Abstract
Genomic testing has become a part of routine oncology care and plays critical roles in diagnosis, prognostic assessment, and treatment selection. Thus, in parallel, the variety of genomic testing providers and sequencing platforms has grown exponentially. Selection of the best-fit panel for each case can be daunting, with many factors to consider. Among them is whether alteration interpretation and therapy/clinical trial matching are included and/or sufficient. In this article, we review some common commercially available sequencing platforms for the genes and types of alterations tested, samples needed, and reporting content provided. We review publicly available resources for a do-it-yourself approach to alteration interpretation when it is not provided or when supplemental research is needed, along with resources to identify genomically matched treatment options that are approved and/or investigational. However, with both commercially provided interpretation and publicly available resources, there are still caveats and limitations that can stem from insufficient or ambiguous nomenclature as well as from the presentation of information. Use cases in which clinical decision making was affected are discussed. After treatment options are identified, it is important to assess the level of evidence for use within the patient’s tumor type and molecular profile. However, numerous level-of-evidence scales have been published in recent years, so we provide a publicly available tool to facilitate interoperability. The level of evidence, along with other factors, such as allelic frequency and copy number, can be used to prioritize treatment options when multiple are identified.
INTRODUCTION
The high-throughput capacity of next-generation sequencing (NGS) and the rapid development of bioinformatics tools have transformed genomic testing and our understanding of cancer. As of May 2019, more than 30,000 genetic tests in the National Institutes of Health Genetic Testing Registry list NGS as their primary test method.1,2 Within recent years, the US Food and Drug Administration (FDA) has approved three NGS-based gene panels: Oncomine Dx Target Test (Thermo Fisher Scientific, Waltham, MA), MSK-IMPACT (Memorial Sloan Kettering Cancer Center, New York, NY), and FoundationOne CDx (Foundation Medicine, Cambridge, MA).3 There is no doubt that we are transitioning to an era during which comprehensive tumor profiling by NGS is routine oncology practice. A recent survey of 1,281 United States oncologists revealed that 75.6% of oncologists used NGS tests to guide treatment decisions in the past 12 months.4 Usage of these tests ranged from decision support for patients with advanced refractory disease to clinical trial eligibility screening and off-label use of FDA-approved drugs. Despite the high adoption rate of NGS tests, studies suggest that many oncologists find it difficult or do not have adequate confidence to interpret NGS results,4-6 which potentially hinders the clinical utility of the results. In this review, we discuss the overall workflow for a clinician to select a platform for genomic testing, interpret the significance of the results, and identify genomically informed treatment options.
OPTIONS FOR NGS
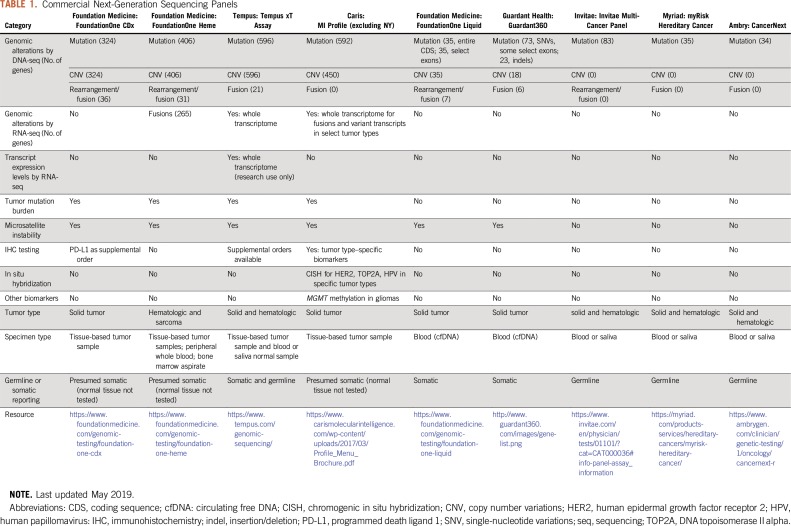
Many larger medical centers offer NGS testing in house for clinical decision making. However, institutions/oncology practices with smaller patient populations thought to benefit from NGS may need to rely on commercial vendors. Even for larger centers, these panels may have specific advantages. There is quite a bit of variability between these platforms. Thus, a summary of commercially available assays aimed at detection of somatic or germline alterations is listed in Table 1.
TABLE 1.
Commercial Next-Generation Sequencing Panels
Clinical Laboratory Improvement Amendments (CLIA) or Good Clinical Laboratory Practice certification is required for any institutional or commercial vendor that offers NGS‐based cancer diagnostic tests. In addition, when an NGS provider is selected, the following specific criteria should be considered. (1) Genes, codons, and alteration types covered by the panel: For example, the FoundationOne Liquid panel does not report gene deletions, and this may be important for tumor types in which loss of tumor suppressor genes, such as PTEN or CDKN2A, is common and may be therapeutically targeted.7,8 (2) Sample available for testing: If insufficient tissue is available, liquid biopsies are an alternative and have the advantage of being minimally invasive, potentially more representative of the entire tumor mutational profile, and possibly preferred when serial testing after treatment is desired.9 (3) Germline or somatic testing: Providers such as Ambry and Myriad offer germline testing with interpretation of variant pathogenicity and guidelines for relative cancer risk. Other assays report somatic or potentially somatic alterations. Notably, an alteration cannot be unequivocally determined to be somatic unless a normal sample is tested alongside; however, most providers who do not test normal tissue take measures to filter out common polymorphisms. (4) Tumor type: Many providers offer cancer type–specific assays, such as FoundationOne Heme, or assays that test for mutations known to predispose to a particular cancer type, such as the Colaris assay for hereditary colorectal and endometrial cancer (Myriad, Salt Lake City, UT). (5) Biomarkers measured: Some panels provide other analytics, such as transcriptome readouts, promoter methylation, and protein expression levels, that cover clinically relevant markers individualized to tumor type or other factors. Benefits include determination of whether a mutation in a tumor suppressor gene leads to loss of expression or detection of immunotherapy markers, such as programmed death ligand 1.10 (6) Interpretation: Commercial vendors that offer interpretation of testing results, as well as do-it-yourself resources, are discussed next.
CONTEXT
Key Objective
How do we operationalize the use of next-generation sequencing and decision support resources to enable high-quality delivery of personalized cancer care to patients on a routine basis?
Knowledge Generated
We reviewed several common commercially available sequencing platforms and their reporting structure, along with publicly available resources for alteration interpretation and identification of genomically matched treatment options. In addition, we identified caveats and limitations that resulted from the lack of standardization of nomenclature and report formatting, and we debuted a public-facing Web site to harmonize level-of-evidence scales among several prevailing standards.
Relevance
Genomic testing plays a critical role in cancer care. However, navigation of the complex landscape of precision oncology is a significant undertaking, which remains a major challenge to clinicians. In this review, we discuss an operational workflow to help a clinician select a genomic testing platform, interpret the significance of results, and identify the genomically informed treatment options.
INTERPRETATION OF ALTERATIONS REPORTED FROM NGS
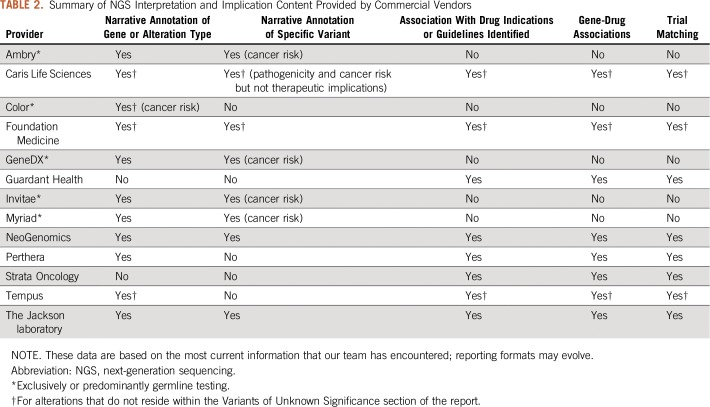
After NGS results are reported, interpretation of the functional and therapeutic significance of the alterations is essential to assess treatment options.11,12 Several commercial vendors offer interpretation bundled with their sequencing panel workflow (Table 2). Germline testing providers, such as Myriad or Ambry, typically report gene and alteration type descriptions (eg, inactivating BRCA1 mutations in general) in addition to alteration-specific (eg, BRCA1 C24R) interpretation for functional significance and cancer risk. Conversely, providers who report somatic mutations vary considerably on the level of interpretation provided, which includes no interpretation, only gene or alteration type summaries, or variant-specific interpretation as well. However, most providers have become consistent in reporting gene-drug associations, FDA indications, potentially National Comprehensive Cancer Network (NCCN) guidelines, and limited clinical trial matching.
TABLE 2.
Summary of NGS Interpretation and Implication Content Provided by Commercial Vendors
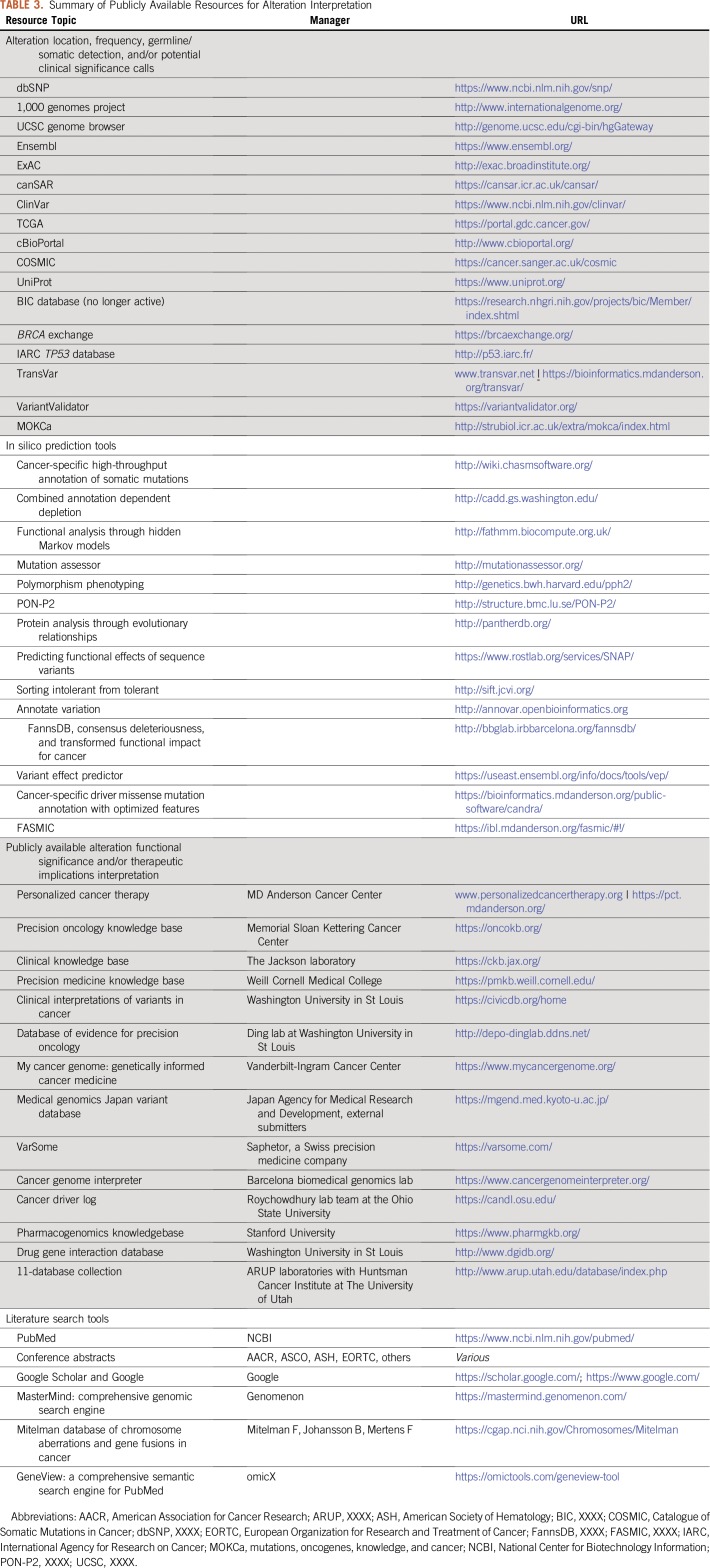
Although some oncologists may choose to conduct their own research for alterations detected on panels that lack sufficient interpretation, the demand of keeping up with ever-evolving data has led several large cancer centers to develop dedicated in-house alteration interpretation teams, such as the Precision Oncology Decision Support team at MD Anderson13 and the Memorial Sloan Kettering OncoKB (Precision Oncology Knowledge Base) team.14 For institutions without such options, in-house alteration interpretation can be performed with the aid of many publicly available resources (Table 3). These tools can assist by identifying exon or protein features (eg, domains, regions, motifs) in which the alteration is located or could be inferred to affect (eg, Ensembl, UniProt, TransVar). In silico prediction tools (eg, SIFT, Polyphen, FASMIC) provide an indication of the alteration’s significance but should be used with strong caution, because methods may not agree and in silico results may not align with experimental observations. Resources that provide alteration frequency, germline/somatic status of previous detections, and potential calls about clinical significance (eg, dbSNP, COSMIC, cBIOPortal, ClinVar) can be used to infer the likelihood that an alteration is a driver event versus a benign polymorphism. Alteration-specific functional and therapeutic significance is provided within several publicly available knowledge bases, including personalizedcancertherapy.org, OncoKB, and JAX-CKB, which provide annotations with references that should be reviewed as the primary source. Finally, tools such as PubMed, Google Scholar, and American Association for Cancer Research or ASCO abstract search engines may be used for direct literature queries.
TABLE 3.
Summary of Publicly Available Resources for Alteration Interpretation
CAVEATS AND LIMITATIONS OF ALTERATION INTERPRETATION
Limitations of NGS testing
Despite the advantages of NGS, one must be cognizant of the inherent limitations. Specific details about tissue sample acquisition, preparation, preservation, and storage that can affect NGS outputs should be predetermined, when possible, and aligned with the specific NGS method and analysis to be used.15 Alteration detection after NGS relies on computational data analysis, which is ever evolving and often varies between users. In most cases, current algorithms are best designed to identify somatic single nucleotide variants (SNVs); small insertions or deletions; and some structural variants/fusions, either relative to patient-matched DNA (when testing normal–tumor pairs) or to a standardized genome (when testing tumor only). In addition, copy number alterations (CNAs) may be estimated on the basis of the number of reads that covers the genetic region.16 Each of these types of aberrations requires a different computational approach, and there are important caveats that should be considered. For example, the fraction of cancer cells within the sample can affect the detection of aberrations, especially SNVs with a low variant allele frequency (VAF) and gene loss CNAs. In addition, the detection of larger insertions or deletions and more complex SNVs can be computationally challenging; thus, methods and calling algorithms intended to assess specific aberrations of these types may be absent or quite variable. Recommendations focused on these issues in clinical NGS development and use are recently published.16,17
Insufficient Nomenclature
Sometimes insufficient information is provided within the sequencing report, which hinders interpretation of the functional and therapeutic significance of alterations. The Association for Molecular Pathology, ASCO, and College of American Pathologists issued a joint recommendation for CLIA-accredited laboratories that included guidelines to properly report alterations.18 Here, we present two real case scenarios in which insufficient data elements hindered interpretation. In case 1, PDGFRB (platelet-derived growth factor receptor beta) rearrangement was reported without specific information on the type of rearrangement or fusion partner. Because PDGFRB fusions that retain the kinase domain and result in a gain of function may be therapeutically targeted,19,20 the treating oncologist was considering PDGFR-targeted therapies as treatment options. Additional inquiry revealed a fusion between PDGFRB and RB1 that did not retain the kinase domain of PDGFRB, so the mutation was not actionable for PDGFRB inhibitors and likely inactivated RB1. Loss of RB1 is relevant as it confers resistance to clinically available cyclin-dependent kinase 4/6 inhibitors.2 In case 2, an NGS provider reported BRCA1 truncation intron 18. Truncating mutations typically are described within coding regions, so this terse nomenclature delayed care recommendation until it was confirmed that this alteration was a genomic deletion that encompassed exons 19 to 23.
Ambiguous Nomenclature
Even sufficient protein nomenclature can lead to ambiguity without the inclusion of genomic coordinates and/or the reference transcript. For example, FGFR1:p.T726A maps to both chr8:g.38271680T>C and chr8:38271773T>C mutations, and chr7:g.116412023A>T maps to both MET:p.Y1021F and MET:p.Y1003F, dependent on the transcripts used in each case. Thus, it is ideal that all nomenclatures reported and used within a knowledge base be normalized to genomic coordinates to support a query. When not provided, the multilevel variant annotator TransVar21 can be used to convert all protein nomenclatures to genomic coordinates and vice versa. This tool was used to identify the correlative MET:p.Y1003 mutation, which multiple publications described as a gain-of-function alteration,22-24 from a query of MET:p.Y1021F. The biologic significance and therapeutic implication of correlative alterations such as these should be judged on a case-by-case basis. If it is concluded that the functional and clinical consequences are identical, then the alterations should be associated with each other to enhance recall.
Potentially Misleading Representation
When a commercial vendor is used for interpretation, all evidence provided should be examined rather than taken as “front-page” recommendations at face value. In a case example, BRCA2 E2981K was reported alongside FDA-approved poly (ADP-ribose) polymerase inhibitors as a therapy with clinical benefit on the front page of a clinical report. Although substantial evidence exists for targeting deleterious BRCA2 mutations with poly (ADP-ribose) polymerase inhibitors,25-27 this particular variant was of unknown functional significance, as described within its detailed description located later in the report. In another case, a patient with equivocal FGF ligand amplifications was not considered for an FGFR (fibroblast growth factor receptor) inhibitor clinical trial, because equivocal amplifications did not meet trial eligibility criteria. However, additional examination of the VUS (variant of unknown significance) section revealed FGFR1 amplification, which was actionable for clinical trial accrual. Thus, an alteration’s location within the report is not definitive for actionability.
SURVEYANCE OF TREATMENT OPTIONS
After actionable alterations are identified, the oncologist must next assess treatment options applicable to the patients’ molecular and clinical profile, including FDA-approved therapies, expert panel recommendations (eg, NCCN), and/or clinical trials. However, not all CLIA-validated panels currently return this information or may do so in a limited fashion. We surveyed several commercial NGS vendors and summarized their coverage of the treatment options in Table 2. Most commercial vendors, as well as dedicated decision support teams, routinely use the following publicly available resources.
FDA-Approved Drugs
The FDA Web site Drugs@FDA28 lists all drugs approved by the agency as well as links to the labels that detail their indications. Each indication must be analyzed thoroughly to determine whether it is indicated for a specific biomarker and for which cancer types. Metadata about the patient’s alteration and the FDA-indicated biomarker must be considered to determine if the biomarkers match. For example, EGFR L747_A750delinsP matches to the erlotinib FDA indication for EGFR exon 19 deletions. If the cancer type(s) in the indication match the patient’s (when disease hierarchy is considered), this treatment option is generally considered to be the one with the highest level of supporting evidence. However, if the cancer types differ, the use of the therapy for this indication would be considered off label.
Standard-of-Care Options
NCCN compiles clinical practice guidelines in oncology by cancer type and is considered an authoritative reference for standard-of-care options within the United States. Compendiums from the perspective of drugs and biologics and biomarkers are also made available for a fee that facilitates searches of the concepts that involve drugs and biomarkers.29,30
Clinical Trials
Within the United States, ClinicalTrials.gov is the most comprehensive registry of clinical trials. To identify relevant trials, users can search by a combination of cancer types, drugs, and/or biomarkers. However, searches like this will only return clinical trials with text that exactly matches the keywords. All of the aforementioned concepts may have synonymous terms that should be accounted for in the search. Furthermore, cancer types are hierarchical in nature, and molecular aberrations as drug targets are usually not explicitly stated in ClinicaTrials.gov—sometimes, not even specifically stated in the protocol—which may compromise recall. To provide more robust and streamlined support, some informatics infrastructure is critical. Zeng et al31 have reviewed related work and presented a generalized framework that addresses the unique needs of precision oncology and aims to interpret NGS results.
PRIORITIZATION OF TREATMENT OPTIONS
In patients who undergo NGS, prioritization of treatment options is an important area to consider. Needs include the following: (1) prioritization of genomically targeted treatment options when more than one exists; (2) prioritization of targets when more than one actionable alteration exists; and (3) identification of optimal therapy, through comparison of expected efficacy of genomically matched therapy with expected outcome of standard-of-care options.
Level of Evidence
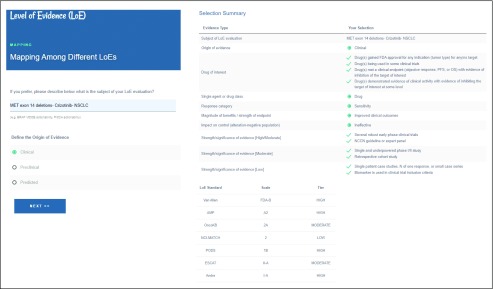
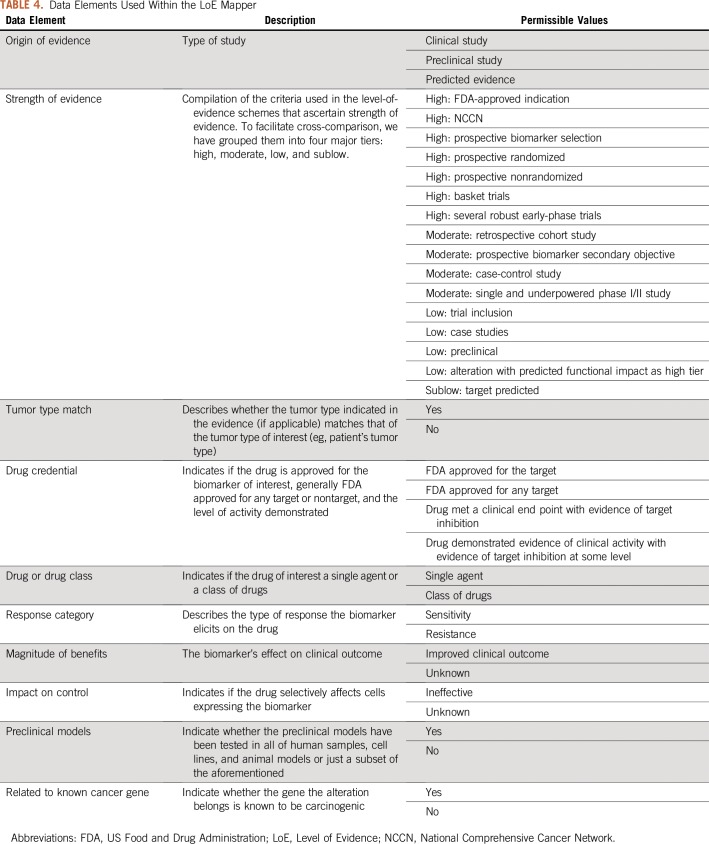
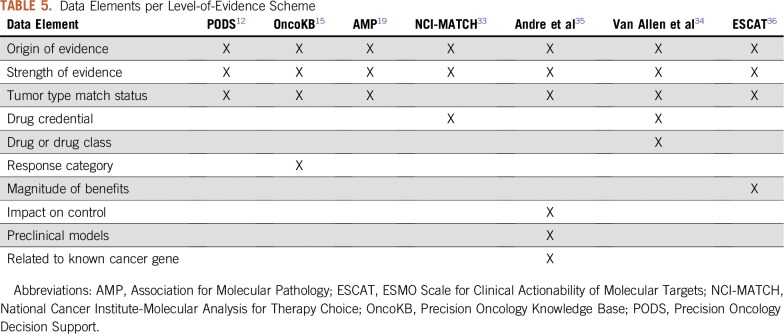
To prioritize treatment options when multiple actionable alterations/therapies are identified, a quantification of their level of evidence (LoE) is necessary. To that effect, several LoE standards have been proposed. Although each has its own merit and unique perspectives, the lack of interoperability strategy that allows for a direct comparison from one to another complicates their utility. Here, we propose an interoperability tool that analyzes all of the unique features of seven existing LoE standards: Precision Oncology Decision Support,11 OncoKB,14 Association for Molecular Pathology,18 NCI-MATCH (National Cancer Institute-Molecular Analysis for Therapy Choice),32 Van Allen et al,33 Andre et al,34 and ESCAT (ESMO Scale for Clinical Actionability for Molecular Targets)35 (Fig 1). To maximize interoperability, we extracted the features that contribute to the LoE assignment of each scheme and developed a Web application (https://pct.mdanderson.org/loe/) that interactively guides the users to provide necessary information of their sources to match the evidence with as many LoE schemes as possible. The data elements considered by the application, along with their permissible values in square brackets, are enumerated in Table 4. Table 5 lists the background of the data element by indicating the LoE schemes that use them for LoE assignment.
FIG 1.
Level of Evidence (LoE) mapper. The LoE mapper provides an interface for a user to provide a description of the evidence found for use of a therapy within the context of a specified disease harboring a specific biomarker. On the basis of the evidence criteria selected, the LoE scale associated with seven standards is displayed along with a tier which we have defined within this article.
TABLE 4.
Data Elements Used Within the LoE Mapper
TABLE 5.
Data Elements per Level-of-Evidence Scheme
VAF and CNA Levels
VAF and CNA levels can help physicians choose treatment options. Priority may be given to the mutation with higher VAF or gene copy number, when a patient has multiple alterations that are otherwise equally targetable. In addition, VAF via sequencing of liquid biopsy is used as a noninvasive method to monitor response to treatment and disease progression. This may provide an earlier measure of patient prognosis compared with imaging-based methods.36-38 When a matched normal sample is not available, the VAF can give insight about the potential that an alteration is a germline mutation.18 Also, an extremely low VAF may warrant additional confirmation of the alteration with orthogonal technologies, such as Sanger sequencing. Low VAF would suggest that an alteration is subclonal, and subclonal alterations are likely inferior therapeutic targets.
Consideration of Alterations Within the Context of One Another
Multiple mutations also can exist in the same driver gene; thus, treatment options must be tailored according to the functions of all mutations. For example, a clinical study reported that first-generation epidermal growth factor receptor (EGFR) inhibitors are not effective in patients who have non–small-cell lung cancer with coexisting EGFR inhibitor–sensitive and –resistant mutations; conversely, the third-generation inhibitor osimertinib demonstrated benefit.39
Feedback and crosstalk between signaling pathways adds another layer of complexity in cancer treatment. In the scenario of concomitant driver mutations, multiple pathways may compensate each other and counteract the effect of a drug that targets one pathway. It is already known that KRAS mutations confer resistance to EGFR-targeted therapy in colon cancer. Recently, it also has been shown that KRAS mutations confer resistance to human epidermal growth factor receptor 2–targeted therapy (trastuzumab/pertuzumab) for HER2-amplified colon cancer.40 It thus is likely that these principles of drug resistance are extrapolatable to other drug classes and to other mitogen-activated protein kinase pathway alterations. Additional data are needed to determine how to best incorporate these principles into clinical decision support.
There has been growing interest in targeting multiple driver alterations, when they exist, to broaden actionability and enhance efficacy. Although this may not be feasible in some cases because of expected toxicity, a pilot study by Sicklick et al41 demonstrated that, in experienced clinical trial units, a combination therapy approach may indeed be feasible and that targeting a larger fraction of alterations may improve disease control rates, progression-free survival, and overall survival.
INCORPORATION OF NGS INTO ROUTINE CLINICAL PRACTICE: CURRENT CHALLENGES AND FUTURE DIRECTIONS
Although NGS is used increasingly, several clinical challenges to implementation of NGS as part of routine care remain. These include the broader context of when NGS should be ordered, especially in tumor types for which there are no drugs approved by the FDA on the basis of a genomic marker. Although some studies were not able to demonstrate an advantage of genomically matched therapy,42 Kopetz et al43 demonstrated with a larger panel that patients with actionable alterations who were treated with matched therapy had improved overall survival compared with patients who were not.
Debate also remains about the utility of fresh biopsies versus archival tissue for NGS, the value of repeat biopsies and NGS after intervening therapy, and the use of liquid biopsies for serial monitoring. However, there is increasing recognition of genomic evolution, mechanisms of acquired resistance, and emerging strategies to overcome these resistance mechanisms. Overall, it is likely that the most compelling alterations therapeutically may be truncal alterations; however, emerging alterations, such as MET amplifications after use of EGFR inhibitors in lung cancer, represent promising therapeutic opportunities.44
For NGS to truly affect outcomes, patients with actionable alterations need access to genomically matched therapies. Thus, it is critical for them have access to a large clinical trial portfolio so that compelling alterations can be acted upon either in the context of standard of care or via investigational therapies. For example, Dumbrava et al45 recently demonstrated that patients with HER2 amplification, even beyond breast and gastric cancer, had improved overall survival if they received human epidermal growth factor receptor 2–targeted therapy.
Finally, to date, most NGS-based decision making has been based on the principle of matching a single gene to a single targeted therapy. It is likely that combination therapies may enhance efficacy by deepening responses and enhancing their durability. Thus, future decision support efforts must be nimble in incorporation of multianalyte information as well as dynamic markers, such as pharmacodynamic markers, or in target inhibition and adaptive response to facilitate selection of optimal monotherapy or combination therapy in a longitudinal continuum.
Footnotes
Supported in part by The Cancer Prevention and Research Institute of Texas (Award No. RP150535), the Sheikh Khalifa Bin Zayed Al Nahyan Institute for Personalized Cancer Therapy, National Center for Advancing Translational Sciences Grant No. UL1 TR000371 (Center for Clinical and Translational Sciences), and the MD Anderson Cancer Center Support Grant No. P30 CA016672.
AUTHOR CONTRIBUTIONS
Conception and design: Jia Zeng, Amber Johnson, Scott Woodman, Funda Meric-Bernstam
Collection and assembly of data: Jia Zeng, Amber Johnson, Md Abu Shufean, Michael Kahle, Dong Yang, Thuy Vu, Shhyam Moorthy, Vijaykumar Holla, Funda Meric-Bernstam
Data analysis and interpretation: Jia Zeng, Amber Johnson, Md Abu Shufean, Dong Yang, Michael Kahle, Thuy Vu, Funda Meric-Bernstam
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/cci/author-center.
Jia Zeng
Stock and Other Ownership Interests: McKesson, Mylan
Dong Yang
Employment: Molecular Health
Thuy Vu
Employment: Roche
Funda Meric-Bernstam
Honoraria: Sumitomo Group, Dialectica
Consulting or Advisory Role: Genentech, Inflection Biosciences, Pieris Pharmaceuticals, Clearlight Diagnostics, Darwin Health, Samsung Bioepis, Spectrum Pharmaceuticals, Aduro Biotech, Origimed, Xencor, Debiopharm Group, Mersana, Seattle Genetics
Research Funding: Novartis, AstraZeneca, Taiho Pharmaceutical, Genentech, Calithera Biosciences, Debiopharm Group, Bayer, Aileron Therapeutics, Puma Biotechnology, CytomX Therapeutics, Jounce Therapeutics, Zymeworks, Curis, Phfizer, eFFECTOR Therapeutics, AbbVie, Boehringer Ingelheim (I), Guardant Health (Inst), Daiichii Sankyo, GlaxoSmithKline
Speakers Bureau: Chugai Pharmaceuticals
No other potential conflicts of interest were reported.
REFERENCES
- 1.Rubinstein WS, Maglott DR, Lee JM, et al. The NIH genetic testing registry: A new, centralized database of genetic tests to enable access to comprehensive information and improve transparency. Nucleic Acids Res. 2013;41:D925–D935. doi: 10.1093/nar/gks1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O’Leary B, Finn RS, Turner NC. Treating cancer with selective CDK4/6 inhibitors. Nat Rev Clin Oncol. 2016;13:417–430. doi: 10.1038/nrclinonc.2016.26. [DOI] [PubMed] [Google Scholar]
- 3.Nagahashi M, Shimada Y, Ichikawa H, et al. Next-generation sequencing–based gene panel tests for the management of solid tumors. Cancer Sci. 2019;110:6–15. doi: 10.1111/cas.13837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Freedman AN, Klabunde CN, Wiant K, et al. Use of next-generation sequencing tests to guide cancer treatment: Results from a nationally representative survey of oncologists in the United States. JCO Precis Oncol. doi: 10.1200/PO.18.00169. 10.1200/PO.18.00169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gray SW, Hicks-Courant K, Cronin A, et al. Physicians’ attitudes about multiplex tumor genomic testing. J Clin Oncol. 2014;32:1317–1323. doi: 10.1200/JCO.2013.52.4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnson LM, Valdez JM, Quinn EA, et al. Integrating next-generation sequencing into pediatric oncology practice: An assessment of physician confidence and understanding of clinical genomics. Cancer. 2017;123:2352–2359. doi: 10.1002/cncr.30581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Álvarez-Garcia V, Tawil Y, Wise HM, et al. Mechanisms of PTEN loss in cancer: It’s all about diversity. Semin Cancer Biol. doi: 10.1016/j.semcancer.2019.02.001. . [epub ahead of print on February 7, 2019] [DOI] [PubMed] [Google Scholar]
- 8.Hamid A, Petreaca B, Petreaca R. Frequent homozygous deletions of the CDKN2A locus in somatic cancer tissues. Mutat Res. 2019;815:30–40. doi: 10.1016/j.mrfmmm.2019.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koessler T, Addeo A, Nouspikel T. Implementing circulating tumor DNA analysis in a clinical laboratory: A user manual. Adv Clin Chem. 2019;89:131–188. doi: 10.1016/bs.acc.2018.12.004. [DOI] [PubMed] [Google Scholar]
- 10.Lee HT, Lee SH, Heo YS. Molecular interactions of antibody drugs targeting PD-1, PD-L1, and CTLA-4 in immuno-oncology. Molecules. 2019;24:E1190. doi: 10.3390/molecules24061190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meric-Bernstam F, Johnson A, Holla V, et al. A decision support framework for genomically informed investigational cancer therapy. J Natl Cancer Inst. 2015;107:djv098. doi: 10.1093/jnci/djv098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson A, Zeng J, Bailey AM, et al. The right drugs at the right time for the right patient: The MD Anderson precision oncology decision support platform. Drug Discov Today. 2015;20:1433–1438. doi: 10.1016/j.drudis.2015.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson A, Khotskaya YB, Brusco L, et al. Clinical use of precision oncology decision support. JCO Precis Oncol. doi: 10.1200/PO.17.00036. 10.1200/PO.17.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chakravarty D, Gao J, Phillips SM, et al. OncoKB: A precision oncology knowledge base. JCO Precis Oncol. doi: 10.1200/PO.17.00011. 10.1200/PO.17.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bohannan ZS, Mitrofanova A. Calling variants in the clinic: Informed variant calling decisions based on biological, clinical, and laboratory variables. Comput Struct Biotechnol J. 2019;17:561–569. doi: 10.1016/j.csbj.2019.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jennings LJ, Arcila ME, Corless C, et al. Guidelines for validation of next-generation sequencing-based oncology panels: A joint consensus recommendation of the Association for Molecular Pathology and College of American Pathologists. J Mol Diagn. 2017;19:341–365. doi: 10.1016/j.jmoldx.2017.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roy S, Coldren C, Karunamurthy A, et al. Standards and guidelines for validating next-generation sequencing bioinformatics pipelines: A joint recommendation of the association for Molecular Pathology and the College of American Pathologists. J Mol Diagn. 2018;20:4–27. doi: 10.1016/j.jmoldx.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 18.Li MM, Datto M, Duncavage EJ, et al. Standards and guidelines for the interpretation and reporting of sequence variants in cancer: A joint consensus recommendation of the Association for Molecular Pathology, American Society of Clinical Oncology, and College of American Pathologists. J Mol Diagn. 2017;19:4–23. doi: 10.1016/j.jmoldx.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.David M, Cross NC, Burgstaller S, et al. Durable responses to imatinib in patients with PDGFRB fusion gene–positive and BCR-ABL–negative chronic myeloproliferative disorders. Blood. 2007;109:61–64. doi: 10.1182/blood-2006-05-024828. [DOI] [PubMed] [Google Scholar]
- 20.Cheah CY, Burbury K, Apperley JF, et al. Patients with myeloid malignancies bearing PDGFRB fusion genes achieve durable long-term remissions with imatinib. Blood. 2014;123:3574–3577. doi: 10.1182/blood-2014-02-555607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou W, Chen T, Chong Z, et al. TransVar: A multilevel variant annotator for precision genomics. Nat Methods. 2015;12:1002–1003. doi: 10.1038/nmeth.3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abella JV, Peschard P, Naujokas MA, et al. Met/hepatocyte growth factor receptor ubiquitination suppresses transformation and is required for HRS phosphorylation. Mol Cell Biol. 2005;25:9632–9645. doi: 10.1128/MCB.25.21.9632-9645.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kong-Beltran M, Seshagiri S, Zha J, et al. Somatic mutations lead to an oncogenic deletion of MET in lung cancer. Cancer Res. 2006;66:283–289. doi: 10.1158/0008-5472.CAN-05-2749. [DOI] [PubMed] [Google Scholar]
- 24.Peschard P, Park M. From Tpr-Met to Met, tumorigenesis and tubes. Oncogene. 2007;26:1276–1285. doi: 10.1038/sj.onc.1210201. [DOI] [PubMed] [Google Scholar]
- 25.Kurnit KC, Coleman RL, Westin SN. Using PARP inhibitors in the treatment of patients with ovarian cancer. Curr Treat Options Oncol. 2018;19:1. doi: 10.1007/s11864-018-0572-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Litton JK, Rugo HS, Ettl J, et al. Talazoparib in patients with advanced breast cancer and a germline BRCA mutation. N Engl J Med. 2018;379:753–763. doi: 10.1056/NEJMoa1802905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Musella A, Bardhi E, Marchetti C, et al. Rucaparib: An emerging PARP inhibitor for treatment of recurrent ovarian cancer. Cancer Treat Rev. 2018;66:7–14. doi: 10.1016/j.ctrv.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 28.2019. Drugs@FDA. FDA-approved drug products. Rockville, MD, US Department of Health and Human Services.
- 29.Biomarkers Compendium NCCN. https://www.nccn.org/Store/Login/Login.aspx?ReturnURL=/professionals/biomarkers/content/ National Comprehensive Cancer Network:
- 30.Drugs and Biologics Compendium NCCN. https://www.nccn.org/store/login/login.aspx?ReturnURL=/professionals/drug_compendium/content National Comprehensive Cancer Network:
- 31.Zeng J, Shufean MA, Khotskaya Y, et al. OCTANE: Oncology clinical trial annotation engine. JCO Clin Cancer Inform. 2019;3:1–11. doi: 10.1200/CCI.18.00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brower V. NCI-MATCH pairs tumor mutations with matching drugs. Nat Biotechnol. 2015;33:790–791. doi: 10.1038/nbt0815-790. [DOI] [PubMed] [Google Scholar]
- 33.Van Allen EM, Wagle N, Stojanov P, et al. Whole-exome sequencing and clinical interpretation of formalin-fixed, paraffin-embedded tumor samples to guide precision cancer medicine. Nat Med. 2014;20:682–688. doi: 10.1038/nm.3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Andre F, Mardis E, Salm M, et al. Prioritizing targets for precision cancer medicine. Ann Oncol. 2014;25:2295–2303. doi: 10.1093/annonc/mdu478. [DOI] [PubMed] [Google Scholar]
- 35.Mateo J, Chakravarty D, Dienstmann R, et al. A framework to rank genomic alterations as targets for cancer precision medicine: The ESMO scale for clinical actionability of molecular targets (ESCAT) Ann Oncol. 2018;29:1895–1902. doi: 10.1093/annonc/mdy263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dawson SJ, Tsui DW, Murtaza M, et al. Analysis of circulating tumor DNA to monitor metastatic breast cancer. N Engl J Med. 2013;368:1199–1209. doi: 10.1056/NEJMoa1213261. [DOI] [PubMed] [Google Scholar]
- 37.de Figueiredo Barros BD, Kupper BEC, Aguiar S. et al. Mutation detection in tumor-derived cell free DNA anticipates progression in a patient with metastatic colorectal cancer. Front Oncol. 2018;8:306. doi: 10.3389/fonc.2018.00306. Jr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lin SY, Huang SK, Huynh KT, et al. Multiplex gene profiling of cell-free DNA in patients with metastatic melanoma for monitoring disease. JCO Precis Oncol. doi: 10.1200/PO.17.00225. 10.1200/PO.17.00225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang B, Xu J, Zhang X, et al. Coexistence of sensitive and resistant epidermal growth factor receptor (EGFR) mutations in pretreatment non–small-cell lung cancer (NSCLC) patients: First or third generation tyrosine kinase inhibitors (TKIs)? Lung Cancer. 2018;117:27–31. doi: 10.1016/j.lungcan.2018.01.006. [DOI] [PubMed] [Google Scholar]
- 40.Meric-Bernstam F, Hurwitz H, Raghav KPS, et al. Pertuzumab plus trastuzumab for HER2-amplified metastatic colorectal cancer (MyPathway): An updated report from a multicentre, open-label, phase 2a, multiple basket study. Lancet Oncol. 2019;20:518–530. doi: 10.1016/S1470-2045(18)30904-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sicklick JK, Kato S, Okamura R, et al. Molecular profiling of cancer patients enables personalized combination therapy: The I-PREDICT study. Nat Med. 2019;25:744–750. doi: 10.1038/s41591-019-0407-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Le Tourneau C, Delord JP, Gonçalves A, et al. Molecularly targeted therapy based on tumour molecular profiling versus conventional therapy for advanced cancer (SHIVA): A multicentre, open-label, proof-of-concept, randomised, controlled phase 2 trial. Lancet Oncol. 2015;16:1324–1334. doi: 10.1016/S1470-2045(15)00188-6. [DOI] [PubMed] [Google Scholar]
- 43.Kopetz S, Shaw KRM, Lee JJ, et al. Use of a targeted exome next-generation sequencing panel offers therapeutic opportunity and clinical benefit in a subset of patients with advanced cancers. JCO Precis Oncol. doi: 10.1200/PO.18.00213. 10.1200/PO.18.00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sequist LA LJ, Han JY, Su WC, et al. TATTON Phase Ib expansion cohort: Osimertinib plus savolitinib for patients with EGFR-mutant, MET-amplified NSCLC after progression on prior third-generation epidermal growth factor receptor tyrosine kinase inhibitor Cancer Res792019 suppl; abstr CT033 [Google Scholar]
- 45.Ileana Dumbrava E, Balaji K, Raghav K, et al. Targeting ERBB2 (HER2) amplification identified by next-generation sequencing in patients with advanced or metastatic solid tumors beyond conventional indications. JCO Precis Oncol. doi: 10.1200/PO.18.00345. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]