Abstract
In the postpartum period, the maternal brain experiences both structural and functional plasticity. While we have a growing understanding of the human maternal brain’s responses to infant stimuli, little is known about the intrinsic connectivity among those regions during the postpartum months. Resting-state functional connectivity (rsFC) provides a measure of the functional architecture of the brain based upon intrinsic functional connectivity i.e. the temporal correlation in blood oxygenation level dependent signal when the brain is not engaged in a specific task. In this study, we used resting-state fMRI to examine how later postpartum months are associated with rsFC and maternal behaviors. We recruited a sample of forty-seven socioeconomically diverse first-time mothers with singleton pregnancies. As the amygdala has been shown to play a critical role in maternal behaviors in the postpartum period, it was chosen as the seed for a seed-based correlation analysis. For the left amygdala, later postpartum months were associated with greater connectivity with the anterior cingulate gyrus, left nucleus accumbens, right caudate, and left cerebellum (p < 0.05, FDR corrected). Further, in an exploratory analysis, we observed indications that rsFC between the left amygdala and left nucleus accumbens was positively associated with maternal structuring during a mother child-interaction. In addition, later postpartum months were associated with greater connectivity between the right amygdala and the bilateral caudate and right putamen. Overall, we provide evidence of relationships between postpartum months and rsFC in the regions involved in salience detection and regions involved in maternal motivation. Greater connectivity between the amygdala and nucleus accumbens may play a role in positive maternal behaviors.
Keywords: maternal, brain, postpartum, resting-state, connectivity
1. Introduction
Functional connectivity analysis has emerged as a critical tool to understand the functional architecture of the human brain. Functional connectivity analyses broadly fall into two categories: task-based (psychophysiological interaction, PPI) and resting state. Task-based functional connectivity involves analyzing correlations in time series across the brain in the context of the participant performing a task. Functional connectivity analysis has been used as a method to study the maternal brain during the postpartum period1, but most studies have used task-based functional connectivity. Resting-state functional magnetic resonance imaging (rsfMRI) measures intrinsic functional connectivity while the participant is at ‘rest’ and not engaged in a specific task. For maternal brain studies, rsfMRI examines baseline functional connectivity between brain regions without the constraints of examining functional connectivity within the specific context of an infant cry or infant picture task. Thus, rsfMRI may be particularly helpful to examine the functional plasticity during the first postpartum year. Thus, the current cross-sectional study examined the resting state functional connectivity (rsFC) of the maternal brain and its associations with the postpartum timing and maternal behaviors.
The early postpartum period is a time of both structural and functional plasticity in the maternal brain. While structural plasticity refers to changes of the physical structure of neurons, functional plasticity refers to neurons or brain regions changing aspects of their function2. In non-human animal studies, structural changes have been observed in the amygdala, prefrontal cortex, hypothalamus, striatum, and medial temporal lobe3, 4. These changes in brain structure of nonhuman animals are critical for the initiation and maintenance of maternal behaviors and have functional roles in maternal motivation5, processing of infant somatosensory information as well as self-monitoring of parental behaviors5. The structural changes that have been observed in animal studies were also observed in similar regions for human mothers in which gray matter volume increased during the early postpartum period in the amygdala, hypothalamus, substantia nigra, prefrontal cortex, and medial temporal lobes1. Further, in a cross-sectional study of first-time mothers, later postpartum months were associated with greater cortical thickness in the superior frontal gyrus extending into the medial and orbitofrontal gyri, lateral occipital gyrus extending into the inferior parietal and fusiform gyri, caudal middle frontal, and precentral gyri6. Interestingly, evidence also exists to the contrary, pregnancy was associated with volumetric reductions, reductions in cortical thickness and surface area in prefrontal and parietal regions involved in social cognition as well as in the hippocampus; the reductions in gray matter remained at a follow-up two years postpartum except for a partial left hippocampal volume recovery7. The contradicting evidence remains unclear, however, pregnancy versus the postpartum period may have unique associations with brain structure that remain to be tested within the same sample.
While several studies have identified regions that are activated in response to infant stimuli8, much less is known about the association between postpartum timing and brain function. Evidence from non-human animal studies suggests increases in functional plasticity during the postpartum period1, 3. During pregnancy, functional plasticity is mediated predominantly by hormonal changes3, however; during the postpartum period, the functional plasticity is primarily driven by the social experience of interacting with the infant9. This functional plasticity supports the mother’s ability to link sensory cues to the underlying needs of the infant and respond in a prompt and appropriate manner10. This experience-dependent plasticity functions to modulate the maternal brain circuitry and facilitate the ongoing interactions with the infant3. However, the relationship between postpartum months and rsFC in the postpartum period remains unclear.
A large-scale network of brain regions of human mothers is strongly activated by infant cues in the postpartum period, however it remains unclear if similar regions’ ‘baseline’ or ‘intrinsic functional connectivity’ is associated with postpartum timing. The rsFC of the amygdala with other brain regions is involved in the maternal motivation/reward system as well as salience detection and emotion regulation. Evidence from non-human animal and human literature suggest the amygdala plays a critical role in detecting the salient value of an infant cues and activating the motivation and affective networks3. Therefore, in task-based functional connectivity analysis of human mothers, the amygdala is often chosen as the seed region. In a study using task-based connectivity, mothers in the postpartum period that were more successful in coordination of their behavior with their infant’s signals, had greater rsFC between the amygdala and medial prefrontal cortex when using the amygdala as a seed region11. The regions that are functionally connected with the amygdala are important for supporting maternal behaviors. In the postpartum period, basolateral amygdala activation is increased, which provides inputs to the nucleus accumbens, a key region involved in motivation12. Brain regions such as the anterior cingulate cortex that are involved in emotion regulation are often active for new mothers listening to their infant’s cry, thought to represent parents managing their own distress when an infant is distressed13. The medial prefrontal cortex, a part of the motivation and affective networks and connected with the amygdala, is also activated in response to infant stimuli14. Overall, studies of functional connectivity using task-based analysis have highlighted the importance of the functional connectivity of the amygdala in response to infant cues.
Few studies have used rsFC to examine the postpartum period in human mothers. In non-human animal work, rsFC has been used to examine the effects of early life social stress on functional connectivity in animal studies, specifically in offspring15; changes in dams exposed to early life social stress showed deficits in maternal caregiving behaviors accompanied by changes in functional connectivity in the prefrontal cortex, nucleus accumbens, hippocampus and somatosensory cortex15. One existing human mother study of the rsfMRI in the postpartum period found that women experiencing postpartum depression had a negative coupling in rsFC between the posterior cingulate cortex and the right amygdala compared to a positive coupling in healthy women16. Another study focusing on postpartum depression, found that women experiencing postpartum depression had attenuated rsFC between the anterior cingulate cortex, amygdala, hippocampus, and dorsolateral prefrontal cortex17. However, the existing studies of rsFC in the postpartum period have focused on postpartum depression; therefore, there is relatively little knowledge concerning rsFC in the postpartum period in women not experiencing clinical levels of depression. Such understanding can guide further investigation of the functional plasticity in maternal brain as well as provide a risk marker for negative postpartum adaptation in the intrinsic functional connectivity throughout the postpartum months.
Further, because structural and functional variations in maternal brain circuitry are hypothesized to support maternal behaviors3, we conducted an exploratory analysis of the association between rsFC in the postpartum period and positive maternal behaviors. Positive maternal behaviors refer to parenting behaviors that are associated with positive outcomes for the infant18. Maternal sensitivity is a positive behavior that is described as being aware of infant cues, interpreting them correctly, and responding appropriately with genuine and appropriate positive affect directed towards the infant19. Maternal sensitivity has implications for later infant social competency20 and cognitive development21. Maternal structuring is another positive maternal behavior that is associated with infant outcomes. Structuring refers to the ability of the mother to scaffold appropriate interactions with her infant in a way that the infant responds positively19. Structuring has been shown to be positively associated with an infant’s ability to encode actions as goal-directed and theory-of-mind capacities22. Evidence from a task-based functional connectivity study suggests that greater functional connectivity between the amygdala and other maternal brain regions is associated with positive maternal behaviors. In a study of women in the postpartum period, the association between task-based functional connectivity while mothers viewed a video of their infant and maternal behaviors was examined11. Mothers with synchronous behavior in a mother-child interaction had greater amygdala and nucleus accumbens functional connectivity with regions involved in social cognition (such as the insula and superior temporal gyrus). However, it is unclear whether FC is associated with positive parental behaviors more generally, and whether non-task based FC is also associated with positive parental behaviors. For this reason, we have examined rsFC and its association with sensitivity and structuring. This understanding would help provide evidence that functional variations in maternal brain circuitry support positive parenting behaviors.
The current study examined whether postpartum months are associated with the strength of the intrinsic functional connectivity in human mothers’ brains. We used rsfMRI to examine the relationship between postpartum months and rsFC. As there is an extensive literature suggesting the functional importance of the amygdala for maternal behaviors in the postpartum period and many task-based functional connectivity studies use the amygdala as a seed region23, 24, we chose it as the seed for a resting-state seed-based correlation analysis. We hypothesized, due to the role of the reward system in maintenance of maternal behaviors and its extensive structural connectivity with the amygdala3, postpartum months will have a positive association with amygdala-nucleus accumbens rsFC. Further, due to its role in both the salience and emotional regulation networks critical for motherhood and connectivity with the amygdala25, we hypothesize that postpartum months will have a positive association with amygdala-anterior cingulate/medial prefrontal cortex and amygdala-anterior insular rsFC. We also explored the association between rsFC and maternal behaviors measured during a mother-child interaction. We hypothesize there will be a positive relationship between the amygdala rsFC and positive maternal behaviors. We focus on two types of positive maternal behaviors: sensitivity and structuring.
Materials and Methods
1.1. Participants
A socioeconomically diverse sample was recruited via brochures and flyers at WIC (Women, Infant, and Children) centers, midwifery clinics in the Denver metro area, and Colorado-state Prenatal Plus programs. All the participants were first time mothers with a biological infant aged 0–10 month. Participants were considered eligible for participation if they were (1) English speaking (2) did not have a current or historical psychiatric/neurological disorder other than anxiety or depression (to keep a controlled but ecologically valid community sampling approach) (3) did not have psychoactive drug use (except antidepressants) (4) pregnancy-related or infant medical illnesses that involved more than a one-night stay in the neonatal intensive care unit (5) did not have MRI contraindications that would prevent scanning. Of 51 participants, 4 participants were excluded after neuroimaging data quality control, thus the final number of the participants included in the analysis was 47. See Table 1 for a description of the demographic characteristics of the sample.
Table 1.
Demographic information for the sample.
| N (%) | Mean ± SD | Range | |
|---|---|---|---|
| Postpartum months at the time of scan | 4.52 ± 1.85 | 0.89 – 9.82 | |
| Maternal age (years) | 25.65 ± 5.29 | 18 – 36 | |
| Maternal race/ethnicity (non-white) | 18 (38.2) | ||
| Caucasian | 29 (61.7) | ||
| Hispanic | 7 (14.9) | ||
| Mixed | 4 (8.5) | ||
| Other | 4 (8.5) | ||
| Infant sex (female) | 31 (66) | ||
| Maternal Sensitivity | 5.14 ± 1.23 | 3 – 7 | |
| Maternal Structuring | 4.75 ± 1.35 | 2 – 7 | |
| Depressive mood (BDI) | 7.08 ± 5.29 | 0 – 26 | |
| Anxious mood (State) | 31.55 ± 7.35 | 20 – 55 | |
| Postpartum months at the time of home visit | 3.63 ± 1.66 | 0.72 – 7.00 | |
| History of depression or anxiety (yes) | 15 (31.3) | ||
| Current intake of antidepressants | 4 (0.08) | ||
| Breastfeeding (and/or daily pump; not formula only) | 46 (97.87) | ||
| Right handedness | 42 (89.36) | ||
| Time away from own infant per week (hours) | 15.76 ± 16.13 | 0 – 50 | |
| Interval between home and MRI visit (months) | 0.91 ± 0.74 | 0.13 – 3.42 | |
| Maternal education (years) | 14.36 ± 2.38 | 9 – 20 | |
| Income-to-needs ratio | 2.65 ± 1.45 | 0.63 – 5.88 | |
1.2. Procedure
Participants interested in the study contacted the lab and it was determined if they met the eligibility criteria. If eligible, a home visit was scheduled in which researchers would visit the home of the participants and conduct several questionnaires and interviews. During the home visit, researchers video recorded a naturalistic mother-child interaction. Mothers were instructed to play naturally for 15 minutes with their infant without involving the use of toys. This interaction took 15 minutes. After the home visit, an fMRI visit was scheduled. Participants visited the Center for Innovation & Creativity at the University of Colorado Boulder for an MRI scan. Mothers received child care and transportation support (if needed) as well as monetary compensation upon completion of each visit.
1.3. Measures
Postpartum Months.
Postpartum months were calculated as the time in months from the birth of the mother’s infant to the fMRI scan (see Table 1 for the mean, SD, and range). Later postpartum months indicated the mother was further into the postpartum period.
Beck Depression Inventory (BDI).
To assess depressive symptoms, we used the BDI26. The BDI is comprised of 21 items in which each item is answered on a scale from 0 (symptoms are absent) to 3 (symptoms are severe). The BDI has been used across diverse demographic backgrounds including the perinatal population27. For the sample, the Cronbach’s alpha on the BDI was 0.80.
State Anxiety.
To assess state anxiety symptoms28, we used the STAI. The STAI uses two 20 item self-report questionnaires to measure temporary feelings of anxiety (‘state anxiety’) and long-standing feelings of anxiety (‘trait anxiety’). We focused on state anxiety as we are interested in the postpartum period and therefore a measure of “present” anxiety is most appropriate. Each item is answered on a 4-point scale from “Almost Never” to “Almost Always”. The STAI has been used extensively to study anxiety symptoms in perinatal populations 29. The Cronbach’s alpha for the for the state scale of the STAI was 0.83.
Emotional Availability.
The Emotional Availability (EA) coding scheme was developed by Zeynep Biringen to assess dyadic qualities of maternal and child behavior. The EA scales measure both parental sensitivity and parental structuring as well as nonintrusiveness and nonhostility. Sensitivity and structuring are considered to be positive parenting behaviors30 and therefore were chosen as the focus of this study due to the previous evidence of the relationship between functional connectivity and positive parenting behaviors. Other studies focusing on positive parenting behaviors have also focused on these scales from the EA coding scheme30.
Parental sensitivity refers to the ability of the parent to be warm and emotionally connected with their child, both in terms of affect and responsiveness. The sensitivity scale is a 7-point Likert scale with a 7 representing a highly sensitive parent, a 4 representing an ‘apparently’ sensitive parent (i.e. they have inappropriately positive affect that is incongruent with their behavior), and a 1 representing a highly insensitive parent. In the context of an infant interaction, highly sensitive parents would be those parents who display positive and appropriate affect when interacting with their infant, and who observe their infant’s cues and respond appropriately, soothing the infant if they are upset and engaging in positive play routines that stimulate the infant. A very low score would be given to parents who display flat or negative affect or who do not respond to their infant appropriately. This may be in the form of being unable to recognize the infant needs to be soothed or being unable to soothe them, mimicking the infant’s distress, or not recognizing when the infant is bored or in need of different stimulation.
Parental structuring refers to the ability of the parent to support learning and exploration without overwhelming the child’s autonomy and in a way to which the child is receptive. The structuring scale is a 7-point Likert scale with a 7 representing optimal structuring, a 4 representing non-optimal but still successful structuring, and a 1 representing completely unstructuring. In the context of infant interaction, a very high structuring score would be given to a parent who uses both physical and verbal modes of play with their infant and monitors the success of each attempt, switching if the infant is unhappy or persisting with successful modes if the infant is still engaged. A low structuring score would be given if a parent is either switching between activities very quickly and the infant feels overstimulated, or if a parent does not actively engage with their child enough to stimulate the infant and bring them into the interaction. Our sample had similar mean scores and ranges (see Table 1) for other studies of adult parents with infants31. Two coders who have been trained by Dr. Biringen watched the videos independently with 19% overlapping and coded the dyadic interaction for sensitivity and structuring behaviors. The ICC of the sample including direct scores and subscales was .856. The ICC for sensitivity direct score and subscales was .890 and the ICC for structuring direct score and subscales was .743.
1.4. MRI Data Acquisition
MRI data was acquired with a 3T Magnetom TIM Trio and 3T Magnetom Prisma FIT scanner with a 32-channel phased-array coil. The scanner underwent an upgrade from Trio to Prisma during the data acquisition of the study. 62.5% of the data was acquired using the Tim Trio scanner and 37.5% was acquired using the Prisma. For the Trio, high resolution T1-weighted magnetization prepared rapid gradient-echo (MPRAGE) images were acquired with the following parameters: 192 sagittal slices, TR = 2530 ms, TE = 1.64 ms, flip angle = 7°, FOV = 256 mm2 and voxel size 1 × 1 × 1 mm. For the Prisma, sequence parameters were 224 sagittal slices, TR = 2400 ms, TE = 2.07 ms, flip angle = 8°, FOV = 256 mm2 and voxel size 0.8 × 0.8 × 0.8 mm. Resting-state data was acquired using a T2*-weighted gradient echo planar imaging sequence with the following parameters: 36 transverse slices, TR = 2300 ms, TE = 27 ms, flip angle = 73°, FOV = 192 mm, slice thickness = 3mm, and voxel size = 3.00 mm isotropic. The resting-state sequence parameters were identical for the Trio and Prisma. During the resting-state acquisition participants were instructed to fixate, with eyes open, upon a cross in the center of the screen for the entire 5-minute duration of the sequence.
1.5. MRI Data Quality Control Procedure
Initial quality control for both the resting-state and structural images was conducted using MRIQC32. The standard individual MRIQC pipeline was run for both the resting-state data and the structural data. For the resting-state data, this pipeline calculates several image quality metrics (IQMs) including mean framewise displacement (FD). FD represents instantaneous head motion by measuring the differentiating head alignment parameters across frames of the functional acquisition. As head motion has been demonstrated to introduce spurious but systematic correlations for resting-state data, we excluded participants from the analysis based upon a mean FD greater than 0.4 mm. Based upon this criterion for mean FD, two participants were excluded from the analysis. This threshold is a common ‘stringent’ threshold uses for FD in resting-state studies33. For the structural data, the pipeline calculates several IQMs as well as provides tools to predict structural image quality using machine learning techniques. T1 structural images are segmented and used to generate masks to remove signals from gray matter, white matter, and cerebrospinal fluid during the preprocessing of the resting-state data. Therefore, we assessed the quality of the structural images prior to the analysis. MRIQC’s random-forests classifier, that uses the ABIDE and DS030 datasets as a training sample, was used to predict the quality labels (0 = accept, 1 = reject) of the structural images for the current study. Based upon the classifier, one structural image was classified as a rejection. Visual quality control confirmed that this image was unusable due to excessive motion artifact, thus the participant was excluded from the study. Last, an additional participant was removed due to an inadequate acquisition coverage of the whole brain bringing the final sample size to 47 participants.
1.6. MRI Data Processing and Analysis
Resting-state analysis was conducted using the standard seed-based approach in the CONN Functional Connectivity Toolbox v17.f in MATLAB R2014b and SPM12. Preprocessing of the functional images was conducted using CONN’s default preprocessing pipeline for volume-based images. Functional data was realigned to the first volume, slice time corrected, normalized into MNI template space, and smoothed with an 8mm full width at half maximum (FWHM) kernel. Outlier detection was performed by the Artifact Detection Tools (ART) software (http://www.nitrc.org/projects/artifact_detect) with conservative settings to detect volumes that had greater head displacement than 2 mm in the x, y, or z direction from the previous image and a z-score of 3 for scan-to-scan changes in global signal. CONN’s default settings for scrubbing was used based upon the outlier’s detected by ART and confound regressor for the motion parameters were created for the first level analysis. Structural images were co-registered to the functional images and segmented into gray matter, white matter and cerebrospinal fluid (CSF). Based upon the image segmentation, the aCompCor method was used to attempt to regress out contributions to the BOLD signal from physiological noise and head motion. The realignment parameters, scrubbing, white matter signal, and CSF signal were used as first-level covariates. The fMRI data was bandpass filtered using the default settings in CONN (0.008–0.09 Hz).
Second-level covariates included the participant’s postpartum months as a main variable of interest, and the outlier volumes as well as maternal age, history of depression or anxiety, and scanner type (binary encoded as 0 for Siemens TIM Trio and 1 for Siemens Prisma) as covariates. We included the number of outlier volumes as detected by the ART toolbox as a part of the standard preprocessing pipeline in CONN. Volumes are determined to be ‘outliers’ if they exceed the outlier parameters set during artifact detection; it is included in several resting-state studies as a second level covariate to mitigate confounds from motion artifact. We included a covariate of whether participants had history of depression or anxiety as these disorders have been associated with variations in rsFC34. A seed-to-voxel analysis was conducted using the standard anatomical seeds available in CONN. For both the left and right amygdala, we examined the main effect of postpartum months using a multiple regression (with the number of outlier volumes, age, history of depression or anxiety, and scanner type as covariates). Statistical testing was performed at the group level with a height threshold of p < 0.001 and a cluster threshold of p < 0.05 (FDR corrected). As post-hoc analysis, we ran a whole-brain regression to test if the relationship between postpartum months and rsFC was still significant when depressive symptoms, state anxiety symptoms, and maternal education were included as covariates as they may be associated with variations in rsFC25. We also conducted an additional post-hoc whole brain analysis after controlling for the demographic characteristics that were associated with the postpartum months. For the associations with maternal behaviors, we extracted the mean rsFC values from each cluster. We used a Pearson correlation via SPSS to examine the association between the extracted values from the significant clusters and the maternal sensitivity/structuring scores.
2. Results
2.1. Demographic Variables
Table 1 includes the descriptive statistics for the sample. Postpartum months were associated with antidepressant use, greater interval between home and fMRI visits, and time away from the participant’s infant (ps < 0.05); see Table 2 for the correlations between demographic variables. Postpartum months did not differ by scanner type, maternal age, or history of depression or anxiety (ps > 0.05). There was no significant relationship between postpartum months and maternal mood as measured by the Beck Depression Inventory and the state anxiety scale of the STAI (ps > 0.05). There was no significant relationship between postpartum months and the maternal sensitivity or structuring; however, for maternal structuring the association was a trend level (r = 0.26, p = 0.07).
Table 2.
Zero-order correlation matrix of demographic variables.
| Variable | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1.Postpartum months at fMRI | - | .12 | .03 | .23 | .01 | −.08 | −.03 | .09 | .10 | .26 | .32* | .25 | .43* | .36* | −.01 | .90** |
| 2. Maternal age | - | −.30* | .04 | .66** | .52** | .10 | .20 | .17 | .16 | .29* | .23 | −.03 | −.03 | .10 | .15 | |
| 3. Maternal race (white = 0, nonwhite = 1) | - | .08 | −.34* | −.14 | −.11 | −.11 | −.00 | −.01 | −.08 | −.25 | −.19 | .33* | .11 | .11 | ||
| 4. Infant sex (female = 0, male = 1) | - | .02 | .06 | −.10 | −.09 | −.05 | −.05 | .10 | .27 | .03 | .13 | −.20 | .23 | |||
| 5. Maternal education | - | .45** | .16 | .12 | .35* | .29* | .11 | .04 | −.11 | −.01 | −.04 | .06 | ||||
| 6. Income-to-needs ratio | - | .02 | −.02 | .19 | .12 | .07 | −.03 | −.02 | 0.2 | −.06 | −.10 | |||||
| 7. Depressive mood (BDI) | - | .71** | .12 | .04 | .18 | .28 | .00 | −.08 | .14 | 0.05 | ||||||
| 8. State anxiety (STATE) | - | .23 | .12 | .13 | .31* | .08 | .01 | .21 | .04 | |||||||
| 9. Sensitivity (EA) | - | .62** | −.00 | −.07 | −.03 | .88 | .01 | .12 | ||||||||
| 10. Structuring (EA) | - | −.07 | −.08 | .09 | .00 | −.04 | .25 | |||||||||
| 11. Antidepressant use (no = 0, 1 = yes) | - | .44* | .33* | .17 | .04 | .20 | ||||||||||
| 12. History of depression or anxiety (no = 0, 1 = yes) | - | .32* | −.13 | .10 | .12 | |||||||||||
| 13. Home visit to scan interval (months) | - | .10 | −.04 | .02 | ||||||||||||
| 14. Hours away from infant | - | −.22 | .34* | |||||||||||||
| 15. Breastfeeding (no = 0, 1 = yes) | - | .00 | ||||||||||||||
| 16. Postpartum months at home visit | - | |||||||||||||||
p < 0.05 (two-tailed)
p < 0.01 (two-tailed)
2.2. Left and Right Amygdala Seeds
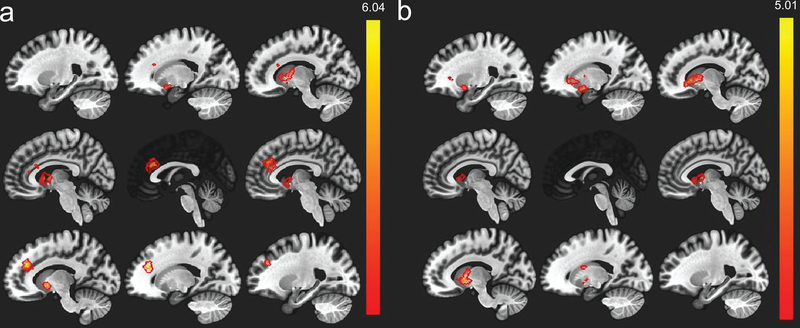
Using a multiple regression with the participant’s outlier volumes, participant age, history of depression or anxiety, and scanner as covariates, there was main effect of postpartum months such that later postpartum months were associated with greater connectivity between the left amygdala and the anterior cingulate gyrus, left nucleus accumbens, right caudate, left cerebellum (Figure 1; Table 3). The anterior cingulate gyrus cluster extended into the paracingulate gyrus, right caudate, and left nucleus accumbens. The left nucleus accumbens cluster extended into the left caudate and left pallidum. The right caudate cluster extended into the right putamen. For the right amygdala, an identical multiple regression was conducted controlling for participant’s outlier volumes, age, history of depression or anxiety, and scanner. There was also a main effect of postpartum months such that later postpartum months were associated with greater functional connectivity between the right amygdala and bilateral caudate. The left caudate cluster extended into the left pallidum. There was also greater connectivity between the right amygdala and right caudate as well as the right amygdala and right pallidum (Table 3). We also examined if there were any negative associations between postpartum months and amygdala functional connectivity. However, there were no significant negative associations. We include a figure of the left and right amygdala rsFC (Supplementary Figure 1) to show the whole-brain connectivity of the amygdala itself.
Figure 1.
(a) Seed-based resting-state functional connectivity for the left amygdala seed, red/orange regions indicate a significant positive association with postpartum months. (b) Right amygdala results for the seed-based analysis.
Table 3.
Cluster information from the whole-brain analysis (height threshold = p < 0.001, cluster threshold = p < 0.05, FDR corrected). (LA = left amygdala, RA = right amygdala, L = left, R = right, ACG = anterior cingulate gyrus, PCG = paracingulate gyrus, NAc = nucleus accumbens,)
| Cluster # | Seed | Cluster Size | MNI X | MNI Y | MNI Z | Cluster p value (FDR) |
Cluster regions |
|---|---|---|---|---|---|---|---|
| 1 | LA | 583 | −16 | 34 | 22 | 0.000001 | ACG, PCG, R caudate, L NAc |
| 2 | LA | 264 | 8 | 6 | 4 | 0.000007 | R Caudate, R Putamen |
| 3 | LA | 137 | −12 | 6 | −4 | 0.000986 | L NAc, L Caudate, L Pallidum |
| 4 | LA | 87 | −36 | −44 | −42 | 0.009639 | Left Cerebellum |
| 5 | RA | 300 | 16 | 18 | 0 | 0.000004 | R Caudate, R Putament |
| 6 | RA | 254 | −10 | −2 | 0 | 0.000001 | L Caudate, L Pallidum |
| 7 | RA | 92 | 20 | 6 | −12 | 0.000652 | R Putamen |
2.3. Post-hoc Whole Brain Regression Analysis
We used multiple regressions to test if the relationship between postpartum months and extracted amygdala rsFC values remained were still significant when depressive symptoms (BDI scores), state anxiety, and maternal education were included as covariates in the whole brain analysis. We tested the whole brain model with all the covariates included (age, outlier volumes, history of depression or anxiety, scanner, depressive symptoms, state anxiety, and maternal education). For the left amygdala, all the clusters from the main analysis remained significant (anterior cingulate, right caudate, left nucleus accumbens, and left cerebellum) and an additional cluster in the right cerebellum was significant. For the right amygdala, with all covariates included, the bilateral caudate was significant; however, the right putamen was no longer significant. We conducted a second post-hoc analysis in which the demographic variables associated with postpartum months were includes as covariates along with outlier volumes, age, history of depression or anxiety, and scanner. For the left amygdala, the results remained consistent with clusters in the anterior cingulate, right caudate, left nucleus accumbens, and an additional cluster in the left paracingulate gyrus. There were no significant clusters for the cerebellum. For the right amygdala, the results remained consistent with clusters in the left and right caudate. Only the putamen cluster from the main analysis was no longer significant.
2.4. Associations with Maternal Behaviors
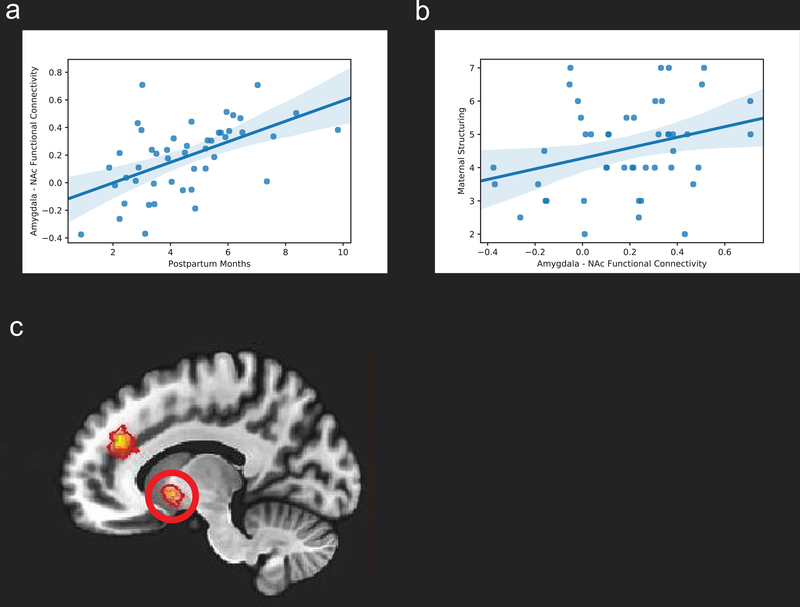
As an exploratory analysis, we examined whether maternal sensitivity and structuring would be positively associated with rsFC between the amygdala and regions involved in salience detection and reward/motivation functioning (reported in Table 3). There was a significant positive association between the left amygdala – left nucleus accumbens functional connectivity values and maternal structuring (r = 0.29, p = 0.042) (Figure 2). There was no significant association between the functional connectivity values of this connectivity and maternal sensitivity. There was no other significant association with maternal sensitivity or structuring. While the association between maternal structuring and left amygdala – left nucleus accumbens functional connectivity was significant, the finding was during an exploratory analysis in which multiple comparisons were uncorrected. Supplementary Table 1 shows the results of each correlation test performed for the exploratory analysis.
Figure 2.
(a) Scatter plot of the association between postpartum months and left amygdala - left nucleus accumbens resting-state functional connectivity. (b) Scatter plot of the association between left amygdala-left nucleus accumbens functional connectivity and maternal structuring. (c) Left nucleus accumbens (circled in red) resting-state functional connectivity with the left amygdala.
Maternal education, but no other demographic variables, was associated with both maternal sensitivity (r = 0.35, p < 0.05) and structuring (r = 0.29, p < 0.05) (Table 2). Therefore, as a post-hoc test, we examined if the association between maternal structuring and left-amygdala – left nucleus accumbens functional connectivity remained when controlling for maternal education. Using a multiple regression, controlling for maternal education, the positive association between maternal structuring and left-amygdala – left nucleus accumbens functional connectivity remained significant (β = 0.31, p = 0.042).
Discussion
In this study, we provide evidence of an association between postpartum timing and resting-state functional connectivity in the brain for a sample of primiparous mothers. When using the left amygdala as the seed region, later postpartum months were associated with greater rsFC with the anterior cingulate gyrus, left nucleus accumbens, right caudate, and left cerebellum. For the right amygdala, later postpartum months were associated greater functional connectivity with the bilateral caudate and right putamen. Further, we explored the association between the amygdala-nucleus accumbens functional connectivity and positive maternal behaviors (sensitivity and structuring). We found indications of a positive association between the amygdala-nucleus accumbens functional connectivity and maternal structuring measured during a mother-child interaction. These findings provide evidence of an association between postpartum months and intrinsic functional connectivity in the brain. Overlapping regions, such as the anterior cingulate cortex and nucleus accumbens, have been identified by animal functional neuroimaging studies to play a role in maternal behaviors in the postpartum period. Here, we extend these findings to primiparous human mothers.
The findings from this study suggests that for new mothers’ brains, even when not engaged in a task using infant cues, women with later postpartum months had greater baseline functional connectivity between brain regions critical for parenting including those involved in salience detection and reward/motivation. These associations remained significant when controlling for potentially confounding variables including a history of depression or anxiety diagnosis, current depressive symptoms, state anxiety, and maternal education. These associations also remained significant when we conducted a second post-hoc analysis with the demographic variables that were associated with postpartum months: antidepressant use, the interval of time between the home visit and scan, and the number of hours spent away from the child. The association between amygdala-nucleus accumbens and maternal structuring may suggest that baseline functional variations in maternal brain circuits is associated with the adjustment to parenthood. This finding, from an exploratory analysis, indicated a relationship between the functional connectivity and maternal structuring; however the finding was not corrected for multiple comparisons (see Supplementary Table 1). These findings may further inform future longitudinal investigations of the functional plasticity in maternal brain and could support future efforts to identify the functional connectivity patterns associated with negative adaptation to motherhood.
For the left amygdala, later postpartum months were associated with greater functional connectivity with the anterior cingulate gyrus. The amygdala and anterior cingulate gyrus are part of a network of brain regions known as the salience network (along with the anterior insula). The salience network is a higher-order system involved in the competitive, context-specific stimulus selection to enhance resources necessary for goal-directed behaviors35. Thus, greater salience network functional connectivity may support salience detection and may help new mothers respond to the infant cues necessary to facilitate proper caregiving behavior1, 3. It is critical for new mothers to effectively select and prioritize infant cues to support specific maternal behavior parents and inhibit behaviors that are not appropriate for caregiving. It is also critical for new mothers to detect infant cues as salient to recognize their babies and promote mother-infant attachment3. Infant cues are complex and multisensory and therefore require allocation of attentional and motivational resources in the brain to respond appropriately to their infant. The anterior cingulate is consistently activated in response to infant stimuli for both own baby versus other baby cries and pictures36 and is hypothesized to not only be involved in salience processing of infant stimuli but also in emotion regulatory processes related to parenting. The current finding suggests later postpartum months was associated with greater rsFC between salience network regions. While we hypothesized later postpartum months would be associated with greater amygdala-insular rsFC, the analysis did not have any significant associations between postpartum months and amygdala-insula functional connectivity. Future studies will be needed to examine the potential role of insula intrinsic functional connectivity in the postpartum period and parenting.
Greater functional connectivity was also found between the left amygdala and left nucleus accumbens. For the right amygdala, there was greater functional connectivity for the left and right caudate. These findings suggest that as postpartum months increased, rsFC between the amygdala and regions involved in maternal motivation and reward also increased. Evidence suggests that maternal behaviors are facilitated through a process in which brain increases the salience of infant stimuli through initial experiences of pleasure and activity in reward/motivation circuitry37. In animal models, the nucleus accumbens is the target of ascending dopaminergic cells from the ventral tegmental area; dopamine is released before and during interactions with the pups and has been hypothesized to increase the salience of pup4. In humans, the nucleus accumbens has been shown to have increased activation to own vs. other infant stimuli for both infant cries and pictures36. Also, mothers that had greater synchrony with their infants had greater nucleus accumbens response to infant cries and greater functional connectivity between the nucleus accumbens and empathy/theory of mind regions11.
Further, we found that postpartum timing was associated with increased amygdala-caudate and amygdala-putamen rsFC. Human neuroimaging studies of mothers suggest that the caudate and putamen also play a role in reward/motivation. For example, mothers experiencing postpartum depression have lower caudate, nucleus accumbens, and medial thalamus activity when hearing their infant cry versus a generic infant cry13. Therefore, it is likely that for human mothers the amygdala, nucleus accumbens, caudate, and putamen may all be involved in reward/motivation. Lastly, postpartum months had a positive association with amygdala-cerebellum rsFC. The role of the cerebellum in the maternal brain is less clear however it has been shown to have greater activation to infant stimuli and may play a role in emotion regulatory processes for mothers38.
To provide support for the hypothesis that functional variations in the brain in the postpartum period support the facilitation of maternal behaviors, we tested the association between the amygdala-nucleus accumbens rsFC and positive maternal behaviors (sensitivity and structuring) in an exploratory analysis. We found an indication of a significant positive association between amygdala-nucleus accumbens functional connectivity and maternal structuring. During the mother-child interaction, mothers with higher amygdala-nucleus accumbens rsFC had higher maternal structuring scores. Maternal structuring involves the caregiver modeling, directing, and controlling the flow of play. This involves supporting learning and exploration without overwhelming the child’s autonomy. This suggests that the greater amygdala-nucleus accumbens functional connectivity associated with later postpartum months may facilitate structuring behavior during unstructured play. The lack of association between amygdala-other neural region functional connectivity and maternal structuring suggests perhaps mesolimbic circuitry is particularly important for facilitating maternal structuring. The analysis was exploratory and had a significance value of p = 0.042, therefore strong conclusions about the relationship between rsFC and maternal structuring (or the lack of associations with maternal sensitivity) should be avoided until further studies designed to answer this question are conducted.
We should note that while the relationship between postpartum months and structuring was not significant, it was at a trend level (p < 0.07). No relationship between postpartum months and sensitivity was identified. Therefore, structuring may have a stronger relationship with postpartum timing, and therefore a stronger relationship with rsFC39. Further, this may suggest maternal sensitivity is relatively stable with regards to its relationship with postpartum months. It may be that structuring is increasing across the postpartum period in response to the increased demand to provide structure as the infant becomes more autonomous. Additionally, structuring behaviors might emerge as the result of increased caregiving experience as mothers learn how to support their baby’s play in ways that are optimal for their infant. The relationship between postpartum months and sensitivity vs. structuring will need to be tested in future studies, as well as longitudinal assessments of sensitivity/structuring to quantify their trajectories over the postpartum period. It is also noteworthy that all the women in this study were primiparous whereas many of the previous neuroimaging studies include both primiparous and multiparous mothers. Therefore, it will be critical to test the associations between postpartum months, rsFC, and positive maternal behaviors in a sample of multiparous women. It is currently unclear whether these relationships extend beyond the transitions from nulliparous to primiparous as well as are evident or exaggerated with each additional birth.
The relationship between postpartum months and rsFC could have several potential underlying mechanisms. In the current study, as in previous work6, the link between postpartum months and cumulative parenting experience was assumed. Therefore, the results should be interpreted with that limitation. Postpartum months may also covary with other factors not related to parenting experience such as hormonal fluctuations, recovery from pregnancy or pregnancy complications, or alterations in stress exposure or occupational demands. Considering these limitations, we suggest that experience-dependent plasticity is one of the potential mechanisms of our findings. In rats, maternal experience is associated with increases in cell survival and dendritic complexity in maternal brain regions such as the nucleus accumbens40. Based on these studies, the human maternal brain may also undergo similar structural plasticity (increased cell survival/dendritic complexity) that can be observed in the form of increased gray matter volume41. This structural plasticity may underlie experience-dependent functional plasticity that supports a new mother’s ability to respond effectively to the demands of her offspring. Therefore, this experience-dependent functional plasticity may result in greater functional connectivity as several studies have shown experience-dependent plasticity is associated with greater rsFC42. This hypothesis would be further supported by demonstrating structural plasticity in the postpartum period for white matter pathways connecting maternal brain regions, however; to our knowledge not been tested. Future studies of the relationship between human structural plasticity (using techniques such as diffusion weighted imaging) and functional plasticity in the maternal brain are needed.
Regarding the underlying mechanisms of the association between postpartum months and rsFC, hormones in the postpartum period may also play a critical role. Animal and human studies find support for the role of oxytocin in the postpartum period3. In rats, females that had higher levels of pup licking/grooming had higher oxytocin receptors in the medial preoptic area, central nucleus of the amygdala, and hypothalamus43. In humans, non-parent women administered oxytocin had increased activation in the anterior cingulate, the orbitofrontal cortex, and the insula44. Further, an oxytocin genetic polymorphism was associated with increased activation of the anterior cingulate, hippocampus, and orbitofrontal cortex44. Overall, these studies suggest a relationship between the postpartum period, oxytocin, and plasticity of the maternal brain. Future studies will be needed to examine the relationships between postpartum timing, oxytocin, and rsFC in new mothers and test it as a potential mechanism. In addition to oxytocin, several other hormones play a role in neural plasticity in the postpartum period. Estrogen has been shown to play a critical role in priming regions of the brain involved in maternal motivation in humans. In a human study, genetic variations of the estrogen receptor gene (lower expression) were associated with harsher parenting45. Further, inferior frontal gyrus functional activity mediated the relationship between the lower expression and harsh parenting45. Several other hormones in addition to oxytocin and estrogen have been shown to fluctuate in the postpartum period and may impact neural plasticity and parenting behaviors including cortisol46, progesterone47, and prolactin48. Future studies can focus on the trajectories of these hormones in the postpartum period and their associations with rsFC.
The findings of the current study are not without limitations. First, the study’s cross-sectional design limits the examination of the temporal relationships among postpartum months, functional connectivity, and maternal structuring. For the rsFC analyses, the design cannot assess whether there was a change in rsFC in the postpartum period as this would require at least two timepoints of MRI data as there is no baseline measurement. Therefore, future studies with longitudinal designs including pre-pregnancy, pregnancy, and multiple postnatal scans will be required to examine functional connectivity changes in the postpartum period. Second, as mentioned, the study could not rule out all factors that are changing during the postpartum period. Factors such as hormonal changes, recovery from delivery, or other postpartum challenges that likely covary with postpartum months could not be disentangled in the current study. Therefore, future studies, with more direct measures of cumulative parenting experience, should be conducted to replicate these findings. Further, our study did not measure the amount of stress that was experienced by the mothers in sample; this will be critical for future studies to examine as stress exposure has been shown to be associated with variations in the maternal brain49. Postpartum months is also a measurement of the age of the child, therefore the rsFC could be related to the changing demands of having an older child, indicating another way of interpreting experience-dependent plasticity in postpartum period. More research is needed to dissociate the amount of experience with infant from increasing child age as it is associated with variations in rsFC thought to be due to experience-dependent plasticity. Similarly, mothers with later postpartum months have older infants which may results in better quality sleep and less fatigue which in turn could be related to variations in functional connectivity.
Most of the women in our study had subclinical levels of depression (87.2%) and anxiety (89.3%). Five women in the study met the cutoff for mild depression (BDI > 13) and one woman met the cutoff for moderate depression (BDI > 19). Five women reported experience state anxiety symptoms the met the cutoff for clinically significant symptoms (STATE > 39). While the BDI and STATE scales are commonly used to assess maternal mood in the postpartum period, it will be critical for future studies to include measures of maternal mood that may have increased sensitivity beyond the BDI and STATE such as the Inventory of Depression and Anxiety symptoms50. Therefore, it will be critical to examine the association between postpartum months, rsFC, and positive parenting behaviors in populations with predominantly clinical levels of symptoms such as women experiencing postpartum depression or anxiety. Another possible limitation of the study is the focus on seed-based functional connectivity. Due to the large literature of the role of the amygdala in maternal behavior, we chose to conduct a seed-based FC as it is more hypothesis driven than other rsFC techniques. This study provides a foundation for data-driven techniques that utilize rsFC data such as ICA and graph theoretical approaches. Lastly, the association between the left amygdala – left nucleus accumbens rsFC and maternal structuring was significant (p = 0.042). Since this analysis was exploratory we did not correct for multiple comparisons for the associations between the rsFC and maternal behavior analysis, however, this finding should be interpreted with caution. This relationship will require studies focusing on the associations between rsFC and maternal behavior to attempt to replicate this finding.
Conclusions
Findings from the current study suggest a positive association between postpartum timing and rsFC in the regions of the brain involved in maternal caregiving specifically amygdala-anterior cingulate gyrus, amygdala-nucleus accumbens, amygdala-caudate, amygdala-putamen, and amygdala-cerebellum connectivity. These findings provide human evidence of observations from animal studies in which the postpartum period involves variations in brain function to support caregiving behaviors. We also found an indication of an association between amygdala-nucleus accumbens and maternal structuring as measured during a mother-child interaction. While interesting, this association was found during an exploratory analysis with uncorrected comparisons and will need further exploration in future studies. The findings help us to understand that in addition to the brain structure, the intrinsic functional connectivity may be associated with postpartum months in human mothers. The findings can further inform future studies of the longitudinal relationships between postpartum timing, rsFC, and maternal behaviors in typical and at-risk populations.
Supplementary Material
Acknowledgements
This work was supported by the National Institute of Health [R01HD090068; R21HD078797; R21DA046556]; the Professional Research Opportunity for Faculty (PROF) and Faculty Research Fund (FRF), University of Denver; NARSAD Independent Investigator Grant, and the Victoria S. Levin Award For Early Career Success in Young Children’s Mental Health Research, Society for Research in Child Development (SRCD). The authors declare that they have no conflicts of interest in the research. The authors thank the families that participated in the study and the individuals that supported the recruitment. The authors also wish to acknowledge Amy Anderson, Lindsay Blanton, Christian Capistrano, Christina Congleton, Tanisha Crosby-Attipoe, Victoria Everts, Rachel Gray, Claire Jeske, Laura Jeske, Daniel Mason, Rebekah Tribble, and Nanxi Xu for research assistance. We thank Samantha Buxbaum for editorial comments on the manuscript. The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1.Kim P, et al. , The maternal brain and its plasticity in humans. 2016. 77: p. 113–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holtmaat A. and Svoboda K, Experience-dependent structural synaptic plasticity in the mammalian brain. Nature Reviews Neuroscience, 2009. 10(9): p. 647. [DOI] [PubMed] [Google Scholar]
- 3.Lonstein JS, et al. , Common and divergent psychobiological mechanisms underlying maternal behaviors in non-human and human mammals. 2015. 73: p. 156–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Champagne FA, et al. , Variations in nucleus accumbens dopamine associated with individual differences in maternal behavior in the rat. Journal of Neuroscience, 2004. 24(17): p. 4113–4123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lonstein JS, Lévy F, and Fleming AS, Common and divergent psychobiological mechanisms underlying maternal behaviors in non-human and human mammals. Hormones and Behavior, 2015. 73: p. 156–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim P, Dufford AJ, and Tribble RC, Cortical thickness variation of the maternal brain in the first 6 months postpartum: associations with parental self-efficacy. Brain Structure and Function, 2018. 223(7): p. 3267–3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoekzema E, et al. , Pregnancy leads to long-lasting changes in human brain structure. Nature Neuroscience, 2017. 20(2): p. 287. [DOI] [PubMed] [Google Scholar]
- 8.Swain JE, et al. , Maternal brain response to own baby‐cry is affected by cesarean section delivery. 2008. 49(10): p. 1042–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pereira, Structural and functional plasticity in the maternal brain circuitry. 2016. 2016(153): p. 23–46. [DOI] [PubMed] [Google Scholar]
- 10.Barba-Müller E, et al. , Brain plasticity in pregnancy and the postpartum period: links to maternal caregiving and mental health. 2018: p. 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Atzil S, Hendler T, and Feldman R, Specifying the neurobiological basis of human attachment: brain, hormones, and behavior in synchronous and intrusive mothers. Neuropsychopharmacology, 2011. 36(13): p. 2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Numan M. and Woodside B.J.B.n., Maternity: neural mechanisms, motivational processes, and physiological adaptations. 2010. 124(6): p. 715. [DOI] [PubMed] [Google Scholar]
- 13.Laurent HK and Ablow JC, The missing link: mothers’ neural response to infant cry related to infant attachment behaviors. Infant Behavior and Development, 2012. 35(4): p. 761–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lorberbaum JP, et al. , A potential role for thalamocingulate circuitry in human maternal behavior. 2002. 51(6): p. 431–445. [DOI] [PubMed] [Google Scholar]
- 15.Nephew BC, et al. , Altered neural connectivity in adult female rats exposed to early life social stress. Behavioural brain research, 2017. 316: p. 225–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chase HW, et al. , Disrupted posterior cingulate–amygdala connectivity in postpartum depressed women as measured with resting BOLD fMRI. Social cognitive and affective neuroscience, 2013. 9(8): p. 1069–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deligiannidis KM, et al. , GABAergic neuroactive steroids and resting-state functional connectivity in postpartum depression: a preliminary study. Journal of psychiatric research, 2013. 47(6): p. 816–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Milliones J, Relationship between perceived child temperament and maternal behaviors. Child Development, 1978: p. 1255–1257. [Google Scholar]
- 19.Biringen Z, Robinson JL, and Emde RN, Appendix B: The emotional availability scales (; an abridged infancy/early childhood version). Attachment & human development, 2000. 2(2): p. 256–270. [DOI] [PubMed] [Google Scholar]
- 20.Leerkes EM, Blankson AN, and O’Brien M, Differential effects of maternal sensitivity to infant distress and nondistress on social‐emotional functioning. Child development, 2009. 80(3): p. 762–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stams G-JJ, Juffer F, and Van IJzendoorn MH, Maternal sensitivity, infant attachment, and temperament in early childhood predict adjustment in middle childhood: The case of adopted children and their biologically unrelated parents. Developmental psychology, 2002. 38(5): p. 806. [DOI] [PubMed] [Google Scholar]
- 22.Licata M, Kristen S, and Sodian B, Mother–child interaction as a cradle of theory of mind: the role of maternal emotional availability. Social Development, 2016. 25(1): p. 139–156. [Google Scholar]
- 23.Swain JE, et al. , Approaching the biology of human parental attachment: Brain imaging, oxytocin and coordinated assessments of mothers and fathers. Brain research, 2014. 1580: p. 78–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haim A, Sherer M, and Leuner B, Gestational stress induces persistent depressive‐like behavior and structural modifications within the postpartum nucleus accumbens. European Journal of Neuroscience, 2014. 40(12): p. 3766–3773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim P, Capistrano C, and Congleton C, Socioeconomic disadvantages and neural sensitivity to infant cry: role of maternal distress. Social cognitive and affective neuroscience, 2016. 11(10): p. 1597–1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beck AT, Steer RA, and Brown GK, Beck depression inventory-II. San Antonio, 1996. 78(2): p. 490–498. [Google Scholar]
- 27.Gotlib IH, et al. , Prevalence rates and demographic characteristics associated with depression in pregnancy and the postpartum. Journal of consulting and clinical psychology, 1989. 57(2): p. 269. [DOI] [PubMed] [Google Scholar]
- 28.Spielberger CD, et al. , The state-trait anxiety inventory. Revista Interamericana de Psicologia/Interamerican Journal of Psychology, 2017. 5(3 & 4). [Google Scholar]
- 29.Hundley V, et al. , Can anxiety in pregnant women be measured using the State-Trait Anxiety Inventory. Midwifery, 1998. 14(2): p. 118–121. [DOI] [PubMed] [Google Scholar]
- 30.Biringen Z, et al. , Maternal representation of the self as parent: Connections with maternal sensitivity and maternal structuring. Attachment & Human Development, 2000. 2(2): p. 218–232. [DOI] [PubMed] [Google Scholar]
- 31.Riva Crugnola C., Ierardi E, and Canevini MP, Reflective functioning, maternal attachment, mind-mindedness, and emotional availability in adolescent and adult mothers at infant 3 months. Attachment & human development, 2018. 20(1): p. 84–106. [DOI] [PubMed] [Google Scholar]
- 32.Esteban O, et al. , MRIQC: Predicting Quality in Manual MRI Assessment Protocols Using No-Reference Image Quality Measures. bioRxiv, 2017: p. 111294. [Google Scholar]
- 33.Jiang L, et al. , Examination of local functional homogeneity in Autism. BioMed research international, 2015. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Greicius MD, et al. , Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biological psychiatry, 2007. 62(5): p. 429–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Menon V, Salience network. 2015. [Google Scholar]
- 36.Swain JE, Baby stimuli and the parent brain: functional neuroimaging of the neural substrates of parent-infant attachment. Psychiatry (Edgmont), 2008. 5(8): p. 28. [PMC free article] [PubMed] [Google Scholar]
- 37.Strathearn L, et al. , What’s in a smile? Maternal brain responses to infant facial cues. Pediatrics, 2008. 122(1): p. 40–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dolan R, A cognitive affective role for the cerebellum. Brain: a journal of neurology, 1998. 121(4): p. 545–546. [DOI] [PubMed] [Google Scholar]
- 39.Salvatori P, et al. , Mother-toddler play interaction in extremely, very low birth weight, and full-term children: a longitudinal study. Frontiers in psychology, 2016. 7: p. 1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Akbari EM, et al. , Experience-dependent cell survival in the maternal rat brain. Behavioral neuroscience, 2007. 121(5): p. 1001. [DOI] [PubMed] [Google Scholar]
- 41.Kim P, et al. , The plasticity of human maternal brain: longitudinal changes in brain anatomy during the early postpartum period. Behavioral neuroscience, 2010. 124(5): p. 695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guerra-Carrillo B, Mackey AP, and Bunge SA, Resting-state fMRI: a window into human brain plasticity. The Neuroscientist, 2014. 20(5): p. 522–533. [DOI] [PubMed] [Google Scholar]
- 43.Champagne F, et al. , Naturally occurring variations in maternal behavior in the rat are associated with differences in estrogen-inducible central oxytocin receptors. Proceedings of the National Academy of Sciences, 2001. 98(22): p. 12736–12741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Riem MM, et al. , Oxytocin effects on complex brain networks are moderated by experiences of maternal love withdrawal. European Neuropsychopharmacology, 2013. 23(10): p. 1288–1295. [DOI] [PubMed] [Google Scholar]
- 45.Lahey BB, et al. , Preliminary genetic imaging study of the association between estrogen receptor-α gene polymorphisms and harsh human maternal parenting. Neuroscience letters, 2012. 525(1): p. 17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fleming AS, Steiner M, and Anderson V, Hormonal and attitudinal correlates of maternal behaviour during the early postpartum period in first-time mothers. Journal of Reproductive and Infant Psychology, 1987. 5(4): p. 193–205. [Google Scholar]
- 47.Bridges RS, A quantitative analysis of the roles of dosage, sequence, and duration of estradiol and progesterone exposure in the regulation of maternal behavior in the rat. Endocrinology, 1984. 114(3): p. 930–940. [DOI] [PubMed] [Google Scholar]
- 48.Rosenblatt J, Psychobiology of maternal behavior: contribution to the clinical understanding of maternal behavior among humans. Acta paediatrica, 1994. 83: p. 3–8. [DOI] [PubMed] [Google Scholar]
- 49.Slattery DA and Neumann ID, No stress please! Mechanisms of stress hyporesponsiveness of the maternal brain. The Journal of physiology, 2008. 586(2): p. 377–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Watson D, et al. , Development and validation of the Inventory of Depression and Anxiety Symptoms (IDAS). Psychological assessment, 2007. 19(3): p. 253. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.