Abstract
BACKGROUND:
Elevated D-dimers in injured patients with paradoxically low fibrinolysis activity measured by viscoelastic assays have been speculated to be “occult” fibrinolysis. However, an alternative explanation is that these patients have previously activated their fibrinolytic system and have shut it down by the time of blood draw, and would gain no benefit in clot strength with tranexamic acid (TXA). We hypothesize that TXA will not increase clot strength in injured patients with low fibrinolytic activity measured by thrombelastography (TEG), despite biomarkers of fibrinolysis activation.
STUDY DESIGN:
Three TEG assays (rapid, tissue plasminogen activator, and functional fibrinogen) were run on trauma patients. The tissue plasminogen activator TEG served as a functional assay to quantify depletion of fibrinolysis inhibitors (DFI). Patients were stratified by DFI vs non-DFI and then by rapid TEG lysis at 30 minutes phenotype cutoffs. Response to TXA was evaluated with functional fibrinogen TEG by calculating percent change in clot strength with the addition of exogenous TXA in the TEG cup.
RESULTS:
Six hundred and thirty patients with a median new injury severity score of 20 were analyzed. Depletion of fibrinolysis inhibitors was present in 116 (18%). The DFI patients had significantly increased D-dimer (p < 0.001) and lower fibrinogen (p < 0.001). The DFI patients had increased rates of massive transfusion (33% vs 3.3%; p < 0.001) and mortality (40% vs 6.2%; p ≤ 0.001). Among DFI patients, TXA significantly improved fibrin clot strength with hyperfibrinolysis (+19% clot strength; p < 0.001) but not with shutdown (+1.2%) or physiologic (−2.5%).
CONCLUSIONS:
Patients with DFI have multiple abnormalities of their coagulation system, but only DFI patients with hyperfibrinolysis have improved fibrin clot strength with TXA. (J Am Coll Surg 2019;229: 92–101.
Early activation of the fibrinolytic system with subsequent inhibition of fibrinolysis after injury was first described by Innes and Sevitt in 1964.1 This phenomenon was subsequently defined as fibrinolysis shutdown, which was also observed in multiple clinical settings in which patients experienced an acute physiologically stress.2 Although animal work has demonstrated the adverse effects of fibrinolysis shutdown driving organ failure and irreversible shock,3 poor outcomes with low fibrinolytic activity after injury were not recognized until 50 years later.4 Viscoelastic assessment of blood demonstrating low fibrinolytic activity has documented increased mortality in adults5–8 and children9 after injury and in the ICU.6
Recently, a sub-phenotype of trauma patients with low fibrinolysis activity measure by thrombelastography (TEG) with a paradoxical sensitivity to tissue plasminogen activator (t-PA) was identified.10 Several additional studies have also described patients with low fibrinolytic activity measured by viscoelastic hemostatic assays (VHA), but elevated levels of D-dimer levels and plasmin antiplasmin levels,6–8 indicating these patients had activated their fibrinolytic system. Some authors have interpreted these findings to suggest VHAs are insensitive to measuring fibrinolysis in trauma patients,6 and specifically these patients harbor an “occult” hyperfibrinolysis.8
However, an alternative explanation for these findings is that patients have previously activated their fibrinolytic system and have shut it down by the time of blood draw, similarly to what was described decades earlier.1 Unlike t-PA and plasmin, which have circulating half-lives of minutes,11,12 D-dimer and plasmin antiplasmin levels remain in the circulation for 12 to 16 hours.13 Therefore, if the classic description of fibrinolysis shutdown is accurate, patients with low fibrinolysis measured by VHA and elevated D-dimer levels would not obtain a hemostatic benefit with TXA. We hypothesize that TXA will not increase fibrin clot strength in injured patients with low fibrinolytic activity measured by TEG, despite biomarkers of fibrinolysis activation.
METHODS
Patients characteristics and outcomes
Adult trauma patients meeting criteria for the highest level of activation at our Level 1 trauma center (Ernest E Moore Shock and Trauma Center at Denver Health) from 2014 to 2018 were included in this analysis. Patients had samples collected under protocols approved by the Colorado Multiple IRB for prospective evaluation of coagulation status in response to trauma. Patient demographics, injury mechanism, laboratory results, and transfusion requirements were recorded by professional research assistants who provide on-site, continuous coverage of the emergency department. Injury severity was calculated by Abbreviated Injury Scale scores for the head/neck, chest, abdomen, and extremities, which was summated as the top 3 scores, irrespective of location, to calculate the New Injury Severity Score (NISS). The patient’s coagulation score was calculated at the time of emergency department admission by the surgical attending using previously published grading scores.14 Massive transfusion was defined as >10 units of RBC within the first 6 hours post injury. Cause of death was adjudicated by the senior investigator (EEM) at the weekly laboratory meetings. The research team was blinded to the patient’s coagulation status when discussing mortality outcomes. Attributable causes of death included lack of mechanical surgical control (exsanguination from inability to achieve surgical bleeding controlled), coagulopathy (ongoing bleeding, despite surgical control), physiologic collapse (continued metabolic failure despite mechanical control of bleeding), traumatic brain injury, and multiple organ failure.
Blood collection
Blood was collected in 3.5-mL tubes containing 3.2% citrate in the prehospital ambulance or on arrival to the emergency department. Professional research assistants performed coagulation assays within 2 hours of blood draw. Additional assays were ordered at the discretion of the treating surgeon and performed by the hospital laboratory.
Viscoelastic assays
All viscoelastic assays were conducted by a team of trained professional research assistants with extensive experience performing TEG assays. Citrated blood samples were analyzed using the TEG 5000 Thrombelastography Hemostasis Analyzer (Haemonetics, Niles, IL). The following indices were obtained from the tracings of the TEG: activated clotting time (minutes), angle (degrees), maximum amplitude (mm), and lysis 30 minutes after MA (LY30, %).
Rapid TEG (kaolin and tissue factor-activated TEG) was conducted according to manufactured recommendations. An additional modified assay to quantify sensitivity to fibrinolysis was done in parallel, termed t-PA challenged TEG. In brief, 500 μL of whole blood was pipetted into a customized vial containing lyophilized t-PA (Thrombo Therapeutics, Inc) to a final concentration of 75 ng/mL, and mixed by gentle inversion. A 340-μL aliquot of this mixture was then transferred to a 37°C TEG cup, preloaded with 20 μL of 0.2 mol/L of CaCl2 without tissue factor or kaolin activator. The differential functional fibrinogen TEG (DIFF-TEG; Thrombo Therapeutics, Inc) assay was also performed to measure the degree of TXA-reversible fibrinolysis in the patient’s blood sample. The DIFF-TEG assay consists of the comparison of 2 channels of Functional Fibrinogen assay (Haemonetics), both of which are run on platelet-inhibited samples containing abciximab, and one channel additionally containing a maximal inhibitor concentration of TXA lyophilized in the TEG cup (180 μg per cup).
Statistical analysis
SPSS, version 23 (IBM) was used for statistical analyses. Clinical and TEG measurements are presented as median and interquartile range (IQR, 25th to 75th percentile). Using receiver operating characteristic curves, the inflection point for massive transfusion with the t-PA TEG LY30 was used to re-identify a cut point for patients who had an increased risk of bleeding, using the Youden index. We used this cut point as an indication of pathologic depletion of fibrinolytic inhibitors, and used the proteomics of 108 patients from an earlier study10 to confirm depletion of fibrinolytic regulators. The methods for quantifying proteins levels have been described previously.15 Fibrin clot strength in res ponse to TXA was assessed based on the percent change of clot strength between the patient’s ex vivo functional fibrinogen with TXA to strength without. The TEG indices and clot strength in response to TXA were contrasted between cohorts using a Mann-Whitney U test (for 2 comparisons) or Kruskal-Wallis test (for 3 or more comparisons). Categorical variables were contrasted by chi-square analysis.
RESULTS
Study population
There were 630 patients included in this study, 46 patients in the prospective database were excluded for not having rapid TEG or t-PA TEG. Median age of the study population was 34 years (IQR 27 to 48 years) and subjects were predominantly male (80%). The majority were severely injured (median NISS 20; IQR 9 to 34) with 55% of patients sustaining blunt trauma. Massive transfusion occurred in 8.7% of patients and the overall mortality was 12%.
Depletion of fibrinolytic inhibitors
Receiver operating characteristic identified a t-PA TEG LY30 >42% as an inflection point for massive transfusion using the Youden index. In the nested cohort of patients with earlier proteomic analysis, patients with a t-PA TEG LY30 higher than this threshold had a reduction in the majority of proteases known to inhibit fibrinolysis (α2 antiplasmin, thrombin activatable fibrinolysis inhibitor, coagulation factor XIII, vitronectin; all, p < 0.001) and depletion of plasminogen (p < 0.001) (Table 1). The DFI patients had significantly elevated D-dimers (14.9; IQR 3.5 to 20.0 vs 1.8; IQR 0.4 to 6.6; p < 0.001) and decreased fibrinogen levels (163; IQR 124 to 2,250 vs 230; IQR 118 to 292; p < 0.001) compared with non-DFI patients in the overall study population indicating that the fibrinolytic system had been activated.
Table 1.
Protein Concentrations in Patients With and Without Depletion of Fibrinolytic Inhibitors
| Protein | Non-DFI, median (range) | DFI, median (range) | p Value |
|---|---|---|---|
| Fibrinogen α | 127.2 (99.59–155.7) | 93.75 (64.90–108.1) | <0.001 |
| Fibrinogen β | 260.8 (206.5–328.0) | 195.3 (131.6–228.6) | 0.001 |
| Fibrinogen γ | 108.0 (89.08–136.6) | 81.23 (54.92–96.88) | 0.002 |
| Plasminogen | 16.09 (13.01–19.73) | 12.80 (8.582–16.65) | 0.005 |
| α2 antiplasmin | 5.334 (4.515–6.168) | 3.850 (2.570–4.896) | <0.001 |
| α2 macroglobulin | 286.6 (215.4–328.9) | 264.3 (157.0–346.5) | 0.569 |
| Factor XIII A | 1.288 (1.006–1.520) | 0.962 (0.742–1.150) | <0.001 |
| Factor XIII B | 2.141 (1.839–2.380) | 1.691 (1.286–2.074) | 0.001 |
| TAFI | 0.309 (0.250–0.350) | 0.240 (0.167–0.293) | 0.001 |
| C-l esterase inhibitor | 14.02 (11.69–16.36) | 11.33 (8.157–14.570) | 0.008 |
| αl anti-trypsin | 129.6 (111.8–151.0) | 110.3 (74.31–143.3) | 0.030 |
| Vitronectin | 25.75 (21.16–29.96) | 19.59 (13.14–24.69) | <0.001 |
| Albumin | 3,093 (2,770–3,529) | 2,665 (1,933–3,388) | 0.466 |
All measurements are in mg/dL.
DFI, depletion of fibrinolysis inhibitors; TAFI, thrombin-activated fibrinolysis inhibitor.
Clinical characteristics of depletion of fibrinolysis inhibitors patients
The t-PA TEG stratified 18% of patients as having DFI. Clinical characteristics of DFI vs non-DFI are listed in Table 2 with no major differences between age and injury mechanism, but patients with DFI were more severely injured, with higher degrees of shock, and all coagulation measurements were more hypocoagulable.
Table 2.
Demographics, Injury Patterns, and Outcomes in Patients With and Without Depletion of Fibrinolytic Inhibitors
| Variable | Non-DFI | DFI | p Value |
|---|---|---|---|
| Age, y, median (IQR) | 33 (26–48) | 37 (26–50) | 0.270 |
| Male, % | 79 | 83 | 0.445 |
| Blunt injury, % | 53 | 62 | 0.069 |
| New Injury Severity Score, median (IQR) | 19 (11–34) | 34 (25–50) | <0.001 |
| Systolic blood pressure, mmHg, median (IQR) | 113 (92–142) | 92 (70–130) | <0.001 |
| Lactate, mmol/L, median (IQR) | 3.4 (2.4–4.8) | 6.5 (3.2–11.3) | <0.001 |
| International normalized ratio of prothrombin time, median (IQR) | 1.1 (1.0–1.2) | 1.4 (1.2–2.0) | <0.001 |
| Partial thromboplastin time, seconds, median (IQR) | 27 (24–30) | 38 (30–51) | <0.001 |
| Platelet count of 100,000, median (IQR) | 257(211–305) | 182 (133–219) | <0.001 |
| Activated clotting time of TEG, s, median (IQR) | 121 (113–128) | 128 (113–152) | <0.001 |
| Angle, degrees, median (IQR) | 73 (69–76) | 66 (57–72) | <0.001 |
| Maximum amplitude, mm, median (IQR) | 64 (60–68) | 54 (43–60) | <0.001 |
| Lysis at 30 min after maximum amplitude, %, median (IQR) | 1.7 (0.8–2.9) | 3.4 (1.3–44.3) | <0.001 |
| Coagulopathy score, median (IQR) | 1 (1–2) | 2 (1–4) | <0.001 |
| Massive transfusion, % | 3.3 | 33 | <0.001 |
| Mortality, % | 6.2 | 40 | <0.001 |
DFI, depletion of fibrinolysis inhibitors; IQR, interquartile range (25th–75th percentile); TEG, thrombelastography.
Sub-phenotypes of fibrinolysis coagulation and blood product requirements
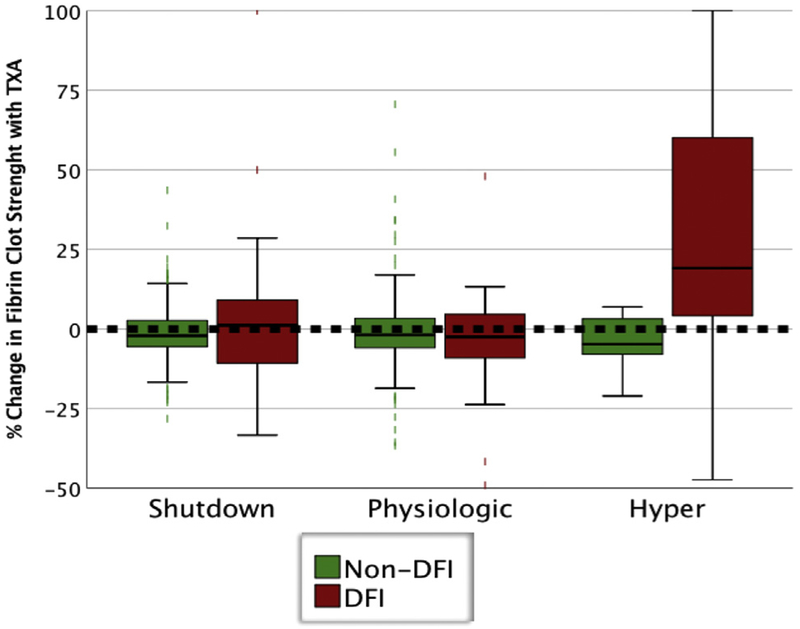
The distribution of fibrinolysis phenotypes differed between DFI and non-DFI patients (p < 0.001). In the DFI cohort, the distributions were 21% shutdown, 41% physiologic, and 37% hyperfibrinolysis. In the non-DFI group, physiologic was the most common (71%), followed by shutdown (23%), and hyperfibrinolysis was present in <5%. Within the DFI phenotype, hyperfibrinolysis patients had lower fibrinogen levels, lower platelet counts, higher international normalized ratio (INR) of prothrombin time, higher partial thromboplastin time, and all TEG indices were more hypocoagulable that other DFI phenotypes (Table 3). In the DFI cohort, only patients with hyperfibrinolysis had an increase in fibrin clot strength with TXA when assessed ex vivo (hyper: 19; IQR 4 to 60, shutdown: 1.2; IQR −11 to 9, physiologic: −2.5; IQR −10 to 4; p < 0.001) (Fig. 1). Hyperfibrinolytic DFI patients had the lowest median systolic blood pressure on arrival to the hospital (70 mmHg; IQR 0 to 114 mmHg) and the shutdown patients were predominantly normotensive (median systolic blood pressure 114 mmHg; IQR 88 to 144 mmHg) (Table 3), with both cohorts being equally severely injured with a median NISS of 43.
Table 3.
Demographics, Injury Patterns, Coagulation Parameters, and Transfusion Outcomes Between Sub-Phenotypes
| Non-DFI | DFI | |||||||
|---|---|---|---|---|---|---|---|---|
| Shutdown | Physiologic | Hyperfibrinolysis | Shutdown | Physiologic | Hyperfibrinolysis | |||
| Blunt, % | 57 | 51 | 52 | 0.845 | 56 | 64 | 65 | 0.769 |
| New Injury Severity Score, median (IQR) | 17 (16–28) | 17 (8–29) | 16 (9–26) | 0.031 | 43 (22–66) | 34 (29–54) | 43 (34–50) | 0.530 |
| Head AIS, median (IQR) | 0 (0–2) | 0 (0–3) | 0 (0–2) | 0.398 | 3 (0–5) | 3 (0–5) | 4 (0–5) | 0.837 |
| Chest AIS, median (IQR) | 0 (0–3) | 0 (0–3) | 0 (0–2) | 0.158 | 3 (3–3) | 3 (1–3) | 2 (2–3) | 0.234 |
| Abdomen AIS, median (IQR) | 0 (0–2) | 0 (0–2) | 1 (0–2) | 0.548 | 2 (0–3) | 3 (1–4) | 0 (0–2) | 0.968 |
| Systolic blood pressure, mmHg, median (IQR) | 118 (91–141) | 128 (97–142) | 141 (126–156) | 0.138 | 113 (88–144) | 86 (65–121) | 70 (0–114) | 0.004 |
| D–dimer, μg/mL, median (IQR) | 7.13 (1.3–20.01) | 1.5 (0.4–6.3) | 1.2 (0.5–4.8) | 0.242 | 13.7 (2.9–20.0) | 10.6 (0.5–19.4) | 20.0 (8.6–20.0) | 0.051 |
| Fibrinogen, mg/dL, median (IQR) | 200 (167–269) | 247 (211–316) | 228 (179–347) | 0.010 | 164 (114–268) | 200 (176–234) | 133 (98–164) | 0.005 |
| International normalized ratio of prothrombin time, median (IQR) | 1.1 (1.0–1.3) | 1.1 (1.0–1.1) | 1.1 (0.9–1.1) | 0.201 | 1.3 (1.1–1.8) | 1.2 (1.1–1.35) | 1.7 (1.4–2.3) | <0.001 |
| Partial thromboplastin time, s, median (IQR) | 27 (25–32) | 26 (24–29) | 23 (21–34) | 0.203 | 37 (28–48) | 30 (26–38) | 47 (39–72) | <0.001 |
| Platelet count of 100,000, median (IQR) | 241 (169–288) | 249 (222–315) | 293 (168–441) | 0.001 | 184 (104–234) | 217 (173–265) | 168 (125–213) | 0.003 |
| Activated clotting time of TEG, s, median (IQR) | 121 (113–128) | 121 (113–128) | 121 (121–132) | 0.303 | 121 (205–156) | 121 (113–136) | 144 (121–175) | 0.002 |
| Angle, degrees, median (IQR) | 73 (67–76) | 73 (69–77) | 71 (66–76) | 0.078 | 67 (55–74) | 69 (64–74) | 60 (50–69) | 0.003 |
| Maximum amplitude, mm, median (IQR) | 66 (64–71) | 68 (65–72) | 66 (61–71) | 0.005 | 53 (47–63) | 57 (55–63) | 45 (32–56) | <0.001 |
| RBC, U, median (IQR) | 0 (0–3) | 0 (0–1) | 0 (0–2) | 0.037 | 0 (0–11) | 4 (0–21) | 8 (4–21) | 0.040 |
| Plasma, U, median (IQR) | 0 (0–2) | 0 (0–1) | 0 (0–2) | 0.007 | 0 (0–6) | 3 (0–7) | 5 (2–13) | 0.034 |
| Cryoprecipitate, U, median (IQR) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0.106 | 0 (0–1) | 0 (0–0) | 0 (0–2) | 0.226 |
| Platelets, U, median (IQR) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0.039 | 0 (0–2) | 0 (0–1) | 1 (0–2) | 0.077 |
| Tranexamic acid, % | 5 | 1 | 4 | 0.006 | 15 | 42 | 42 | 0.685 |
| Massive transfusion, % | 6 | 3 | 0 | 0.240 | 28 | 27 | 42 | 0.287 |
AIS, Abbreviated Injury Severity Scale score; DFI, depletion of fibrinolysis inhibitors, IQR, interquartile range (25th–75th percentile); TEG, thromboelastography.
Figure 1.
Change in clot strength based on the differential functional fibrinogen thrombelastography. DFI, depletion of fibrinolysis inhibitors
In the non-DFI cohort patients in shutdown had a higher NISS, lower fibrinogen level, lower platelet counts, and lower TEG maximum amplitude compared with other phenotypes (Table 3). Transfusions were infrequent in the non-DFI cohort, but shutdown patients had higher RBC count and plasma transfusions at 6 hours compared with other phenotypes (Table 3). There was no significant increase in fibrin clot strength with TXA when assessed ex vivo in the non-DFI cohort (hyper: −4%; IQR −8 to 3, shutdown: −2%; IQR −5 to 3; physiologic: −2%; IQR −5 to 4; p < 0.549) (Fig. 1).
Fibrinolysis sub-phenotype outcomes
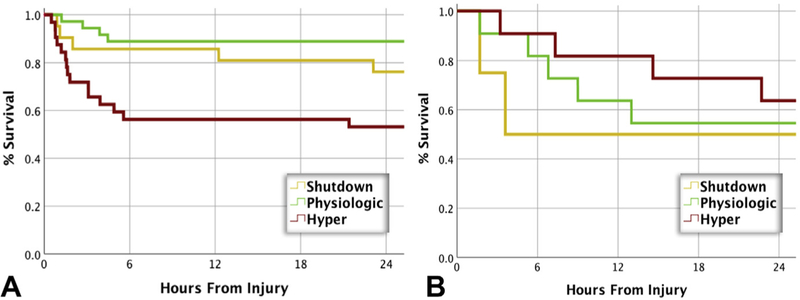
In the DFI cohort, hyperfibrinolytic patients had fewer ICU and ventilator-free days when compared with the other cohorts (Table 4). These patients received more units of RBCs and plasma (Table 3). Although not significant, the hyperfibrinolytic DFI phenotype had almost double the rate of massive transfusion (42% vs 27% shutdown and 28% physiologic; p= 0.287). The use of TXA was also more common, but not significant in the hyperfibrinolysis (42%) and physiologic (42%) group compared with shutdown (15%; p 0.685). The hyperfibrinolytic DFI cohort had a higher rate of deep vein thrombosis, and the shutdown cohort had the highest rate of pulmonary embolism, but neither were statistically significant (Table 4). The mortality showed a U-shaped distribution, with hyperfibrinolysis having a 51% mortality rate and shutdown elevated (36%) compared with physiologic (31%), but did not reach significance (Table 4). There were no significant differences in causes of mortality between the phenotypes, but nearly one- quarter of patients with hyperfibrinolysis died from physiologic exhaustion, and only 8% per the other phenotype. Dying from coagulopathy occurred in <10% of the total study population. In patients who did not receive TXA, the survival time was significantly shorter in patients with hyperfibrinolysis compared with other cohorts (p 0.007). In patients who received TXA, no survival time differences were appreciated (Fig. 2). Within DFI phenotypes, there was a shorter survival time in patients with physiologic fibrinolysis who received TXA (p = 0.043), which was not significant for shutdown, but limited to only 2 patients who received TXA. The outcomes of survivors in the DFI cohort were different, with fewer than half of the shutdown and hyperfibrinolytic patients returning home, and nearly 70% of physiologic survivors were discharged home.
Table 4.
Demographics, Injury Patterns, Coagulation Parameters, and Transfusion Outcomes Between Sub-Phenotypes
| Non-DFI | DFI | |||||||
|---|---|---|---|---|---|---|---|---|
| Shutdown | Physiologic | Hyperfibrinolysis | Shutdown | Physiologic | Hyperfibrinolysis | |||
| ICU-free, d, median (IQR) | 24 (15–26) | 26 (23–28) | 25 (22–27) | <0.001 | 15 (0–23) | 19 (0–25) | 0 (0–16) | 0.006 |
| Ventilator–free, d, median (IQR) | 26 (17–28) | 27 (26–28) | 27 (26–28) | <0.001 | 17 (0–25) | 24 (0–27) | 0 (0–22) | 0.005 |
| Length of stay, d, median (IQR) | 9 (4–17) | 6 (2–12) | 9 (3–16) | 0.020 | 11 (2–28) | 7 (2–15) | 3 (1–18) | 0.299 |
| Deep vein thrombosis, % | 0.8 | 1.9 | 4 | 0.567 | 0 | 0.5 | 4.7 | 0.180 |
| Pulmonary embolism, % | 1.7 | 1.6 | 0 | 0.999 | 8 | 2 | 0 | 0.165 |
| Disposition, % | 0.015 | 0.032 | ||||||
| Home % | 68 | 77 | 68 | 43 | 70 | 30 | ||
| Rehab/skilled nursing facility % | 17 | 16 | 20 | 25 | 18 | 35 | ||
| Long-term acute care % | 15 | 7 | 12 | 32 | 12 | 35 | ||
| Mortality, % | 12.5 | 4.3 | 0 | 0.003 | 36.0 | 31.0 | 51.0 | 0.141 |
| Mechanical bleeding, % | 0 | 0.5 | 0 | 0.999 | 4.0 | 2.0 | 4.6 | 0.831 |
| Coagulopathy, % | 1.7 | 0.3 | 0 | 0.268 | 8.0 | 4.2 | 7.0 | 0.785 |
| Physiologic exhaustion, % | 0.8 | 0 | 0 | 0.281 | 8.0 | 8.3 | 23.2 | 0.105 |
| Traumatic brain injury, % | 9.2 | 3.0 | 0 | 0.010 | 12.0 | 14.5 | 16.2 | 0.944 |
| Multiple organ failure, % | 0.8 | 0.5 | 0 | 0.630 | 4.0 | 2.0 | 0 | 0.691 |
DFI, depletion of fibrinolysis inhibitors; IQR, interquartile range (25th–75% percentile).
Figure 2.
Differences in survival times between patients with depletion of fibrinolysis inhibitors stratified by fibrinolysis phenotype and whether they (A) did not receive tranexamic acid (TXA) or (B) did receive TXA. Patients who did not receive TXA hyperfibrinolysis had the highest mortality, and in the cohort of patients who received TXA mortality was higher in the shutdown cohort. Contrasting phenotypes, only TXA (p = 0.043) was associated with increased mortality in the physiologic cohort.
In the non-DFI cohort, the shutdown phenotype had fewer ICU and ventilator-free days compared with other phenotypes (Table 4). The rates of deep vein thrombosis and pulmonary embolism were low in this overall cohort, with no differences between phenotypes, although the hyperfibrinolysis phenotype had the highest rate of deep vein thrombosis (4%). The mortality rate was the highest in the shutdown group compared with other phenotypes (Table 4). Traumatic brain injury was the most common cause of death in patients with shutdown, which was significantly higher than other phenotypes. Bleeding-related mortality was low in this overall study population, with no differences between phenotypes. Survivor dispositions were significantly different between groups with both shutdown and hyperfibrinolysis having lower rates of patients returning home, compared with physiologic, and higher rates of patients transferred to long-term care facilities.
DISCUSSION
In a cohort of trauma patients meeting the highest level of trauma activation at our Level I trauma center, 18% of patients had an elevated LY30 t-PA TEG, consistent with depletion of fibrinolysis inhibitor deficiency. Depletion of fibrinolysis inhibitors was associated with increased D-dimers, hypocoagulability, massive transfusion, and increased mortality. However, within the DFI cohort, only trauma patients with an rapid TEG with an LY30 >3% gained an increase in fibrin clot strength with TXA when tested ex vivo. Those patients with lower LY30s within the DFI cohort and without DFI demonstrated no increase in clot strength with TXA. Patients with DFI and the lowest LY30 consistent with fibrinolysis shutdown, harbor additional coagulation abnormalities, including prolonged INR, decreased platelets, and low fibrinogen, but do not have evidence of excessive fibrinolytic activity at the time of their blood draw. Although this shutdown cohort had the lowest transfusion requirements, the overall mortality was not lower than patients with physiologic fibrinolysis, and the majority of these patients who survived their injury did not return home.
Our study’s description of trauma patients harboring low fibrinolytic activity measured by viscoelastic assays and elevated D-dimer levels is consistent with what Innes and Sevitt1 described nearly half a century earlier using the euglobulin lysis test and D-dimer levels. Although low fibrinolysis causing morbidity in animal shock models3,16 and human trauma patients,17 fibrinolysis shutdown in trauma was not mentioned in an article until 1985,18 and increased morbidity associated with shutdown was not published until 2014.4 Contemporary trauma literature has defined shutdown as a low TEG LY305–7,9,19 or low maximum lysis7,8 with the rotational thromboelastometry (ROTEM) device, but this does not account for additional laboratory measurement confirming earlier activation of the fibrinolytic system.
Evidence that not all trauma patients with low TEG LY30 had activated their fibrinolytic system emerged when sub-phenotypes of “shutdown” were identified.10 This earlier study used a cutoff of t-PA sensitivity to stratify patients based on healthy volunteer data, and not based on a clinical end point, such as massive transfusion, which was used in this study. Subsequently, 2 additional studies have indicated sub-phenotypes of fibrinolysis shutdown.6,8 The first study in the US2 used D-dimer, plasmin antiplasmin levels, and TEG LY30 to stratify patients into low, moderate, and high fibrinolysis levels in a cohort of trauma patients who were receiving blood products from the PROPPR study.19 This study identified that 94% of the population with a shutdown level LY30 had abnormally elevated D-dimer levels, supporting that these patients were in fibrinolysis shutdown due to previous fibrinolysis activation. This patient population also harbored an elevated INR, low platelet count, and received a median of 9 U RBCs during resuscitation. However, there was significantly less bleeding than hyperfibrinolytic trauma patients who received a median of 15 U of blood. In this study, the shutdown cohort had a higher mortality rate than the physiologic fibrinolysis cohort (17% vs 11%), which were both considerably lower than hyperfibrinolytic patients (51%). These data indicate that the majority of trauma patients who are receiving blood product resuscitation with low LY30 have shut down their fibrinolytic system, but are still at risk of bleeding from other coagulation abnormalities. These associations were also appreciated in our study and in both studies from Canada7 and Europe.8
A European study speculated that low fibrinolysis measured by ROTEM and high D-dimer levels are a sign of occult hyperfibrinolysis at the site of injury, rather than shutdown.8 This “occult fibrinolysis” cohort of patients had a prolonged INR, but a median of 0 RBC units transfused, which was significantly lower than patient with hyperfibrinolysis and elevated D-dimer levels (median 6 units). Both of these cohorts had elevated mortality compared with patients with normal fibrinolytic activity (7% vs occult 30% and hyperfibrinolysis 59%). The occult cohort also had a delayed mortality, with a median time of 2 days compared with hyperfibrinolysis, who died within 24 hours of injury. It is difficult to reconcile that the “occult” fibrinolysis cohort was bleeding to death from unmeasured fibrinolysis. The DFI shutdown cohort described in our study has many similarities to the occult lysis phenotype described in Europe, as these patients harbor additional coagulation abnormalities and have an elevated mortality rate compared with patients with normal fibrinolysis. Our study also indicates that these patients have a lack of excessive fibrinolytic activity, as their ex vivo TEG with TXA failed to improve fibrin clot strength (Fig. 1). Collectively, there is no evidence that TXA use in the shutdown cohort has a benefit in reducing blood transfusions or mortality.
A recent study from Toronto has speculated that fibrinolysis shutdown is protective to counter hypocoagulability.7 Using a multivariate regression analysis, the unadjusted mortality in patients with shutdown was not significant. However, a much larger study with more than 2,500 trauma patients with blood samples obtained within an hour from injury failed to identify a survival benefit in severely injury patients after controlling for confounding variables.5 It is known that even hyperfibrinolytic trauma patients will transition to low fibrinolytic activity after resuscitation, and typically do this within 2 hours of injury.20 An early surge in plasminogen activator inhibitor-1 (PAI-1) after injury was described in 198518 and speculated to occur by investigators decades earlier.1 Our data support that hypotension drives pathologic fibrinolysis, as the median SBP of DFI hyperfibrinolytic patients was 70 mmHg, which is consistent with animal models,21,22 and previous clinical observations.4,5 Conversely, those patients with normal blood pressure but who were severely injured were more likely to be DFI with shutdown. This population was likely hyperfibrinolytic at one point, but with normalization of blood pressure had endogenously suppressed fibrinolytic activity without TXA.
This transition to fibrinolysis resistance is likely a physiologic response to severe injury, as it has been described that >80% of severely injured trauma patients have t-PA resistance within the first 24 hours of injury,20 but adverse outcomes are not appreciated until patients retain low fibrinolytic activity beyond 24 hours.23,24 This has clinical significance as blood draws obtained more than 2 hours from injury do not necessarily reflect the trauma patient’s acute fibrinolytic phenotype. Evidence for this is demonstrated in patient with blood samples obtained up to 12 hours from injury, in which fibrinolysis shutdown is identified in >50% of patients,4,23 and for samples obtained within an hour, the prevalence rate is 20% to 40%.5,9,10 Consequently, combing patient samples to define a single phenotype from admission beyond the first 2 hours poses the risk of misclassifying patients with a mixture of acute fibrinolysis shutdown that appears to be pathologic, with a physiologic shutdown that occurs after resuscitation and does not appear to be pathologic, unless it persists beyond 24 hours.23,24 Arguable because most patients transition into fibrinolysis shutdown, empiric TXA could be harmless. However, TXA has been associated with prolonged fibrinolysis shutdown.20,25 Therefore, delivering TXA to trauma patients who do not gain clot strength has an unclear benefit with a concern for causing harm. As suggested previously, the potential vulnerable population is patients with physiologic levels of fibrinolysis,26 which our study illustrated in the DFI physiologic cohort (Fig. 2). These findings add to the growing literature on the selective use of TXA in trauma based on fibrinolytic status.27,28 An important consideration to take into account is that TXA does not replace fibrinolytic inhibitors. Tranexamic acid binds to plasminogen, causing a confirmation change preventing its binding to fibrin.29 In the absence of t-PA, the inhibitory effects of TXA to convert plasminogen into plasmin are limited, and para- doxically can increase plasmin generation when urokinase is present,30 which has been associated with increased intracranial bleeding in experimental traumatic brain injury models.31
Limitations of this study include ex vivo effects of TXA on fibrin clot strength. The in vivo effects remain speculative, just as the hypothesis of occult fibrinolysis.8 Local clot mediators of fibrinolysis are nearly impossible to assess in human subjects, and there could be upregulation of endothelial receptors, such as S100A10, which were found in higher concentrations in circulation from the European study.8 However, this is also a systemic measurement of a receptor that lines the endothelium and, when knocked out in a mouse, causes microvascular thrombosis.32 Therefore, it remains unclear whether this S100A10 is pathologic or protective after trauma, and with its upregulation associated with minimal blood loss, it is difficult to suggest it requires inhibition with a known protective role of the microvasculature. Although in vitro studies have suggested TXA has a protective role of the endothelium,33,34 additional in vitro data now demonstrate that TXA can be proinflammatory and activate complement when there is a lack of a t-PA,35 so speculation from in vitro data needs to be interpreted with caution. Another limitation of this study is defining a cut point with the t-PA TEG to stratify patients for DFI and create sub-phenotypes. We had previously used a t-PA TEG LY30 >95th percentile of healthy controls to define these sub-phenotypes of shutdown.10 However, the proteomic data from this study did not define a clear pattern associated with a specific phenotype or cause of death. With our refined definition of sub-phenotypes of fibrinolysis, it appears that we have identified a cohort of patients who have maximal benefit from TXA (DFI hyperfibrinolysis), and a cohort of patients who might be harmed (DFI physiologic). This study supports the earlier analysis that an LY30 of >3% is not adequate to determine who benefits from TXA,36 as only a portion of these patients are DFI and are at risk of massive bleeding.
Early TXA use in hypotensive patients (systolic blood pressure <75 mmHg) is the only cohort of patients who had a survival advantage in the CRASH II trial.37 However, it has been difficult to identify the correct patient population in prospective randomized controlled trials in trauma who need blood product resuscitation. This was evident in CRASH II, in which fewer than half of patients received an RBC transfusion, which we also appreciated in our recent prehospital plasma cohort, with enrollment criteria designed to capture patients who would require blood product resuscitation.38 Ultimately, a multicenter clinical trial is needed to determine whether goal-directed TXA used in a mature trauma system with VHA capacity can improve outcomes. To date, a single- center randomized controlled trial using VHA reduced mortality by nearly 50%,39 which is a larger reduction in mortality than CRASH II37 and retrospective analysis in both MATTERs (Military Application of Tranexamic Acid in Trauma Emergency Resuscitation) studies.40,41
CONCLUSIONS
Patients with DFI exhibited high rates of massive transfusion and mortality. Although these patients have multiple abnormalities of their coagulation system, with evidence of earlier activation of fibrinolysis, only DFI patients with hyperfibrinolysis have improved fibrin clot strength with TXA treatment. These data indicate that DFI is a risk factor for coagulopathy, but these patients might have adverse outcomes with routine antifibrinolytic therapy.
Support:
This study was supported in part by National Institute of General Medical Sciences (NIGMS) grant: T32-GM008315, and National Heart, Lung and Blood Institute (NHLBI) grant: 1UM1HL120877. Dr Sauaia receives grant money from the NIH and the Department of Defense and receives support for travel from the American Association for the Surgical Trauma.
Disclosure Information: This study received research support from Haemonetics. Drs HB Moore, EE Moore, and Chapman have shared intellectual property with Heamonetics through Thrombo Therapeutics, Inc. Drs Chapman, HB Moore, and EE Moore hold provisional patents for viscoelastic assays of fibrinolysis.
Abbreviations and Acronyms
- DFI
depletion of fibrinolysis inhibitors
- INR
international normalized ratio
- IQR
interquartile range
- LY30
lysis 30 minutes after maximum amplitude
- NISS
New Injury Severity Score
- TEG
thrombelastography
- t-PA
tissue plasminogen activator
- TXA
tranexamic acid
- VHA
viscoelastic hemostatic assay
Footnotes
Publisher's Disclaimer: Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIGMS or NHLBI.
Disclosures outside the scope of this work: Dr Chapman is a board member and CEO of Thrombo Therapeutics, Inc. Dr HB Moore is a paid consultant to Instrument Laboratories and is a board member and President of Thrombo Therapeutics, Inc. Drs HB Moore and EE Moore hold stock options in Thrombo Therapeutics. Dr Hansen is a paid employee of Omix Technologies and receives patent royalties from the University of California. All other authors have nothing to disclose.
Presented at the Western Surgical Association 126th Scientific Session, San Jose del Cabo, Mexico, November 2018.
REFERENCES
- 1.Innes D, Sevitt S. Coagulation and fibrinolysis in injured patients. J Clin Pathol 1964;17:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chakrabarti R, Hocking ED, Fearnley GR. Reaction pattern to three stressesdelectroplexy, surgery, and myocardial infarctiond—of fibrinolysis and plasma fibrinogen. J Clin Pathol 1969;22:659–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hardaway RM, Drake DC. Prevention of “irreversible” hemorrhagic shock with fibrinolysin. Ann Surg 1963;157:39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moore HB, Moore EE, Gonzalez E, et al. Hyperfibrinolysis, physiologic fibrinolysis, and fibrinolysis shutdown: the spectrum of postinjury fibrinolysis and relevance to antifibrinolytic therapy. J Trauma Acute Care Surg 2014;77:811–817; discussion 817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moore HB, Moore EE, Liras IN, et al. Acute fibrinolysis shutdown after injury occurs frequently and increases mortality: a multicenter evaluation of 2,540 severely injured patients. J Am Coll Surg 2016;222:347–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cardenas JC, Wade CE, Cotton BA, et al. TEG Lysis shutdown represents coagulopathy in bleeding trauma patients: analysis of the PROPPR cohort. Shock 2019;51:273–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gomez-Builes JC, Acuna SA, Nascimento B, et al. Harmful or physiologic: diagnosing fibrinolysis shutdown in a trauma cohort with rotational thromboelastometry. Anesth Analg 2018;127:840–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gall LS, Vulliamy P, Gillespie S, et al. The S100A10 pathway mediates an occult hyperfibrinolytic subtype in trauma patients. Ann Surg 2018. March 19 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 9.Leeper CM, Neal MD, McKenna C, et al. Abnormalities in fibrinolysis at the time of admission are associated with deep vein thrombosis, mortality, and disability in a pediatric trauma population. J Trauma Acute Care Surg 2017;82:27–34. [DOI] [PubMed] [Google Scholar]
- 10.Moore HB, Moore EE, Huebner BR, et al. Fibrinolysis shutdown is associated with a fivefold increase in mortality in trauma patients lacking hypersensitivity to tissue plasminogen activator. J Trauma Acute Care Surg 2017;83:1014–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Einarsson M, Smedsrod B, Pertoft H. Uptake and degradation of tissue plasminogen activator in rat liver. Thromb Haemost 1988;59:474–479. [PubMed] [Google Scholar]
- 12.Lijnen HR, Collen D. Mechanisms of physiological fibrinolysis. Baillieres Clin Haematol 1995;8:277–290. [DOI] [PubMed] [Google Scholar]
- 13.Ruhl H, Berens C, Winterhagen A, et al. Label-free kinetic studies of hemostasis-related biomarkers including d-dimer using autologous serum transfusion. PLoS One 2015;10: e0145012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Neal MD, Moore HB, Moore EE, et al. Clinical assessment of trauma-induced coagulopathy and its contribution to postinjury mortality: a TACTIC proposal. J Trauma Acute Care Surg 2015;79:490–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dzieciatkowska M, D’Alessandro A, Hill RC, et al. Plasma QconCATs reveal a gender-specific proteomic signature in apheresis platelet plasma supernatants. J Proteomics 2015; 120:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hardaway RM, Burns JW. Mechanism of action of fibrinolysin in the prevention of irreversible hemorrhagic shock. Ann Surg 1963;157:305–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cafferata HT, Aggeler PM, Robinson AJ, et al. Intravascular coagulation in the surgical patient: its significance and diagnosis. Am J Surg 1969;118:281–291. [DOI] [PubMed] [Google Scholar]
- 18.Kluft C, Verheijen JH, Jie AF, et al. The postoperative fibrinolytic shutdown: a rapidly reverting acute phase pattern for the fast-acting inhibitor of tissue-type plasminogen activator after trauma. Scand J Clin Lab Invest 1985;45:605–610. [DOI] [PubMed] [Google Scholar]
- 19.Holcomb JB, Tilley BC, Baraniuk S, et al. Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: the PROPPR randomized clinical trial. JAMA 2015;313:471–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moore HB, Moore EE, Gonzalez E, et al. Reperfusion shutdown: delayed onset of fibrinolysis resistance after resuscitation from hemorrhagic shock is associated with increased circulating levels of plasminogen activator inhibitor-1 and postinjury complications. Blood 2016;128:206. [Google Scholar]
- 21.Moore HB, Moore EE, Lawson PJ, et al. Fibrinolysis shutdown phenotype masks changes in rodent coagulation in tissue injury versus hemorrhagic shock. Surgery 2015;158:386–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Macko AR, Moore HB, Cap AP, et al. Tissue injury suppresses fibrinolysis after hemorrhagic shock in nonhuman primates (rhesus macaque). J Trauma Acute Care Surg 2017;82: 750–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meizoso JP, Karcutskie CA, Ray JJ, et al. Persistent fibrinolysis shutdown is associated with increased mortality in severely injured trauma patients. J Am Coll Surg 2017; 224:575–582. [DOI] [PubMed] [Google Scholar]
- 24.Leeper CM, Neal MD, McKenna CJ, et al. Trending fibrinolytic dysregulation: fibrinolysis shutdown in the days after injury is associated with poor outcome in severely injured children. Ann Surg 2017;266:508–515. [DOI] [PubMed] [Google Scholar]
- 25.Meizoso JP, Dudaryk R, Mulder MB, et al. Increased risk of fibrinolysis shutdown among severely injured trauma patients receiving tranexamic acid. J Trauma Acute Care Surg 2018; 84:426–432. [DOI] [PubMed] [Google Scholar]
- 26.Moore HB, Moore EE, Huebner BR, et al. Tranexamic acid is associated with increased mortality in patients with physiological fibrinolysis. J Surg Res 2017;220:438–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moore EE, Moore HB, Gonzalez E, et al. Rationale for the selective administration of tranexamic acid to inhibit fibrinolysis in the severely injured patient. Transfusion 2016;56[Suppl 2]: S110–S114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roberts I Fibrinolytic shutdown: fascinating theory but randomized controlled trial data are needed. Transfusion 2016; 56 [Suppl 2]:S115–S118. [DOI] [PubMed] [Google Scholar]
- 29.Markus G, Priore RL, Wissler FC. The binding of tranexamic acid to native (Glu) and modified (Lys) human plasminogen and its effect on conformation. J Biol Chem 1979;254: 1211–1216. [PubMed] [Google Scholar]
- 30.Christensen U Urokinase-catalysed plasminogen activation. Effects of ligands binding to the AH-site of plasminogen. Biochim Biophys Acta 1988;957:258–265. [DOI] [PubMed] [Google Scholar]
- 31.Hijazi N, Abu Fanne R, Abramovitch R, et al. Endogenous plasminogen activators mediate progressive intracerebral hemorrhage after traumatic brain injury in mice. Blood 2015;125: 2558–2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Surette AP, Madureira PA, Phipps KD, et al. Regulation of fibrinolysis by S100A10 in vivo. Blood 2011;118:3172–3181. [DOI] [PubMed] [Google Scholar]
- 33.Diebel LN, Martin JV, Liberati DM. Early tranexamic acid administration ameliorates the endotheliopathy of trauma and shock in an in vitro model. J Trauma Acute Care Surg 2017;82:1080–1086. [DOI] [PubMed] [Google Scholar]
- 34.Diebel ME, Martin JV, Liberati DM, et al. The temporal response and mechanism of action of tranexamic acid in endothelial glycocalyx degradation. J Trauma Acute Care Surg 2018;84:75–80. [DOI] [PubMed] [Google Scholar]
- 35.Barrett CD, Moore HB, Kong YW, et al. Tranexamic acid mediates proinflammatory and anti-inflammatory signaling via complement C5a regulation in a plasminogen activator-dependent manner. J Trauma Acute Care Surg 2019;86:101–107. [DOI] [PubMed] [Google Scholar]
- 36.Harvin JA, Peirce CA, Mims MM, et al. The impact of tranexamic acid on mortality in injured patients with hyperfibrinolysis. J Trauma Acute Care Surg 2015;78: 905–909; discussion 909–911. [DOI] [PubMed] [Google Scholar]
- 37.Collaborators C- T, Shakur H, Roberts I, et al. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): a randomised, placebo-controlled trial. Lancet 2010;376:23–32. [DOI] [PubMed] [Google Scholar]
- 38.Moore HB, Moore EE, Chapman MP, et al. Plasma-first resuscitation to treat haemorrhagic shock during emergency ground transportation in an urban area: a randomised trial. Lancet 2018;392:283–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gonzalez E, Moore EE, Moore HB, et al. Goal-directed hemostatic resuscitation of trauma-induced coagulopathy: a pragmatic randomized clinical trial comparing a viscoelastic assay to conventional coagulation assays. Ann Surg 2016;263: 1051–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morrison JJ, Dubose JJ, Rasmussen TE, et al. Military Application of Tranexamic Acid in Trauma Emergency Resuscitation (MATTERs) study. Arch Surg 2012;147:113–119. [DOI] [PubMed] [Google Scholar]
- 41.Morrison JJ, Ross JD, Dubose JJ, et al. Association of cryoprecipitate and tranexamic acid with improved survival following wartime injury: findings from the MATTERs II study. JAMA Surg 2013;148:218–225. [DOI] [PubMed] [Google Scholar]