Abstract
Background
Knee osteoarthritis (KOA) is the most prevailing form of joint disease. Despite the importance of minimally invasive therapeutic methods of KOA, there is a lack of evidence to compare intraarticular hyaluronic acid injection vs traditional dextrose prolotherapy.
Objective
The aim was to compare the therapeutic effects of prolotherapy with hypertonic dextrose vs hyaluronic acid on function and pain in KOA cases.
Materials and methods
One hundred and four KOA patients were enrolled and randomly assigned into two groups, each containing 52 patients. The hyaluronic acid (HA) group were treated by 2.5 mL of hyaluronic acid intraarticulary, and the hypertonic dextrose (HD) group received 10 mL of 12.5% dextrose periarticulary. Injections were repeated three times with 1-week intervals. Pain intensity, measured by visual analog scale, and knee function, scaled by the Western Ontario and McMaster university arthritis index scores were compared between the two groups before and 3 months after intervention. Pain and function of the knee improved significantly (P<0.001) in all patients. However, significantly more symptom relief was found in the HA over the HD group. Prolotherapy with hypertonic dextrose and intraarticular injection of hyaluronic acid results in the same pain reduction and symptom relief as a noninvasive therapeutic method of KOA.
Conclusion
These results recommended intraarticular hyaluronic acid rather than prolotherapy by hypertonic dextrose for KOA symptoms relief.
Keywords: prolotherapy, periarticular, intraarticular, dextrose, hyaluronic, knee osteoarthritis
Introduction
Knee osteoarthritis (KOA) is the most prevailing form of joint disease, the symptoms of which involve nearly 15% of adults over 60.1 An estimated 80% of them are affected by movement limitations, and a quarter are disabled to carry out daily activities.2
KOA treatment begins by nonpharmacological treatments, focuses on joint-unloading therapies, and includes weight loss, exercise, physiotherapy, and orthotic devices.3,4 Pharmacological add-on regimens such as acetaminophen, nonsteroidal anti-inflammatory drugs (NSAIDs), cyclooxygenase 2 (COX2) inhibitors, and disease-modifying drugs were prescribed regularly for short-term pain management, if symptoms do not.5,6
Conservative therapy at final session includes intraarticular injections by corticosteroids, hyaluronic acid (HA), blood-derived products, and mesenchymal stem cells.7
Prolotherapy, introduced recently as a peri- or intraarticular injection-based treatment to pain relief and symptoms improvement of KOA. However, the mechanism of action is not well understood. Small amounts, maximum 6 mL, of an irritant solution, usually hypertonic dextrose, were injected at painful tendons and ligaments.8 Prolotherapy is an injection-based treatment for chronic musculoskeletal pain.9 The mechanism of action is probably multifactorial and seems to work via fibroblast stimulation and proliferation of vascular, deposition of dense collagen, and growth of cartilage.10 Moreover, dextrose solutions may have high sensorineural analgesic effects as recommended recently by the effect of epidural injection of dextrose in the treatment of chronic non-surgical low back pain.11 It may treat KOA by targeting structural dysfunction, dropping nociceptive drive, and diminishing peripheral sensitization.12
Although considerable trials support the therapeutic effects of hyaluronic acid on KOA pain and symptoms, there is still insufficient evidence to permit a conclusion concerning the effect of this treatment, if any, on the progression of osteoarthritis in humans. The therapeutic benefits of hyaluronic acid are increasingly being considered, especially for musculoskeletal disorders including KOA. However evidence on the efficacy of this therapy on KOA patients and its mechanism of action is unknown.13
The aim of the authors was to compare two different and common therapeutic modalities in terms of their effect on pain relief of knee osteoarthritis. In the current randomized clinical trial, we aimed to compare the effects of prolotherapy with hypertonic dextrose vs hyaluronic acid on function and pain in KOA cases.
Materials and methods
The study was reviewed and approved by the Ethics Committee of the Shahid Beheshti University of Medical Sciences. Also, his trial was conducted in accordance with the Declaration of Helsinki. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee. Information about the study was given comprehensively both orally and in written form to all patients or their accompanying adult. They gave informed consent in writing prior to inclusion in the study. The clinical trial number for this study is IRCT20130518013364N6.
During a 14-month study, from February 2016 to April 2017, 104 patients with mild-to-moderate KOA, grade II or more, were enrolled. Diagnosis of KOA knee was diagnosed according to American College of Rheumatology Criteria,14 and grade was determined according to Kellgren-Lawrence grade.15 All patients were aged between 50–75 years and had experienced less than 30 minutes of morning stiffness. Patients were excluded if they met any exclusion criteria, such as severe underlying diseases like diabetes16 and/or hypothyroidism,17 immune suppression or deficiency, serious local infectious or inflammatory knee disease, anticoagulant drug history during the last 3 months, lateral knee compartment involvement,18 being a candidate for knee joint replacement, any intraarticular injection based treatment as prolotherapy during the last year, and opioid drugs addiction, as studies revealed a strong association between them and KOA. KOA was diagnosed upon clinical examinations and radiographic documents in standing position. At the beginning, all patients gave their written informed consent.
To allocate patients into two groups, blocked randomization in a 1:1 ratio was used.19 Random Allocation Software was used to sequence. Random sequence was concealed by sequentially numbered, opaque sealed envelopes (SNOSE) technique.20
Two previous RCTs guided a priori sample size calculations.21,22 Therefore, 32 participants per arm would provide 80% power to detect a 20% difference in mean composite WOMAC scores between two groups at an alpha level of 5%.
Therefore, 104 eligible cases were randomly divided into two groups, the hyaluronic acid (HA) group and the hypertonic dextrose prolotherapy (HD) group, each of which contained 52 patients.
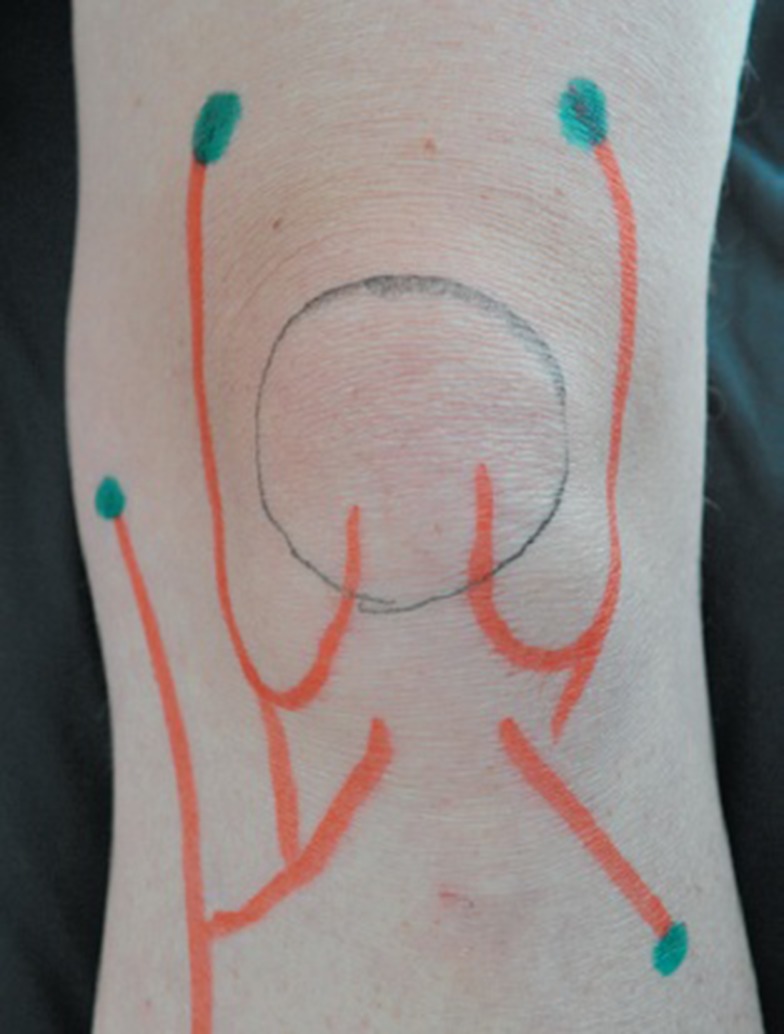
Before the main injections, lidocaine 2% was used as local anesthetic. For the HA group, 2.5 mL of hyaluronic acid was injected intraarticulary via the inferomedial of patella. The HD group received 10 mL of 12.5% hypertonic dextrose through four point injections, two points at superolateral of patella, one point at the medial knee joint line and another point was at the anterior of fibula head, via a fan wise technique, 2.5 cc for each point (Figure 1). All injections were done by a 23-G needle subcutaneously under ultrasound guidance. The injections were done, three times within 2 weeks, at days 0, 7, and 14.
Figure 1.
Fan wise approach for hypertonic dextrose injection.
Both groups completed a 100 Scales questionnaire, the Western Ontario and McMaster University Arthritis Index (WOMAC), in which higher Points show better knee status. Also, the pain intensity was measured by visual analog scale, VAS (a 10-cm colored ruler) which was scaled 0 to 10. In this, 0 indicates “no pain” and 10 reflects the “worst pain” felt ever. At the first injection time and 3 months later, the pain intensity and the WOMAC scores were obtained for all patients and compared intra and between groups.
Statistical analysis
SPSS version 20 was used to analyses the data. Student’s t-test was used to compare the VAS and WOMAC before and after intervention and to compare between two groups.
Results
The demographic data, such as gender, age, and Body mass index, which are detailed in Table 1, were obtained and compared between the two groups. There is no statistically significant difference between the two groups.
Table 1.
Demographic data between two groups
| HD | HA | P | ||
|---|---|---|---|---|
| Age (years) | 61.2±11.5 | 63.7±12.2 | 0.42 | |
| Gender | Male | 29 | 33 | 0.78 |
| Female | 25 | 21 | ||
| BMI | 30.7±1.2 | 29.5±1.3 | 0.64 | |
Abbreviations: HD, hypertonic dextrose; HA, hyaluronic acid.
Pain level and knee function (WOMAC score) were gathered and compared between the two groups, before and after treatment. Post-treatment, pain level and WOMAC scores significantly improved in both groups (P<0.001) (Table 2). Moreover, between-groups comparison of pain intensity and WOMAC scores at 3 months after injection had shown significantly more improvement with hyaluronic acid (Table 2).
Table 2.
Pain intensity and WOMAC score comparison between two groups, pre- and post-injection
| HD | HA | P (between groups) | ||
|---|---|---|---|---|
| Pain intensity (VAS) | Before | 7.8±1.4 | 8.2±1.7 | — |
| After | 2.5±1.1 | 2.1±0.6 | 0.02 | |
| P (Within group) | 0.02 | 0.03 | — | |
| WOMAC Score | Before | 52.7±9.8 | 55.9±10.4 | — |
| After | 83.7±12.7 | 88.5±15.6 | <0.001 | |
| P (Within group) | 0.01 | 0.02 | — | |
Abbreviations: HD, hypertonic dextrose; HA, hyaluronic acid; WOMAC, McMaster University Arthritis Index.
Discussion
Our results show that both prolotherapy with hypertonic dextrose and pre-articular injection of hyaluronic acid have positive therapeutic effects, as a nonoperative regimen in patients with KOA. However, intraarticular hyaluronic acid appears to have most advantages when compared to classic prolotherapy with hypertonic dextrose. Prolotherapy as an injection-based technique is shown to relieve pain and some other symptoms of chronic musculoskeletal disorders such as KOA. However, the baseline chains of action that lead to pain relief and disease modification are not yet clearly understood, although this method is being increasingly used worldwide.23
Osteoarthritis, a mild inflammation process, which is characterized as a complex of degradative and reparative disease in the articular cartilage and subchondral bone usually, according to marginal osteophyte. In order to avoid unwanted general effects, therefore, local therapeutic regimens and methods have been considered recently.
Hyaluronic acid, a macro polysaccharide constructed by a long chain of disaccharides, which has a very high-water binding capacity, plays a main role in the viscosity of the synovial fluid. Water absorbent properties of hyaluronic acid build it as a physiological factor in the trophic status of cartilage. Hyaluronic acid of the synovial fluid is the main cause of viscous acts of this lubricant fluid, especially during slow movement of the joint, and also has a protective role during rapid movement, like running.24 Parenteral hyaluronic acid as a viscosupplementation is novel, and probably effective for OA symptoms modification. Replacement of hyaluronic acid as a main goal of this method may could return and maintain the viscosity of the synovial fluid at normal level.25
Upon In recent trials, a clinical positive effect of treatment by hyaluronic acid has been reported compared with the Placebo group and also vs local corticosteroids. In a short time, during the first few weeks, the analgesic effects of hyaluronic acid are reported as the same as local corticosteroids. However hyaluronic acid demonstrates more sustained advantages of up to 2 months.26
Hyaluronic acid as an intraarticular approach is relatively safe; however, some side-effects have been reported. In some cases, acute synovitis in associated with joint swelling, which remains up to 3 weeks.27 The most reported adverse event was pain at the injection site, with relief spontaneously. Other side-effects included post-injection “flares”, hemarthrosis, muscle pain, and pseudogout, which occurred rarely.27–33 Lussier et al27 evaluated 336,oderate KOA patients in a period of 2.5 years and proposed that the incidence of post-injection adverse events of hylan G-F 20 was low and the injection technique affected the rate of side-effects. They suggested that a medial approach when the knee is flexed partially is most related to transient side-effects. In sequence straight knee medial approach and straight knee lateral approach were supposed to be less related to adverse events. Our results have shown no serious adverse events; however, all patient in this trial were treated through straight knee medial.
Some controversial comments about the effects of injection based hyaluronic acid on the KOA path remain.
Maillefert et al33 investigated the modifying effects of Hyalgan through a 1-year follow-up trial study, used by an arthroscopic score. This study proved hyaluronic acid superiority over placebo to modify the severity of osteoarthritis lesions.
Henderson et al34 treated 91 KOA patients by intra-articular 750 kD hyaluronic acid. They suggested no significant therapeutic effect over placebo by 5 weeks after injection.
Another prospective control trial enrolled 52 KOA cases and compared the therapeutic effects of intra-articular hyaluronic acid vs placebo. This found no statistically significant difference during a 5-week follow-up.35
The clinical benefit of hyaluronic acid in comparison to placebo was proved in the majority of clinical trials.
The beneficial events of prolotherapy with hypertonic dextrose have been considered recently. Also, there is some dissension. Reeves and Hassanein21 found a significant decrease in pain, knee swelling, and bulking episodes, and also an improvement in knee function among KOA patients treated by intra-articular 10% dextrose. Hashemi et al36 compared hypertonic dextrose with intraarticular ozone on 80 mild-to-moderate KOA pateints through a prospective 3-month trial. They proved that prolotherapy with either dextrose or prolozone results in the same pain reduction and knee functional improvement in patients with mild-to-moderate KOA. Rezasoltani et al37 compared the effect of periarticular vs intraarticular prolotherapy on pain and disability in patients with knee OA. They proved that periarticular prolotherapy has comparable effects on pain and disability due to knee OA to intraarticular injections, while avoiding risks of complications.
Upon our knowledge, unfortunately there is no evidence comparing therapeutic effects of prolotherapy with hypertonic dextrose and prearticular injection of hyaluronic acid. However, our findings are in accordance with previous studies in supporting the therapeutic advantages of prolotherapy with dextrose or hyaluronic acid.
In conclusion, we discussed that both Intraarticular injection-based techniques, hypertonic dextrose and hyaluronic acid could significantly improve knee function, range of motion and decrease pain among patients with moderate KOA. However, our study suggested that hyaluronic acid was more effective than hypertonic dextrose in KOA pain and symptoms control. More trials are recommended to find the more efficient technique.
Disclosure
The authors declare no conflicts of interest in the present study.
References
- 1.WHO. Osteoarthritis. Update on background paper 6.12; 2013. Available from: http://appswhoint/medicinedocs/en/m/. Accessed January 28, 2013.
- 2.WHO. Chronic rheumatic conditions 2015. Available from: http://wwwwhoint/chp/topics/rheumatic/en/. Accessed July 21, 2016.
- 3.Hunter DJ, Felson DT. Osteoarthritis. BMJ. 2006;332(7542):639–642. doi: 10.1136/bmj.332.7542.639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fibel KH, Hillstrom HJ, Halpern BC. State-of-the-art management of knee osteoarthritis. World J Clin Cases. 2015;3(2):89–101. doi: 10.12998/wjcc.v3.i2.89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bobacz K. Pharmacologic treatment of hand-, knee- and hip-osteoarthritis. Wien Med Wochenschr. 2013;163(9–10):236–242. doi: 10.1007/s10354-013-0203-7 [DOI] [PubMed] [Google Scholar]
- 6.Bannuru RR, Schmid CH, Kent DM, Vaysbrot EE, Wong JB, McAlindon TE. Comparative effectiveness of pharmacologic interventions for knee osteoarthritis: a systematic review and network meta-analysis. Ann Intern Med. 2015;162(1):46–54. doi: 10.7326/M14-1231 [DOI] [PubMed] [Google Scholar]
- 7.Ayhan E, Kesmezacar H, Akgun I. Intraarticular injections (corticosteroid, hyaluronic acid, platelet rich plasma) for the knee osteoarthritis. World J Orthop. 2014;5(3):351–361. doi: 10.5312/wjo.v5.i3.351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rabago D, Best TM, Beamsley M, Patterson J. A systematic review of prolotherapy for chronic musculoskeletal pain. Clin J Sport Med. 2005;15(5):376–380. [DOI] [PubMed] [Google Scholar]
- 9.Rabago D, Slattengren A, Zgierska A. Prolotherapy in primary care practice. Prim Care. 2010;37(1):65–80. doi: 10.1016/j.pop.2009.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reeves KD, Sit RW, Rabago DP. Dextrose Prolotherapy: a narrative review of basic science, clinical research, and best treatment recommendations. Phys Med Rehabil Clin N Am. 2016;27(4):783–823. doi: 10.1016/j.pmr.2016.06.001 [DOI] [PubMed] [Google Scholar]
- 11.Maniquis-Smigel L, Reeves KD, Rosen HJ, et al. analgesic effect of caudal 5% dextrose in water in chronic low back pain. a randomized controlled trial of epidural injection. Anesth Pain Med. 2016;7:e42550. doi: 10.5812/aapm.42550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sit RWS, Wu RWK, Reeves KD, et al. Efficacy of intra-articular hypertonic dextrose prolotherapy versus normal saline for knee osteoarthritis: a protocol for a triple-blinded randomized controlled trial. BMC Complement Altern Med. 2018;18(1):157. doi: 10.1186/s12906-018-2317-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dougados M. Sodium hyaluronate therapy in osteoarthritis: arguments for a potential beneficial structural effect. Semin Arthritis Rheum. 2000;30(2 Suppl 1):19–25. [DOI] [PubMed] [Google Scholar]
- 14.Altman R, Hochberg M, Moskowitz R, Schnitzer T. American College of Rheumatology Subcommittee on Osteoarthritis Guidelines. Recommendations for the medical management of osteoarthritis of the hip and knee: 2000 update. Arthritis Rheum. 2000;43(9):1905–1915. doi: [DOI] [PubMed] [Google Scholar]
- 15.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16(4):494–502. doi: 10.1136/ard.16.4.494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Williams MF, London DA, Husni EM, Navaneethan S, Kashyap SR. Type 2 diabetes and osteoarthritis: a systematic review and meta-analysis. J Diabetes Complications. 2016;30(5):944–950. doi: 10.1016/j.jdiacomp.2016.02.016 [DOI] [PubMed] [Google Scholar]
- 17.McLean RM, Podell DN. Bone and joint manifestations of hypothyroidism. Semin Arthritis Rheum. 1995;24(4):282–290. [DOI] [PubMed] [Google Scholar]
- 18.Wise BL, Niu J, Yang M, et al. Patterns of compartment involvement in tibiofemoral osteoarthritis in men and women and in whites and African Americans. Arthritis Care Res (Hoboken). 2012;64(6):847–852. doi: 10.1002/acr.21606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kernan WN, Viscoli CM, Makuch RW, Brass LM, Horwitz RI. Stratified randomization for clinical trials. J Clin Epidemiol. 1999;52(1):19–26. doi: 10.1016/s0895-4356(98)00138-3 [DOI] [PubMed] [Google Scholar]
- 20.Schulz KF, Grimes DA. Allocation concealment in randomised trials: defending against deciphering. Lancet. 2002;359(9306):614–618. doi: 10.1016/S0140-6736(02)07750-4 [DOI] [PubMed] [Google Scholar]
- 21.Reeves KD, Hassanein K. Randomized prospective double-blind placebo-controlled study of dextrose prolotherapy for knee osteoarthritis with or without ACL laxity. Altern Ther Health Med. 2000;6(2):68–80. [PubMed] [Google Scholar]
- 22.Yelland MJ, Glasziou PP, Bogduk N, Schluter PJ, McKernon M. Prolotherapy injections, saline injections, and exercises for chronic low-back pain: a randomized trial. Spine. 2004;29(1):9–16. doi: 10.1097/01.BRS.0000105529.07222.5B [DOI] [PubMed] [Google Scholar]
- 23.Linetsky F, Botwin K, Gorfine L, et al. Regenerative injection therapy (RIT): effectiveness and appropriate usage. Position paper by The Florida Academy of Pain Medicine (FAPM). 2001:1–12.
- 24.Balazs EA, Denlinger JL. Viscosupplementation: a new concept in the treatment of osteoarthritis. J Rheumatol Suppl. 1993;39:3–9. [PubMed] [Google Scholar]
- 25.Brandt KD, Smith GN Jr., Simon LS. Intraarticular injection of hyaluronan as treatment for knee osteoarthritis: what is the evidence? Arthritis Rheum. 2000;43(6):1192–1203. doi: [DOI] [PubMed] [Google Scholar]
- 26.Altman RD, editor. Intra-articular sodium hyaluronate in osteoarthritis of the knee. Seminars in arthritis and rheumatism; 2000; Elsevier. doi: 10.1053/sarh.2000.0248 [DOI] [PubMed] [Google Scholar]
- 27.Lussier A, Cividino AA, McFarlane CA, Olszynski WP, Potashner WJ, De Medicis R. Viscosupplementation with hylan for the treatment of osteoarthritis: findings from clinical practice in Canada. J Rheumatol. 1996;23(9):1579–1585. [PubMed] [Google Scholar]
- 28.O’Hanlon D. Acute local reactions after intraarticular hylan for osteoarthritis of the knee. J Rheumatol. 1996;23(5):945–946. [PubMed] [Google Scholar]
- 29.Pullman-mooar S, Mooar P, Sieck M, Clayburne G, Schumacher HR. Are there distinctive inflammatory flares of synovitis after hyalan GF intra-articular injections? Arthritis Rheum. 1999;42(9):S295. [Google Scholar]
- 30.Scale D, Wobig M, Wolpert W. Viscosupplementation of osteoarthritic knees with hylan: a treatment schedule study. Curr Ther Res. 1994;55(3):220–232. doi: 10.1016/S0011-393X(05)80166-3 [DOI] [Google Scholar]
- 31.Luzar MJ, Altawil B. Pseudogout following intraarticular injection of sodium hyaluronate. Arthritis Rheum. 1998;41(5):939–940. doi: [DOI] [PubMed] [Google Scholar]
- 32.Listrat V, Ayral X, Patarnello F, et al. Arthroscopic evaluation of potential structure modifying activity of hyaluronan (Hyalgan) in osteoarthritis of the knee. Osteoarthr Cartilage. 1997;5(3):153–160. [DOI] [PubMed] [Google Scholar]
- 33.Maillefert JF, Hirschhorn P, Pascaud F, Piroth C, Tavernier C. Acute attack of chondrocalcinosis after an intraarticular injection of hyaluronan. Rev Rhum Engl Ed. 1997;64(10):593–594. [PubMed] [Google Scholar]
- 34.Henderson E, Smith E, Pegley F, Blake D. Intra-articular injections of 750 kD hyaluronan in the treatment of osteoarthritis: a randomised single centre double-blind placebo-controlled trial of 91 patients demonstrating lack of efficacy. Ann Rheum Dis. 1994;53(8):529–534. doi: 10.1136/ard.53.8.529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dahlberg L, Lohmander LS, Ryd L. Intraarticular injections of hyaluronan in patients with cartilage abnormalities and knee pain. A one-year double-blind, placebo-controlled study. Arthritis Rheum. 1994;37(4):521–528. doi: 10.1002/art.1780370412 [DOI] [PubMed] [Google Scholar]
- 36.Hashemi M, Jalili P, Mennati S, et al. The effects of prolotherapy with hypertonic dextrose versus prolozone (Intraarticular Ozone) in patients with knee osteoarthritis. Anesth Pain Med. 2015;5(5):e27585. doi: 10.5812/aapm.27585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rezasoltani Z, Taheri M, Mofrad MK, Mohajerani SA. Periarticular dextrose prolotherapy instead of intra-articular injection for pain and functional improvement in knee osteoarthritis. J Pain Res. 2017;10:1179–1187. doi: 10.2147/JPR.S127633 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- WHO. Osteoarthritis. Update on background paper 6.12; 2013. Available from: http://appswhoint/medicinedocs/en/m/. Accessed January 28, 2013.