Abstract
Stress is well-known to inhibit a variety of reproductive processes, including the suppression of episodic Gonadotropin releasing hormone (GnRH) secretion, typically measured via downstream luteinizing hormone (LH) secretion. Since pulsatile secretion of GnRH and LH are necessary for proper reproductive function in both males and females, and stress is common for both human and animals, understanding the fundamental mechanisms by which stress impairs LH pulses is of critical importance. Activation of the hypothalamic-pituitary-adrenal axis, and its corresponding endocrine factors, is a key feature of the stress response, so dissecting the role of stress hormones, including corticotrophin releasing hormone (CRH) and corticosterone, in the inhibition of LH secretion has been one key research focus. However, some evidence suggests that stress hormone alone are not sufficient for the full inhibition of LH caused by stress, implicating the additional involvement of other hormonal or neural signaling pathways in this process (including inputs from the brainstem, amygdala, parabrachial nucleus, and dorsomedial nucleus). Moreover, different stress types, such as metabolic stress (hypoglycemia), immune stress, and psychosocial stress, appear to suppress LH secretion via partially unique neural and endocrine pathways. The mechanisms underlying the suppression of LH pulses in these models offer interesting comparisons and contrasts, including the specific roles of amygdaloid nuclei and CRH receptor types. This review focuses on the most recent and emerging insights into endocrine and neural mechanisms responsible for the suppression of pulsatile LH secretion in mammals, and offers insights in important gaps in knowledge and future directions.
Keywords: Luteinizing Hormone, Gonadotropin, Reproduction, Stress, GnRH, Kisspeptin, Kiss1, RFRP3, Rfrp
INTRODUCTION
Stress is defined as a real or perceived threat to homeostasis; without an adequate and appropriate physiological response to stressful stimuli, detrimental health or even death will occur (1). An important feature of the body’s stress response is a core set of physiological processes that is initiated regardless of which stressful stimulus is applied. Among these is the activation of the hypothalamic-pituitary-adrenal (HPA) axis and the suppression of the hypothalamic-pituitary-gonadal (HPG) axis (2). This makes physiologic sense as reproduction is energetically costly and inhibition of reproduction during stressful periods is beneficial for conserving energy. Indeed, there is clear evidence that stress impacts numerous tissues and processes necessary for reproduction [stress effects on the gonads and reproductive tract have been reviewed recently (3–5)]; here, we will focus on discussing recent and emerging insights into the neural and endocrine mechanisms by which stress impairs pulsatile luteinizing hormone (LH) secretion. Although follicle stimulating hormone (FSH) is a clinically and physiologically important gonadotropin, discussion of this hormone is beyond the scope of this review as its secretory patterns have been recently reviewed recently (6–8), including in another article in this Special Issue.
LH is secreted from anterior pituitary gonadotrope cells and acts to govern sex steroid synthesis and secretion, as well as ovulation, by the gonads (ovaries and testes). The pattern of LH secretion is driven primarily by upstream secretion of gonadotropin releasing hormone (GnRH) from the brain. GnRH itself is typically secreted in pulses, occurring approximately every 20 min to 120 min, depending on the species and hormonal milieu. There is now general agreement that the guiding neural mechanism controlling GnRH pulses—the GnRH pulse generator—is formed by an afferent population of neurons in the arcuate nucleus of the hypothalamus (ARC) that express the neuropeptides kisspeptin, neurokinin B, and dynorphin (KNDy cells; ARCKiss1 cells) (9, 10). Kisspeptin acts on GnRH cells (11) or GnRH axon terminals in rodents (12) to stimulate GnRH secretion. Each pulse of GnRH stimulates a corresponding pulse of LH from gonadotrope cells of the anterior pituitary gland (13). Thus, suppressed gonadotropin secretion following stress could be the result of impairment of either ARCKiss1 cells, GnRH cells, or gonadotropes (or some combination of these sites).
Broadly speaking, the majority of the published work in this field addresses one of three main questions: 1) Where in the body (primarily, the brain) is the proximate stressor detected? 2) How is this stress signal transmitted in the brain (i.e. what are the neural substrates and which signaling molecules are involved)? 3) What is the site of impaired gonadotropin secretion (i.e. ARCKiss1 cells, GnRH cells, or gonadotropes)? Conceptually, these questions represent three phases of the neural process by which stress impairs gonadotropin secretion.
ELEMENTS OF THE HPA AXIS
During the response to stress, the HPA axis is robustly and rapidly activated, resulting in elevated secretion of corticotropin-releasing hormone (CRH) in the brain, adrenocorticotropin-releasing hormone (ACTH) from the pituitary, and glucocorticoids (corticosterone in rodents, cortisol in larger mammals) from the adrenals (2). These stress hormones represent common mediators by which diverse types of stress might suppress LH. This section will highlight evidence supporting the sufficiency of HPA axis signaling molecules in the suppression of LH secretion in response to stress.
The neuropeptide CRH is produced in several brain regions, including hypophysiotropic neurons in the paraventricular nucleus (PVN) (14, 15). Early reports that intracerebroventricular (ICV) injection of CRH suppressed LH secretion in rats (16) and monkeys (17–19), and that CRH receptor antagonists prevented stress-induced suppression of LH (20–22), supported the hypothesis that CRH is necessary and sufficient for suppression of gonadotropin secretion during stress. However, follow-up studies reported highly variable responses to CRH, including either no effect (23, 24) or even stimulatory actions (23, 25). Many factors may account for these diverse effects, such as dose, route of administration, CRH preparation, species, and gonad (and hence, sex steroid) status of the experimental model. The inconsistent effects of CRH on LH secretion (inhibitory vs. stimulatory vs. no effect) are in contrast to the robust suppression of LH that is observed during numerous models of stress, which supports the hypothesis that additional signaling molecules are involved in the suppression of LH during stress. It should be noted that there are occasional reports of increased LH secretion during stress (26–29); these events are largely associated with surge-type LH secretion rather than pulsatile LH secretion. An additional complication is that the CRH antagonists used in these early studies (e.g., α-helical CRH and [D-Phe12, Nle21,38]hCRF-[21–41]) act on both CRHR1 (high-affinity receptor for CRH) and CRHR2 (low affinity receptor for CRH; displaying 70% homology to CRHR1) (30, 31). This makes interpretation of the discrepant results complex and challenging. More recently, a specific CRHR1 antagonist partially reversed the inhibitory effect of acute psychosocial stress, but not immune or metabolic stress, on LH in female rats (32), which indicates that CRH acting via CRHR1 may be necessary for the response to some acute stress types, but not others.
Arginine vasopressin (AVP) is co-secreted with CRH during stress (33) and acts synergistically with CRH to induce ACTH secretion, though the relative importance of these two neuropeptides varies by species (34). ICV administration of AVP suppressed LH secretion in rats (35) and OVX rhesus monkeys (20, 36), but stimulated LH secretion in gonad-intact baboons (37). Intriguingly, Knobil’s group reported that AVP infusion caused a slight increase in frequency of ARC multi-unit activity (MUA) volleys (neuron electrical activity), despite lower mean LH levels in peripheral blood. Similarly, bath application of AVP increased intracellular ARCKiss1 calcium concentrations in female mice (38). Thus, in females there may be a stimulatory effect of AVP on ARCKiss1 cells that can occur with lower LH concentrations, due to factors such as gonadal status or responsiveness of the GnRH cell or gonadotrope to highfrequency ARCKiss1 stimulation. An important caveat to these studies is that AVP is synthesized in multiple brain regions to modulate numerous physiological processes; thus, exogenous AVP may target multiple cell-types and brain regions. Indeed, AVP synapses onto GnRH cells have also been described (39), though function and regional source of these connections is unknown. It should be noted that AVP treatment of dispersed pituitary cells from OVX rats did not alter in vitro LH release (40).
Peripheral administration of ACTH did not decrease LH secretion in OVX monkeys (41). Moreover, scant expression of MC2R, the receptor for ACTH, outside of the adrenal gland (42) has likely further dampened enthusiasm for investigating a role for this signaling molecule in stress-induced suppression of gonadotropin secretion.
The inhibitory effects of glucocorticoids on reproductive function have been extensively studied in numerous species, and several key differences have been reported. In castrated male monkeys, chronic treatment with hydrocortisone acetate (rapidly converted to cortisol) suppressed pulsatile LH secretion, but did not alter the LH response to exogenous GnRH (43). Similarly, in OVX pigs, chronic hydrocortisone acetate suppressed LH pulse frequency, but not in animals with pituitary stalk transections given exogenous GnRH (44), which implies a hypothalamic site of action because LH pulse frequency is modulated by neural (rather than pituitary) mechanisms. However, glucocorticoid-treated pigs have reduced LH response to GnRH challenge, indicating a pituitary effect as well (45). These data are consistent with a gonadal steroid-independent suppression of GnRH secretion by glucocorticoids, with minimal effect directly on pituitary function, at least in these species. In contrast, OVX sheep given sustained cortisol treatment displayed suppressed LH pulse amplitude, but not frequency, via a reduction in pituitary sensitivity to GnRH, indicating a pituitary effect in this species (46–49). Conversely, in the presence of gonadal steroids, GnRH and LH pulse frequency are suppressed by sustained cortisol treatment (50, 51). This estradiol-dependent suppression of GnRH and LH pulse frequency in sheep is similar to the recent report in female mice that showed an inhibitory effect of chronic corticosterone on LH pulse frequency in OVX mice only when they were also treated with estradiol (52). Intriguingly, in both sheep and mice, the majority of ARCKiss1 neurons contain glucocorticoid receptor (GR) (52, 53), which raises the possibility of direct action in these cells. In contrast, in OVX and estradiol replaced rats, a similar chronic corticosterone treatment did not alter either LH pulse amplitude or pulse frequency (54). Perhaps relatedly, it was reported that virtually no ARCKiss1 (<3%) cells contain GR in rats (55).
REACTIVE STRESS
Reactive stress is induced by stimuli that present an actual physical challenge to homeostasis; thus, the body is ‘reacting’ to internal stimuli. The most studied are immune/inflammatory and metabolic stress, but also include hemorrhage, osmotic stress and visceral or somatic pain. Immune stress is often modeled by injection of lipopolysaccharide (LPS), a non-replicative component of the gram negative cell wall that induces a nearly-full immune response. Models of metabolic stress include: 1) insulin-induced hypoglycemia (IIH), in which a bolus insulin injection causes rapid peripheral glucose uptake, resulting in hypoglycemia; or 2) injection of 2-deoxyglucose, which models glucoprivation by inhibiting glucose-6-phosphate isomerase, acutely interfering with ATP production.
How and where is the proximate stressor detected?
Technically, all cells respond to changes in glucose concentrations (i.e. metabolically slowing as energy is depleted). However, there are several populations of neurons that display acute changes in firing rate in response to physiological changes in blood glucose concentrations. One important region is the area postrema of the brainstem (56). Lesion of the area postrema completely prevents suppression of LH pulses caused by IIH in rats (57), which supports the hypothesis that low blood glucose is primarily detected within this specialized hindbrain region.
Detection of immune stress is comparatively more diffuse. In experimental conditions, LPS activates the innate immune system via toll-like receptor 4, which is expressed throughout the body including several brain regions (58). Toll-like receptor 4 activation causes secretion of proinflammatory cytokines, including interleukin-1β (IL-1β) and tumor necrosis factor α (TNFα). Blockade of either of these cytokine receptors partially reversed the inhibition of LH pulses by LPS treatment (59, 60), suggesting that these cytokines contribute to the inhibitory effect of LPS on LH secretion. Receptors for IL-1β and TNFα are expressed in numerous tissues and cell-types, including the area postrema (61, 62). Like other circumventricular areas, the area postrema has a rarefied blood-brain barrier, and therefore is prime for sensing circulating cytokines. Lesion of the area postrema blunted the ACTH rise following intravenous administration of IL-1β (63), which raises the possibility that other stress-sensitive endocrine systems, such as the network controlling LH, are also modulated by the detection of circulating cytokines via actions within the area postrema. Whether vagal afferents contribute to the suppression of LH following peripheral administration of inflammatory cytokines (64), as they do for induction of ACTH secretion, is unknown.
What are the neural substrates and which signaling molecules are involved?
The majority of the neural projections from the area postrema are to medullary and pontine nuclei (65), including dense innervation of the nucleus of the solitary tract (NTS), which includes the A2 population of norepinephrine neurons (A2NE) (66). Activation of A2NE neurons has been demonstrated in response to a variety of stressors, including metabolic (67) and immune stress (68), and A2NE neurons project broadly throughout the hypothalamus, including to the PVN (69). Interestingly, NE microinjection into the PVN suppressed pulsatile LH secretion in rats (70). Ablation of NE cells that project to the PVN prevented the disruption of estrous cycles caused by glucoprivation in rats (71), supporting the possibility that a brainstem NE neuron to PVN circuit is necessary for suppression of LH during metabolic stress, and possibly other stress types. It is important to note that this neuron ablation technique lesioned other NE cell populations, in addition to the brainstem A2NE neurons, and reduced NE fiber density in other hypothalamic regions, complicating final interpretation.
Several lines of evidence support the hypothesis that CRHR signaling contributes the suppression of LH. The suppression of LH caused by NE injection into the PVN was reversed by central administration of a non-selective CRH receptor antagonist in rats (70), which is consistent with the finding that a different non-selective CRH receptor antagonist prevented the suppression of LH during IIH in rhesus monkeys (72). Importantly, pharmacological blockade of CRHR2, but not CRHR1, prevented the suppression of pulsatile LH in estradiol-treated rats during either metabolic or immune stress (32). Together, these data support the hypothesis that NE release in the PVN (presumably from A2 neurons) suppresses LH secretion via downstream CRHR2 signaling. In support of this idea, central administration of urocortin 2 (UCN2; a potent and specific agonist of CRHR2) suppressed LH pulse frequency in rats (73). Thus, CRHR2 activation is both necessary and sufficient for the suppression of LH during two types of reactive stress. ARCKiss1 cells receive direct synaptic innervation from several neuron types in the PVN, but not from CRH neurons in the PVN (74). Ultimately, a multi-synaptic brainstem to ARCKiss1 pathway is supported by a recent wheat germ agglutinin tracing study (75). However, the precise cellular intermediates are not completely resolved.
Another signaling molecule of interest is calcitonin gene related peptide (CGRP), which is produced in neurons of the parabrachial nucleus. These neurons are innervated by A2NE cells (76) and activated during a variety of stress types (77). Furthermore, central administration of CGRP suppressed LH pulses (78). Interestingly, a CGRP receptor antagonist reversed the inhibitory effect of IIH on LH pulses (78), indicating a critical role for this signaling molecule in metabolic stress-induced suppression of LH. The neural sites of action for CGRP is an outstanding question.
Several lines of evidence support the hypothesis that cells the dorsomedial nucleus (DMN) and amygdala may also be important for the response to immune stress. First, RFRP-3, a peptide made in the DMN, suppressed LH concentrations in rodents (79, 80) and sheep (81). Second, LPS induced c-Fos expression (a marker of neuronal activation) in the DMN in several species (82), and in sheep, the RFRP-3 cells were activated by LPS (83). Similarly, in rats, Rfrp mRNA abundance was increased in micropunches of the DMN following high dose LPS treatment (84). Though Rfrp expression, cell number, or cell activity was not reduced by 12 hr of fasting (79), it remains possible that only more extreme stress regulates these cells because a high, but not a low dose of LPS altered Rfrp mRNA levels (84). Thus, future work will be needed to determine if RFRP-3 cells are also regulated by metabolic stress, and if this neuropeptide (or the cells that produce it) is necessary for the suppression of LH during reactive stress types. Since RFRP-3 stimulates corticosterone secretion and CRH neuron activation (85), it is possible that RFRP-3 is upstream of HPA axis activation. Indeed, mechanisms for RFRP-3 cell activation are a significant outstanding question. Immune stress also induced c-Fos in the amygdala, though the phenotype of these cells is unknown (86). Excitotoxic lesion of the central amygdala partially reversed the inhibitory effects of LPS, but not of IIH or restraint stress (87), which implicates the central amygdala in the suppression of LH during immune stress and further demonstrates stressor-specific activation of different neural pathways.
What is the site of impaired LH secretion?
Metabolic stress suppressed LH pulse frequency in numerous species (72, 88–90) and inhibited MUA volleys in the MBH of monkeys (72), which is consistent with actions on ARCKiss1 cells. Kiss1 mRNA in ARC punches was reduced following IIH (54), though the rapid cessation of LH pulses observed during metabolic stress (often within 20 min), is unlikely to be mediated by an inhibition of peptide synthesis, which occurs on the order of hours vs. minutes. The possibility that metabolic stress impairs GnRH cell function, independent from ARCKiss1, is supported by the finding that a CRHR2 agonist suppressed GnRH cell firing in brain slice preparation (91), though this effect is unlikely to be mediated directly on GnRH neurons because few GnRH cells contain CRH receptors (92). Metabolic stress does not appear to alter pituitary function as LH responses to exogenous GnRH were similar during euglycemic control periods and IIH in rats (93) and monkeys (94). Moreover, in rats with area postrema lesions, pulsatile LH secretion patterns during IIH and non-stress conditions are identical (57), which supports the hypothesis that low blood sugar (or high insulin) does not directly impair ARCKiss1 cells, GnRH neurons, or gonadotropes, but rather activates neural afferents to indirectly suppress ARCKiss1 (or possibly GnRH) neurons.
Immune stress presents an interesting contrast to metabolic stress in that LPS suppressed both hypothalamic and pituitary function. An inhibitory action of LPS in the hypothalamus is supported by a reduction in the frequency of MUA volleys in goats (95) and a suppression in the frequency of GnRH pulses in sheep (96). Additionally, LPS reduced Kiss1 mRNA in micropunches of the ARC in rats (54). An inhibitory action at the pituitary level was demonstrated in sheep, whereby lower doses of LPS suppressed LH secretion without altering GnRH pulses (97). This inhibition of pituitary function in response to LPS was shown to occur independent of the action of cortisol (49), but rather via a prostaglandin-dependent pathway (97). In contrast to sheep, rats treated with LPS exhibit low LH without a reduction in the response to exogenous GnRH (98), indicating possible species differences in the role of altered pituitary function in response to stress-induced mediators.
PSYCHOSOCIAL STRESS
How and where is the proximate stressor detected?
Psychosocial stress is psychogenic in nature and characterized by the perception of a threat to homeostasis, which is experience dependent. Acute psychosocial stress is often modeled with physical restraint, typically including isolation from cage or pen mates and/or exposure to predator smells or sounds (e.g. fox urine or dog barking). Thus, sensory inputs (particularly olfactory inputs in rodents) and cognitive processes likely activate limbic brain regions resulting in the neuroendocrine responses to psychosocial stress.
What are the neural substrates and which signaling molecules are involved?
Assessment of c-Fos distribution following restraint stress has implicated several brain regions, including the DMN and amygdala in the response to psychosocial stress (99). A study in gonad-intact male rats reported an increase in Rfrp mRNA (expressed in the DMN) following restraint stress (100). In OVX females, restraint induced a 20% increase in Rfrp cell number 3 hr after restraint stress, but not at earlier time points (101). In contrast, no change in Rfrp cell number was observed in castrated males over the same observation period (102). Interestingly and perhaps more importantly, acute increases in Rfrp cell activation, as assessed by cfos co-labeling, were observed after 45 min of restraint stress in both gonadectomized male and female mice (101, 102). These findings support the hypothesis that psychosocial stress increases RFRP-3 signaling, which is inhibitory on the reproductive axis. Indeed, a functional role for RFRP-3 in the suppression of LH is supported by the observation that knock-down of Rfrp in the DMN restored fertility in female rats exposed to repeated restraint stress (103). Rfrp is likely regulated by an acute rise in glucocorticoids, as these cells contain glucocorticoid receptor, and adrenalectomy with low dose corticosterone replacement prevented the upregulation of Rfrp in gonad-intact male rats (100). However, in contrast to the rodent studies, restraint stress did not alter RFRP-3 cell number or activation in OVX sheep, though this stress paradigm produced only a modest suppression of LH secretion in that study (104).
The amygdala is part of the limbic system and is well known for its role in fear and emotion processing. This brain region is therefore is an interesting potential target for stress regulation of reproductive processes. While the central amygdala may regulate immune stress as previously described, lesions of the medial amygdala partially prevented restraint stress-induced suppression of LH in female rats (87). Since lesion of the medial amygdala also prevented the induction of c-Fos expression in the PVN (105), it is possible that neuropeptides produced in the PVN, such as CRH, are important for the inhibitory effect of restraint stress on LH. It also suggests that the medial amygdala may be upstream of the PVN in the psychosocial stress neural circuitry.
Specific blockade of CRHR1 partially prevented the suppression of LH during restraint stress in rats (32). However, CRH knock-out mice have normal suppression of LH during restraint stress (106). This disparity could reflect compensatory changes in whole-body knock-out animals during development, actions of the other CRHR1 agonist urocortin 1, or species differences. Similar to the physical stressors (immune and metabolic), CRHR2 blockade also partially prevented restraint stress-induced suppression of LH (73). Thus, it appears activation of CRHR2 represents a commonality between psychosocial and physical stressors. It will be interesting to determine whether the neural source of endogenous CRHR2 ligands (i.e. the population of cells that produce the peptides) are also common between stress types.
What is site of impaired LH secretion?
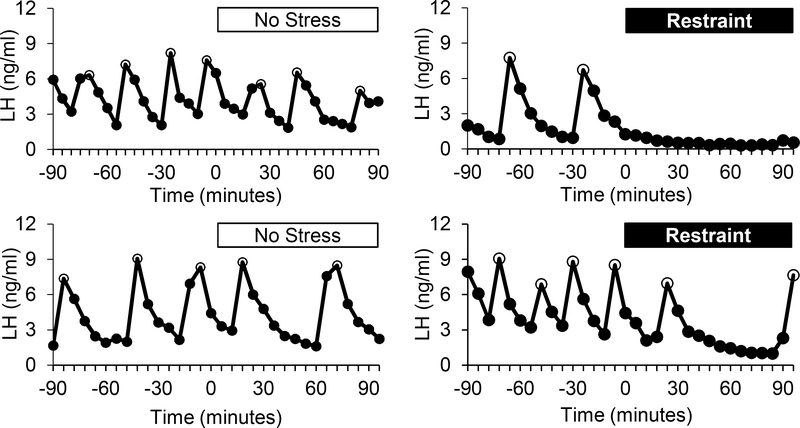
Restraint stress suppressed LH pulse frequency in several species, including mice (101), rats (107), and sheep (108), which implies a hypothalamic site of action as discussed above. Strong evidence supports a mediatory role of ARCKiss1 cells in the suppression of LH pulse frequency in response to restraint stress in female mice. In OVX mice, restraint elicited a slowing of pulse frequency demonstrated by a lengthening of interpulse interval from 20 min to 58 min during the 90 min stress period (Figure 1). Associated with this reduction in pulse frequency was a 50% reduction in the percentage of Kiss1 cells that contained cfos at 45, 90 and 180 minutes of restraint stress, thus demonstrating suppression of ARCKiss1 cell activation (101). In contrast, castrated male mice had a more modest suppression (~30%) in the percentage of ARCKiss1 cells containing cfos at 3hr of restraint stress, but not at earlier times (45 or 90 min) (102). These observations provide correlative evidence that restraint stress impairs LH via inhibition of ARCKiss1 cells. Whether the possible sex difference in Kiss1 cell suppression following restraint stress reflects alternate mechanisms operating in the sexes or is a consequence of the technique used to assess cell activation (cfos induction) is unknown. Consistent with an action upon the ARCKiss1 cell population, ARC Kiss1 mRNA abundance was suppressed by 1hr of restraint stress in estradiol-treated OVX rats (note: neural tissue was collected 5 hr after release from restraint, 6 hr after initiation of the restraint) (54). However, in castrate male and OVX female mice, no change in the number of Kiss1 cells was observed during 3 hr of continuous restraint (101, 102). Several differences exist in these experimental paradigms, including timing of tissue collection, steroid status, and species, as well as mRNA quantification technique.
Figure 1.
Representative LH pulse patterns in OVX mice exposed to no stress (left) or restraint stress for 90 minutes (right). Open circles represent LH pulses. [Note, these are unpublished LH pulse patterns from a published study. (101)]
In addition to the effects of acute psychosocial stress on reproductive brain circuits, evidence in some species support the hypothesis that pituitary function is also affected. For example, OVX ewes treated with gonadal steroids displayed a reduction in LH pulse amplitude during restraint and isolation stress (108). Transport stress reduced LH pulse frequency in intact ewes during the mid-follicular phase (109), and suppressed the LH response to GnRH injections (110). In OVX sheep, an acute layered psychosocial stressor model (isolation, restraint, blindfolding and barking dog sounds), suppressed both GnRH pulse amplitude and LH pulse amplitude (111). This suppression of just the latter is reversed by the GR antagonist RU486 (112, 113) suggesting an action of cortisol on the gonadotrope cell. In contrast to these inhibitory effects on LH pulse amplitude in sheep, in castrated male mice pulse amplitude during restraint stress was increased (102), which may reflect the release of a larger stored pool of LH during each pulse. Considering that acute effects of glucocorticoids on LH in rodents remains unclear, analysis of LH secretion in a GnRH-clamp experiment during restraint stress would be necessary to determine whether there is any suppression of pituitary function in rodents during this stress type.
CONCLUSIONS
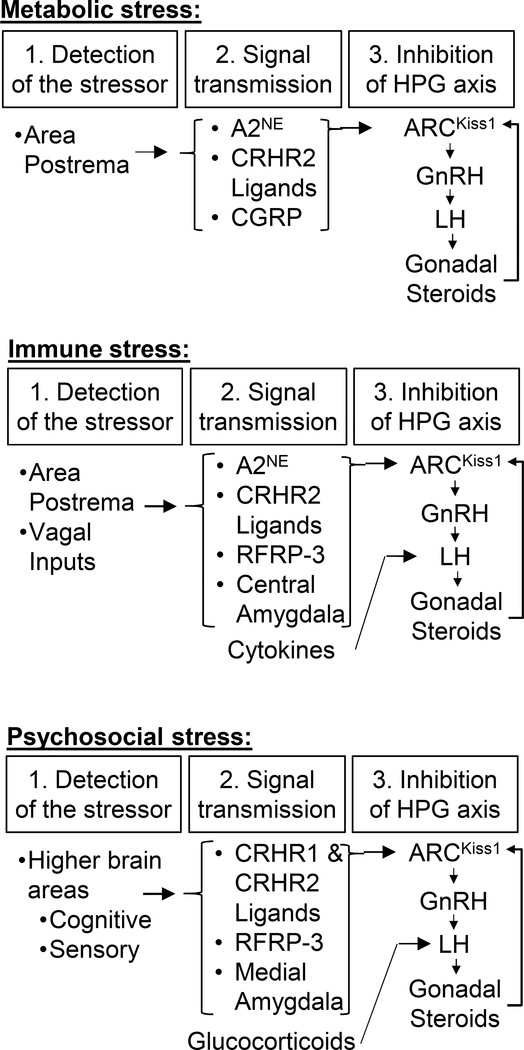
The inhibitory actions of many of the elements of the HPA axis on pulsatile LH secretion have been studied extensively and appear to contribute to some, but not all, of the inhibitory effects of stress. It is becoming increasingly evident that different types of stress suppress LH secretion through distinct mechanisms, which is summarized in Figure 2. CRH/CRHR1 signaling presents in interesting example of a mechanism contributing to some (psychosocial) but not other (immune and metabolic) stress types. The specific role of CRH and the ligands for CRHR2 in the suppression of LH during stress remain interesting and important questions. During reactive stress types, a brainstem to hypothalamus circuit ultimately resulting in suppression of ARCKiss1 cells is activated; the role of the peptides RFRP-3 and CGRP, as well as contributions from the amygdala remain interesting additions to this neurocircuit. For psychosocial stress it is apparent that the inputs from the amygdala, RFRP-3, as well as CRHR1 and CRHR2 ligands, all contribute to the suppression of ARCKiss1 cells. Thus, numerous signaling molecules and brain regions contribute to the suppression of LH during stress (Figure 2); an important challenge moving forward will be to integrate the actions of these diverse molecules and form ordered pathways for their effects on reproduction.
Figure 2:
Schematic for neural pathways by which reactive stress (metabolic and immune) and psychosocial stress impair reproductive hormone secretion in mammals.
Acknowledgments
Grant Support: The authors are supported by NSF grant 1OS-1457226 and NIH grants R01 HD090161, R01 HD086100, P50 HD012303, T32 HD007203 and F32 HD096811.
Footnotes
Disclosure Statement: The authors have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hart BL. Biological basis of the behavior of sick animals. Neuroscience and biobehavioral reviews. 1988;12(2):123–37. [DOI] [PubMed] [Google Scholar]
- 2.Smith SM, Vale WW. The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues in clinical neuroscience. 2006;8(4):383–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gilbert RO. Symposium review: Mechanisms of disruption of fertility by infectious diseases of the reproductive tract. Journal of dairy science. 2019;102(4):3754–65. [DOI] [PubMed] [Google Scholar]
- 4.Prasad S, Tiwari M, Pandey AN, Shrivastav TG, Chaube SK. Impact of stress on oocyte quality and reproductive outcome. Journal of biomedical science. 2016;23:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Belhadj Slimen I, Najar T, Ghram A, Abdrrabba M. Heat stress effects on livestock: molecular, cellular and metabolic aspects, a review. Journal of animal physiology and animal nutrition. 2016;100(3):401–12. [DOI] [PubMed] [Google Scholar]
- 6.Whirledge S, Cidlowski JA. Glucocorticoids, stress, and fertility. Minerva endocrinologica. 2010;35(2):109–25. [PMC free article] [PubMed] [Google Scholar]
- 7.Stamatiades GA, Carroll RS, Kaiser UB. GnRH-A Key Regulator of FSH. Endocrinology. 2019;160(1):57–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Breen KM, Mellon PL. Influence of stress-induced intermediates on gonadotropin gene expression in gonadotrope cells. Molecular and cellular endocrinology. 2014;385(1–2):71–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moore AM, Coolen LM, Porter DT, Goodman RL, Lehman MN. KNDy Cells Revisited. Endocrinology. 2018;159(9):3219–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herbison AE. The Gonadotropin-Releasing Hormone Pulse Generator. Endocrinology. 2018;159(11):3723–36. [DOI] [PubMed] [Google Scholar]
- 11.Merkley CM, Coolen LM, Goodman RL, Lehman MN. Evidence for Changes in Numbers of Synaptic Inputs onto KNDy and GnRH Neurones during the Preovulatory LH Surge in the Ewe. Journal of neuroendocrinology. 2015;27(7):624–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krajewski SJ, Anderson MJ, Iles-Shih L, Chen KJ, Urbanski HF, Rance NE. Morphologic evidence that neurokinin B modulates gonadotropin-releasing hormone secretion via neurokinin 3 receptors in the rat median eminence. J Comp Neurol. 2005;489(3):372–86. [DOI] [PubMed] [Google Scholar]
- 13.Clarke IJ, Cummins JT. The temporal relationship between gonadotropin releasing hormone (GnRH) and luteinizing hormone (LH) secretion in ovariectomized ewes. Endocrinology. 1982;111(5):1737–9. [DOI] [PubMed] [Google Scholar]
- 14.Peng J, Long B, Yuan J, Peng X, Ni H, Li X, et al. A Quantitative Analysis of the Distribution of CRH Neurons in Whole Mouse Brain. Frontiers in neuroanatomy. 2017;11:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zimmerman EA, Stillman MA, Recht LD, Antunes JL, Carmel PW, Goldsmith PC. Vasopressin and corticotropin-releasing factor: an axonal pathway to portal capillaries in the zona externa of the median eminence containing vasopressin and its interaction with adrenal corticoids. Annals of the New York Academy of Sciences. 1977;297:405–19. [DOI] [PubMed] [Google Scholar]
- 16.Rivier C, Vale W. Influence of corticotropin-releasing factor on reproductive functions in the rat. Endocrinology. 1984;114(3):914–21. [DOI] [PubMed] [Google Scholar]
- 17.Williams CL, Nishihara M, Thalabard JC, Grosser PM, Hotchkiss J, Knobil E. Corticotropin-releasing factor and gonadotropin-releasing hormone pulse generator activity in the rhesus monkey. Electrophysiological studies. Neuroendocrinology. 1990;52(2):133–7. [DOI] [PubMed] [Google Scholar]
- 18.Olster DH, Ferin M. Corticotropin-releasing hormone inhibits gonadotropin secretion in the ovariectomized rhesus monkey. The Journal of clinical endocrinology and metabolism. 1987;65(2):262–7. [DOI] [PubMed] [Google Scholar]
- 19.Gindoff PR, Ferin M. Endogenous opioid peptides modulate the effect of corticotropin-releasing factor on gonadotropin release in the primate. Endocrinology. 1987;121(3):837–42. [DOI] [PubMed] [Google Scholar]
- 20.Chen MD, Ordog T, O’Byrne KT, Goldsmith JR, Connaughton MA, Knobil E. The insulin hypoglycemia-induced inhibition of gonadotropin-releasing hormone pulse generator activity in the rhesus monkey: roles of vasopressin and corticotropin-releasing factor. Endocrinology. 1996;137(5):2012–21. [DOI] [PubMed] [Google Scholar]
- 21.Rivier C, Rivier J, Vale W. Stress-induced inhibition of reproductive functions: role of endogenous corticotropin-releasing factor. Science (New York, NY). 1986;231(4738):607–9. [DOI] [PubMed] [Google Scholar]
- 22.Tsukahara S, Tsukamura H, Foster DL, Maeda KI. Effect of corticotropin-releasing hormone antagonist on oestrogen-dependent glucoprivic suppression of luteinizing hormone secretion in female rats. Journal of neuroendocrinology. 1999;11(2):101–5. [DOI] [PubMed] [Google Scholar]
- 23.Caraty A, Miller DW, Delaleu B, Martin GB. Stimulation of LH secretion in sheep by central administration of corticotrophin-releasing hormone. Journal of reproduction and fertility. 1997;111(2):249–57. [DOI] [PubMed] [Google Scholar]
- 24.Petraglia F, Sutton S, Vale W, Plotsky P. Corticotropin-releasing factor decreases plasma luteinizing hormone levels in female rats by inhibiting gonadotropin-releasing hormone release into hypophysial-portal circulation. Endocrinology. 1987;120(3):1083–8. [DOI] [PubMed] [Google Scholar]
- 25.Tilbrook AJ, Canny BJ, Stewart BJ, Serapiglia MD, Clarke IJ. Central administration of corticotrophin releasing hormone but not arginine vasopressin stimulates the secretion of luteinizing hormone in rams in the presence and absence of testosterone. The Journal of endocrinology. 1999;162(2):301–11. [DOI] [PubMed] [Google Scholar]
- 26.Traslavina GA, Franci CR. Divergent roles of the CRH receptors in the control of gonadotropin secretion induced by acute restraint stress at proestrus. Endocrinology. 2012;153(10):4838–48. [DOI] [PubMed] [Google Scholar]
- 27.Tarin JJ, Hamatani T, Cano A. Acute stress may induce ovulation in women. Reproductive biology and endocrinology : RB&E. 2010;8:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ciechanowska M, Lapot M, Antkowiak B, Mateusiak K, Paruszewska E, Malewski T, et al. Effect of short-term and prolonged stress on the biosynthesis of gonadotropin-releasing hormone (GnRH) and GnRH receptor (GnRHR) in the hypothalamus and GnRHR in the pituitary of ewes during various physiological states. Animal reproduction science. 2016;174:65–72. [DOI] [PubMed] [Google Scholar]
- 29.Brann DW, Putnam CD, Mahesh VB. Corticosteroid regulation of gonadotropin secretion and induction of ovulation in the rat. Proceedings of the Society for Experimental Biology and Medicine Society for Experimental Biology and Medicine (New York, NY). 1990;193(3):176–80. [DOI] [PubMed] [Google Scholar]
- 30.Rivier J, Gulyas J, Kirby D, Low W, Perrin MH, Kunitake K, et al. Potent and long-acting corticotropin releasing factor (CRF) receptor 2 selective peptide competitive antagonists. Journal of medicinal chemistry. 2002;45(21):4737–47. [DOI] [PubMed] [Google Scholar]
- 31.Reul JM, Holsboer F. On the role of corticotropin-releasing hormone receptors in anxiety and depression. Dialogues in clinical neuroscience. 2002;4(1):31–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li XF, Bowe JE, Kinsey-Jones JS, Brain SD, Lightman SL, O’Byrne KT. Differential role of corticotrophin-releasing factor receptor types 1 and 2 in stress-induced suppression of pulsatile luteinising hormone secretion in the female rat. Journal of neuroendocrinology. 2006;18(8):602–10. [DOI] [PubMed] [Google Scholar]
- 33.Whitnall MH, Mezey E, Gainer H. Co-localization of corticotropin-releasing factor and vasopressin in median eminence neurosecretory vesicles. Nature. 1985;317(6034):248–50. [DOI] [PubMed] [Google Scholar]
- 34.Antoni FA. Vasopressinergic control of pituitary adrenocorticotropin secretion comes of age. Frontiers in neuroendocrinology. 1993;14(2):76–122. [DOI] [PubMed] [Google Scholar]
- 35.Rivier C, Vale W. Effects of corticotropin-releasing factor, neurohypophyseal peptides, and catecholamines on pituitary function. Federation proceedings. 1985;44(1 Pt 2):189–95. [PubMed] [Google Scholar]
- 36.Shalts E, Xia L, Xiao E, Ferin M. Inhibitory effect of arginine-vasopressin on LH secretion in the ovariectomized rhesus monkey. Neuroendocrinology. 1994;59(4):336–42. [DOI] [PubMed] [Google Scholar]
- 37.Koyama T, Hagino N. The effect of vasopressin on LH release in baboons. Hormone and metabolic research = Hormon- und Stoffwechselforschung = Hormones et metabolisme. 1983;15(4):184–6. [DOI] [PubMed] [Google Scholar]
- 38.Schafer D, Kane G, Colledge WH, Piet R, Herbison AE. Sex- and sub region-dependent modulation of arcuate kisspeptin neurones by vasopressin and vasoactive intestinal peptide. Journal of neuroendocrinology. 2018;30(12):e12660. [DOI] [PubMed] [Google Scholar]
- 39.Thind KK, Boggan JE, Goldsmith PC. Interactions between vasopressin- and gonadotropin-releasing-hormone-containing neuroendocrine neurons in the monkey supraoptic nucleus. Neuroendocrinology. 1991;53(3):287–97. [DOI] [PubMed] [Google Scholar]
- 40.Ono N, Bedran de Castro J, Khorram O, McCann SM. Role of arginine vasopressin in control of ACTH and LH release during stress. Life sciences. 1985;36(18):1779–86. [DOI] [PubMed] [Google Scholar]
- 41.Xiao EN, Ferin M. The inhibitory action of corticotropin-releasing hormone on gonadotropin secretion in the ovariectomized rhesus monkey is not mediated by adrenocorticotropic hormone. Biology of reproduction. 1988;38(4):763–7. [DOI] [PubMed] [Google Scholar]
- 42.Chhajlani V Distribution of cDNA for melanocortin receptor subtypes in human tissues. Biochemistry and molecular biology international. 1996;38(1):73–80. [PubMed] [Google Scholar]
- 43.Dubey AK, Plant TM. A suppression of gonadotropin secretion by cortisol in castrated male rhesus monkeys (Macaca mulatta) mediated by the interruption of hypothalamic gonadotropin-releasing hormone release. Biol Reprod. 1985;33(2):423–31. [DOI] [PubMed] [Google Scholar]
- 44.Estienne MJ, Barb CR, Kesner JS, Kraeling RR, Rampacek GB. Luteinizing hormone secretion in hypophysial stalk-transected gilts given hydrocortisone acetate and pulsatile gonadotropin-releasing hormone. Domestic animal endocrinology. 1991;8(3):407–14. [DOI] [PubMed] [Google Scholar]
- 45.Pearce GP, Paterson AM, Hughes PE. Effect of short-term elevations in plasma cortisol concentration on LH secretion in prepubertal gilts. Journal of reproduction and fertility. 1988;83(1):413–8. [DOI] [PubMed] [Google Scholar]
- 46.Breen KM, Stackpole CA, Clarke IJ, Pytiak AV, Tilbrook AJ, Wagenmaker ER, et al. Does the type II glucocorticoid receptor mediate cortisol-induced suppression in pituitary responsiveness to gonadotropin-releasing hormone? Endocrinology. 2004;145(6):2739–46. [DOI] [PubMed] [Google Scholar]
- 47.Breen KM, Karsch FJ. Does season alter responsiveness of the reproductive neuroendocrine axis to the suppressive actions of cortisol in ovariectomized ewes? Biology of reproduction. 2006;74(1):41–5. [DOI] [PubMed] [Google Scholar]
- 48.Breen KM, Davis TL, Doro LC, Nett TM, Oakley AE, Padmanabhan V, et al. Insight into the neuroendocrine site and cellular mechanism by which cortisol suppresses pituitary responsiveness to gonadotropin-releasing hormone. Endocrinology. 2008;149(2):767–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Debus N, Breen KM, Barrell GK, Billings HJ, Brown M, Young EA, et al. Does cortisol mediate endotoxin-induced inhibition of pulsatile luteinizing hormone and gonadotropin-releasing hormone secretion? Endocrinology. 2002;143(10):3748–58. [DOI] [PubMed] [Google Scholar]
- 50.Oakley AE, Breen KM, Clarke IJ, Karsch FJ, Wagenmaker ER, Tilbrook AJ. Cortisol reduces gonadotropin-releasing hormone pulse frequency in follicular phase ewes: influence of ovarian steroids. Endocrinology. 2009;150(1):341–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Oakley AE, Breen KM, Tilbrook AJ, Wagenmaker ER, Karsch FJ. Role of estradiol in cortisol-induced reduction of luteinizing hormone pulse frequency. Endocrinology. 2009;150(6):2775–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kreisman M, McCosh R, Tian K, Song C, Breen K. Estradiol enables chronic corticosterone to inhibit pulsatile LH secretion and suppress Kiss1 neuronal activation in female mice. Neuroendocrinology. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Oakley AE. Central Inhibitory Actions of Glucocorticoids on Reproductive Function: Permissive Role of Estradiol: University of Michagan; 2008. [Google Scholar]
- 54.Kinsey-Jones JS, Li XF, Knox AM, Wilkinson ES, Zhu XL, Chaudhary AA, et al. Down-regulation of hypothalamic kisspeptin and its receptor, Kiss1r, mRNA expression is associated with stress-induced suppression of luteinising hormone secretion in the female rat. Journal of neuroendocrinology. 2009;21(1):20–9. [DOI] [PubMed] [Google Scholar]
- 55.Takumi K, Iijima N, Higo S, Ozawa H. Immunohistochemical analysis of the colocalization of corticotropin-releasing hormone receptor and glucocorticoid receptor in kisspeptin neurons in the hypothalamus of female rats. Neuroscience letters. 2012;531(1):40–5. [DOI] [PubMed] [Google Scholar]
- 56.Funahashi M, Adachi A. Glucose-responsive neurons exist within the area postrema of the rat: in vitro study on the isolated slice preparation. Brain research bulletin. 1993;32(5):531–5. [DOI] [PubMed] [Google Scholar]
- 57.Cates PS, O’Byrne KT. The area postrema mediates insulin hypoglycaemia-induced suppression of pulsatile LH secretion in the female rat. Brain research. 2000;853(1):151–5. [DOI] [PubMed] [Google Scholar]
- 58.Vaure C, Liu Y. A comparative review of toll-like receptor 4 expression and functionality in different animal species. Frontiers in immunology. 2014;5:316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yoo MJ, Nishihara M, Takahashi M. Tumor necrosis factor-alpha mediates endotoxin induced suppression of gonadotropin-releasing hormone pulse generator activity in the rat. Endocrine journal. 1997;44(1):141–8. [DOI] [PubMed] [Google Scholar]
- 60.Ebisui O, Fukata J, Tominaga T, Murakami N, Kobayashi H, Segawa H, et al. Roles of interleukin-1 alpha and -1 beta in endotoxin-induced suppression of plasma gonadotropin levels in rats. Endocrinology. 1992;130(6):3307–13. [DOI] [PubMed] [Google Scholar]
- 61.Ericsson A, Liu C, Hart RP, Sawchenko PE. Type 1 interleukin-1 receptor in the rat brain: distribution, regulation, and relationship to sites of IL-1-induced cellular activation. The Journal of comparative neurology. 1995;361(4):681–98. [DOI] [PubMed] [Google Scholar]
- 62.Nadeau S, Rivest S. Effects of circulating tumor necrosis factor on the neuronal activity and expression of the genes encoding the tumor necrosis factor receptors (p55 and p75) in the rat brain: a view from the blood-brain barrier. Neuroscience. 1999;93(4):1449–64. [DOI] [PubMed] [Google Scholar]
- 63.Lee HY, Whiteside MB, Herkenham M. Area postrema removal abolishes stimulatory effects of intravenous interleukin-1beta on hypothalamic-pituitary-adrenal axis activity and c-fos mRNA in the hypothalamic paraventricular nucleus. Brain research bulletin. 1998;46(6):495–503. [DOI] [PubMed] [Google Scholar]
- 64.Ishizuka Y, Ishida Y, Kunitake T, Kato K, Hanamori T, Mitsuyama Y, et al. Effects of area postrema lesion and abdominal vagotomy on interleukin-1 beta-induced norepinephrine release in the hypothalamic paraventricular nucleus region in the rat. Neuroscience letters. 1997;223(1):57–60. [DOI] [PubMed] [Google Scholar]
- 65.van der Kooy D, Koda LY. Organization of the projections of a circumventricular organ: the area postrema in the rat. The Journal of comparative neurology. 1983;219(3):328–38. [DOI] [PubMed] [Google Scholar]
- 66.Cunningham ET Jr., Miselis RR, Sawchenko PE. The relationship of efferent projections from the area postrema to vagal motor and brain stem catecholamine-containing cell groups: an axonal transport and immunohistochemical study in the rat. Neuroscience. 1994;58(3):635–48. [DOI] [PubMed] [Google Scholar]
- 67.Paranjape SA, Briski KP. Recurrent insulin-induced hypoglycemia causes site-specific patterns of habituation or amplification of CNS neuronal genomic activation. Neuroscience. 2005;130(4):957–70. [DOI] [PubMed] [Google Scholar]
- 68.Buller KM, Xu Y, Day TA. Indomethacin attenuates oxytocin and hypothalamic-pituitary-adrenal axis responses to systemic interleukin-1 beta. Journal of neuroendocrinology. 1998;10(7):519–28. [DOI] [PubMed] [Google Scholar]
- 69.Sawchenko PE, Swanson LW. The organization of noradrenergic pathways from the brainstem to the paraventricular and supraoptic nuclei in the rat. Brain research. 1982;257(3):275–325. [DOI] [PubMed] [Google Scholar]
- 70.Tsukamura H, Nagatani S, Cagampang FR, Kawakami S, Maeda K. Corticotropin-releasing hormone mediates suppression of pulsatile luteinizing hormone secretion induced by activation of alpha-adrenergic receptors in the paraventricular nucleus in female rats. Endocrinology. 1994;134(3):1460–6. [DOI] [PubMed] [Google Scholar]
- 71.I’Anson H, Sundling LA, Roland SM, Ritter S. Immunotoxic destruction of distinct catecholaminergic neuron populations disrupts the reproductive response to glucoprivation in female rats. Endocrinology. 2003;144(10):4325–31. [DOI] [PubMed] [Google Scholar]
- 72.Chen MD, O’Byrne KT, Chiappini SE, Hotchkiss J, Knobil E. Hypoglycemic ‘stress’ and gonadotropin-releasing hormone pulse generator activity in the rhesus monkey: role of the ovary. Neuroendocrinology. 1992;56(5):666–73. [DOI] [PubMed] [Google Scholar]
- 73.Li XF, Bowe JE, Lightman SL, O’Byrne KT. Role of corticotropin-releasing factor receptor-2 in stress-induced suppression of pulsatile luteinizing hormone secretion in the rat. Endocrinology. 2005;146(1):318–22. [DOI] [PubMed] [Google Scholar]
- 74.Yeo SH, Kyle V, Blouet C, Jones S, Colledge WH. Mapping neuronal inputs to Kiss1 neurons in the arcuate nucleus of the mouse. PLoS One. 2019;14(3):e0213927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Deura C, Minabe S, Ikegami K, Inoue N, Uenoyama Y, Maeda KI, et al. Morphological analysis for neuronal pathway from the hindbrain ependymocytes to the hypothalamic kisspeptin neurons. The Journal of reproduction and development. 2019;65(2):129–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Roman CW, Derkach VA, Palmiter RD. Genetically and functionally defined NTS to PBN brain circuits mediating anorexia. Nature communications. 2016;7:11905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Palmiter RD. The Parabrachial Nucleus: CGRP Neurons Function as a General Alarm. Trends in neurosciences. 2018;41(5):280–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Li XF, Bowe JE, Mitchell JC, Brain SD, Lightman SL, O’Byrne KT. Stress-induced suppression of the gonadotropin-releasing hormone pulse generator in the female rat: a novel neural action for calcitonin gene-related peptide. Endocrinology. 2004;145(4):1556–63. [DOI] [PubMed] [Google Scholar]
- 79.Poling MC, Shieh MP, Munaganuru N, Luo E, Kauffman AS. Examination of the influence of leptin and acute metabolic challenge on RFRP-3 neurons of mice in development and adulthood. Neuroendocrinology. 2014;100(4):317–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kriegsfeld LJ, Mei DF, Bentley GE, Ubuka T, Mason AO, Inoue K, et al. Identification and characterization of a gonadotropin-inhibitory system in the brains of mammals. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(7):2410–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Clarke IJ, Sari IP, Qi Y, Smith JT, Parkington HC, Ubuka T, et al. Potent action of RFamide-related peptide-3 on pituitary gonadotropes indicative of a hypophysiotropic role in the negative regulation of gonadotropin secretion. Endocrinology. 2008;149(11):5811–21. [DOI] [PubMed] [Google Scholar]
- 82.Rivest S, Laflamme N. Neuronal activity and neuropeptide gene transcription in the brains of immune-challenged rats. Journal of neuroendocrinology. 1995;7(7):501–25. [DOI] [PubMed] [Google Scholar]
- 83.Clarke IJ, Bartolini D, Conductier G, Henry BA. Stress Increases Gonadotropin Inhibitory Hormone Cell Activity and Input to GnRH Cells in Ewes. Endocrinology. 2016;157(11):4339–50. [DOI] [PubMed] [Google Scholar]
- 84.Iwasa T, Matsuzaki T, Tungalagsuvd A, Munkhzaya M, Kawami T, Niki H, et al. Hypothalamic Kiss1 and RFRP gene expressions are changed by a high dose of lipopolysaccharide in female rats. Hormones and behavior. 2014;66(2):309–16. [DOI] [PubMed] [Google Scholar]
- 85.Kim JS, Brownjohn PW, Dyer BS, Beltramo M, Walker CS, Hay DL, et al. Anxiogenic and Stressor Effects of the Hypothalamic Neuropeptide RFRP-3 Are Overcome by the NPFFR Antagonist GJ14. Endocrinology. 2015;156(11):4152–62. [DOI] [PubMed] [Google Scholar]
- 86.Hare AS, Clarke G, Tolchard S. Bacterial lipopolysaccharide-induced changes in FOS protein expression in the rat brain: correlation with thermoregulatory changes and plasma corticosterone. Journal of neuroendocrinology. 1995;7(10):791–9. [DOI] [PubMed] [Google Scholar]
- 87.Lin Y, Li X, Lupi M, Kinsey-Jones JS, Shao B, Lightman SL, et al. The role of the medial and central amygdala in stress-induced suppression of pulsatile LH secretion in female rats. Endocrinology. 2011;152(2):545–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Oltmanns KM, Fruehwald-Schultes B, Kern W, Born J, Fehm HL, Peters A. Hypoglycemia, but not insulin, acutely decreases LH and T secretion in men. J Clin Endocrinol Metab. 2001;86(10):4913–9. [DOI] [PubMed] [Google Scholar]
- 89.Clarke IJ, Horton RJ, Doughton BW. Investigation of the mechanism by which insulin-induced hypoglycemia decreases luteinizing hormone secretion in ovariectomized ewes. Endocrinology. 1990;127(3):1470–6. [DOI] [PubMed] [Google Scholar]
- 90.Goubillon ML, Thalabard JC. Insulin-induced hypoglycemia decreases luteinizing hormone secretion in the castrated male rat: involvement of opiate peptides. Neuroendocrinology. 1996;64(1):49–56. [DOI] [PubMed] [Google Scholar]
- 91.Phumsatitpong C, Moenter SM. Estradiol-Dependent Stimulation and Suppression of Gonadotropin-Releasing Hormone Neuron Firing Activity by Corticotropin-Releasing Hormone in Female Mice. Endocrinology. 2018;159(1):414–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jasoni CL, Todman MG, Han SK, Herbison AE. Expression of mRNAs encoding receptors that mediate stress signals in gonadotropin-releasing hormone neurons of the mouse. Neuroendocrinology. 2005;82(5–6):320–8. [DOI] [PubMed] [Google Scholar]
- 93.Howland BE. Effect of glucoprivation induced by 2-deoxy-D-glucose on serum gonadotropin levels, pituitary response to GnRH and progesterone-induced release of luteinizing hormone in rats. Hormone and metabolic research = Hormon- und Stoffwechselforschung = Hormones et metabolisme. 1980;12(10):520–3. [DOI] [PubMed] [Google Scholar]
- 94.Lujan ME, Krzemien AA, Van Vugt DA. Hypoglycemia does not affect gonadotroph responsiveness to gonadotropin-releasing hormone in rhesus monkeys. Endocrine. 2003;21(2):109–14. [DOI] [PubMed] [Google Scholar]
- 95.Takeuchi Y, Nagabukuro H, Kizumi O, Mori Y. Lipopolysaccharide-induced suppression of the hypothalamic gonadotropin-releasing hormone pulse generator in ovariectomized goats. The Journal of veterinary medical science. 1997;59(2):93–6. [DOI] [PubMed] [Google Scholar]
- 96.Battaglia DF, Bowen JM, Krasa HB, Thrun LA, Viguie C, Karsch FJ. Endotoxin inhibits the reproductive neuroendocrine axis while stimulating adrenal steroids: a simultaneous view from hypophyseal portal and peripheral blood. Endocrinology. 1997;138(10):4273–81. [DOI] [PubMed] [Google Scholar]
- 97.Williams CY, Harris TG, Battaglia DF, Viguie C, Karsch FJ. Endotoxin inhibits pituitary responsiveness to gonadotropin-releasing hormone. Endocrinology. 2001;142(5):1915–22. [DOI] [PubMed] [Google Scholar]
- 98.He D, Sato I, Kimura F, Akema T. Lipopolysaccharide inhibits luteinizing hormone release through interaction with opioid and excitatory amino acid inputs to gonadotropin-releasing hormone neurones in female rats: possible evidence for a common mechanism involved in infection and immobilization stress. Journal of neuroendocrinology. 2003;15(6):559–63. [DOI] [PubMed] [Google Scholar]
- 99.Kellogg CK, Awatramani GB, Piekut DT. Adolescent development alters stressor-induced Fos immunoreactivity in rat brain. Neuroscience. 1998;83(3):681–9. [DOI] [PubMed] [Google Scholar]
- 100.Kirby ED, Geraghty AC, Ubuka T, Bentley GE, Kaufer D. Stress increases putative gonadotropin inhibitory hormone and decreases luteinizing hormone in male rats. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(27):11324–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yang JA, Song CI, Hughes JK, Kreisman MJ, Parra RA, Haisenleder DJ, et al. Acute Psychosocial Stress Inhibits LH Pulsatility and Kiss1 Neuronal Activation in Female Mice. Endocrinology. 2017;158(11):3716–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yang JA, Hughes JK, Parra RA, Volk KM, Kauffman AS. Stress rapidly suppresses in vivo LH pulses and increases activation of RFRP-3 neurons in male mice. The Journal of endocrinology. 2018;239(3):339–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Geraghty AC, Muroy SE, Zhao S, Bentley GE, Kriegsfeld LJ, Kaufer D. Knockdown of hypothalamic RFRP3 prevents chronic stress-induced infertility and embryo resorption. eLife. 2015;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Papargiris MM, Rivalland ET, Clarke IJ, Smith JT, Pereira A, Tilbrook AJ. Evidence that RF-amide related peptide-3 is not a mediator of the inhibitory effects of psychosocial stress on gonadotrophin secretion in ovariectomised ewes. Journal of neuroendocrinology. 2011;23(3):208–15. [DOI] [PubMed] [Google Scholar]
- 105.Dayas CV, Buller KM, Day TA. Neuroendocrine responses to an emotional stressor: evidence for involvement of the medial but not the central amygdala. The European journal of neuroscience. 1999;11(7):2312–22. [DOI] [PubMed] [Google Scholar]
- 106.Jeong KH, Jacobson L, Widmaier EP, Majzoub JA. Normal suppression of the reproductive axis following stress in corticotropin-releasing hormone-deficient mice. Endocrinology. 1999;140(4):1702–8. [DOI] [PubMed] [Google Scholar]
- 107.Li XF, Edward J, Mitchell JC, Shao B, Bowes JE, Coen CW, et al. Differential effects of repeated restraint stress on pulsatile lutenizing hormone secretion in female Fischer, Lewis and Wistar rats. Journal of neuroendocrinology. 2004;16(7):620–7. [DOI] [PubMed] [Google Scholar]
- 108.Tilbrook AJ, Canny BJ, Serapiglia MD, Ambrose TJ, Clarke IJ. Suppression of the secretion of luteinizing hormone due to isolation/restraint stress in gonadectomised rams and ewes is influenced by sex steroids. The Journal of endocrinology. 1999;160(3):469–81. [DOI] [PubMed] [Google Scholar]
- 109.Dobson H, Tebble JE, Phogat JB, Smith RF. Effect of transport on pulsatile and surge secretion of LH in ewes in the breeding season. Journal of reproduction and fertility. 1999;116(1):1–8. [DOI] [PubMed] [Google Scholar]
- 110.Phogat JB, Smith RF, Dobson H. Effect of transport on pituitary responsiveness to exogenous pulsatile GnRH and oestradiol-induced LH release in intact ewes. Journal of reproduction and fertility. 1999;116(1):9–18. [DOI] [PubMed] [Google Scholar]
- 111.Pierce BN, Hemsworth PH, Rivalland ET, Wagenmaker ER, Morrissey AD, Papargiris MM, et al. Psychosocial stress suppresses attractivity, proceptivity and pulsatile LH secretion in the ewe. Hormones and behavior. 2008;54(3):424–34. [DOI] [PubMed] [Google Scholar]
- 112.Wagenmaker ER, Breen KM, Oakley AE, Tilbrook AJ, Karsch FJ. Psychosocial stress inhibits amplitude of gonadotropin-releasing hormone pulses independent of cortisol action on the type II glucocorticoid receptor. Endocrinology. 2009;150(2):762–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Breen KM, Oakley AE, Pytiak AV, Tilbrook AJ, Wagenmaker ER, Karsch FJ. Does cortisol acting via the type II glucocorticoid receptor mediate suppression of pulsatile luteinizing hormone secretion in response to psychosocial stress? Endocrinology. 2007;148(4):1882–90. [DOI] [PubMed] [Google Scholar]