Abstract
Objectives:
The goals of treating recurrent platinum-resistant ovarian cancer are palliative, aimed at reducing symptoms and improving progression free survival. A prospective trial was conducted to determine the prevalence and severity of symptoms, and associated care needs.
Methods:
Eligible women included those with persistent or recurrent platinum-resistant ovarian cancer with an estimated life expectancy of at least 6 months. The Needs at the End-of-Life Screening Tool (NEST), FACIT-Fatigue (FACIT-F), NCCN-FACT Ovarian Symptom Index [NFOSI-18]; Disease Related Symptoms (DRS), Treatment Side Effects (TSE), and Function / Well Being (F/WB)] were collected at study entry, 3 and 6 months.
Results:
We enrolled 102 evaluable patients. Initiation of Do Not Resuscitate (DNR) discussions increased over time from 28% at study entry to 37% at 6 months. At study entry, the most common disease-related symptoms were fatigue (92%), worry (89%), and trouble sleeping (76%); 73% reported being “bothered by treatment side effects”, which included nausea (41%) and hair loss (51%) neither of which changed over time. The most common NEST unmet needs were in the symptom dimension. The social dimension was associated with F/WB (p=0.002) and FACIT-F (p=0.006); symptoms were associated with DRS (p=0.04), TSE (p=0.03), and FACIT-F (p=0.04); existential was not associated with any of the patient-reported symptoms; therapeutic was associated with F/WB (p=0.02).
Conclusions:
In patients nearing the end of life, there are significant associations between disease and treatment related symptoms and unmet patient needs, which do not change substantially over time. Careful exploration of specific end-of-life care needs can improve patient-centered care and QOL.
Keywords: quality of life, symptoms, care needs, ovarian cancer
INTRODUCTION
Women with advanced ovarian cancer are living longer due to tumor reductive surgery, chemotherapy advances, new monoclonal antibodies, and multidisciplinary care [1]. Despite advances in clinical research, the majority of ovarian cancer patients diagnosed with advanced disease will eventually develop recurrence, become resistant to platinum agents and receive palliative treatment. Palliative care is specifically intended to improve the symptoms associated with terminal cancer. Such symptoms include physical, social, and psychological aspects of coping over the entire continuum of care [2]. Nevertheless, the relationships between the patient’s unmet needs, disease, treatment-related symptoms, and quality of life (QOL) have not been well described for women with recurrent ovarian cancer.
Symptom management, the core of palliative care, is an integral part of cancer care throughout the course of disease. Gynecologic cancer symptoms are multi-factorial in character as the primary cancer frequently metastasizes to other abdominal organs [3]. Consequently, recurrent ovarian cancer patients may be on chemotherapy for prolonged periods of time [4], making it difficult to discern if symptoms are related to the disease or treatment side effects. Nevertheless, symptom management and disease monitoring require vigilance.
Patients with advanced incurable cancer have a diverse range of needs [5]. Although a number of advances have been made in the understanding and treatment of cancer-related symptoms and QOL these advances have not necessarily translated into an understanding of the full range of social, existential, and therapeutic needs [6]. Examples of unmet needs include financial hardship, struggles with relationships, and personal goals. Unveiling these issues in an ovarian cancer population remains unexplored.
The multidimensional trajectory of recurrent platinum resistant or refractory ovarian cancer patients has not been previously described [7]. This prospective observational study assessed the care needs, symptoms, and QOL in patients with platinum resistant or platinum refractory ovarian, fallopian and peritoneal cancers on chemotherapy and those not on active treatments. The purpose of this study was to identify significant symptoms and needs to establish optimal targets for future intervention research.
METHODS
Patients
This study was approved by the Institutional Review Board of the participating hospitals. Eligible patients had persistent or recurrent epithelial ovarian, peritoneal or fallopian tube cancer that was platinum-resistant. Platinum-resistance was defined as less than 6 months from the date of the first platinum therapy to date of first evidence of recurrent or persistent disease per imaging, physical exam, or CA-125. Patients were eligible for the study whether or not they were receiving anticancer treatment. The patient’s life expectancy was to be at least 6 months from date of enrollment, and the patient’s consent was required.
Measures
Patient demographic and clinical data were obtained at study entry. Disease status, current cancer therapy, and performance status were collected at study entry, 3 and 6 months. Those patients on cancer therapy were evaluated for the presence of measurable disease at study entry using Response Evaluation Criteria in Solid Tumors’ (RECIST) guidelines version 1.1 [8].
Patient-reported outcome measures (PROs) were collected at study entry, 3 and 6 months. Unmet needs were measured with the Needs at the End-of-Life Screening Tool (NEST). NEST is classified and aggregated into 4 dimensions, which include Social including: social Needs, Existential, Symptoms, and Therapeutic [9–11]. The tool consists of 13 questions: the social dimension includes financial, access to care, having someone close, and care-giving needs; the existential dimension includes distress, spirituality, settledness, and purpose; the symptom dimension includes physical and mental symptoms; the therapeutic dimension includes patient-clinical relationship, information, and goals of care. Each question evaluates the extent to which distinct needs are being met from the patient’s perspective. Cut scores exist for each of the 13 needs with a score above cutoffs considered an unmet need. A domain score was also calculated using proration, if more than 50% of domain items were answered.
QOL was measured with the Functional Assessment of Cancer Therapy – Ovarian (FACT-O) [12]. Fatigue was measured with the FACIT-F (fatigue) subscale [13], neurotoxicity with the FACT/GOG-Ntx-4 subscale [14], and abdominal discomfort with the FACT/GOG-AD subscale [15]. Items were scored using a 5-point scale (0 = not at all; 1=a little bit; 2 = somewhat; 3 = quite a bit; 4 = very much). According to the FACIT measurement system, a subscale score was the summation of the individual item scores if more than 50% of subscale items were answered. Negative statements (or questions) were reversed prior to score calculation. When unanswered items existed, a subscale score was prorated by multiplying the mean of the answered item scores by the number of items in the subscale. A total FACT-O score is the sum of the subscale scores if more than 80% of the FACT-O items provide valid answers.
Ovarian cancer symptoms were also evaluated with the NCCN-FACT Ovarian Symptom Index-18 (NFOSI-18), which includes subscales assessing disease-related symptoms-physical (DRS-P), disease-related symptoms-emotional (DRS-E), treatment side effects (TSE), and function / well-being (F/WB) which is designed specifically to measure symptoms in patients with advanced ovarian cancer. For negative statements (or questions), reversal was performed prior to score calculation. A higher score indicates better QOL/functioning or fewer symptoms/side effects for the FACT-O, the FACT/GOG-Ntx-4 subscale, the FACT/GOG-Ad subscale, the FACIT-Fatigue subscale, and NFOSI-18 subscales. DNR (Do Not Resuscitate) information was gathered during study entry, 3, and 6 months.
Statistical analysis
The prevalence and severity of patient-reported symptoms were measured with the NFOSI-18, defined as the percentage of patients with any degree of the symptom (e.g. item score >=1). A severe symptom was defined as the percentage of patients reporting a symptom as “quite a bit” or “very much” (item score >= 3). The unmet needs for care were summarized as the percentage of patients who scored above the cut score for each distinct category. The association between the NFOSI-18 subscale scores and physical and mental health care needs was explored by fitting a linear mixed model adjusting for patient’s age and performance status at study entry, and assessment time. The association between the NEST subscale scores and NFOSI-18 subscale scores and patient-reported outcomes (PROs) was explored by fitting a linear mixed model adjusting for patient’s age at study entry, and assessment time. All the p-values reported were not adjusted for multiple testing. Therefore, conclusions should be moderated by the exploratory nature of this study.
All analyses were performed using the SAS software, version 9.3 (SAS Institute Inc., Cary, NC).
RESULTS
Between June 2011 and October 2013, 103 eligible platinum-resistant ovarian, peritoneal or fallopian tube cancer patients were enrolled in GOG 267. One patient relocated and could not be contacted. Of the 102 remaining patients, 65 completed all 3 assessments, 21 completed only baseline assessments (19 patients were too sick or died, 1 declined, and 1 was lost to follow-up), 15 completed the first 2 assessments (14 patients died and 1 declined), and 1 patient completed the baseline and third assessment. Overall assessment compliance by living patients at 3 and 6 months was 93% and 94%, respectively. The characteristics of 102 patients are displayed in Table 1. The mean age was 63 with 89% being white and 96% of patients with a provider-rated PS of 0-1. Most (74%) had progression of disease at enrollment and 83% were on chemotherapy.
Table 1:
Patient characteristics at baseline.
| Characteristic | Category | N | % |
|---|---|---|---|
| Age (yrs) | Mean | 63.6 | |
| Age Group | <50 | 14 | 14 |
| 50-59 | 21 | 21 | |
| 60-69 | 39 | 38 | |
| 70-79 | 22 | 22 | |
| >=80 | 6 | 6 | |
| Race | White | 91 | 89 |
| Black/African American | 6 | 6 | |
| Other/Unknown | 5 | 5 | |
| Performance Status | 0 | 58 | 57 |
| 1 | 40 | 39 | |
| 2+ | 4 | 4 | |
| Marital Status | Single | 15 | 15 |
| Married/living with partner | 62 | 61 | |
| Divorced | 13 | 13 | |
| Widowed | 12 | 12 | |
| Employment | Employed or self employed | 38 | 37 |
| Not employed or disabled | 24 | 23.5 | |
| Retired | 39 | 38 | |
| Other | 1 | 1 | |
| Disease Status | Progression | 76 | 74.5 |
| Stable | 15 | 15 | |
| Partial regression | 4 | 4 | |
| Unknown | 1 | 1 | |
| Assessment not done | 6 | 6 | |
| Therapy | Chemotherapy | 85 | 83 |
| Radiation therapy | 1 | 1 | |
| Other care | 2 | 2 | |
| None | 14 | 14 |
Greater than 90% of patients resided at home (96, 92, 93% at time points 0, 3 and 6 months, respectively). The majority of patients received assistance by a family member (73, 73 and 68% at each time point, respectively). Assistance needed within the transportation and personal domains was approximately 30% over the 3 time points. At study entry 28% of patients (n=29) already had a DNR discussion with their provider and this increased to 37% at 6 months. A fitted linear mixed model was applied and suggests that the initiation of a DNR discussion was not associated with any of the PRO outcomes reported in this study.
Patient-reported symptoms and quality of life
Ovarian cancer symptoms in the NFOSI-18 subscales of physical, functional, emotional and side effect did not change significantly over assessment times. The most common disease-related symptoms of any severity were fatigue (92% at baseline, 95% at 3 months, and 91% at 6 months respectively), worry about worsening condition (89% at baseline, 80% at 3 months, and 89% at 6 months respectively), trouble sleeping (76% at baseline, 81% at 3 months, and 86% at 6 months respectively), pain (69% at baseline, 65% at 3 months, and 68% at 6 months respectively), and abdominal problems (65% at baseline, 57% at 3 months, and 59% at 6 months respectively) (Figure 1). More than 70% of the patients were bothered by treatment side effects, for example nausea (41% at baseline, 43% at 3 months, and 48% at 6 months respectively), and hair loss (51% at baseline, 43% at 3 months, and 45% at 6 months respectively). With respect to symptom severity, as measured by proportions of patients experiencing ‘Quite a bit’ or ‘Very much’ of the symptom, fatigue, abdominal symptoms, pain and emotional distress (e.g., worry and discontent) exceeded a 20% threshold as a dominant and severe symptom (Figure 2).
Figure 1.
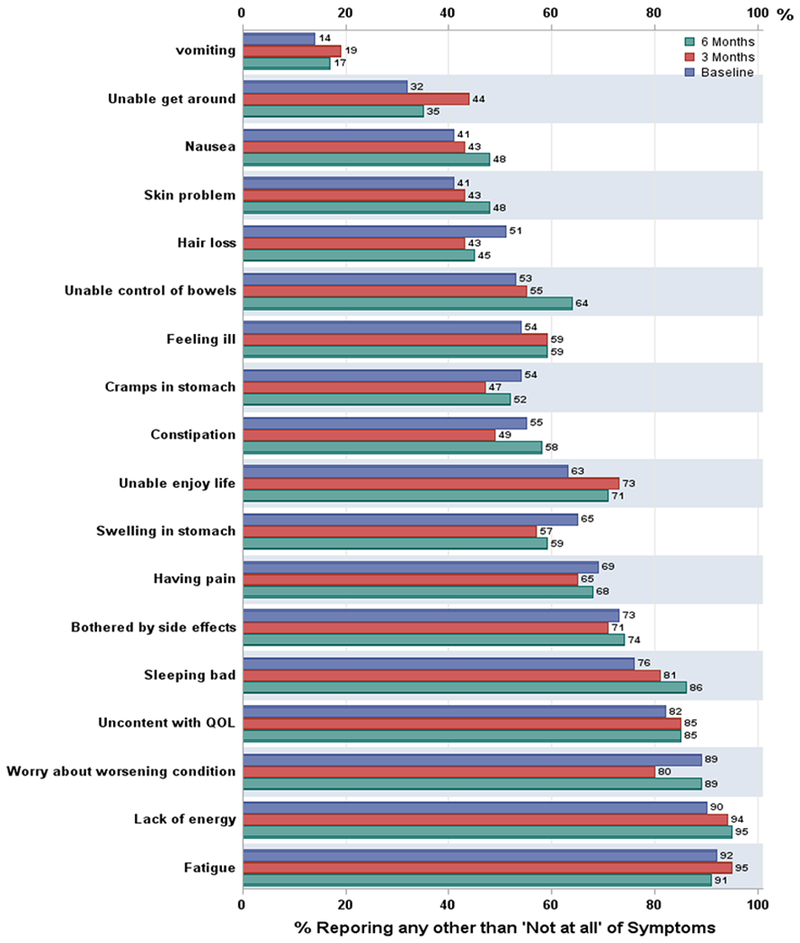
Percentage of patients reporting any other than ‘not at all’ of the FOSI-18 symptoms Baseline is blue bar, 3 months is red bar, 6 months is green bar.
Figure 2.
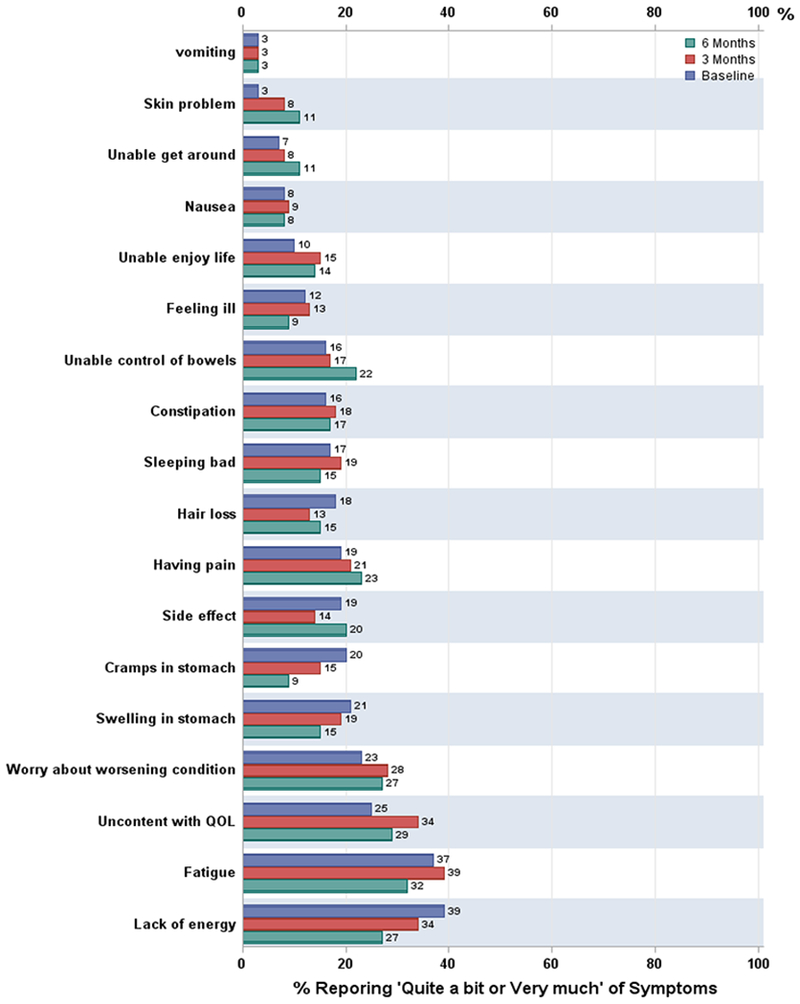
Percentage of patients reporting ‘Quite a bit/very much’ of the FOSI-18 symptoms Baseline is blue bar, 3 months is red bar, 6 months is green bar.
Patient-reported unmet needs and associations
The most common unmet need (NEST) over time was in the symptom dimension compared with the social, existential, and therapeutic dimensions. The 4 dimensions of needs did not change over time. There were 5 dominant areas within the dimensions in which there were unmet needs (Figure 3). Those included the areas of caregiving, mental health care, financial, illness distress, and physical health care needs. The largest change over time was financial support with 45% at study entry, 59% at 3 months, and 61% at 6 months having needs.
Figure 3.
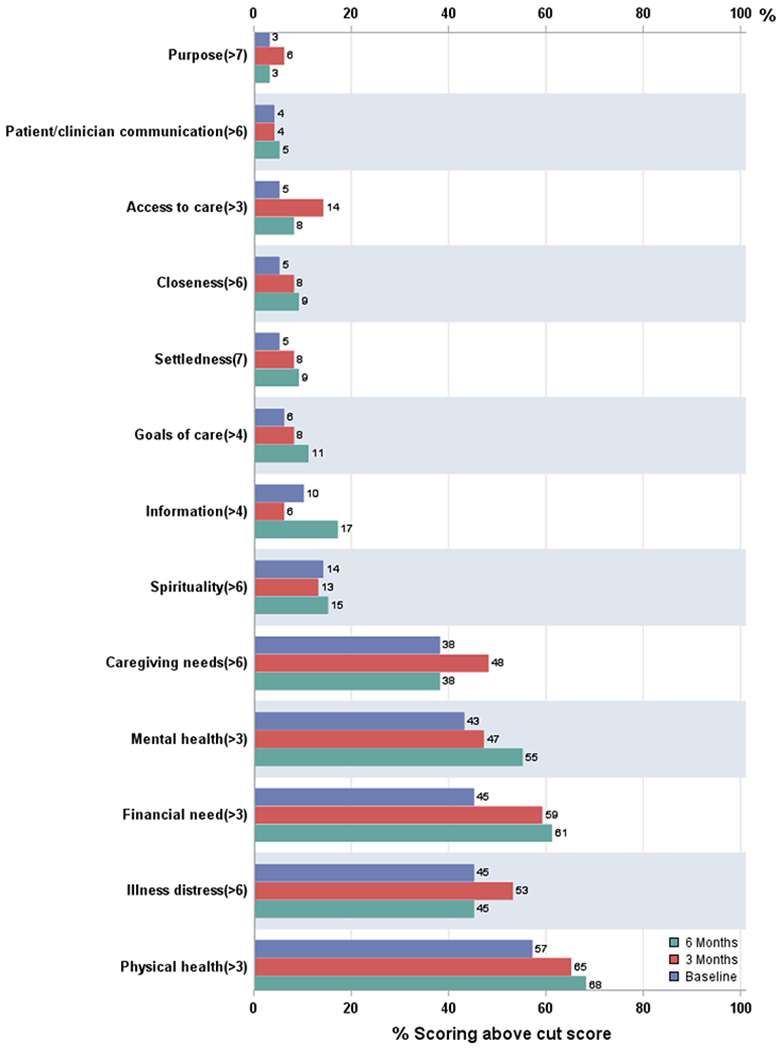
Percentage of patients reporting unmet needs. Baseline is blue bar, 3 months is red bar, 6 months is green bar.
There are associations between care needs (NEST) in the symptom care dimension and the NFOSI-18 symptom subscale scores (Figure 4, A and B). After adjusting for age, performance status at study entry and repeated measures over time, patients reporting significant physical and mental health care needs (>cut score) also reported more disease-related physical symptoms (p<0.001 for physical health care needs and p=0.006 for mental health care needs), more disease-related emotional symptoms (p=0.02 for physical health care needs and p<0.001 for mental health care needs), more treatment side effects (p<0.001 for physical health care needs and mental health care needs respectively), and compromised functional well-being (p<0.001 for physical and mental health care needs respectively).
Figure 4.
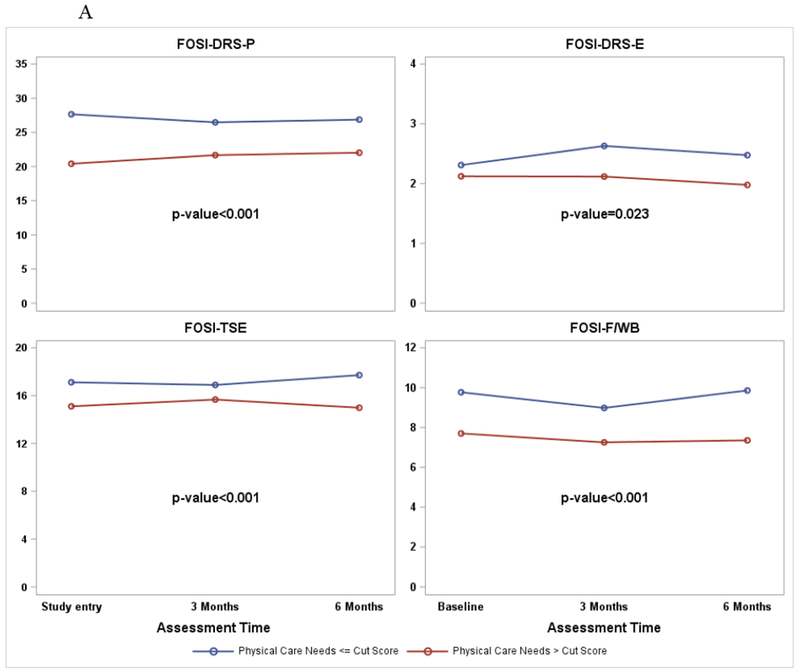
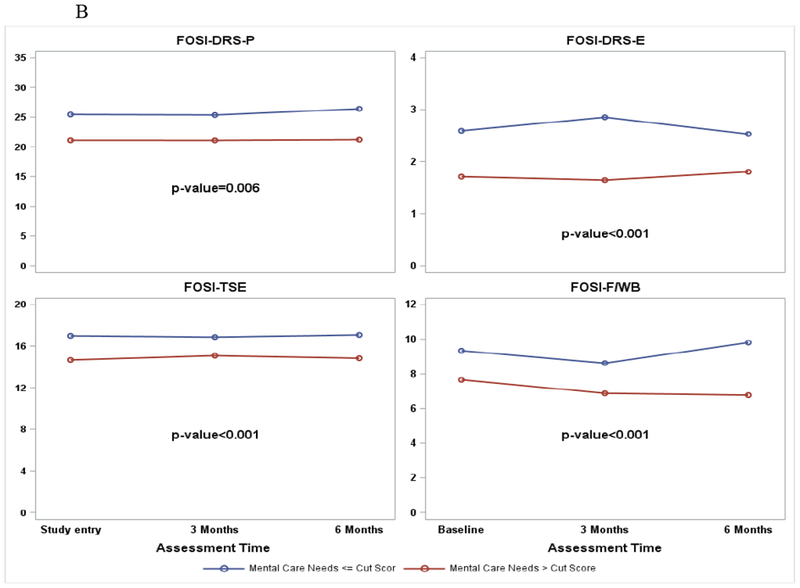
A. NFOSI-18 Subscale score by physical care need cut score. P-values are for the associations between the NFOSI-18 subscale scores and physical health care needs explored with a linear mixed model with adjustment for age, performance status at baseline and assessment time.
B. NFOSI-18 Subscale score by mental care need cut score. P-values are for the associations between the NFOSI-18 subscale scores and mental health care needs explored with a linear mixed model with adjustment for age, performance status at baseline and assessment time.
The associations between care needs (NEST) dimensions (social, existential, symptoms and therapeutic), NFOSI-18 subscales (DRS, TSE, F/WB), and PROs (FACT/GOG-Ad subscale, FACT/GOG-Ntx subscale, FACIT-Fatigue subscale) were explored. The NEST score of the social domain was found to be associated with F/WB (p=0.002) and FACIT-F (p=0.006); the NEST symptom dimension was associated with DRS (p=0.04), TSE (p=0.03), FACT/GOG-Ad (p=0.04), FACT/GOG-Ntx (p=0.006), and FACIT-F (p=0.04); the NEST existential dimension was not associated with any of the patient-reported symptoms; the NEST therapeutic dimension was associated with F/WB (p=0.02) only. There were, therefore, significant unmet needs in disease, treatment related patient symptoms and QOL.
DISCUSSION
This is the first study to prospectively describe QOL, disease and treatment related symptoms, and unmet needs in patients with recurrent, platinum resistant ovarian cancer. Approximately 14% of patients died within 3 months and 30% died within 6 months, confirming that this patient population has a poor clinical prognosis. The most common disease-related symptoms in these patients were fatigue, worry, and trouble sleeping. In addition, the majority of patients reported being bothered by treatment side effects, which was corroborated by distress reported from nausea, skin problems, and hair loss. There were significant and stable correlations between disease and treatment related symptoms, QOL and patient’s unmet needs which were stable over time. In essence, both disease and treatment-related symptoms were prominent, and can be linked to specific care needs expressed by this population.
This study revealed significant unmet needs in the existential dimension. The existential dimension explores the meaning of the patient’s illness, spirituality, personal relationships, and the sense of purpose in life [10]. A substantial majority of advanced cancer patients receiving palliative care consider themselves spiritual or religious [16]. Vallurupalli et al., surveyed patients receiving palliative radiation therapy for advanced cancer and 84% indicated reliance on religion or spirituality to cope with cancer [17]. In addition, they found that patient’s spiritually and religious coping were associated with improved QOL. Since research examining ovarian cancer patient’s existential perceptions is lacking, this study supports the importance of communication on this topic between the clinician and patient.
The literature examining financial distress in palliative cancer patients is minimal. Emanuel et al. interviewed nearly 1,000 patients with a terminal illness and found substantial care needs for transportation, nursing care, homemaking and personal care [18]. Many spent 10% of their household income on their terminal illness needs while others had family members that took out loans or mortgages, spent their savings or obtained additional employment to help with global financial issues. Using the NEST tool, Grudzen et al., assessed palliative needs in seriously ill patients accessing the emergency department, in which 42% had cancer, and revealed that over half of the patients had significant needs in the finance category [19]. Others have reported on the financial strain during cancer treatment revealing significant burden and lower QOL [20, 21]. In our sample, at 6 months, 61% of patients reported substantial financial needs requiring support.
Consistent with the literature, symptoms of fatigue, worry, nausea and sleep disturbance are significant issues in ovarian cancer patients as the disease advances [4, 22, 23]. Beesley et al., in a population-based study of ovarian cancer patients, found that in patients with platinum-resistant disease, retreatment with chemotherapy did not significantly improve or diminish their QOL as one quarter had increases and one third had decreases [24]. As with our results, they also found that the social domain was the highest QOL component with platinum-resistant or refractory disease. While our study population had a large compromise in emotional well being, Sjoquist et al. saw their lowest score in the functional domain [23]. Not surprisingly, similar domain trends in QOL have been seen during adjuvant treatment for ovarian cancer [25]. While other authors have found correlations between the social, functional and fatigue domains, this is the first study revealing all three within platinum-resistant disease [22,25]
The American Society of Clinical Oncology (ASCO) and the National Comprehensive Cancer Network (NCCN) recommend that providers engage in advance care planning to discuss advanced and recurrent cancer patient’s goals, expectations and palliative care with not only the patient but also with the family [26]. Documents such as advance directives or DNR can be generated from such discussions. Research has shown that informational pamphlets can help facilitate discussions, increase DNR orders and decrease hospital deaths in cancer patients [27]. We found improvement in DNR orders between study entry at 28% to 37% at 6 months, however, opportunity for further progress remains. While our study was limited to assessing a DNR order, others have found that the majority of advanced or recurrent ovarian cancer patients do not have any documentation of advance care planning [28]. There continues to be a need for both better understanding of the dynamics of palliative chemotherapy and care between physicians and patients in addressing end-of-life care planning [29]. Not surprisingly, there was significant attrition in the study, secondary to death as physicians have a tendency to initiate DNR orders close to the end of life. [28,29]
The strengths of the study include its prospective design with state-of-the science patient-reported outcome measures within a national cancer cooperative group. It is noteworthy that the study outpaced accrual expectations, highlighting indications nationally that the topic of unmet needs and symptom management for recurrent ovarian cancer patients is viewed as a priority. Although data trends reveal that symptoms were relatively stable over the 6 months, we recognize that those who deteriorated at the 3 and 6-month assessment intervals are therefore not represented in this data set (see accompanying manuscript). The stability of these scores do, however, reflect the symptom profile and needs of those whose disease might be stable or who are responding to cancer or palliative care treatment, or both. While this statement is speculative, in the absence of clinical data, one can surmise that a sizeable proportion of women (>50%) who entered the study, with an estimated prognosis of 6 months or more, continued to maintain an overall reasonable quality of life. Nevertheless, documentation of their unmet needs suggests that more can be done to address physical, functional, emotional and existential and spiritual concerns. Importantly, a DNR order was used as a surrogate marker for end-of-care planning; however we recognize that discussions between the physician and the patient may have been held but not captured within the study so this marker of 37% is likely an underestimate of meaningful discourse.
This study has provided insight into advanced, recurrent ovarian cancer patients unmet needs and their complex associations with disease and treatment-related symptoms and QOL. Although it is beyond the scope of this manuscript to predict who was or was not benefitting from palliative chemotherapy, future longitudinal studies with this population will be able to document varying levels of responsiveness to cancer treatments despite an overall poor prognosis. These results can help future researchers decide which PRO tools are best for a given study or application. The fact that they are significantly related to one another provides reassurance that selecting one or another instrument will be informative and reasonably comprehensive. The FACT-O and its abbreviated, more symptom-focused NFOSI, remain good choices for clinical trials and longitudinal assessment of QOL. On the other hand, NEST might be the better choice for a focused study of needs assessment in patients with very advanced or progressive disease. Finally, the challenge for clinicians is how to help individuals with significant symptoms and gauge whether palliative and supportive treatments are helping or hurting the amelioration of unmet health needs and the enhancement of patient QOL.
RESEARCH HIGHLIGHTS.
In recurrent ovarian cancer, the most common unmet need is in the symptom dimension.
The most common symptom is fatigue.
Nearing the end of life there are associations between symptoms, unmet need, and QOL.
Acknowledgments
This study was supported by National Cancer Institute grants to the Gynecologic Oncology Group (GOG) Administrative Office (CA 27469), the Gynecologic Oncology Group Statistical Office (CA 37517), NRG Oncology SDMC (1U10 CA180822) and NRG Operations (U10CA180868). The following Gynecologic Oncology Group member institutions participated in the primary treatment studies: University of Oklahoma Health Sciences Center, Women and Infants Hospital, Ohio State University Comprehensive Cancer Center, University of Virginia, Carolinas Medical Center/Levine Cancer Institute, Metro-Minnesota CCOP, University of California Medical Center at Irvine-Orange Campus, Wayne State University/Karmanos Cancer Institute, Froedtert and the Medical College of Wisconsin, Washington University School of Medicine, University of Wisconsin Hospital and Clinics, Baystate Medical Center, Cancer Research for the Ozarks NCORP, Mainline Health CCOP, University of New Mexico, University of Massachusetts Memorial Health Care, Case Western Reserve University, The Hospital of Central Connecticut, Evanston CCOP-NorthShore University Health System, Michigan Cancer Research Consortium Community Clinical Oncology Program, Northern Indiana Cancer Research Consortium, Northwestern University, University of Colorado Cancer Center – Anschutz Cancer Pavilion, Rush University Medical Center, State University of New York Downstate Medical Center, MD Anderson Cancer Center, University of Chicago and Wichita CCOP.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICTS OF INTEREST
Dr. David Cella receives money from GOG for travel to meetings for the study or other purposes.
Dr. Joan Walker receives money from Mateon for consultancy, as well as travel/accommodations/meeting expenses unrelated to activities listed.
Dr. Susan Modesitt receives grant money for the University of Virginia.
Dr. Matthew Boente receives money from Genentech Inc. for consultancy, employment and stock/stock options. He also receives money from Genentech Inc. and Clovis Inc. for payment for lectures including service on speakers bureaus, as well as travel/accommodations/meeting expenses unrelated to activities listed.
Dr. Lari Wenzel receives money from NRG Oncology Group for support for travel to meetings for the study or other purposes.
All other co-authors having no conflicts of interest to declare.
REFERENCES
- 1.Cliby WA, Powell MA, Al-Hammadi N, Chen L, Philip Miller J, Roland PY, et al. Ovarian cancer in the United States: contemporary patterns of care associated with improved survival. Gynecol Oncol 2015; 136(1):11–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Radwany SM, von Gruenigen VE. Palliative and end-of-life care for patients with ovarian cancer. Clin Obstet Gynecol. 2012; 55(1):173–184. [DOI] [PubMed] [Google Scholar]
- 3.Donovan KA, Donovan HS, Celia D, Gaines ME, Penson RT, Plaxe SC, et al. Recommended patient-reported core set of symptoms and quality-of-life domains to measure in ovarian cancer treatment trials. J Natl Cancer Inst 2014; 106(7):1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Friedlander ML, Stockler M, O’Connell R, Voysey M, Oza A, Gillies K, et al. Symptom burden and outcomes of patients with platinum resistant/refractory recurrent ovarian cancer: a reality check: results of stage 1 of the gynecologic cancer intergroup symptom benefit study. Int J Gynecol Cancer 2014; 24(5):857–864. [DOI] [PubMed] [Google Scholar]
- 5. http://www.cancer.gov/cancertopics/pdq/supportivecare/transitiontoEOLcare/healthprofessional.
- 6.Levy MH, Adolph MD, Back A, Block S, Codada SN, Dalai S, et al. Palliative Care. J Natl Compr Cancer Netw. 2012; 10(10):1284–1309. [DOI] [PubMed] [Google Scholar]
- 7.Donovan KA, Greene PG, Shuster JL, Partridge EE, Tucker DC. Treatment preferences in recurrent ovarian cancer. Gynecol Oncol. 2002; 86: 200–211. [DOI] [PubMed] [Google Scholar]
- 8.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009; 45:228–247. [DOI] [PubMed] [Google Scholar]
- 9.Emanuel EJ, Fairclough DL, Slutsman J, Alpert H, Baldwin D, Emanuel LL. Assistance from family members, friends, paid care givers, and volunteers in the care of terminally ill patients. N Engl J Med. 1999; 341: 956–963. [DOI] [PubMed] [Google Scholar]
- 10.Emanuel LL, Alpert HR, Emanuel EE. Concise screening questions for clinical assessments of terminal care: the needs near the end-of-life care screening tool. J Palliat Med. 2001; 4:465–474. [DOI] [PubMed] [Google Scholar]
- 11.Scandrett KG, Reitschuler-Cross EB, Nelson L, Sanger JA, Feigon M, et al. Feasibility and effectiveness of the NEST13+as a screening tool for advanced illness care needs. J Palliat Med. 2010; 13:161–169. [DOI] [PubMed] [Google Scholar]
- 12.Basen-Engquist K, Bodurka-Bevers D, Fitzgerald MA, Webster K, Cella D, Shaohua H, et al. Reliability and validity of the functional assessment of cancer therapy-ovarian. J Clin Oncol 2001; 19:1809–1817. [DOI] [PubMed] [Google Scholar]
- 13.Yellen SB, Cella DF, Webster K, Blendowski C, Kaplan E. Measuring fatigue and other anemia-related symptoms with the functional assessment of cancer therapy (FACT) measurement system. J Pain Symptom Manage. 1997; 13:63–74. [DOI] [PubMed] [Google Scholar]
- 14.Huang HQ, Brady MF, Cella D, Fleming G. Validation and reduction of FACT/GOG-Ntx subscale for platinum/paclitaxel-induced neurologic symptoms: a Gynecologic Oncology Group study. Int J Gynecol Cancer. 2007; 17:387–393. [DOI] [PubMed] [Google Scholar]
- 15.Wenzel L, Huang HQ, Cella D, Walker JL, Armstrong DK; Gynecologic Oncology Group. Validation of FACT/GOG-AD subscale for ovarian cancer-related abdominal discomfort: a Gynecologic Oncology Group study. Gynecol Oncol. 2008;110(1):60–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Delgado-Guay MO, Hui D, Parsons HA, Govan K, De la Cruz M, Thorney S, et al. , Spirituality, religiosity, and spiritual pain in advanced cancer patients. J Pain Symptom Manage. 2011; 41 (6):986–994. [DOI] [PubMed] [Google Scholar]
- 17.Vallurupalli M, Lauderdale K, Balboni MJ, Phelps AC, Block SD, Ng AK, et al. The role of spirituality and religious coping in the quality of life of patients with advanced cancer receiving palliative radiation therapy. J Support Oncol. 2012; 10 (2):81–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Emanuel EJ, Fairclough DL, Slutsman J, Emanuel LL. Understanding economic and other burdens of terminal illness: the experience of patients and their caregivers. Ann Intern Med. 2000; 132(6):451–459. [DOI] [PubMed] [Google Scholar]
- 19.Grudzen CR, Richardson LD, Morrison M, Cho E, Morrison RS. Palliative care needs of seriously ill, older adults presenting to the emergency department. Acad Emerg Med. 2010; 17(11):1253–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kale HP, Carroll NV. Self-reported financial burden of cancer care and is effect on physical and mental health-related quality of life among US cancer survivors. Cancer. 2016; 122(8):283–289. [DOI] [PubMed] [Google Scholar]
- 21.Fenn KM, Evans SB, McCorkle R, DiGiovanna MP, Pusztai L, Sanft T, et al. Impact of financial burden of cancer on survivors’ quality of life. J Oncol Pract. 2014; 10(5):332–338. [DOI] [PubMed] [Google Scholar]
- 22.Price MA, Bell ML, Sommeijer DW, Friedlander M, Stockler MR, Defazio A, et al. Physical symptoms, coping styles and quality of life in recurrent ovarian cancer: A prospective population-based study over the last year of life. Gynecol Oncol. 2014; 130:162–168. [DOI] [PubMed] [Google Scholar]
- 23.Sjoquist KM, Friedlander ML, O’Connell RL, Voysey M, King MT, Stockler MR, et al. Hope, quality of life, and benefit from treatment in women having chemotherapy for platinum-resistant/refractory recurrent ovarian cancer: the gynecologic cancer intergroup symptom benefit study. Oncologist. 2013; 18(11):1221–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beesley VL, Green AC, Wyld DK, O’Rourke P, Wockner LF, DeFazio A, et al. Quality of life and treatment response among women with platinum-resistant versus platinum-sensitive ovarian cancer treated for progression: a prospective analysis. Gynecol Oncol. 2014; 132(1 ):130–136. [DOI] [PubMed] [Google Scholar]
- 25.von Gruenigen VE, Huang HQ, Gil KM, Gibbons HE, Monk BJ, Rose PG, et al. Assessment of factors that contribute to decreased quality of life in Gynecologic Oncology Group ovarian cancer trials. Cancer. 2009; 115(20):4857–4864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Calton BA, Alvarez-Perez A, Portman DG, Ramchandran KJ, Sugalski J, Rabow MW. The Current State of Palliative Care for Patients Cared for at Leading US Cancer Centers: The 2015 NCCN Palliative Care Survey. J Natl Compr Canc Netw. 2016; 14(7):859–866. [DOI] [PubMed] [Google Scholar]
- 27.Stein RA, Sharpe L, Bell ML, Boyle FM, Dunn SM, Clarke SJ. Randomized controlled trial of structured intervention to facilitate end-of-life decision making in patients with advanced cancer. J Clin Oncol. 2013; 31:3403–3410. [DOI] [PubMed] [Google Scholar]
- 28.Brown AJ, Sun CC, Prescott LS, Taylor JS, Ramondetta LM, Bodurka DC. Missed opportunities: Patterns of medical care and hospice utilization among ovarian cancer patients. Gynecol Oncol. 2014; 135:244–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.von Gruenigen V, Daly B, Gibbons H, Hutchins J, Green A. Indicators of survival duration in ovarian cancer and implications for aggressiveness of care. Cancer. 2008; 112: 2221–2227. [DOI] [PubMed] [Google Scholar]


