Abstract
Purpose:
Quality of life (QOL) for patients with oropharyngeal squamous cell cancer is negatively affected by conventional radiation (RT) owing to radiation exposure to normal tissues. Proton therapy, via pencil beam scanning (PBS), can better spare many of these tissues, and may thereby improve QOL.
Patients and Methods:
Patient-reported outcomes were prospectively collected from patients treated from April 2013 to April 2015. Patients were treated with PBS or intensity-modulated radiation therapy (IMRT) via volumetric arc therapy after transoral robotic surgery. Validated QOL questionnaires were collected before RT, and 3, 6, and 12 months post RT.
Results:
Sixty-four patients were treated with adjuvant RT after transoral robotic surgery, 33 (52%) with volumetric arc therapy, and 31 (48%) with PBS. Both groups were similar in terms of age, site, stage, and dose delivered. Patients receiving PBS had significantly less dose to many normal structures than those receiving IMRT. These dosimetric advantages with PBS were reflected in higher scores in head and neck specific, as well as general, QOL measures. Most notable was significantly less xerostomia with PBS, on multiple patient-reported outcomes at multiple timepoints (6 and 12 months).
Conclusion:
Pencil beam scanning, when compared to IMRT, confers a significant dosimetric advantage to many normal organs at risk, with a corresponding benefit in multiple patient-reported QOL parameters in patients receiving adjuvant RT for oropharyngeal squamous cell cancer.
Keywords: oropharyngeal cancer, quality of life, proton therapy, intensity-modulated radiation therapy
Introduction
The increasing incidence of oropharyngeal squamous cell carcinoma (OPSCC) in the United States and Europe has been driven largely by increasing rates of oral human papilloma virus (HPV) infection [1]. Given the generally favorable prognosis of patients with HPV-related OPSCC [2], there is an expanding focus on decreasing the toxicity of treatment while preserving efficacy [3].
Curative treatment of OPSCC commonly involves radiation with or without chemotherapy after surgery or in the definitive, nonoperative setting. The clinical benefits of elective treatment of the neck in OPSCC are well established [4], but comprehensive nodal irradiation can lead to significant and sometimes severe short- and long-term toxicities. This is an especially important issue for patients with HPV-positive OPSCC who are relatively young and healthy, often cured of their disease, and will live for decades beyond their treatment. Even the most technically advanced forms of conformal radiation therapy, such as intensity-modulated radiation treatment (IMRT), still cause acute grade-3 side effects in many patients [5]. Acute radiation toxicities in turn can then lead to late toxicities that can negatively impact quality of life (QOL) [6, 7]. Efforts to reduce toxicity and improve patient QOL are therefore of paramount importance.
Proton beam therapy (PBT), with its unique physical beam characteristics (Bragg peak), consistently results in improvements in dosimetry and normal tissue sparing [8–12]. However, translation of dosimetric benefits to improved clinical outcomes has been limited in the setting of head and neck cancers. This study is the first of its kind to report an association between dosimetric advantages and subsequent clinical outcomes in patients receiving postoperative radiation therapy in the treatment of oropharynx cancer, and the first to report late outcomes at 12 months.
Materials and Methods
Patients
This is an institutional review board–approved study of 64 patients with OPSCC, treated at the University of Pennsylvania (between 2013 and 2015) initially with transoral robotic surgery and selective neck dissection, followed by adjuvant (n = 64) radiation, with or without chemotherapy (according to standard indications) [13]. Whether patients received PBT or IMRT via volumetric modulated arc therapy (VMAT) was determined solely on the basis of insurance approval for each patient.
Treatment Planning and Dosimetric Comparisons
At time of treatment planning, normal structures representing organs at risk were contoured for all patients. Contouring followed previously published guidelines [14]. All patients received treatment to the primary site and bilateral neck, with standard, accepted postoperative fractionation and doses of 60 to 66 Gy [15, 16], and with target delineation of primary and secondary echelon nodal regions, based on published and commonly used guidelines [17]. Treatment planning for both PBT and VMAT was performed via Eclipse version 11 (Varian Medical Systems, Palo Alto, California). Proton beam therapy was planned and delivered via a pencil beam scanning (PBS), single-field uniform dose technique. Dosimetric data for all structures were extracted from Eclipse, with dose-volume histogram (DVH) data exported into the R software package version 3.2.1 (R Foundation for Statistical Computing, Auckland, New Zealand).
Assessment of Patient-Reported QOL Outcomes
Patient-reported QOL questionnaires were prospectively obtained at initial radiation therapy consultation (pretreatment) and at 3, 6, and 12 month follow-up intervals and included the following: European Organization for Research and Therapy of Cancer (EORTC) QLQ-30 version 3 EORTC OLO-H&N35 and the Groningen (GRIX) Xerostomia, Work Status, and Performance Status Scale–Head and Neck Cancer questionnaires. All questionnaires had previously been externally validated [18, 19]. The EORTC questionnaire was used to determine General Health Domain, Physical and Role Function, overall xerostomia, dental issues, head and neck pain, and fatigue scores. The GRIX questionnaire was used to analyze separate components of dry mouth such as differentiating between day and night xerostomia and separate subscales for sticky saliva. As per the EORTC and GRIX guidelines, composite scores examining specific domains were linearly converted to a 0 to 100 scale, with a difference of 10 in the composite scale considered to be clinically significant [20].
Statistical Analysis
All statistical analyses were completed by using Stata, version 13.1 (StataCorp LP, College Station, Texas). Patient characteristics were compared by using a t test for continuous and Fisher exact test for discrete variables. Dosimetric comparisons of salivary structures were performed by using a t test with correction for multiple comparisons using the Bonferroni method, ascribing a P value of .004 as statistically significant. For patient-reported outcomes (PROs) with continuous variables t test was used, while Wilcoxon rank sum test was used for comparison of categorical variables reported on the PROs. For parameters with binary outcomes, Fisher exact test was used. Associations were noted as statistically significant with a 2-sided P value <.05.
Results
Patient and disease characteristics were similar between both groups (PBS vs. IMRT) of patients, including age, sex, stage, nodal status, radiation dose, and receipt of concurrent systemic therapy (Table 1).
Table 1.
Patient characteristics.
|
VMAT (n = 33) |
PBS (n = 31) |
P
value |
|
| Age, y, mean | 58 | 60 | .365 |
| Sex | .561 | ||
| Male | 27 (82%) | 27 (87%) | |
| Female | 6 (18%) | 4 (13%) | |
| Primary site | .800 | ||
| Tonsil | 20 (61%) | 20 (65%) | |
| Base of tongue | 13 (39%) | 11 (35%) | |
| Stage | .796 | ||
| I-III | 5 (15%) | 4 (13%) | |
| IVA | 28 (85%) | 27 (87%) | |
| T stage | .272 | ||
| Tis, T1, T2 | 32 (97%) | 28 (90%) | |
| T3 | 1 (3%) | 3 (10%) | |
| Nodal status | .195 | ||
| N0 | 1 (3%) | 2 (6%) | |
| N1-N2b | 29 (88%) | 29 (94%) | |
| N2c-N3 | 3 (9%) | 0 (0%) | |
| Median dose, Gy | 62.6 | 61.7 | .288 |
| Chemotherapy | .110 | ||
| None | 19 (58%) | 19 (61%) | |
| Cisplatin | 13 (39%) | 7 (23%) | |
| Cetuximab | 1 (3%) | 5 (16%) |
Abbreviations: VMAT, volumetric arc therapy; PBS, pencil beam scanning.
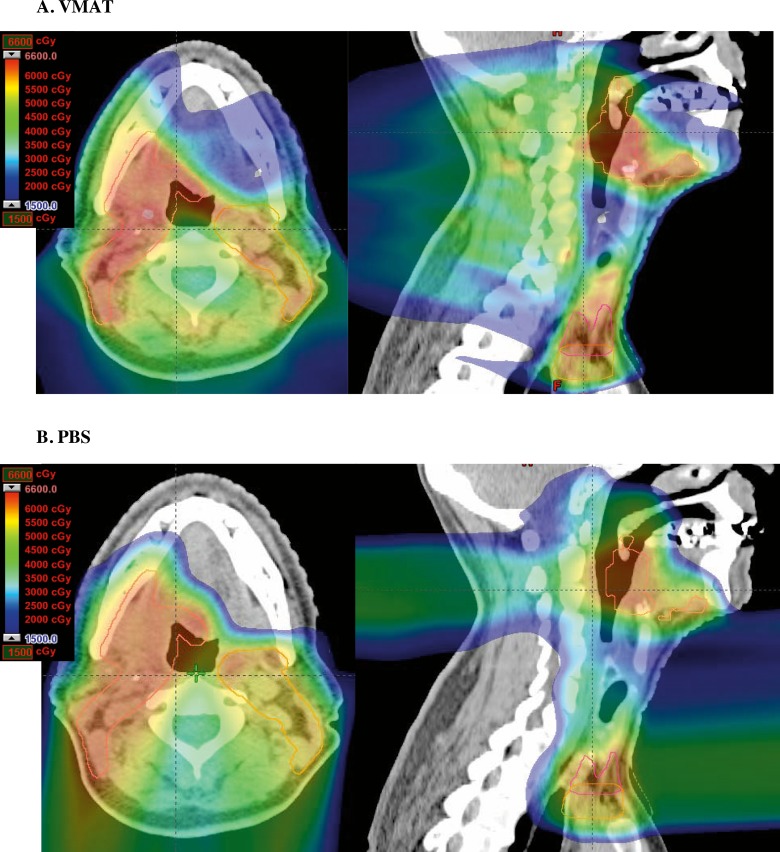
Representative radiation plans for a patient treated with adjuvant radiation for OPSCC with VMAT and PBS are shown in Figure 1, with significant sparing of the anterior oral cavity with PBS. Mean radiation dose was significantly lower for PBS than VMAT patients for all salivary structures other than the ipsilateral parotid and ipsilateral submandibular glands, with the contralateral parotid and submandibular glands, ipsilateral and contralateral buccal mucosa, upper and lower lips, and oral tongue receiving one-third to 10-fold lower mean dose with PBS (Table 2). When combining all structures known to produce saliva in the oral cavity and defining this as a separate composite structure, PBS resulted in significantly lower mean dose to the composite oral cavity structure than VMAT (35.1 Gy VMAT vs. 21.2 Gy PBS, P < .0001). Both VMAT and PBS patients had a 0% rate of percutaneous endoscopic gastrostomy tube dependence at 6 months.
Figure 1.
VMAT versus PBS plan comparison: axial (left) and sagittal (right) slices of representative radiation plans for adjuvant radiation therapy in a patient with T1N2aM0 stage IVA base of tongue carcinoma status post transoral robotic surgery and neck dissection, showing VMAT (A) and PBS (B) radiation plans (60 Gy in 30 fractions) for the same patient. The PBS plan demonstrates lower dose to oral cavity structures than VMAT. Abbreviations: PBS, pencil beam scanning; VMAT, volumetric arc therapy.
Table 2.
Dosimetric comparison of all patients by treatment cohort, representing OARs important for salivary function.
| Patients (n = 64) |
VMAT (n = 33), Gy |
PBS (n = 31), Gy |
P
value |
|
Mean dose (95% CI) | |||
| Ipsilateral parotid | 32.83 (29.43, 36.32) | 35.29 (32.09, 38.48) | .295 |
| Contralateral parotid | 20.78 (18.34, 23.22) | 11.90 (9.71, 14.09) | <.0001a |
| Ipsilateral submandibular | 60.11 (59.40, 60.81) | 60.52 (59.82, 61.22) | .398 |
| Contralateral submandibular | 38.01 (32.58, 43.43) | 28.75 (24.12, 33.38) | .0107 |
| Ipsilateral sublingual | 42.69 (36.72, 46.04) | 32.47 (27.68, 37.26) | .0009a |
| Contralateral sublingual | 36.03 (32.37, 39.69) | 7.54 (4.85, 10.22) | <.0001a |
| Ipsilateral buccal | 25.97 (23.85, 28.09) | 9.48 (5.94, 13.02) | <.0001a |
| Contralateral buccal | 18.84 (13.66, 18.10) | 1.34 (0.70, 1.99) | <.0001a |
| Hard palate | 15.86 (13.71, 18.00) | 5.66 (2.55, 8.77) | <.0001a |
| Soft palate | 36.82 (33.90, 39.72) | 30.59 (26.05, 35.13) | .202 |
| Tongue | 39.94 (37.45, 42.44) | 25.98 (23.04, 28.91) | <.0001a |
| Upper lip | 10.38 (8.81, 11.95) | 1.40 (0, 3.09) | <.0001a |
| Lower lip | 20.38 (18.26, 22.50) | 2.82 (0.35, 5.29) | <.0001a |
| Oral cavity | 35.11 (33.14, 37.08) | 21.23 (18.82, 23.64) | <.0001a |
Abbreviations: OARs, organs at risk; VMAT, volumetric arc therapy; PBS, pencil beam scanning; CI, confidence interval.
Note: Mean dose with standard error of the mean is represented for these structures.
Statistically significant results (ascribed at P = .0036 using Bonferroni correction for multiple comparisons).
Patients treated with PBS reported significantly less head and neck pain on the EORTC scale than patients treated with VMAT at 12 months (22.0 VMAT vs. 8.3 PBS, P = .01; Table 3).
Table 3.
Patient-reported outcomes representing quality-of-life parameters for patients treated with VMAT or PBS for OPSCC.
|
3 months |
6 months |
12 months |
|||||||
|
VMAT |
PBS |
P
value |
VMAT |
PBS |
P
value |
VMAT |
PBS |
P
value |
|
| Fatigue | 26.50 | 26.50 | .63 | 20.47 | 8.50 | .07 | 22.22 | 4.86 | .17 |
| H&N pain | 28.85 | 25.00 | .34 | 18.86 | 8.33 | .08 | 21.97 | 8.33 | .011a |
| Painkiller use (%) | 35.71 | 30.77 | 1.00 | 21.05 | 16.67 | 1.00 | 36.36 | 17.65 | .38 |
| Xerostomia | 47.62 | 50.00 | .96 | 52.63 | 39.58 | .14 | 54.55 | 23.53 | .003a |
| Moderate-severe dry mouth | 57.14 | 50.00 | 1.00 | 63.16 | 22.22 | .02a | 50.00 | 11.76 | .038a |
| Xerostomia day | 43.06 | 41.20 | .81 | 39.20 | 25.80 | .038a | 33.33 | 19.61 | .06 |
| Xerostomia night | 47.01 | 33.33 | .11 | 35.10 | 22.80 | .042a | 30.56 | 17.65 | .10 |
| Sticky saliva day | 38.46 | 29.37 | .38 | 20.47 | 15.43 | .60 | 22.22 | 17.65 | .31 |
| Sticky saliva | 45.24 | 48.72 | .81 | 26.32 | 27.08 | .90 | 39.39 | 27.45 | .38 |
| Dental problems | 19.05 | 0.00 | .016a | 17.54 | 1.96 | .048a | 21.21 | 5.88 | .13 |
| Physical function | 87.62 | 88.10 | .83 | 89.47 | 97.04 | .006a | 87.88 | 96.86 | .24 |
| Role function | 70.24 | 80.77 | .43 | 76.32 | 96.30 | .0008a | 78.79 | 97.92 | .041a |
| Global health | 66.03 | 69.05 | .41 | 73.15 | 83.33 | .09 | 72.73 | 81.86 | .13 |
Abbreviations: VMAT, volumetric arc therapy; PBS, pencil beam scanning: OPSCC, oropharyngeal squamous cell cancer; H&N, head and neck.
Statistically significant results.
To determine whether the improvement in dosimetric endpoints to oral cavity salivary structures correlated with patient-reported xerostomia, we used PROs from the EORTC and GRIX xerostomia scales (higher score indicating worse xerostomia). Pencil beam scanning conferred a significant benefit in general EORTC xerostomia scores at 12-month follow-up (54.6 VMAT vs. 23.5 PBS, P = .003; Table 3). While PBS patients showed improvement in xerostomia endpoints over time from 3 to 12 months, VMAT patients reported stable or worsening xerostomia outcomes over this period in all cohorts. There was a benefit favoring PBS over VMAT in rates of moderate to severe general xerostomia as reported on the EORTC questionnaire starting at 6 and 12 months (63.2 VMAT vs. 22.2 PBS at 6 months, P = .02; 50 VMAT vs. 11.8 PBS at 12 months, P = .038; Table 3). Similar findings favored PBS over VMAT in a more granular assessment that included daytime and nighttime xerostomia on the GRIX questionnaire, with no significant differences in terms of sticky saliva. In addition to fewer reported problems with xerostomia, patients receiving PBS reported significantly lower rates of posttreatment dental problems than VMAT patients overall (17.5 VMAT vs. 2.0 PBS at 6 months, P = .048; Table 3).
Global health status scores on the EORTC questionnaire showed improvement (higher score is better) between 3 and 6 months post treatment in those receiving PBS versus IMRT. Patients treated with PBS had a trend toward improved global health status scores at 6 months (73.2 VMAT vs. 83.3 PBS, P = .09; Table 3). There were also significant differences favoring protons in terms of role function at 6 and 12 months (76.3 VMAT vs. 96.3 PBS at 6 months, P = .0008; 78.8 VMAT vs. 97.9 PBS at 12 months, P = .041; Table 3).
Discussion
Our study of patients who received adjuvant radiation therapy for OPSCC shows that improved dosimetric sparing of organs at risk with PBS confers an advantage to clinical toxicity outcomes during follow-up of up to 1 year. A previously published study of 81 patients treated for OPSCC suggested a benefit favoring PBS compared to IMRT at about 6 weeks after treatment [21]. Our study contributes to the existing literature, focusing on a homogeneous patient population (postoperative oropharynx cancer requiring adjuvant radiation therapy), and is the first to report patient outcomes at 12 months.
High-quality studies that examine the potential clinical benefit associated with new technologies such as proton therapy are clearly needed. While there is no debate that the physical characteristics of proton radiation can spare organs at risk, published data demonstrating that these dosimetric gains translate to clinical gains are limited. Efforts are underway to formally address this question via a prospective, randomized trial [22]. Results from such studies will not be available for many years. Preliminary information on clinical outcomes and gains associated with proton therapy is important to help current patients, physicians, and payers make the best treatment decisions. Our study fulfills this need by using prospectively collected and well-validated PRO questionnaires over the course of a year post RT.
There are limitations to our study. Although PROs were collected prospectively, our patients were not assigned treatment (PBS vs. IMRT) via a prospective, randomized controlled trial, but rather via insurance approval. Therefore, even though the groups of patients receiving PBS versus IMRT were similar and well balanced, there exists the possibility for unrecognized bias via unmeasured confounders; however, given that both groups were of similar patient characteristics (Table 1) and that patients with HPV-positive OPSCC tend to be homogeneous from a sociodemographic standpoint, we believe that our comparison of the groups, while perhaps not optimal, is still valid. We also acknowledge that current randomized trials of proton therapy versus IMRT for this population will better address such concerns. We also recognize that the total number of patients analyzed is fewer than the number that would be evaluated in a multi-center, randomized trial. We have tried to control for this by focusing our analysis on a relatively homogeneous group of patients treated at a single institution after transoral robotic surgery. However, our limited number of patients allows us to only suggest association between dosimetric gains to organs at risk and improved patient outcomes, rather than demonstrate direct correlation. Again, we are confident that current and future prospective efforts (such as those via prospective trials) will be able to better demonstrate such correlations.
In conclusion, we found that the receipt of PBS, when compared to IMRT, yielded not only dosimetric gains, but also gains in toxicity-specific outcomes, extending to a year out from RT. Patient outcomes are key measures to evaluate and support the application and adoption of a new technology. Our results showing improvements in patient-reported QOL are especially relevant in HPV-related head and neck squamous cell carcinoma in which mitigation of long-term toxicity is of utmost importance given the expected high rates of disease control. Ultimately, our study is but one contribution to the call for clinical data to support the broader use of proton therapy. We, and other centers, will continue work in a collaborative manner to generate additional data to answer these critical questions.
ADDITIONAL INFORMATION AND DECLARATIONS
Conflicts of Interest: The authors have no conflicts of interest to disclose.
References
- 1.Marur S, D'Souza G, Westra WH, Forastiere AA. HPV-associated head and neck cancer: a virus-related cancer epidemic. Lancet Oncol. 2010;11:781–9. doi: 10.1016/S1470-2045(10)70017-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tan PF, Westra WH, Chung CH, Jordan RC, Lu C, Kim H, Axelrod R, Silverman CC, Redmond KP, Gillison ML. Human papillomavirus and survival of patients with oropharyngeal cancer. New Engl J Med. 2010;363:24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O'Sullivan B, Huang SH, Siu LL, Waldron J, Zhao H, Perez-Ordonez B, Weinreb I, Kim J, Ringash J, Bayley A, Dawson LA, Hope A, Cho J, Irish J, Gilbert R, Gullane P, Hui A, Liu FF, Chen E, Xu W. Deintensification candidate subgroups in human papillomavirus-related oropharyngeal cancer according to minimal risk of distant metastasis. J Clin Oncol. 2013;31:543–50. doi: 10.1200/JCO.2012.44.0164. [DOI] [PubMed] [Google Scholar]
- 4.Lindberg R. Distribution of cervical lymph node metastases from squamous cell carcinoma of the upper respiratory and digestive tracts. Cancer. 1972;29:1446–9. doi: 10.1002/1097-0142(197206)29:6<1446::aid-cncr2820290604>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 5.Nutting CM, Morden JP, Harrington KJ, Urbano TG, Bhide SA, Clark C, Miles EA, Miah AB, Newbold K, Tanay M, Adab F, Jefferies SJ, Scrase C, Yap BK, A'Hern RP, Sydenham MA, Emson M, Hall E. Parotid-sparing intensity modulated versus conventional radiotherapy in head and neck cancer (PARSPORT): a phase 3 multicentre randomised controlled trial. Lancet Oncol. 2011;12:127–36. doi: 10.1016/S1470-2045(10)70290-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Langendijk JA, Doornaert P, Verdonck-de Leeuw IM, Leemans CR, Aaronson NK, Slotman BJ. Impact of late treatment-related toxicity on quality of life among patients with head and neck cancer treated with radiotherapy. J Clin Oncol. 2008;26:3770–6. doi: 10.1200/JCO.2007.14.6647. [DOI] [PubMed] [Google Scholar]
- 7.van der Laan HP, Bijl HP, Steenbakkers RJ, van der Schaaf A, Chouvalova O. Vemer-van den Hoek JG, Gawryszuk A, van der Laan BF, Oosting SF, Roodenburg JL, Wopken K, Langendijk JA. Acute symptoms during the course of head and neck radiotherapy or chemoradiation are strong predictors of late dysphagia. Radiother Oncol. 2015;115:56–62. doi: 10.1016/j.radonc.2015.01.019. [DOI] [PubMed] [Google Scholar]
- 8.Taheri-Kadkhoda Z, Bjork-Eriksson T, Nill S, Wilkens JJ, Oelfke U, Johansson KA, Huber PE, Munter MW. Intensity-modulated radiotherapy of nasopharyngeal carcinoma: a comparative treatment planning study of photons and protons. Radiat Oncol. 2008;3:4. doi: 10.1186/1748-717X-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lomax AJ, Goitein M, Adams J. Intensity modulation in radiotherapy: photons versus protons in the paranasal sinus. Radiother Oncol. 2003;66:11–8. doi: 10.1016/s0167-8140(02)00308-0. [DOI] [PubMed] [Google Scholar]
- 10.Simone CB, II, Ly D, Dan TD, Ondos J, Ning H, Belard A, O'Connell J, Miller RW, Simone NL. Comparison of intensity-modulated radiotherapy, adaptive radiotherapy, proton radiotherapy, and adaptive proton radiotherapy for treatment of locally advanced head and neck cancer. Radiother Oncol. 2011;101:376–82. doi: 10.1016/j.radonc.2011.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lomax AJ, Bortfeld T, Goitein G, Debus J, Dykstra C, Tercier PA, Coucke PA, Mirimanoff RO. A treatment planning inter-comparison of proton and intensity modulated photon radiotherapy. Radiother Oncol. 1999;51:257–71. doi: 10.1016/s0167-8140(99)00036-5. [DOI] [PubMed] [Google Scholar]
- 12.Mock U, Georg D, Bogner J, Auberger T, Potter R. Treatment planning comparison of conventional, 3D conformal, and intensity-modulated photon (IMRT) and proton therapy for paranasal sinus carcinoma. Int J Radiat Oncol Biol Phys. 2004;58:147–54. doi: 10.1016/s0360-3016(03)01452-4. [DOI] [PubMed] [Google Scholar]
- 13.Bernier J, Cooper JS, Pajak TF, van Glabbeke M, Bourhis J, Forastiere A, Ozsahin EM, Jacobs JR, Jassem J, Ang KK, Lefebvre JL. Defining risk levels in locally advanced head and neck cancers: a comparative analysis of concurrent postoperative radiation plus chemotherapy trials of the EORTC (#22931) and RTOG (# 9501) Head Neck. 2005;27:843–50. doi: 10.1002/hed.20279. [DOI] [PubMed] [Google Scholar]
- 14.van de Water TA, Bijl HP, Westerlaan HE, Langendijk JA. Delineation guidelines for organs at risk involved in radiation-induced salivary dysfunction and xerostomia. Radiother Oncol. 2009;93:545–52. doi: 10.1016/j.radonc.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 15.Bernier J, Domenge C, Ozsahin M, Matuszewska K, Lefebvre JL, Greiner RH, Giralt J, Maingon P, Rolland F, Bolla M, Cognetti F, Bourhis J, Kirkpatrick A, van Glabbeke M. Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. New Engl J Med. 2004;350:1945–52. doi: 10.1056/NEJMoa032641. [DOI] [PubMed] [Google Scholar]
- 16.Cooper JS, Pajak TF, Forastiere AA, Jacobs J, Campbell BH, Saxman SB, Kish JA, Kim HE, Cmelak AJ, Rotman M, Machtay M, Ensley JF, Chao KS, Schultz CJ, Lee N, Fu KK. Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. New Engl J Med. 2004;350:1937–44. doi: 10.1056/NEJMoa032646. [DOI] [PubMed] [Google Scholar]
- 17.Eisbruch A, Gregoire V. Balancing risk and reward in target delineation for highly conformal radiotherapy in head and neck cancer. Semin Radiat Oncol. 2009;19:43–52. doi: 10.1016/j.semradonc.2008.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, Filiberti A, Flechtner H, Fleishman SB, de Haes JC, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–76. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 19.Beetz I, Burlage FR, Bijl HP, Hoegen-Chouvalova O, Christianen ME, Vissink A, van der Laan BF, de Bock GH, Langendijk JA. The Groningen Radiotherapy-Induced Xerostomia questionnaire: development and validation of a new questionnaire. Radiother Oncol. 2010;97:127–31. doi: 10.1016/j.radonc.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 20.Osoba D, Rodrigues G, Myles J, Zee B, Pater J. Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol. 1998;16:139–44. doi: 10.1200/JCO.1998.16.1.139. [DOI] [PubMed] [Google Scholar]
- 21.Sio TT, Lin HK, Shi Q, Gunn GB, Cleeland CS, Lee JJ, Hernandez M, Blanchard P, Thaker NG, Phan J, Rosenthal DI, Garden AS, Morrison WH, Fuller CD, Mendoza TR, Mohan R, Wang XS, Frank SJ. Intensity modulated proton therapy versus intensity modulated photon radiation therapy for oropharyngeal cancer: first comparative results of patient-reported outcomes. Int J Radiat Oncol Biol Phys. 2016;95:1107–14. doi: 10.1016/j.ijrobp.2016.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.M.D. Anderson Cancer Center. Randomized trial of intensity-modulated proton beam therapy (IMPT) versus intensity-modulated photon therapy (IMRT) for the treatment of oropharyngeal cancer of the head and neck. ClinicalTrials.gov identifier: NCT01893307. https://clinicaltrials.gov/ct2/show/NCT01893307 Accessed May 17, 2018.