sec between adjacent trains) with force recorded with a Kipp-Zonen chart recorder (Bohemia, NY, USA). Muscle specific force generation was determined using the method of Close (Close, 1972). The total weight of the costal diaphragm was determined by adding the weight of the strips used for the force frequency curve to the weight of the remaining muscle. Diaphragm tissue not used for force frequency determinations was frozen, stored at −80°C and subsequently used for assays.
Calpain Activity Assay
A commercially available kit (Abcam, Cambridge, MA) was used to assay calpain activity as previously reported (Supinski et al., 2015). This kit utilizes a proprietary set of buffers for tissue preparation and assay. Use of this particular assay allows one to determine the level of calpain activity present in the tissue prior to homogenization. In brief, samples were prepared in extraction buffer according to the manufacturer’s specifications. Sample protein levels were then assessed and sample containing 100 μg of protein was diluted to a final volume of 85 μl with extraction buffer. In parallel, an additional aliquot of each sample (100 μg of protein) was mixed with 1 μl of calpain inhibitor and sample volume brought to 85 μl with extraction buffer. Extraction buffer alone was used as a blank. All mixtures were placed in a 96 will plate, and then reaction buffer (10 μl) and calpain substrate (5 μl) were added to wells. An initial reading was taken, plates were incubated in the dark at 37°C for one hour and a final fluorescent reading was made (excitation 400 nm, emission 505 nm). The difference in increases in fluorescent activity over time for a given sample in the presence and absence of the specific calpain inhibitor was taken as an index of calpain enzyme activity.
Caspase Activity Assay
To assess caspase 3 activity, diaphragm muscle homogenates (100 μg of protein) were added to assay buffer and a caspase-3-specific fluorogenic substrate (30 μM N-acetyl-Asp-Glu-Val-Asp-7-amino-4-methylcoumarin, Ac-DEVD-AMC) as previously described in detail (Supinski and Callahan, 2014; Supinski et al., 2010; Supinski et al., 2009b). Duplicate determinations were made with muscle homogenate, assay buffer, Ac-DEVD-AMC, and a specific caspase-3 inhibitor (DEVD-CHO, 20 nM). After addition of substrate, a baseline fluorescent measurement of AMC was immediately performed using a Molecular Devices spectrofluorophotometer (excitation frequency of 360 nm and an emission frequency of 460 nm). This measurement was repeated after 0.5 h of incubation at 30°C. The increase in fluorescence from the initial reading to the final reading was calculated to obtain the raw increase in fluorescence; the increase in reading for the DEVD-CHO duplicate was subtracted from the raw reading to determine the caspase 3 specific increase in fluorescence for a given sample. AMC standards were used to calibrate fluorescent measurements.
20S Proteasome Subunit Activity Assay
Proteasome activity in diaphragm homogenates was measured using the Calbiochem 20S Proteasome Subunit Activity kit assay (Calbiochem, San Diego, CA) as previously reported (Supinski and Callahan, 2014; Supinski et al., 2009a) following the manufacturer’s protocol with AMC standards used to calibrate fluorescent measurements.
Protein Determination by Western Blotting
Western blots were used to compare diaphragm protein modifications among the experimental groups. For these determinations, muscles were first homogenized in buffer (Callahan and Supinski, 2014) and total protein levels in the diaphragm homogenates were determined using the Bradford assay (BioRad, Hercules, CA). Muscle homogenates were diluted with loading buffer, equal amounts of proteins loaded onto Tris-glycine polyacrylamide gels and subjected to electrophoresis (Novex Minicell II, Carlsbad, CA) as previously described (Callahan and Supinski, 2014). Proteins were then transferred to polyvinylidene fluoride membranes and incubated over night at 4°C with primary antibodies of interest. For detection of protein modifications, we used an anti-4-hydroxynonenal antibody (Abcam, Cambridge, MA) and an anti-nitrotyrosine antibody (Millipore Sigma, Burlington, MA). To assess changes in protein carbonyls, we employed the OxyBlot™ Protein Oxidation Detection Kit (Millipore Sigma, Burlington, MA) following the manufacturer’s instructions. Membranes were washed, incubated with horseradish peroxidase-conjugated secondary antibodies and antibody binding detected on film using enhanced chemiluminescence (Western Lightning, Perkin Elmer, Boston, MA). Densitometry was performed using a Microtek scanner (Carson, CA) and UN-SCAN-IT software (Silk Scientific, Orem, UT). To verify equal loading, membranes were reprobed with antibodies to GAPDH (Origene Technologies, Inc., Rockville, MD). We examined the following protein modifications: 4-hydroxynonenal, nitrotyrosine, and protein carbonyl formation (OxyBlot™) as previously reported (Callahan and Supinski, 2014).
Statistical Analysis
A one way ANOVA was used to compare parameters (e.g. calpain activity) between the various experimental groups. A repeated measures ANOVA was used to compare variables (e.g. force) over time across groups of CLP animals treated with and without taurine, with post-hoc testing (Tukey Test) to determine differences between groups. A p value of less than 0.05 was used to indicate statistical significance.
Results
Diaphragm Specific Force Generation at 24 Hours After Induction of Sepsis
As shown in Figure 1, CLP sepsis induced massive reductions in diaphragm force generation at 24 hours. Maximum specific force in sham controls averaged 22.4 ± 0.7 N/cm2 and 7.0±1.1 N/cm2 in CLP animals (p<0.001). Taurine administration completely prevented CLP induced reductions in maximum diaphragm force, with force for the CLP + taurine group averaging 21.3 ±1.4 N/cm2, a value equal to sham controls (20.3 ±1.0 N/cm2) and substantially higher than the level observed for the CLP group (p<0.001).
Figure 1.
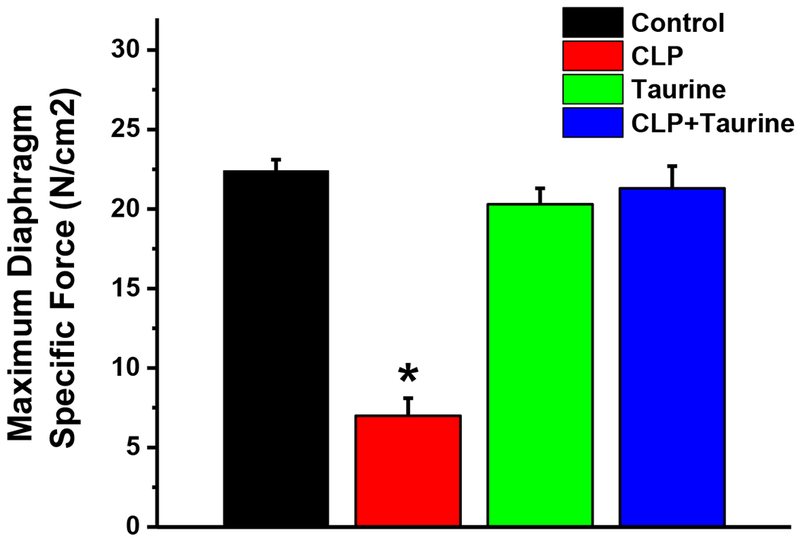
Maximum diaphragm specific force generation in (a) sham operated controls (black), (b) cecal ligation puncture (CLP, red), (c) sham operated + taurine (Taurine, green); and (d) CLP + taurine treated animals (blue). Data represents group mean average ± 1 SEM. Maximum specific force was significantly lower for the CLP group compared to the control group (p<0.001). Taurine treatment prevented CLP induced reductions in force, with maximum force for the CLP + taurine group similar to the control group and significantly higher than the CLP group (p<0.001). Maximum force for the sham + taurine group was similar to controls. * indicates statistical significance.
A similar pattern was observed for force generation in response to the entire range of stimulation frequencies tested (Figure 2). Specifically, CLP sepsis induced large reductions in force generation at all stimulation frequencies tested (p<0.001 for each comparison), decreasing diaphragm force by 72, 71, 70, 66, 68, and 68%, respectively, at 1, 10, 20, 50, 100 and 150 Hz frequencies. Taurine administration ablated CLP induced reductions of diaphragm force, with force for the CLP + taurine group similar to the control group at stimulation frequencies from 1-150 Hz (NS for each comparison).
Figure 2.
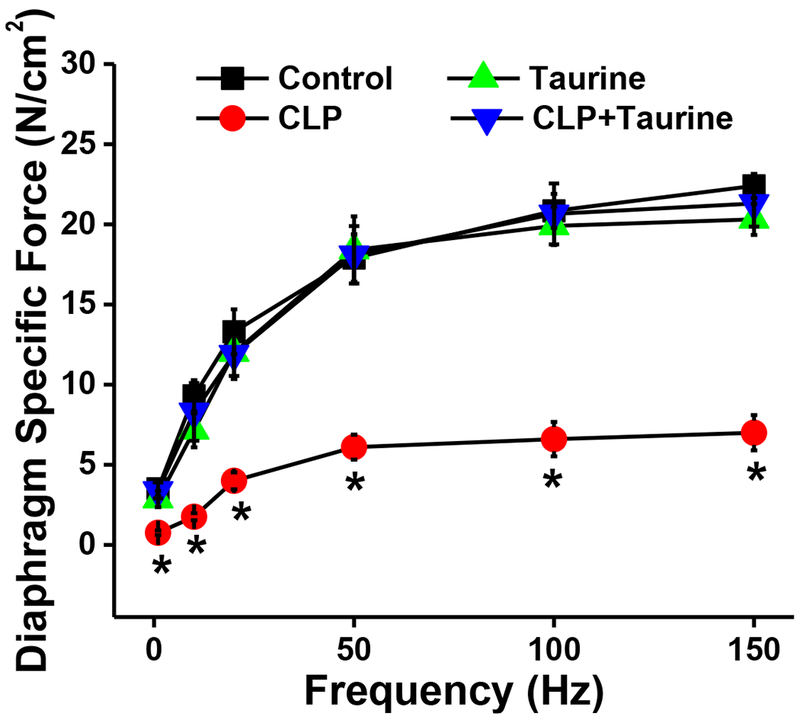
Force-frequency curves for control (black symbols), CLP (red symbols), sham + taurine (green symbols) and CLP + taurine (blue symbols) groups. Data represents group mean average ± 1 SEM. Force generation at all stimulation frequencies was significantly lower for the CLP group when compared to the other three groups (p<0.001 at all stimulation frequencies for this comparison). * indicates statistical significance.
Proteolytic Pathway Activation
Previous work (Supinski et al., 2015, 2016a; Supinski et al., 2009b) has shown that three major proteolytic pathways are activated in the diaphragm in response to sepsis, i.e. the calpain, caspase and proteasomal pathways. In keeping with this past work, we found that calpain activity, measured on diaphragm homogenates using an in vitro assay, was markedly higher for samples from CLP animals than controls (Figure 3, p=0.005 for this comparison). Taurine administration prevented this CLP induced increase in calpain activity, with diaphragm calpain activity for the CLP + taurine group similar to controls and significantly lower than levels for CLP (p=0.005 for this latter comparison). Calpain activity for the sham + taurine group was similar to levels in sham controls.
Figure 3.
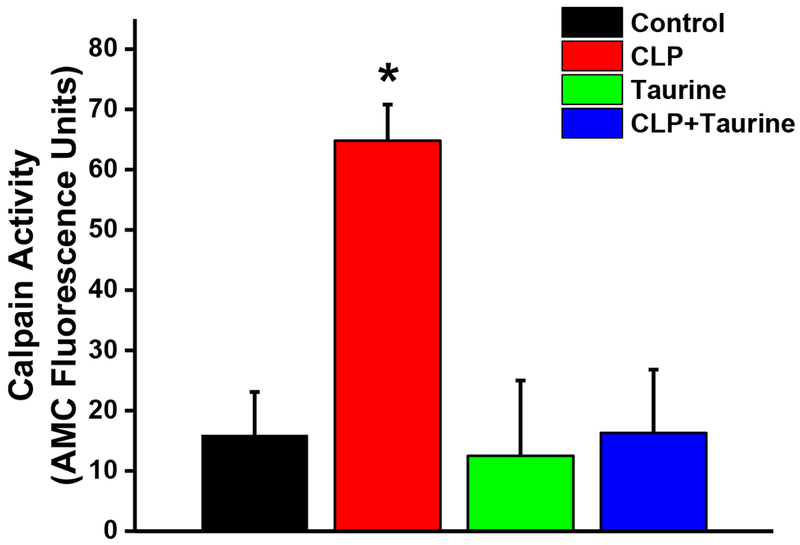
Calpain activity levels measured on diaphragm samples for sham controls (black), cecal ligation puncture (CLP, red), sham operated + taurine (Taurine, green), and (d) CLP + taurine treated animals (blue). CLP induced a large increase in calpain activity (p=0.005 compared to controls) and taurine administration prevented the CLP induced increase in diaphragm calpain activity (p=0.005 for comparison of the CLP to the CLP + taurine group). * indicates statistical significance.
Similarly, CLP induced large increases in diaphragm caspase activity levels (p=0.002 for comparison of controls to CLP, Figure 4) and taurine administration prevented these CLP induced increases in diaphragm caspase 3 activity (p=0.035 for comparison of CLP and CLP + taurine). Caspase activity for the sham + taurine group was similar to levels for the control group.
Figure 4.
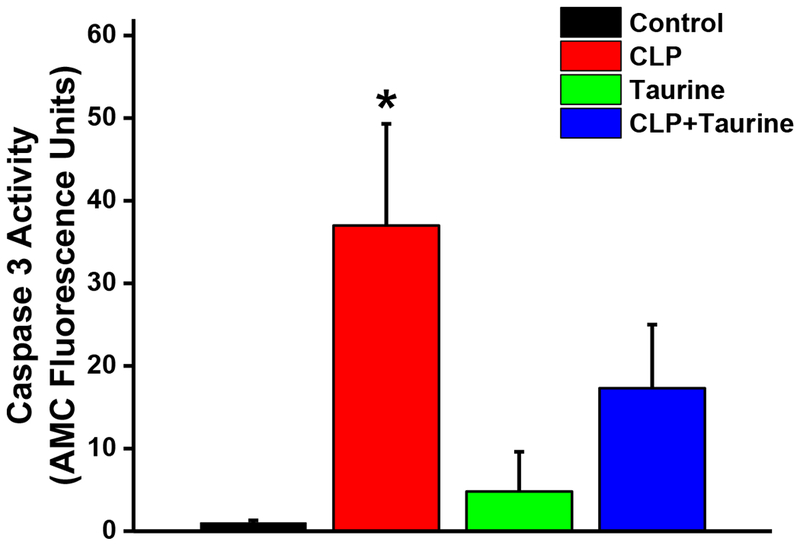
Caspase 3 activity levels in diaphragm samples from control (black), CLP (red), sham + taurine (green), and CLP + taurine (blue) groups. CLP induced a large increase in caspase 3 activity (p=0.002 compared to controls) and taurine administration prevented this CLP induced increase in diaphragm caspase 3 activity (p=0.035 for comparison of the CLP to the CLP + taurine group). * indicates statistical significance.
CLP also increased 20S proteasomal activity (Figure 5, p=0.004 for comparison of diaphragm from sham vs. CLP) and taurine administration prevented CLP induced increases in proteasomal activity (p=0.008 for comparison of CLP to CLP + taurine). Proteasomal activity was similar in control and taurine treated sham animals.
Figure 5.
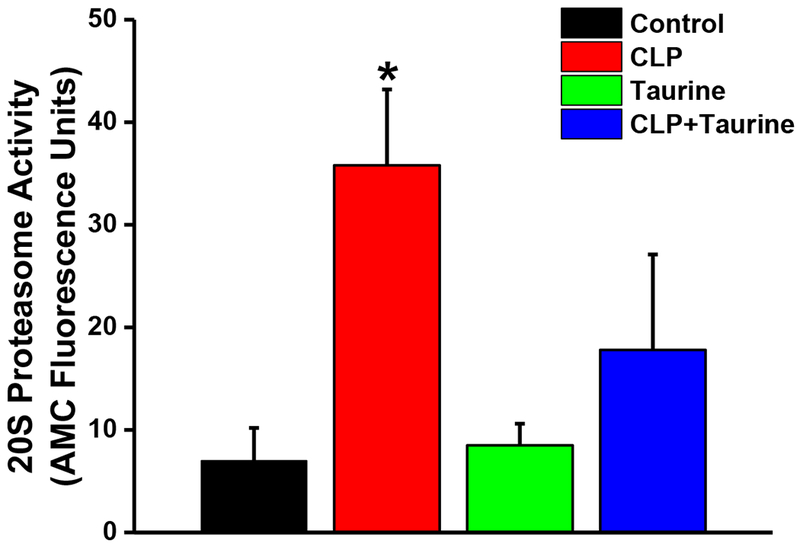
20S Proteasomal activity in diaphragm samples from control (black), CLP (red), sham + taurine (green) and CLP + taurine (blue) groups. CLP induced a significant increase in proteasomal activity (p=0.004 compared to controls) and taurine administration prevented this CLP induced increase in diaphragm proteasomal activity (p=0.008 for comparison of the CLP to the CLP + taurine group). Data shown represents group mean average ± 1 SEM with * indicating statistical significance.
Markers of Diaphragm Oxidative Protein Modification
CLP sepsis is associated with increased production of superoxide by skeletal muscle mitochondria (Supinski et al., 2015), so it seemed reasonable to postulate that diaphragms from CLP animals would manifest higher levels of free-radical mediated protein modifications, including increased levels of 4-hydroxynonenal, nitrotyrosine, and protein carbonyl modified proteins. In addition, since taurine is known to suppress oxidative stress, it seemed possible that taurine would prevent sepsis induced diaphragm protein modifications. As shown in Figure 6, we did not observe CLP induced increases in either 4-hydroxynonenal, nitrotyrosine, or protein carbonyl side-group modifications in diaphragm proteins (NS for comparison of diaphragm levels of these modifications between control and CLP treated groups). We also found that taurine administration did not alter levels of these side-groups when administered to sham or CLP animals.
Figure 6.
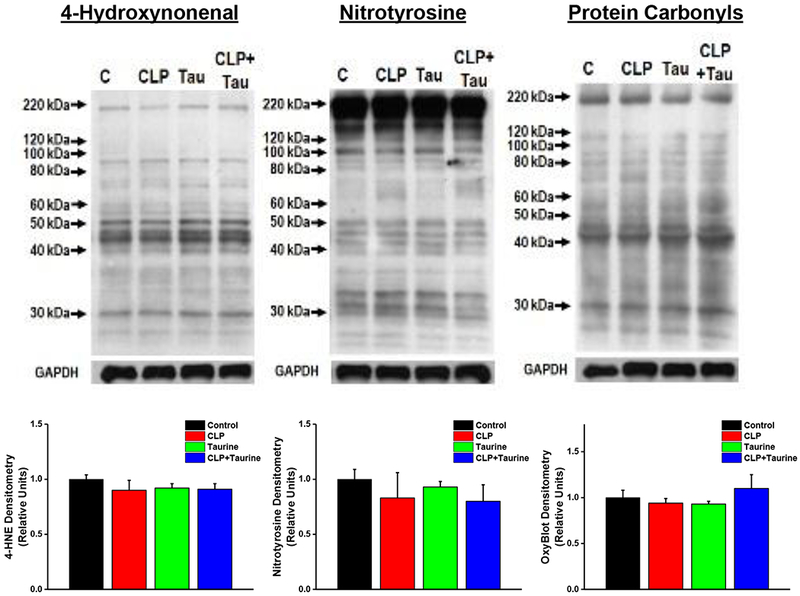
Oxidative mediated protein modifications, namely, 4-hydroxynonenal adducts (left), nitrotyrosine modifications (center) and protein carbonyl modifications (i.e. OxyBlot, right) for diaphragm samples from control, CLP, taurine and CLP + taurine groups. Top panels present representative Western blots for these modifications and bottom panels represent group mean data ± 1 SEM of protein densitometry for the modifications in each experimental group: control (black), CLP (red), sham + taurine (green) and CLP + taurine (blue) . CLP induced sepsis did not induce significant changes in the levels of any of these modifications and administration of taurine to sham or CLP operated animals did not alter levels of these three protein modifications.
Effects of CLP Sepsis and Taurine Administration Over 72 Hours
In clinical situations, sepsis and respiratory failure usually evolve over days rather than hours. We therefore thought it important to determine if repeated doses of taurine would prevent progression of diaphragm weakness over days in our animal model of CLP induced sepsis. As shown in Figure 7, we found that CLP induced severe reductions in diaphragm strength by 24 hours (i.e. 68% reductions from baseline levels) and these persisted for 72 hours after the induction of sepsis. Taurine administration prevented sepsis induced reduction in diaphragm force at all time points up to 72 hours, with diaphragm specific force generation for CLP + taurine animals significantly higher than levels for CLP animals at 24, 48, and 72 hour groups (p<0.01 for each comparison).
Figure 7.
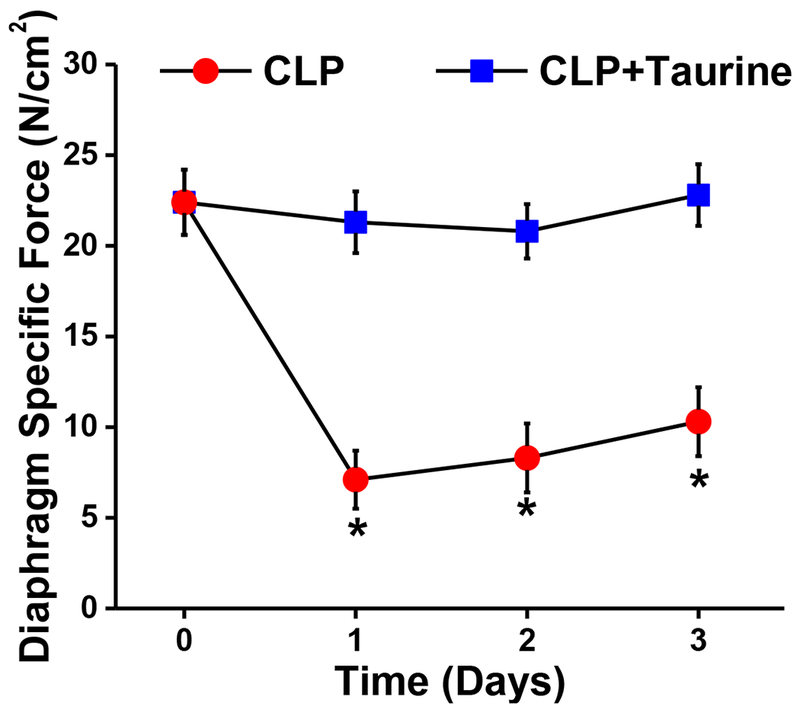
Maximum diaphragm specific force is presented as a function of time for CLP (red) and CLP + taurine treated animals (blue). The CLP + taurine group was treated with taurine, 75 mg/kg/d until sacrifice. Subgroups of animals were sacrificed at 24 hours, 48 hours, and 72 hours after sham or CLP surgeries. Saline treated animals sacrificed immediately after injections served to provide time zero control measurements. CLP treated animals had large reductions in diaphragm specific force generation by 24 hours and these reductions in force persisted at 48 hours and 72 hours after study inclusion. Taurine treatment ablated CLP induced reductions in muscle force generation, with force for the CLP + taurine group remaining the same as the control, non-septic group at 24 hours, 48 hours and 72 hours after study inclusion (p<0.01 for comparison of the two groups at each time point).
Discussion
More than 800,000 patients require sustained mechanical ventilation in medical intensive care units each year in the United States (Ward and Chong, 2015). Unfortunately, a substantial proportion of these patients die, and also, those that survive often require a long duration of mechanical ventilation before they can be successfully weaned from life support. Importantly, many of these patients are infected and recent clinical studies indicate that infection induced diaphragm weakness is a major risk factor for death and sustained mechanical ventilation (Demoule et al., 2013; Supinski and Callahan, 2013; Supinski et al., 2016b). As a result, it is reasonable to speculate that pharmacological treatments capable of preventing or reversing infection induced diaphragm weakness may be useful therapies to shorten the duration of mechanical ventilation and reduce mortality in critical illness.
The purpose of the present study was to test the hypothesis that it is possible to ameliorate infection mediated diaphragm weakness by administration of pharmacological quantities of taurine, a small molecular weight organic acid that is thought to be required for optimal muscle function. The rationale for choosing taurine as a potential therapy is based on results from recent laboratory studies using animal models of infection that have identified the cellular pathways and signaling processes which mediate the development of infection induced diaphragm weakness. This recent work found that systemic infection induces cytokine mediated neutral sphingomyelinase 2 (nSMase 2) activation in the diaphragm (Supinski et al., 2015). Cytokine mediated activation of nSMase 2, in turn, elicits alterations in the distribution of skeletal muscle calcium levels (i.e. increases in cytosolic and mitochondrial calcium concentrations), activation of the calpain proteolytic pathway, and depletion of myosin heavy chain protein (Supinski et al., 2015, 2016a). In support of these concepts, this previous work found that administration of inhibitors of nSMase to intact septic animals prevented the development of sepsis induced diaphragm weakness and also blocked activation of the calpain proteolytic enzyme system. In parallel, pharmacological inhibition of nSMase in isolated muscle cells prevented cytokine induced calpain activation and blocked cytokine mediated loss of contractile protein (i.e. myosin heavy chain).
Based on this previous work, it should be theoretically possible to attenuate infection induced diaphragm weakness by employing treatments that facilitate sarcoplasmic reticulum calcium uptake, thereby preventing cytokine mediated cytosolic and mitochondrial calcium overload and calpain activation. Recent studies suggest that taurine can augment sarcoplasmic reticulum calcium uptake and retention (Bakker and Berg, 2002; De Luca et al., 1996; Dutka et al., 2014; Henry and MacCormack, 2018; Scicchitano and Sica, 2018), making taurine administration a potential means of blocking infection induced diaphragm calpain activation and weakness. The findings of the present study are consistent with this theory. Specifically, our work indicates that taurine administration prevents CLP sepsis induced diaphragm calpain activation and diaphragm weakness, preserving the ability of the diaphragm to generate near normal levels of force in response to a wide range of excitation stimulation frequencies (1-150 Hz). Our observation that taurine blocked calpain activation and improved muscle function is consistent with previous observations examining the effects of taurine on skeletal muscle. Specifically, as reviewed by DeLuca et al, taurine supplementation is known to increase the amino acid content in skeletal muscle, increase muscle force generation, and produce resistance to the development of fatigue (De Luca et al., 2015). These effects occur in parallel with an effect of taurine to increase sarcoplasmic reticulum calcium retention, increasing calcium availability for contraction (Dutka et al., 2014).
We also found that CLP induced an increase in diaphragm caspase 3 activity and taurine administration blocked CLP induced diaphragm caspase 3 activation. This finding is consistent with two previous reports. First, Song et al. found that taurine prevented caspase 3 activation and caspase mediated apoptosis in retinal epithelial cells in response to glucose exposure (Song et al., 2012). In addition, Nagai et al. found that taurine administration reduced hepatic active caspase 3 formation in response to doxorubicin administration (Nagai et al., 2016). We also found that taurine reduced diaphragm 20S proteasomal activity following induction of CLP, a finding in keeping with several previous papers that indicate taurine can inhibit components of the proteasomal degradation system. For example, one study found that taurine reduced Murphy, an ubiquitin–proteasome pathway enzyme, in skeletal muscle in the hind limb unloading animal model of muscle inactivity (Pierno et al., 2012). Importantly, the present study found that all three of the major skeletal muscle proteolytic enzyme systems, i.e. calpain, caspase, and the proteasome, are activated in sepsis and are inhibited by taurine administration.
Sepsis is also associated with enhanced mitochondrial free radical generation in muscle and taurine has previously been shown to inhibit free radical generation in some animal and cell based models (Goodman et al., 2009; Heidari et al., 2019; Niu et al., 2018; Schaffer et al., 2014; Seidel et al., 2018; Silva et al., 2011; Supinski et al., 1993; Wu et al., 1999). For these reasons, we also examined diaphragm muscle samples for evidence of formation of several different markers of free radical mediated protein modifications, i.e. nitrotyrosine, 4-hydroxynonenal and protein carbonyl side group formation. For diaphragm cellular homogenates as a whole, however, we were unable to detect quantitatively significant increases in diaphragm protein modifications for any of these markers in response to CLP. We also did not detect an effect of taurine to reduce modified proteins in the diaphragm. It is possible that the techniques we employed were too insensitive, and that there may have been selective modification of a small number of critical proteins by sepsis that may have affected cellular function but may have escaped detection by our methodology (Sellares et al., 2007). The failure of taurine to quantitatively reduce levels of these particular protein modifications, however, is not without precedent, and other studies have reported that taurine may not alter levels of free radical mediated protein modifications (Zheng and Bizzozero, 2010). Since we were unable to detect effects of CLP to induce free radical mediated alterations in diaphragm proteins and were also unable to detect an effect of taurine to reduce 4-nitrotyrosine, 4-hydroxynonenal and protein carbonyl modifications, it is possible that taurine administration modulated intracellular redox status through other mechanisms (Colovic et al., 2018; Seidel et al., 2018; Shimada et al., 2015). However, based on the findings in the current study, it would appear that a major mechanism by which taurine preserved diaphragm force was to reduce activation of proteolytic enzymes.
The current study is the first to demonstrate an effect of taurine to preserve skeletal muscle function in an animal model of sepsis. There have been, however, other studies of the relationship of taurine to organ function in sepsis, with evidence suggesting that taurine may attenuate a number of forms of tissue damage. For example, taurine plasma levels have been reported to correlate with the degree of pulmonary vascular damage in response to sepsis in human patients, with higher taurine levels associated with improved pulmonary artery hemodynamics and lower levels of lung dysfunction (Chiarla et al., 2000b). Other reports indicate lower taurine levels are associated with the development of sepsis induced pancreatitis and cardiac damage (Janssen et al., 1983; Roth et al., 1985). The mechanisms by which taurine protects these other organs from injury are not entirely clear, but cytokine induced apoptosis has been observed in a wide variety of organs in sepsis, including the heart, thymus, intestine, kidney, and brain (Chiarla et al., 2000a; Nakagawa and Yuan, 2000; Redmond et al., 1998). In theory, the ability of taurine to attenuate activation of several of the upstream cellular triggers of apoptosis (e.g. caspase and calpain) may represent a common process by which this agent can prevent damage to multiple organs.
The initial experimental paradigm we employed in this study was to examine the ability of taurine administration to prevent diaphragm weakness at 24 hours after cecal ligation puncture induced sepsis. While this was a convenient way of determining if taurine has any ability to prevent sepsis induced reductions in diaphragm function, this experimental paradigm is very different from the circumstances under which patients develop sepsis and die. Specifically, the effects of sepsis on organ function usually evolve over days in patients, and death often occurs at a significant interval after the inciting infectious event. In keeping with this concept, it is likely that sepsis induced diaphragm dysfunction may persist for days in critically patients, reducing the ability to wean patients from mechanical ventilation (Supinski and Callahan, 2013; Supinski et al., 2016b). For these reasons, we performed additional experiments in which we examined the effects of sepsis on diaphragm function at 48 and 72 hours after induction of CLP. Importantly, we found that our model of sepsis produced long lasting diaphragm weakness and, also, that continued administration of taurine results in consistent preservation of diaphragm strength. These latter data may have special clinical relevance, because it indicates that it is possible to elicit sustained prevention of sepsis induced diaphragm dysfunction through prolonged administration of taurine.
Limitations
There are several potential limitations to the techniques and protocols employed in this study. First, there is no perfect way of completely replicating the precise manner in which infected patients are cared for in the critical care environment in animal models. In the intensive care unit, nurses provide constant monitoring of vital signs and other key parameters (i.e. electrolyte status, volume status, urine output, mentation, oxygenation, ventilation, etc.) and make rapid adjustments in fluid administration rates, electrolyte replacement, vasoactive drug administration, and numerous other therapeutic agents in response to changes in physiological status. While we did provide initial fluid resuscitation to all animals in the study, and we employed a relatively low grade form of CLP to induce sepsis, it was not possible for our animal laboratory to provide the level of care available in an intensive care unit.
Secondly, the current study focused on the effect of taurine administration on diaphragm function, and we did not explicitly evaluate the effects of this agent on the function of other organ systems. Based on previous reports, it is possible that taurine may also have produced salutary effects on other organs and future studies will be needed to explore such a possibility. However, the fact that our recent clinical studies identify diaphragm function as a key parameter influencing the major outcomes in critically ill medical intensive care unit patients (Supinski and Callahan, 2013; Supinski et al., 2016b) supports the focus chosen for the present work.
Finally, the translation of findings from the current animal study to the human condition warrants further consideration as the effects of taurine supplementation in humans may demonstrate different effects than in rodents (Spriet and Whitfield, 2015). For example, most human studies examining the effects of either acute or chronic taurine administration have focused on changes in muscle performance, with results showing that taurine has minimal to modest effects (Waldron et al., 2018). An important point, however, is that most of these studies examined well trained individuals and/or athletes, while more significant effects have been reported in untrained subjects or in patients with chronic disease, suggesting that taurine supplementation is more likely to improve muscle function in these populations (De Luca et al., 2015; Schaffer and Kim, 2018). In addition, existing data from human studies support the efficacy of taurine supplementation for a variety of clinical conditions, including evidence that oral taurine supplementation increases exercise capacity in congestive heart failure, lowers blood pressure, reduces portal hypertension, diminishes body mass index, and reduces inflammatory markers in obesity (Ahmadian et al., 2017a; Inam et al., 2018; Sun et al., 2016; Wen et al., 2019). Other reported beneficial effects of taurine supplementation in humans include treatment of MELAS, a mitochondrial disorder, protection from cardiac dysrhythmias, and attenuation of atherosclerosis (Ahmadian et al., 2017b; Schaffer and Kim, 2018). These studies, therefore, provide supportive data that oral taurine supplementation is effective in human disease.
In the case of critical illness induced skeletal muscle dysfunction, voluminous data from critically ill patients with sepsis indicate skeletal muscle is profoundly altered as a result of infection. Several studies have also examined plasma taurine concentrations in critically ill patients, indicating these levels fall dramatically at the onset of critical illness, and continue to fall during illness (Chiarla et al., 2000a; Su et al., 2015; Vermeulen et al., 2016). Moreover, mortality was higher in patients with the lowest plasma concentrations of taurine, and reduced taurine levels were associated with severity of organ failure, prolonged duration of mechanical ventilation and prolonged length of stay in the intensive care unit (Vermeulen et al., 2016). Albeit indirect, this data suggests that there may be a role for taurine administration in critical illness.
Many septic patients who are admitted to the intensive care unit with sepsis also require mechanical ventilation. Ventilator induced diaphragm dysfunction is thought to also play a role in diaphragm weakness in patients. Interestingly, both animal and human data indicate that the many of the pathophysiologic mechanisms that have been identified to be responsible for producing mechanical ventilation induced diaphragm dysfunction (VIDD) in animals and humans (such as proteolytic pathway activation) should theoretically be inhibited by taurine, suggesting that taurine could be an effective treatment for either or both conditions. Nonetheless, in the clinical situation of sepsis, the timing and dosing of taurine administration may prove to be critical and the question as to whether taurine administration can prevent or improve diaphragm weakness in septic patients who undergo mechanical ventilation can only be determined in future clinical trials.
Conclusion
In summary, we found that CLP sepsis induced diaphragm weakness, caspase activation, calpain activation, and proteasome activation. Moreover, taurine administration blocked all of these effects of sepsis, and most importantly, completely preserved diaphragm force generation. To our knowledge, this is the first study to show that taurine administration can ameliorate sepsis induced diaphragm weakness. Clinically, sepsis induced diaphragm weakness has been shown to be a major cause of prolonged mechanical ventilation and death in critically ill patients. Unfortunately, no therapies have been identified for this devastating complication of critical illness. Our results suggest that taurine supplementation could represent a useful therapy to prevent diaphragm weakness in the clinical setting. Additional animal and human studies are needed to evaluate this possibility.
Highlights.
Diaphragm weakness is a major clinical problem in critically ill mechanically ventilated patients.
Sepsis is a major risk factor for the development of diaphragm weakness.
Taurine administration improves sepsis-induced diaphragm dysfunction.
Administration of taurine to critically ill patients may improve diaphragm function, reduce duration of mechanical ventilation and reduce mortality.
Acknowledgments
Grants
Dr. Supinski is supported by R01HL113494 and R01HL141356 from the National Heart Lung and Blood Institute of the National Institutes of Health and by 5I01BX002132 from the Department of Veterans Affairs. Dr. Callahan is supported by R01HL112085 and R01HL141356 from the National Heart, Lung and Blood Institute of the National Institutes of Health. Dr. Schroder is supported by R01HL141356 from the National Heart Lung and Blood Institute of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmadian M, Dabidi Roshan V, Ashourpore E, 2017a. Taurine Supplementation Improves Functional Capacity, Myocardial Oxygen Consumption, and Electrical Activity in Heart Failure. Journal of dietary supplements 14, 422–432. [DOI] [PubMed] [Google Scholar]
- Ahmadian M, Roshan VD, Aslani E, Stannard SR, 2017b. Taurine supplementation has anti-atherogenic and anti-inflammatory effects before and after incremental exercise in heart failure. Therapeutic advances in cardiovascular disease 11, 185–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker AJ, Berg HM, 2002. Effect of taurine on sarcoplasmic reticulum function and force in skinned fast-twitch skeletal muscle fibres of the rat. The Journal of physiology 538, 185–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birdsall TC, 1998. Therapeutic applications of taurine. Alternative medicine review : a journal of clinical therapeutic 3, 128–136. [PubMed] [Google Scholar]
- Borck PC, Vettorazzi JF, Branco RCS, Batista TM, Santos-Silva JC, Nakanishi VY, Boschero AC, Ribeiro RA, Carneiro EM, 2018. Taurine supplementation induces long-term beneficial effects on glucose homeostasis in ob/ob mice. Amino acids 50, 765–774. [DOI] [PubMed] [Google Scholar]
- Callahan LA, Supinski GS, 2014. Hyperglycemia-induced diaphragm weakness is mediated by oxidative stress. Critical care (London, England) 18, R88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiarla C, Giovannini I, Siegel JH, Boldrini G, Castagneto M, 2000a. The relationship between plasma taurine and other amino acid levels in human sepsis. The Journal of nutrition 130, 2222–2227. [DOI] [PubMed] [Google Scholar]
- Chiarla C, Giovannini I, Siegel JH, Boldrini G, Castagneto M, 2000b. Taurine and pulmonary hemodynamics in sepsis. Amino acids 18, 389–397. [DOI] [PubMed] [Google Scholar]
- Close RI, 1972. Dynamic properties of mammalian skeletal muscles. Physiological reviews 52, 129–197. [DOI] [PubMed] [Google Scholar]
- Colovic MB, Vasic VM, Djuric DM, Krstic DZ, 2018. Sulphur-containing Amino Acids: Protective Role Against Free Radicals and Heavy Metals. Current medicinal chemistry 25, 324–335. [DOI] [PubMed] [Google Scholar]
- De Carvalho FG, Galan BSM, Santos PC, Pritchett K, Pfrimer K, Ferriolli E, Papoti M, Marchini JS, de Freitas EC, 2017. Taurine: A Potential Ergogenic Aid for Preventing Muscle Damage and Protein Catabolism and Decreasing Oxidative Stress Produced by Endurance Exercise. Frontiers in physiology 8, 710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Luca A, Pierno S, Camerino DC, 1996. Effect of taurine depletion on excitation-contraction coupling and Cl- conductance of rat skeletal muscle. European journal of pharmacology 296, 215–222. [DOI] [PubMed] [Google Scholar]
- De Luca A, Pierno S, Camerino DC, 2015. Taurine: the appeal of a safe amino acid for skeletal muscle disorders. Journal of translational medicine 13, 243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demoule A, Jung B, Prodanovic H, Molinari N, Chanques G, Coirault C, Matecki S, Duguet A, Similowski T, Jaber S, 2013. Diaphragm dysfunction on admission to the intensive care unit. Prevalence, risk factors, and prognostic impact-a prospective study. American journal of respiratory and critical care medicine 188, 213–219. [DOI] [PubMed] [Google Scholar]
- Dutka TL, Lamboley CR, Murphy RM, Lamb GD, 2014. Acute effects of taurine on sarcoplasmic reticulum Ca2+ accumulation and contractility in human type I and type II skeletal muscle fibers. Journal of applied physiology (Bethesda, Md. : 1985) 117, 797–805. [DOI] [PubMed] [Google Scholar]
- Froger N, Cadetti L, Lorach H, Martins J, Bemelmans AP, Dubus E, Degardin J, Pain D, Forster V, Chicaud L, Ivkovic I, Simonutti M, Fouquet S, Jammoul F, Leveillard T, Benosman R, Sahel JA, Picaud S, 2012. Taurine provides neuroprotection against retinal ganglion cell degeneration. PloS one 7, e42017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman CA, Horvath D, Stathis C, Mori T, Croft K, Murphy RM, Hayes A, 2009. Taurine supplementation increases skeletal muscle force production and protects muscle function during and after high-frequency in vitro stimulation. Journal of applied physiology (Bethesda, Md. : 1985) 107, 144–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidari R, Behnamrad S, Khodami Z, Ommati MM, Azarpira N, Vazin A, 2019. The nephroprotective properties of taurine in colistin-treated mice is mediated through the regulation of mitochondrial function and mitigation of oxidative stress. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie 109, 103–111. [DOI] [PubMed] [Google Scholar]
- Henry EF, MacCormack TJ, 2018. Taurine protects cardiac contractility in killifish, Fundulus heteroclitus, by enhancing sarcoplasmic reticular Ca(2+) cycling. Journal of comparative physiology. B, Biochemical, systemic, and environmental physiology 188, 89–99. [DOI] [PubMed] [Google Scholar]
- Inam UL, Piao F, Aadil RM, Suleman R, Li K, Zhang M, Wu P, Shahbaz M, Ahmed Z, 2018. Ameliorative effects of taurine against diabetes: a review. Amino acids 50, 487–502. [DOI] [PubMed] [Google Scholar]
- Ito T, Oishi S, Takai M, Kimura Y, Uozumi Y, Fujio Y, Schaffer SW, Azuma J, 2010. Cardiac and skeletal muscle abnormality in taurine transporter-knockout mice. Journal of biomedical science 17 Suppl 1, S20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito T, Yoshikawa N, Inui T, Miyazaki N, Schaffer SW, Azuma J, 2014a. Tissue depletion of taurine accelerates skeletal muscle senescence and leads to early death in mice. PloS one 9, e107409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito T, Yoshikawa N, Schaffer SW, Azuma J, 2014b. Tissue taurine depletion alters metabolic response to exercise and reduces running capacity in mice. Journal of amino acids 2014, 964680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang H, Lee S, Choi SL, Kim HY, Baek S, Kim Y, 2017. Taurine Directly Binds to Oligomeric Amyloid-beta and Recovers Cognitive Deficits in Alzheimer Model Mice. Advances in experimental medicine and biology 975 Pt 1, 233–241. [DOI] [PubMed] [Google Scholar]
- Janssen HF, Lombardini JB, Lust RM, 1983. Cardiac taurine levels during endotoxemia. Proceedings of the Society for Experimental Biology and Medicine. Society for Experimental Biology and Medicine (New York, N.Y.) 172, 407–411. [DOI] [PubMed] [Google Scholar]
- Khalil RM, Abdo WS, Saad A, Khedr EG, 2018. Muscle proteolytic system modulation through the effect of taurine on mice bearing muscular atrophy. Molecular and cellular biochemistry 444, 161–168. [DOI] [PubMed] [Google Scholar]
- Nagai K, Fukuno S, Oda A, Konishi H, 2016. Protective effects of taurine on doxorubicin-induced acute hepatotoxicity through suppression of oxidative stress and apoptotic responses. Anti-cancer drugs 27, 17–23. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Yuan J, 2000. Cross-talk between two cysteine protease families. Activation of caspase-12 by calpain in apoptosis. The Journal of cell biology 150, 887–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu X, Zheng S, Liu H, Li S, 2018. Protective effects of taurine against inflammation, apoptosis, and oxidative stress in brain injury. Molecular medicine reports 18, 4516–4522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierno S, Liantonio A, Camerino GM, De Bellis M, Cannone M, Gramegna G, Scaramuzzi A, Simonetti S, Nicchia GP, Basco D, Svelto M, Desaphy JF, Camerino DC, 2012. Potential benefits of taurine in the prevention of skeletal muscle impairment induced by disuse in the hindlimb-unloaded rat. Amino acids 43, 431–445. [DOI] [PubMed] [Google Scholar]
- Redmond HP, Stapleton PP, Neary P, Bouchier-Hayes D, 1998. Immunonutrition: the role of taurine. Nutrition (Burbank, Los Angeles County, Calif.) 14, 599–604. [DOI] [PubMed] [Google Scholar]
- Roth E, Muhlbacher F, Karner J, Steininger R, Schemper M, Funovics J, 1985. Liver amino acids in sepsis. Surgery 97, 436–442. [PubMed] [Google Scholar]
- Schaffer S, Kim HW, 2018. Effects and Mechanisms of Taurine as a Therapeutic Agent. Biomolecules & therapeutics 26, 225–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffer SW, Jong CJ, Ito T, Azuma J, 2014. Effect of taurine on ischemia-reperfusion injury. Amino acids 46, 21–30. [DOI] [PubMed] [Google Scholar]
- Schaffer SW, Jong CJ, Ramila KC, Azuma J, 2010. Physiological roles of taurine in heart and muscle. Journal of biomedical science 17 Suppl 1, S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scicchitano BM, Sica G, 2018. The Beneficial Effects of Taurine to Counteract Sarcopenia. Current protein & peptide science 19, 673–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidel U, Huebbe P, Rimbach G, 2018. Taurine: A Regulator of Cellular Redox Homeostasis and Skeletal Muscle Function. Molecular nutrition & food research, e1800569. [DOI] [PubMed] [Google Scholar]
- Sellares J, Mas S, Melo E, Sanchez F, Marin J, Gea J, Barreiro E, 2007. Oxidative stress time course in the rat diaphragm after freezing-thawing cycles. Respiratory physiology & neurobiology 155, 156–166. [DOI] [PubMed] [Google Scholar]
- Shimada K, Jong CJ, Takahashi K, Schaffer SW, 2015. Role of ROS Production and Turnover in the Antioxidant Activity of Taurine. Advances in experimental medicine and biology 803, 581–596. [DOI] [PubMed] [Google Scholar]
- Silva LA, Silveira PC, Ronsani MM, Souza PS, Scheffer D, Vieira LC, Benetti M, De Souza CT, Pinho RA, 2011. Taurine supplementation decreases oxidative stress in skeletal muscle after eccentric exercise. Cell biochemistry and function 29, 43–49. [DOI] [PubMed] [Google Scholar]
- Song MK, Roufogalis BD, Huang TH, 2012. Reversal of the Caspase-Dependent Apoptotic Cytotoxicity Pathway by Taurine from Lycium barbarum (Goji Berry) in Human Retinal Pigment Epithelial Cells: Potential Benefit in Diabetic Retinopathy. Evidence-based complementary and alternative medicine : eCAM 2012, 323784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spriet LL, Whitfield J, 2015. Taurine and skeletal muscle function. Current opinion in clinical nutrition and metabolic care 18, 96–101. [DOI] [PubMed] [Google Scholar]
- Su L, Li H, Xie A, Liu D, Rao W, Lan L, Li X, Li F, Xiao K, Wang H, Yan P, Li X, Xie L, 2015. Dynamic changes in amino acid concentration profiles in patients with sepsis. PloS one 10, e0121933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Q, Wang B, Li Y, Sun F, Li P, Xia W, Zhou X, Li Q, Wang X, Chen J, Zeng X, Zhao Z, He H, Liu D, Zhu Z, 2016. Taurine Supplementation Lowers Blood Pressure and Improves Vascular Function in Prehypertension: Randomized, Double-Blind, Placebo-Controlled Study. Hypertension (Dallas, Tex. : 1979) 67, 541–549. [DOI] [PubMed] [Google Scholar]
- Supinski G, Nethery D, DiMarco A, 1993. Effect of free radical scavengers on endotoxin-induced respiratory muscle dysfunction. The American review of respiratory disease 148, 1318–1324. [DOI] [PubMed] [Google Scholar]
- Supinski GS, Alimov AP, Wang L, Song XH, Callahan LA, 2015. Neutral sphingomyelinase 2 is required for cytokine-induced skeletal muscle calpain activation. American journal of physiology. Lung cellular and molecular physiology 309, L614–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supinski GS, Alimov AP, Wang L, Song XH, Callahan LA, 2016a. Calcium-dependent phospholipase A2 modulates infection-induced diaphragm dysfunction. American journal of physiology. Lung cellular and molecular physiology 310, L975–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supinski GS, Callahan LA, 2013. Diaphragm weakness in mechanically ventilated critically ill patients. Critical care (London, England) 17, R120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supinski GS, Callahan LA, 2014. beta-hydroxy-beta-methylbutyrate (HMB) prevents sepsis-induced diaphragm dysfunction in mice. Respiratory physiology & neurobiology 196, 63–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supinski GS, Vanags J, Callahan LA, 2009a. Effect of proteasome inhibitors on endotoxin-induced diaphragm dysfunction. American journal of physiology. Lung cellular and molecular physiology 296, L994–l1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supinski GS, Vanags J, Callahan LA, 2010. Eicosapentaenoic acid preserves diaphragm force generation following endotoxin administration. Critical care (London, England) 14, R35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supinski GS, Wang L, Song XH, Moylan JS, Callahan LA, 2014. Muscle-specific calpastatin overexpression prevents diaphragm weakness in cecal ligation puncture-induced sepsis. Journal of applied physiology (Bethesda, Md. : 1985) 117, 921–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supinski GS, Wang W, Callahan LA, 2009b. Caspase and calpain activation both contribute to sepsis-induced diaphragmatic weakness. Journal of applied physiology (Bethesda, Md. : 1985) 107, 1389–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supinski GS, Westgate P, Callahan LA, 2016b. Correlation of maximal inspiratory pressure to transdiaphragmatic twitch pressure in intensive care unit patients. Critical care (London, England) 20, 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrill JR, Pinniger GJ, Graves JA, Grounds MD, Arthur PG, 2016. Increasing taurine intake and taurine synthesis improves skeletal muscle function in the mdx mouse model for Duchenne muscular dystrophy. The Journal of physiology 594, 3095–3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thirupathi A, Freitas S, Sorato HR, Pedroso GS, Effting PS, Damiani AP, Andrade VM, Nesi RT, Gupta RC, Muller AP, Pinho RA, 2018. Modulatory effects of taurine on metabolic and oxidative stress parameters in a mice model of muscle overuse. Nutrition (Burbank, Los Angeles County, Calif.) 54, 158–164. [DOI] [PubMed] [Google Scholar]
- Vermeulen MA, van Stijn MF, Visser M, Lemmens SM, Houdijk AP, van Leeuwen PA, Oudemans-van Straaten HM, 2016. Taurine Concentrations Decrease in Critically Ill Patients With Shock Given Enteral Nutrition. JPEN. Journal of parenteral and enteral nutrition 40, 264–272. [DOI] [PubMed] [Google Scholar]
- Waldron M, Patterson SD, Tallent J, Jeffries O, 2018. The Effects of an Oral Taurine Dose and Supplementation Period on Endurance Exercise Performance in Humans: A Meta-Analysis. Sports medicine (Auckland, N.Z.) 48, 1247–1253. [DOI] [PubMed] [Google Scholar]
- Ward NS, Chong DH, 2015. Critical Care Beds and Resource Utilization: Current Trends and Controversies. Seminars in respiratory and critical care medicine 36, 914–920. [DOI] [PubMed] [Google Scholar]
- Wen C, Li F, Zhang L, Duan Y, Guo Q, Wang W, He S, Li J, Yin Y, 2019. Taurine is Involved in Energy Metabolism in Muscles, Adipose Tissue, and the Liver. Molecular nutrition & food research 63, e1800536. [DOI] [PubMed] [Google Scholar]
- Wu QD, Wang JH, Fennessy F, Redmond HP, Bouchier-Hayes D, 1999. Taurine prevents high-glucose-induced human vascular endothelial cell apoptosis. The American journal of physiology 277, C1229–1238. [DOI] [PubMed] [Google Scholar]
- Zhao H, Qu J, Li Q, Cui M, Wang J, Zhang K, Liu X, Feng H, Chen Y, 2018. Taurine supplementation reduces neuroinflammation and protects against white matter injury after intracerebral hemorrhage in rats. Amino acids 50, 439–451. [DOI] [PubMed] [Google Scholar]
- Zheng J, Bizzozero OA, 2010. Traditional reactive carbonyl scavengers do not prevent the carbonylation of brain proteins induced by acute glutathione depletion. Free radical research 44, 258–266. [DOI] [PMC free article] [PubMed] [Google Scholar]


