Introduction
As the first particle therapy center in Austria, MedAustron is currently set up for operation with an expected start of clinical operation in 2016. MedAustron is designed as a dual-particle facility using proton and carbon ion beams for clinical and nonclinical research. Almost all patients will be enrolled in clinical study protocols. It will be possible to treat up to 1200 patients per year at full operation.
Medical Technical Equipment
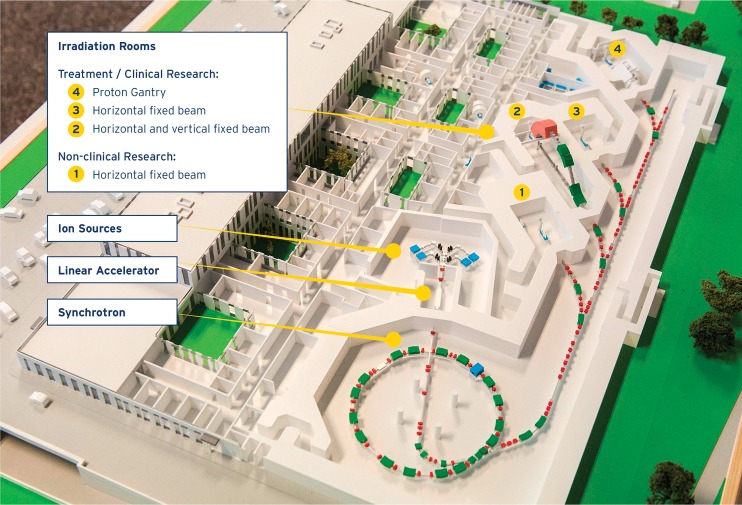
The major medical technical equipment components are not turnkey solutions but are developed and tailored for MedAustron. The particles are accelerated by a synchrotron to energies from 60 to 250 MeV for protons and 120 to 400 MeV/u for carbon ions, and a quasi-discrete spot scanning technique is used for treatment [1]. The accelerator design is a further development of the machine installed at CNAO in Pavia [2], which in turn originated from the Proton-Ion Medical Machine Study [3]. To scan the tumor in depth, 255 energies per particle are provided with expected nominal intensities of 2 × 1010 and 4 × 108 particles per spill of protons and carbon ions, respectively. A total of 4 irradiation rooms will be in operation: 1 nonclinical research room and 3 clinical rooms. A layout of the facility is shown in Figure 1. The clinical rooms are equipped as follows: 1 with a horizontal beam line, 1 with a horizontal and a vertical beam line, and 1 with a proton gantry. The fixed beam lines can all be operated with both protons and carbon ions. At the beginning, 2 treatment rooms with a horizontal beam line will be put into operation. The center is expected to be fully operational, with all beam lines commissioned for the intended particles, by 2019. The sequence of commissioning and therefore the availability of specific beam lines highly impact the spectrum of treatable tumors.
Figure 1.
Layout of the MedAustron facility with accelerator components, and clinical and nonclinical research irradiation rooms indicated.
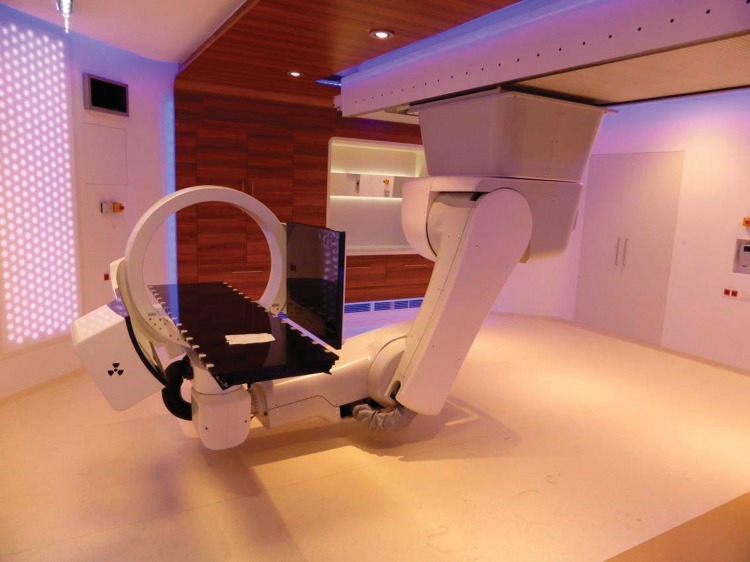
Every treatment room is equipped with a patient alignment system comprising a ceiling-mounted robot and a couch-based imaging ring system equipped with an x-ray source and an independently movable flat panel detector providing cone-beam computed tomography (Figure 2). The robot has 7 degrees of freedom and facilitates nonisocentric treatments to reduce the air gap. An ad hoc developed setup simulation and collision detection software, which is also interfaced with the treatment planning system (TPS), guarantees an efficient planning workflow that reduces lateral dose gradients while ensuring a smooth fraction delivery. The imaging ring system provides pretreatment and intrafraction planar- and cone-beam computed tomography imaging. The completely new design of this imaging ring system not only allows cone-beam computed tomography and planar imaging but also fluoroscopy, dual-energy imaging, as well as image stitching [4]. While ensuring accurate patient positioning and verification in the first place, it will support 4D treatment techniques in the future, mitigating intrafraction motion effects. Its innovative features and flexible implementation allow envisioning the development of further techniques and extended clinical use.
Figure 2.
Ceiling-mounted robot for nonisocentric treatment equipped with imaging ring system for image-guided radiation therapy.
At MedAustron, treatment planning will be accomplished with the TPS RayStation [5]. This TPS is extended and customized by following MedAustron specifications to include scanned proton and carbon ion beams as new treatment modalities. Key features of the TPS for particle therapy include patient-specific Hounsfield unit-density scaling, proton and carbon ion Monte Carlo dose engines, beam-specific margins, machine parameter and treatment time optimization, robust optimization, multicriteria optimization, dose tracking, adaptive re-planning and 4D treatment planning. They are backed up by extensive template and scripting functionalities. For carbon ions, multiple relative-biological-effectiveness models are available, including LEM-I and LEM-IV. Accessibility of model parameters and their organ-specific assignment will allow sensitivity studies.
Since all the major system components discussed above (ie, beam delivery system, patient alignment system, and TPS) have been specifically developed for MedAustron needs, the complexity of integration is quite high. For an efficient facility startup, it is therefore intended to reduce complexity and focus in the initial phase on treatment with protons only. After the specified performance of all key components has been established, the focus will be shifted to the safe implementation of carbon ions in early 2017.
Clinical Study Protocols for Carbon Ion Treatment
Carbon ion treatment will be available in 2017 and clinical studies for highly radioresistant malignancies will then be initiated. The spectrum of planned indications includes adenoid cystic carcinomas, head and neck cancers, pancreas cancer, rectum cancer recurrences, and bone and soft tissue sarcomas and is mostly based on the experience of 2 decades of patient treatment and research at the National Institute of Radiological Science (Chiba, Japan) [6]. For some tumor entities such as chordomas or chondrosarcomas, the role of protons in comparison to carbon ions still needs to be defined in more detail. There are only limited reports comparing the outcome between proton and carbon ion beam treatment in the literature [7]. The need for further research in this area is also reflected by the initiation of trials at other European institutes [8, 9]. Corresponding research activities are already in preparation and will start when both beam qualities are available at MedAustron.
Corresponding study protocols for both beam qualities will be prepared, such as prospective observational studies with the endpoint of tumor control, overall and progression-free survival, as well as side effects and quality of life.
Specific goals will be outlined to:
define dose-volume histogram parameters for the target and relevant organs at risk;
correlate the dose-volume histogram parameters with outcome;
establish dose-response curves for tumor control, morbidity, and quality of life (patient reported symptoms);
develop predictive models for clinical outcome, including dosimetric, clinical, imaging, and biological parameters;
establish an image-guided adaptive radiotherapy concept for ion-beam therapy;
determine how that ion-beam therapy prolongs survival with acceptable quality of life.
Evaluation will thereby focus on commonly used criteria, such as local tumor control assessed by the response evaluation criteria in solid tumors (RECIST) or side effects classified according to the Common Terminology Criteria for Adverse Events (CTCAE), but also we will analyze these aspects in more detail [10, 11]. Important research results will be the correlation of the dose-volume histogram parameters with side effects and their predictive value for generating dose-response curves. Further research fields will be the health-related quality of life and its connection to dose-volume histogram parameters. The evaluation of carbon ion treatment as a part of multimodal treatment concepts (including chemotherapy and targeted therapy strategies) will provide further opportunities for increasing the therapeutic ratio and will add an additional research field at MedAustron.
ADDITIONAL INFORMATION AND DECLARATIONS
Conflicts of Interest: The authors have no conflicts to declare.
References
- 1.Osmić F, Koschik A, Urschütz P, Benedikt M. Status of MedAustron—The Austrian Ion Therapy and Research Centre [abstract] Dresden, Germany: 2014. Presented at: 5th International Particle Accelerator Conference. Abstract WEPRO081. [Google Scholar]
- 2.Rossi S. The status of CNAO. Eur Phys J Plus. 2011;126:78. [Google Scholar]
- 3.Bryant PJ, Badano L, Benedikt M, Crescenti M, Holy P, Maier AT, Pullia M, Reimoser S, Rossi S, Borri G, Knaus P, Gramatica F, Pavlovic M, Weisser L. Proton-Ion Medical Machine Study (PIMMS) Onkologie. 2000. p. 340.
- 4.Deutschmann H, Neuner M, Steininger P, Pinzger M, Buck M, Sedlmayer F. Robotic positioning and imaging. Strahlenther Onkol. 2013;189:185. doi: 10.1007/s00066-013-0358-6. [DOI] [PubMed] [Google Scholar]
- 5.RaySearch Laboratories. RayStation. 2015 http://www.raysearchlabs.com/en/RayStation/ Accessed June 26, 2015.
- 6.Kamada T, Tsujii H, Blakely EA, Debus J, De Neve W, Durante M, Jäkel O, Mayer R, Orecchia R, Pötter R, Vatnitsky S, Chu WT. Carbon ion radiotherapy in Japan: an assessment of 20 years of clinical experience. Lancet Oncol. 2015;16:e93–100. doi: 10.1016/S1470-2045(14)70412-7. [DOI] [PubMed] [Google Scholar]
- 7.Suit H, DeLaney T, Goldberg S, Paganetti H, Clasie B, Gerweck L, Niemierko A, Hall E, Flanz J, Hallman J, Trofimov A. Proton vs carbon ion beams in the definitive radiation treatment of cancer patients. Radiother Oncol. 2010;95:3–22. doi: 10.1016/j.radonc.2010.01.015. [DOI] [PubMed] [Google Scholar]
- 8.Nikoghosyan AV, Karapanagiotou-Schenkel I, Munter MW, Jensen AD, Combs SE, Debus J. Randomised trial of proton vs. carbon ion radiation therapy in patients with chordoma of the skull base, clinical phase III study HIT-1-Study. BMC Cancer. 2010;10:607. doi: 10.1186/1471-2407-10-607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nikoghosyan AV, Rauch G, Munter MW, Jensen AD, Combs SE, Kieser M, Debus J. Randomised trial of proton vs. carbon ion radiation therapy in patients with low and intermediate grade chondrosarcoma of the skull base, clinical phase III study. BMC Cancer. 2010;10:606. doi: 10.1186/1471-2407-10-606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Common Terminology Criteria for Adverse Events (CTCAE) v4.0. 2010 http://evs.nci.nih.gov/ftp1/CTCAE/About.html Accessed June 26, 2015.
- 11.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]