Abstract
Background
One of the most common reasons for poor medication adherence and associated treatment failure of triple therapy is adverse drug effect (ADEs) of medications.
Objective
Assessment of ADEs and associated factors during H. pylori eradication therapy.
Method
Consented H. pylori positive adult outpatients on standard triple therapy (proton pump inhibitor, amoxicillin and clarithromycin) were involved in this facility based follow up study from May 2016 to April 2018 at Bahir Dar city in Ethiopia. Pre-developed questionnaire and formats were used to collect sociodemographic, medical information, and patient practice data before, during, and after therapy. Bivariate and backward stepwise multivariate logistic regression was used to analyze data. P-value < 0.05 at 95%CI was considered as significant.
Result
A total of 421 patients were involved in the study. Almost 80% of the patients were urban residents. Mean (±SD) age and body weight of patients were 30.63 (± 10.74) years and 56.79 (± 10.17) kg, respectively. ADE was reported from 26.1% of the patients and of all the reported ADEs, more than 85% was manifested with gastrointestinal symptoms which include gastrointestinal discomfort(39.1%), nausea (13.6%), constipation(12.7%), diarrhea(12.9%) and anorexia(10%). Determinants of self-reported ADEs among patients in the present study were body mass index above 25 (AOR: 2.55; 95%CI (1.21–5.38), p = 0.014), duration of acid-pepsin disorder more than 3weeks (AOR: 3.57; 95%CI (1.63–7.81), p = 0.001), pain feeling during long interval between meals (AOR: 2.14; 95%CI (1.19–3.84), p = 0.011), and residence in urban area (AOR: 1.95; 95% CI (1.04–3.67), p = 0.038).
Conclusion
Significant proportion of patients reported ADEs which commonly manifested with gastrointestinal symptoms. Consideration of patients’ body mass index, duration of the disorder, period of the day when patients feel pain, and patients’ area of residence could help to reduce ADEs experienced during H. pylori eradication therapy.
Introduction
Approximately two thirds of the world’s population is infected with Helicobacter pylori (H. pylori), making it the most widespread infection in the world[1]. Infection with H. pylori is a major cause of upper gastrointestinal diseases which includes peptic ulcer disease, chronic gastritis, gastric cancer, and mucosa-associated lymphoid tissue lymphoma[2–4].
According to 1996 Maastricht I consensus guideline recommendation, worldwide accepted H. pylori eradication therapy is always a multidrug regimen[5–7]. Within this multidrug regimen, there are different aspects of H. pylori eradication regimens that differ in the duration and the composition of drugs exist in the available guidelines and one of which is the standard triple therapy that consists of a combination of two antibiotics and an acid-suppressant drug[8,9].
Eradication of H. pylori infection in patients receiving medications can often be difficult because of different pushing factors. One of the commonly reported determinants of eradication failure is poor patient adherence to a multidrug regimen usually due to adverse drug effects of medications. Other determinants being the chosen regimen type and antibiotic resistance. Sociodemographic variations of patients, duration of peptic ulcer, cigarette smoking, genetics, and presence of other chronic diseases are also reported to affect eradication therapy[10–12]. Effort has been made to improve efficacy and safety of H. pylori eradication therapy through exploring new first-line treatments, investigating antibiotic resistance rates, evolution of the use of adjunctive therapies, and patient counselling and follow-up, however failure of H. pylori eradication therapy is the prevailing problem in clinical practice[13–20].
As it is true in many other pharmacotherapies, adverse drug effects (ADEs) are one the most common factors that affect the quality of the H. pylori eradication therapy. Although ADEs during H. pylori eradication therapy have been described as well tolerated, the therapy may be associated with significant adverse effects usually revealed with gastrointestinal symptoms that could bring about poor adherence of patients to medications leading to eradication failure[21–23]. Factors which might increase the risk of occurrence of ADEs include; extremes of age, gender, multiple drugs, disease state, past history of ADEs or allergy, genetic, factors, large doses and other patient sociodemographic and medical variables[10–12].
Previous studies including ours showed that adverse drug effect is one of the factors that influences H. pylori eradication rate in patients receiving standard triple therapy with proton pump inhibitor, amoxicillin and clarithromycin[24,25]. In Ethiopia, there are no studies done on the determinant factors for the occurrence of ADEs during H. pylori eradication therapy with the standard triple therapy. Thus the present study was amid to assess self-reported adverse drug effects and its associated factors during H. pylori eradication therapy in patients taking standard triple therapy.
Methods
Ethical issues
The study was approved by the Institutional Review Board of College of Medicine and Health Sciences, Bahir Dar University (Reference No: BCS/171/08). Permission was sought from the health institutions after presentation of the ethical approval. Written consent was obtained from each volunteer adult outpatients fulfilling inclusion criteria (S1 Text). All the drugs used in eradication therapy were approved by Food, Medicine, Healthcare Administration and Control Authority (FMHACA) of Ethiopia and the treatment protocol is as per national General Hospital Guideline. Patients were informed about the benefits and risks of the study as well as their full right to withdraw from the study at any time in point without jeopardizing the care. Moreover, privacy and confidentiality were maintained through anonymity and restricting data access.
Study design and setting
Facility based prospective follow up study was conducted from May 2016 to April 2018 in Bahir Dar, the capital city of Amhara Regional State, located 565 kilometers Northwest of Addis Ababa, the capital of Ethiopia. The study was conducted to assess adverse drug effects during H. pylori eradication therapy with standard triple therapy as part of the study that assess H. pylori eradication rate at two healthcare institutions namely Adinas General Hospital and Kidanemihret Higher Clinic both found in Bahir Dar city. The healthcare institutions were communicated officially through submitting letter of approval of the study protocol offered from Institutional Review Board of College of Medicine and Health Sciences at Bahir Dar University with reference No: BCS/171/08.
Patients on standard triple therapy
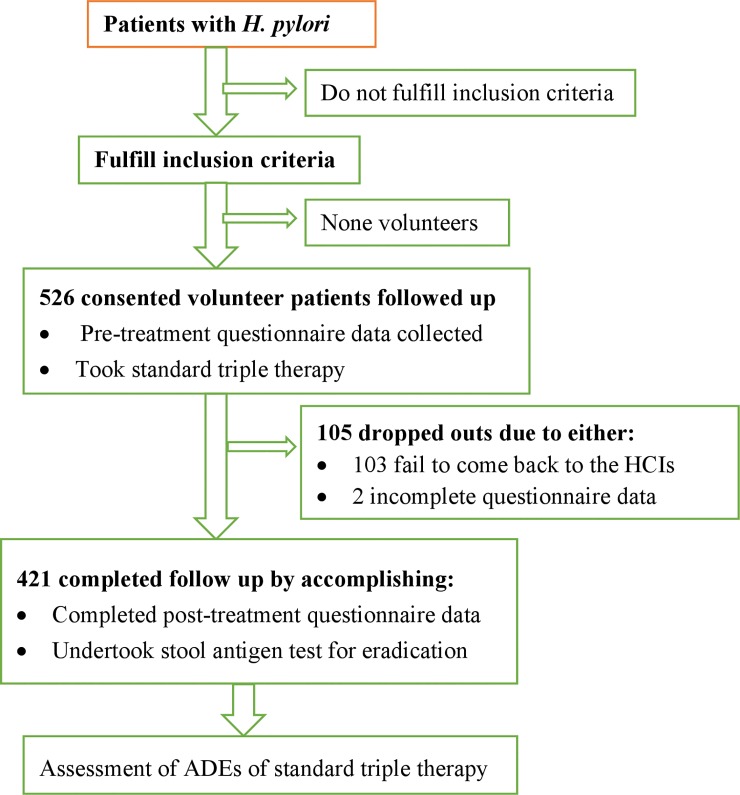
Of the total 526 consented H. pylori positive patients, this study was conducted on 421 patients who completed follow up (Fig 1). All of them were adult outpatients (age ≥18 years) living in rural and urban settings and voluntarily agreed to give written consent. Those who were seriously sick or referred from other facilities as well as those who do not speak the local language (Amharic) were excluded from the study. Assessment of adverse drug effects experienced by patients were made following proton pump inhibitor (PPI)-based standard triple therapy with a regimen of PPI (omeprazole 40 mg or pantoprazole 40 mg, twice/day for 15 to 30 days), clarithromycin (500 mg), and amoxicillin (1000 mg), each twice/day for 10 or 14 days.
Fig 1. Flow chart depicting sequences of the study.
HCIs: healthcare institutions.
Data collection and management
Structured questionnaire developed from the literature was used to collect data in both the recruitment and the follow up period (S1 Table). The questionnaire was developed in English and translated into the local language Amharic and then back to English. Pre-test of the questionnaire was done on 5% of the sample size in another healthcare institution in the study area to ensure whether the questionnaire was able what it was intended to capture and modification of questions were made accordingly. Patients’ sociodemographic and medical information was collected during the first encounter. Data related to adverse drug effects, and also added-on homemade traditional remedies was collected during the follow up period on phone call and during their second encounter to check for eradication of H. pylori infection at the respective healthcare institutions (Fig 1). Both primary diagnosis as well as eradication of H. pylori after 4–6 weeks therapy was confirmed by a stool antigen test (S2 Table), which is recommended by both European and Japanese guidelines conducted according to the Manufacturer’s recommendation (SD BIOLINE H. pylori Ag, Standard Diagnostics, Inc. Korea)[26]. Data was collected by trained clinical pharmacists and nurses. Data accuracy and consistency was assured by the study team on daily basis. Besides collecting data through completion of structured questionnaire, stool sample was collected during the second encounter of patients to determine the success or failure of H. pylori eradication therapy.
Data analysis procedures
Data were entered and analyzed using SPSS statistical package version 21.0. Descriptive statistics such as percentages, means and standard deviations were used to describe data. Bivariate and multivariable logistic regressions were used to identify predictors of adverse drug effects of the standard triple-therapy. Those variables with a p-value in bivariate 0.25 were retained for multivariable logistic regression based on scientific recommendations[27]. The Hosmer-lemeshow test was checked to assess the model fattiness to conduct binary multiple logistic regression. All variables which fulfill Hosmer-Lemeshow were retained for multivariable logistic regression. Backward stepwise logistic regression model was used during multivariable logistic regression to control confounding effect. Odds ratio with 95% confidence intervals was calculated for each of the independent variables using P-value < 0.05 as the level of significance.
Results
Sociodemographic and medical characteristics of patients
A total of 421 patients were able to come back to the healthcare institution to confirm eradication success or failure and provide complete information related to adverse effects they had experienced during their eradication therapy. The mean age (SD) of patients was 30.63 (± 10.74) years, which ranges from 18–86. Nearly 90% of patients were under 45 years old. The mean weight of patients was 56.71 (±10.19) kg. The mean body mass index of all patients was 21.09 (±4.16).
As shown in Table 1, two-third of the patients were females and majority (80%) of the patients were urban dwellers. Close to two-third (63.4%) of them were married and a sizable proportion (42%) of them attended college education or above. Occupation wise, around 38% of the patients were employees of government and private sectors with monthly paid salary and around 62% of patients were engaged in their own income generating activities that include housewives, merchants, farmers, students, and daily laborers. Majority (86%) of the patients were followers of Ethiopian Orthodox Church.
Table 1. Sociodemographic and medical information of patients participated in the study of adverse drug effects of standard triple therapy in selected healthcare institutions at Bahir Dar City Administration, May 2016 to April 2018.
(N = 421).
| Variable and their categories | Frequency and percentage | Self-report on ADEs | Self-reported ADEs in % | |
|---|---|---|---|---|
| Yes (n = 110) | No (n = 311) | |||
| Sex | ||||
| Female | 276(65.6) | 80 | 196 | 29.0 |
| Male | 145(34.4) | 30 | 115 | 20.7 |
| Body mass index | ||||
| <20 | 151(35.9) | 35 | 116 | 23.2 |
| 20–25 | 222(52.7) | 56 | 166 | 25.2 |
| >25 | 48(11.4) | 19 | 29 | 39.6 |
| Age in years | ||||
| 18–24 | 125(29.7) | 33 | 92 | 26.4 |
| 25–34 | 172(40.9) | 40 | 132 | 23.3 |
| 35–44 | 75(17.8) | 23 | 52 | 30.7 |
| ≥ 45 | 49(11.6) | 14 | 35 | 28.6 |
| Residence | ||||
| Urban | 336(79.8) | 95 | 241 | 28.3 |
| Rural | 85(20.2) | 15 | 70 | 17.5 |
| Patents' Zonal address | ||||
| Bahir Dar city | 169(40.1) | 45 | 124 | 26.6 |
| West Gojjam | 96(22.8) | 22 | 74 | 22.9 |
| South Gondar | 62(14.7) | 15 | 47 | 24.2 |
| Awi zone | 52(12.4) | 16 | 36 | 30.1 |
| Others zones | 42(10.0) | 12 | 30 | 28.6 |
| Marital status | ||||
| Single | 145(34.5) | 34 | 111 | 23.4 |
| Married | 267(63.4) | 73 | 194 | 27.3 |
| Divorced/Widowed | 9(2.1) | 3 | 6 | 33.3 |
| Occupation | ||||
| Employee | 159 (37.8) | 46 | 113 | 28.9 |
| Non-employee | 262(62.2) | 64 | 198 | 24.4 |
| Educational status | ||||
| Grade 1–8 and below | 141(33.5) | 35 | 106 | 24.8 |
| Grade 9–12 | 104(24.7) | 30 | 74 | 28.8 |
| College and above | 176(41.8) | 45 | 131 | 25.6 |
| Time duration of the disorder | ||||
| ≤ 3weeks | 110(15.9) | 9 | 101 | 8.2 |
| >3weeks | 354(84.1) | 101 | 253 | 28.5 |
| Presence of other disease(s) | ||||
| Yes | 108(25.6) | 25 | 83 | 23.1 |
| No | 313(74.3) | 85 | 228 | 27.2 |
| Self-reported alcohol intake | ||||
| Yes | 237(56.3) | 52 | 185 | 21.9 |
| No | 184(43.7) | 58 | 126 | 31.5 |
| Pain feeling period in the day | ||||
| After meal | 217(51.5) | 46 | 171 | 21.2 |
| Persistent in the day | 122(29.0) | 35 | 87 | 28.7 |
| Long interval b/n meals | 82(19.5) | 29 | 53 | 35.4 |
| Use of Flaxseed or Fenugreek | ||||
| Yes | 135(32.1) | 35 | 100 | 25.9 |
| No | 286(67.9) | 75 | 211 | 26.2 |
| Triple therapy regimen durations | ||||
| 10 day | 279(66.3) | 79 | 200 | 28.3 |
| 14 day | 142(33.7) | 31 | 111 | 21.8 |
| Self-reported regimen completion | ||||
| Yes | 397(94.3) | 100 | 297 | 25.2 |
| No | 24(5.7) | 10 | 14 | 41.7 |
| Disease symptom resolution | ||||
| Yes | 355(84.3) | 91 | 264 | 25.6 |
| No | 66(15.7) | 19 | 47 | 28.8 |
As summarized in Table 1, almost 85% of them said that they had been living with symptoms of acid-pepsin disorder for more than 3 weeks. Almost half of the patients responded that they feel pain after meal, while 29% reported that the pain feeling persists throughout the day. About a fourth (25.6%) of the patients have responded the presence of other chronic diseases and patients response on alcohol intake before receiving triple therapy was 56.3%. One-quarter of patients reported presence of other chronic diseases of which renal impairment was the highest representing 45.4% of the cases. Almost a third (32.1%) of patients reported that they had taken diets traditionally believed to have healing effect on gastritis and peptic ulcer disease like Fenugreek and Flaxseed together with the triple therapy. Nearly two-third (66.3%) of patients received standard triple therapy for 10 days duration and the remaining for 14 days. The overall H. pylori eradication rate (success of the therapy) was 90% which was a bit lower than self-reported regimen completion 94.3%. However percentage of patients who reported symptom resolution of the disorder was 84.3%.
As shown in Table 1 the contribution of females and males to the reported ADEs of patients was 29.0% and 20.7% respectively. Similarly the contribution of some variable among others were; urban and rural (28.3% vs. 17.5%), disorder duration up to 3 and above three weeks (8.2% vs. 28.5%), history of alcohol intake and no intake (21.9% vs. 31.5%), 10 days and 14 days regimen (28.1% vs. 21.8%), used and not used Fenugreek or Flaxseed (25.9% vs.26.2%), symptom resolved and unresolved (25.6 vs. 28.8%) and eradication success and failure (23.7% vs. 47.6%).
As indicated in Table 2, almost a fourth (26.1%) of the patients responded that they had experienced one or more adverse drug effects while taking medications. Of the overall reported ADEs, more than 85% was gastrointestinal type which includes gastrointestinal discomfort(39.1%), nausea(13.6%), constipation(12.7%), diarrhea(12.9%) and anorexia(10%).
Table 2. Frequency and percentage of self-reported adverse drug effects of patients received standard triple therapy in selected healthcare institutions at Bahir Dar city, May 2016 to April 2018.
(N = 421).
| Self-reported ADEs | Relative Frequency and percentage of self-reported ADEs (n = 110) | Overall percentage of self-reported ADEs (N = 421) |
|---|---|---|
| GI discomfort | 43(39.1) | 10.2 |
| Nausea | 15(13.6) | 3.6 |
| Headache and drowsiness | 15(13.6) | 3.6 |
| Constipation | 14(12.7) | 3.3 |
| Diarrhea | 12(10.9) | 2.8 |
| Anorexia | 11(10.0) | 2.6 |
| Over all | 110(100) | 26.1 |
Factors associated with adverse drug effects
Bivariate and multiple logistic regression analysis is shown in Table 3. On bivariate logistic regression analysis, the following variables were significantly associated with self-reported ADEs on receiving standard triple therapy: body mass index >25 (COR: 2.17 95%CI (1.01–4.33), p = 0.028); urban area residence (COR: 1.84 95%CI (1.00–3.37), p = 0.049), disease duration more than 3 weeks (COR: 2.57 95%CI (1.23–5.39), p = 0.012), history of pain feeling during long interval between meals (COR: 2.03 95%CI (1.16–3.56), p = 0.013), and history of no alcohol intake (COR: 1.24 95%CI (0.74–2.07), p = 0.027).
Table 3. Binary and multiple logistic regression analysis for factors associated with self-reported adverse drug effects on receiving standard triple therapy in selected healthcare institutions at Bahir Dar city, May 2016 to April 2018.
(N = 421).
| Variable Categories | ADEs | Crude odds ratio* | Adjusted odds ratio** | |
|---|---|---|---|---|
| Yes | No | |||
| Sex | ||||
| Female | 80 | 196 | 1.57(0.97–2.52)g | |
| Male | 30 | 115 | 1.00 | |
| Body mass index | ||||
| <20 | 35 | 116 | 1.00 | |
| 20–25 | 56 | 166 | 1.12(0.69–1.82) | |
| >25 | 19 | 29 | 2.17(1.01–4.33)a | 2.55(1.21–5.34)1 |
| Age in years | ||||
| 18–24 | 33 | 92 | 0.90(0.41–1.99) | |
| 25–34 | 40 | 132 | 0.76(0.42–1.54) | |
| 35–44 | 23 | 52 | 1.11(0.50–2.43) | |
| ≥45 | 14 | 35 | 1.00 | |
| Residence | 1.95(1.04–3.67)2 | |||
| Urban | 95 | 24 | 1.84(1.00–3.37)b | |
| Rural | 15 | 170 | 1.00 | |
| Zonal address | ||||
| Bahir Dar city | 45 | 124 | 1.00 | |
| West Gojjam | 22 | 74 | 0.82(0.46–1.47) | |
| South Gondar | 15 | 47 | 0.88(0.45–1.73) | |
| Awi zone | 16 | 36 | 1.23(0.62–2.42) | |
| Other zones | 12 | 30 | 1.10(0.52–2.34) | |
| Marital status | ||||
| Single | 34 | 111 | 1.00 | |
| Married | 73 | 194 | 1.23(0.76–1.96) | |
| Divorced/Widowed | 3 | 6 | 1.63(0.39–6.90) | |
| Occupation | 46 | |||
| Employee | 64 | 113 | 1.26(0.81–1.96) | |
| Non-employee | 198 | 1.00 | ||
| Educational status | ||||
| Grade 1–8 and below | 35 | 106 | 0.96(0.62–1.73) | |
| Grade 9–12 | 30 | 74 | 1.18(0.49–1.46) | |
| College and above | 45 | 131 | 1.00 | |
| Time duration of the disorder | ||||
| ≤ 3weeks | 9 | 101 | 1.00 | |
| >3weeks | 101 | 253 | 2.57(1.23–5.39)c | 3.57(1.63–7.81)3 |
| Pain feeling period in the day | ||||
| After meal | 46 | 171 | 1.00 | |
| Persistent in the day | 35 | 87 | 1.36(0.90–2.48) | |
| Long interval b/n meals | 29 | 53 | 2.03(1.16–3.56)d | 2.14(1.19–3.84)4 |
| Presence of other disease(s) | ||||
| Yes | 25 | 83 | 0.81(0.48–1.35) | |
| No | 85 | 228 | 1.00 | |
| Self-reported alcohol intake | ||||
| Yes | 52 | 185 | 1.00 | |
| No | 58 | 126 | 1.64(1.06–2.54)e | |
| Regimen durations | ||||
| 10day | 79 | 200 | 1.00 | |
| 14day | 31 | 111 | 0.71(0.44–1.14)f | |
| Use of Flaxseed or Fenugreek | ||||
| Yes | 35 | 100 | 1.00 | |
| No | 75 | 211 | 1.02(0.64–1.61) | |
| Self-reported regimen completion | ||||
| Yes | 100 | 297 | 1.00 | |
| No | 10 | 14 | 2.12(0.91–4.93)f | |
| Disease symptom resolution | ||||
| Yes | 91 | 264 | 1.00 | |
| No | 19 | 47 | 1.17(0.65–2.10) | |
*P values for binary logistic regression:
a = 0.028
b = 0.049
c = 0.012
d = 0.013
e = 0.027
f<0.25 but not significant.
**P values for multivariate logistic regression
1 = 0.014
2 = 0.038
3 = 0.001
4 = 0.011
As indicated in Table 3 above, on multivariable binary logistic regression model analysis; body mass index more than 25, duration of acid-pepsin disorder more than 3 weeks, history of pain feeling during long interval between meals, and urban area residence were significantly contributing factors for self-reported ADEs in patients on standard triple therapy. Patients with body mass index more than 25 were 2.55 (AOR: 2.55; 95%CI (1.21–5.38), p = 0.014) times more likely to report ADEs on receiving standard triple therapy compared to patients with body mass index less than 20. Patients with duration of acid-pepsin disorder more than 3weeks were 3.57 (AOR: 3.57; 95%CI (1.63–7.61), p = 0.001) times more likely to report ADEs compared with patients who stayed up to 3weeks. Patients with history of pain feeling during long interval between meals were 2.14 (AOR: 2.14; 95%CI (1.19–3.84), p = 0.011) times more likely to report ADEs compared to patients who feel pain after meal. Patients living in urban areas were 1.95 (AOR: 1.95; 95% CI (1.04–3.67), p = 0.038) times more likely to report ADEs compared to patients whose eradication therapy was successful.
Discussion
Eradication therapy of H. pylori infections have proven to be difficult for different reasons. Assessment of patient and pathogen related factors could insight ways of improving treatment outcome. We evaluated self-reported ADEs that occurred during standard triple therapy and associated risk conditions of the patient that could bring about occurrence of ADEs in H. pylori eradication therapy. Many factors affect the occurrence of ADEs during H. pylori eradication therapy regimens[28]. Understanding the different effects of these factors on ADEs enables healthcare professionals to choose most appropriate medications and give the best advice to patients[23,29].
Of 421 patients who were able to comeback for after 4–6 weeks completion of eradication therapy 110 (26.13%) reported their experience of one or more types of ADEs. These adverse effects were mild, with no documented serious adverse events. The most commonly reported ADEs were manifested with gastrointestinal symptoms which include; gastrointestinal discomfort, nausea, vomiting, diarrhea and constipation. Similar finding of gastrointestinal dominance have been reported in other studies elsewhere[23,30,31]. Self-reported ADEs in the present study was comparable 19%[32], higher than 10% -18% [33–36] and lower than 36%-76% [37–40] reported previously. The variability could be due to different factors such as duration of triple therapy, socio-demographic differences of patients, duration and severity of the disease, pharmacogenetic variability among patients, and drug combinations and possible interactions among these factors.
Predictors of self-reported ADEs of H. pylori eradication standard triple therapy identified in this study were female sex, urban area of residence, patient history of more than three months duration with the disease, pain feeling during long interval between meals, and residence in urban areas.
Body mass index higher than 25 was predictor of self-reported ADEs during triple therapy which could be due to more stomach distension in obese patients [41] directly linked to gastrointestinal symptoms and/or slow gastric emptying in obese patients[42] facilitating significant alteration of gastrointestinal microflora which can be responsible for reported gastrointestinal ADEs. On the other hand it could be also possible to associate higher ADEs in obese patients with slow elimination of drugs specially clarithromycin that has high tissue concentrations as reported previously ([43,44]. Negative impact of higher body mass index on H. pylori eradication therapy has been reported[45] which might be linked with ADEs that reduce drug intake. In addition higher H. pylori infection rate has been reported[1,46] in obese people. To have a better understanding of H. pylori infection and its eradication therapy in relation to body mass index needs further studies as it was also suggested elsewhere [47].
Patients who have been feeling pain during long interval between meals were more likely to report ADEs of medications compared with those who feel pain after meal. This could be due to reporting of disease symptoms as medication adverse effects because both conditions produce upper abdominal pain or discomfort. Similarly H. pylori positive patients with history of acid-pepsin disorder more than 3 weeks were more likely to report ADEs compared with those less than 3 weeks. Lower reporting of ADEs in patients with active ulcer could be relative tolerance of drugs’ effects compared with more severe pain of the active ulcer and more reporting of ADEs during inactive ulcer could also be relative severity of ADEs than inactive ulcer induced pain. Although the same study is not found, study conducted on eradication of H. pylori in patients with active and inactive ulcers reported better rate of eradication in patients with active ulcer[48]. Parallel to this higher adherence rate has been found among the patients with acute conditions compared to those with chronic diseases[49]. Although there was no similar study that reported the effect of residence on H. pylori eradication with standard triple therapy some controversial reports exist in previously reported therapies[50–52]. The difference could be due to better awareness of urban patients to pay attention and reporting adverse drug effects than rural counter parts possibly associated with their differences in several aspects.
The other variable which was significant in predicting self-reported ADEs on bivariate but not on multivariate logistic regression includes history of free from self-reported alcohol intake. Patients who responded no alcohol intake prior to therapy were more likely to report ADEs (p value = 0.027) than those reported alcohol intake. This could be due to fast elimination of drugs in patients taking alcohol because of its documented inducer effect[53,54].
There was a tendency of weak association female sex and self- reported ADEs during H. pylori eradication with triple therapy in this study. Although there was no similar study that reported female sex as a risk factor in development of ADEs during triple therapy, in many other therapies reviewers indicated more ADEs in females that could be due to anatomical and physiological differences such as lower bodyweight and organ size, more body fat, different gastric motility and lower glomerular filtration rate or more attentiveness to recall and report physical illness or symptom perceptions[12,55,56]. Although patients were given the same dose with the common name adult, the mean weight of females and males was 55.2(SD = 10.1) and 59.6 (SD = 9.7) respectively in this study.
There was no difference in self-reported adverse drug effects between 10 days and 14 days triple therapy regimen in this study which is similar to reported study elsewhere[10,33,57]. Following eradication therapy 84.3% of patients reported disease symptom resolution which was a bit lower than reported 91%[57]. Self-reported regimen completion was 94.3% in this study which was comparable with 95.7[58] and a bit lower than 99.8% reported elsewhere[59] and it has no significant influence on self-reported adverse effects. The response of patients showed that 84.3% of patients achieved complete resolution of acid-pepsin disorder symptoms. This value was lower than the 90% H. pylori eradication rate which could suggest that H. pylori eradication may not bring about complete symptom resolution. On the contrary acid-pepsin disorder symptoms may be resolved in patients with eradication failure.
Limitation
Self- reported ADEs in this study could be different from the actual or reported values due to cultural and awareness differences of patients in developing and developed countries.
Conclusion
Significant proportion of patients on standard triple therapy to eradicate H. pylori reported ADEs mostly manifested with gastrointestinal symptoms. Reduction of these ADEs of medications should take into account of patients’ body mass index, duration of the disorder, period of the day when patients feel pain and patients’ area of residence which could help to improve H. pylori eradication. Use of traditional homemade remedies prepared from Flaxseed or Fenugreek lacks to reduce the risk of ADEs during standard triple therapy and thus the healthcare practice shall not be influenced until this traditional practice will to be proven otherwise.
Supporting information
(SAV)
(PDF)
(PDF)
(PDF)
Acknowledgments
The authors would like to acknowledge Bahir Dar University and Addis Ababa University for funding this research project. We would like to thank Adinas General Hospital and Kidanemihret Higher Clinic for allowing data collection in their healthcare institutions. We would like to thank Abebe Fetene and Kibret Ayalew for their administrative support during data collection. Finally, we thank volunteer patients for their participation in this study project.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
The principal investigator, EG, has got finantial and material support from Bahir Dar and Addis Ababa Universsities for this work as any of graduate program students. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Kouitcheu Mabeku LB, Noundjeu Ngamga ML, Leundji H (2018) Potential risk factors and prevalence of Helicobacter pylori infection among adult patients with dyspepsia symptoms in Cameroon. BMC Infect Dis 18: 278 10.1186/s12879-018-3146-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Labigne A, de Reuse H (1996) Determinants of Helicobacter pylori pathogenicity. Infect Agents Dis 5: 191–202. [PubMed] [Google Scholar]
- 3.McColl KE (2010) Clinical practice. Helicobacter pylori infection. N Engl J Med 362: 1597–1604. 10.1056/NEJMcp1001110 [DOI] [PubMed] [Google Scholar]
- 4.Suerbaum S, Michetti P (2002) Helicobacter pylori infection. N Engl J Med 347: 1175–1186. 10.1056/NEJMra020542 [DOI] [PubMed] [Google Scholar]
- 5.Vakil N (1998) Treatment of Helicobacter pylori infection. Am J Ther 5: 197–201. 10.1097/00045391-199805000-00011 [DOI] [PubMed] [Google Scholar]
- 6.Bardhan KD (1996) Triple therapy as a cure for Helicobacter pylori infection. Eur J Gastroenterol Hepatol 8 Suppl 1: S27–30. [DOI] [PubMed] [Google Scholar]
- 7.Malfertheiner P, Megraud F, O'Morain C, Hungin AP, Jones R, Axon A, et al. (2002) Current concepts in the management of Helicobacter pylori infection—the Maastricht 2–2000 Consensus Report. Aliment Pharmacol Ther 16: 167–180. [DOI] [PubMed] [Google Scholar]
- 8.Howden CW, Hunt RH (1998) Guidelines for the management of Helicobacter pylori infection. Ad Hoc Committee on Practice Parameters of the American College of Gastroenterology. Am J Gastroenterol 93: 2330–2338. 10.1111/j.1572-0241.1998.00684.x [DOI] [PubMed] [Google Scholar]
- 9.Malfertheiner P, Megraud F, O'Morain C, Bazzoli F, El-Omar E, Graham D, et al. (2007) Current concepts in the management of Helicobacter pylori infection: the Maastricht III Consensus Report. Gut 56: 772–781. 10.1136/gut.2006.101634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abbasinazari M, Sahraee Z, Mirahmadi M (2013) The Patients' Adherence and Adverse Drug Reactions (ADRs) which are Caused by Helicobacter pylori Eradication Regimens. J Clin Diagn Res 7: 462–466. 10.7860/JCDR/2013/4673.2799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alomar MJ (2014) Factors affecting the development of adverse drug reactions (Review article). Saudi Pharm J 22: 83–94. 10.1016/j.jsps.2013.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Graham DY, Lew GM, Malaty HM, Evans DG, Evans DJ Jr., Klein PD, et al. (1992) Factors influencing the eradication of Helicobacter pylori with triple therapy. Gastroenterology 102: 493–496. 10.1016/0016-5085(92)90095-g [DOI] [PubMed] [Google Scholar]
- 13.Al-Eidan FA, McElnay JC, Scott MG, McConnell JB (2002) Management of Helicobacter pylori eradication—the influence of structured counselling and follow-up. Br J Clin Pharmacol 53: 163–171. 10.1046/j.0306-5251.2001.01531.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bazzoli F, Zagari M, Pozzato P, Varoli O, Fossi S, Ricciardiello L, et al. (1998) Evaluation of short-term low-dose triple therapy for the eradication of Helicobacter pylori by factorial design in a randomized, double-blind, controlled study. Aliment Pharmacol Ther 12: 439–445. 10.1046/j.1365-2036.1998.00330.x [DOI] [PubMed] [Google Scholar]
- 15.Dore MP, Bibbo S (2019) Role of Probiotics in Helicobacter pylori Eradication: Lessons from a Study of Lactobacillus reuteri Strains DSM 17938 and ATCC PTA 6475 (Gastrus(R)) and a Proton-Pump Inhibitor. 2019: 3409820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu Q, Qi D, Kang J, Jin Y, Liu W, Gao W, et al. (2015) Efficacy of real-time PCR-based detection of Helicobacter pylori infection and genotypic resistance-guided quadruple therapy as the first-line treatment for functional dyspepsia with Helicobacter pylori infection. Eur J Gastroenterol Hepatol 27: 221–225. 10.1097/MEG.0000000000000186 [DOI] [PubMed] [Google Scholar]
- 17.Lu M, Yu S, Deng J, Yan Q, Yang C, Xia G, et al. (2016) Efficacy of Probiotic Supplementation Therapy for Helicobacter pylori Eradication: A Meta-Analysis of Randomized Controlled Trials. PLoS One 11: e0163743 10.1371/journal.pone.0163743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park CS, Lee SM, Park CH, Koh HR, Jun CH, Park SY, et al. (2014) Pretreatment antimicrobial susceptibility-guided vs. clarithromycin-based triple therapy for Helicobacter pylori eradication in a region with high rates of multiple drug resistance. Am J Gastroenterol 109: 1595–1602. 10.1038/ajg.2014.222 [DOI] [PubMed] [Google Scholar]
- 19.Tepes B, O'Connor A, Gisbert JP, O'Morain C (2012) Treatment of Helicobacter pylori infection 2012. Helicobacter 17 Suppl 1: 36–42. [DOI] [PubMed] [Google Scholar]
- 20.Graham DY, Shiotani A (2008) New concepts of resistance in the treatment of Helicobacter pylori infections. Nat Clin Pract Gastroenterol Hepatol 5: 321–331. 10.1038/ncpgasthep1138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.B P, AS, Quereshi JM, JJ E (2017) Current Status of H. pylori Infection Treatment. Journal of Applied Pharmaceutical Science 7. [Google Scholar]
- 22.Kwok A, Lam T, Katelaris P, Leong RW (2008) Helicobacter pylori eradication therapy: indications, efficacy and safety. Expert Opin Drug Saf 7: 271–281. 10.1517/14740338.7.3.271 [DOI] [PubMed] [Google Scholar]
- 23.Shakya Shrestha S, Bhandari M, Thapa SR, Shrestha R, Poudyal R, Purbey B, et al. (2016) Medication Adherence Pattern and Factors affecting Adherence in Helicobacter Pylori Eradication Therapy. Kathmandu Univ Med J (KUMJ) 14: 58–64. [PubMed] [Google Scholar]
- 24.Abuhammour A, Dajani A, Nounou M, Zakaria M (2016) Standard triple therapy versus sequential therapy for eradication of Helicobacter pylori in treatment naive and retreat patients. Arab J Gastroenterol 17: 131–136. 10.1016/j.ajg.2016.07.001 [DOI] [PubMed] [Google Scholar]
- 25.Gebeyehu E, Nigatu D, Engidawork E (2019) Helicobacter pylori eradication rate of standard triple therapy and factors affecting eradication rate at Bahir Dar city administration, Northwest Ethiopia: A prospective follow up study. 14: e0217645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shimoyama T (2013) Stool antigen tests for the management of Helicobacter pylori infection. World J Gastroenterol 19: 8188–8191. 10.3748/wjg.v19.i45.8188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sperandei S (2014) Understanding logistic regression analysis. Biochem Med (Zagreb) 24: 12–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jaka H, Mueller A, Kasang C, Mshana SE (2019) Predictors of triple therapy treatment failure among H. pylori infected patients attending at a tertiary hospital in Northwest Tanzania: a prospective study. BMC Infect Dis 19: 447 10.1186/s12879-019-4085-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ksiądzyna D, Szandruk M, Szeląg A (2012) Treatment prospects of Helicobacter pylori infection. Gastroenterology Review 2: 70–77. [Google Scholar]
- 30.Lee M, Kemp JA, Canning A, Egan C, Tataronis G, Farraye FA (1999) A randomized controlled trial of an enhanced patient compliance program for Helicobacter pylori therapy. Arch Intern Med 159: 2312–2316. 10.1001/archinte.159.19.2312 [DOI] [PubMed] [Google Scholar]
- 31.Zhou YQ, Xu L, Wang BF, Fan XM, Wu JY, Wang CY, et al. (2012) Modified Sequential Therapy Regimen versus Conventional Triple Therapy for Helicobacter Pylori Eradication in Duodenal Ulcer Patients in China: A Multicenter Clinical Comparative Study. Gastroenterol Res Pract 2012: 405425 10.1155/2012/405425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arkkila PE, Seppala K, Kosunen TU, Sipponen P, Makinen J, Rautelin H, et al. (2005) Helicobacter pylori eradication as the sole treatment for gastric and duodenal ulcers. Eur J Gastroenterol Hepatol 17: 93–101. 10.1097/00042737-200501000-00018 [DOI] [PubMed] [Google Scholar]
- 33.Chen YI, Fallone CA (2015) A 14-day course of triple therapy is superior to a 10-day course for the eradication of Helicobacter pylori: A Canadian study conducted in a 'real world' setting. Can J Gastroenterol Hepatol 29: e7–10. 10.1155/2015/659390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hori K, Takagawa T, Hida N, Nakamura S (2017) Safety of One-Week, First-Line, Standard Triple Therapy for Helicobacter Pylori Eradication in a Japanese Population. Curr Drug Saf. [DOI] [PubMed] [Google Scholar]
- 35.Silva FM, Zaterka S, Eisig JN, Chehter EZ, Chinzon D, Laudanna AA (2001) Factors affecting Helicobacter pylori eradication using a seven-day triple therapy with a proton pump inhibitor, tinidazole and clarithromycin, in Brazilian patients with peptic ulcer. Rev Hosp Clin Fac Med Sao Paulo 56: 11–16. 10.1590/s0041-87812001000100003 [DOI] [PubMed] [Google Scholar]
- 36.Sun WH, Ou XL, Cao DZ, Yu Q, Yu T, Hu JM, et al. (2005) Efficacy of omeprazole and amoxicillin with either clarithromycin or metronidazole on eradication of Helicobacter pylori in Chinese peptic ulcer patients. World J Gastroenterol 11: 2477–2481. 10.3748/wjg.v11.i16.2477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Masjedizadeh A, Zaeemzadeh N, Mard SA, Vanani GS (2015) Comparing the efficacy of four different protocols for eradicating of Helicobacter pylori infection in Ahvaz, southwest Iran. Prz Gastroenterol 10: 94–99. 10.5114/pg.2015.49001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ramas M, Donday MG, McNicholl AG, Gisbert JP (2017) Efficacy and safety of rifaximin associated with standard triple therapy (omeprazole, clarithromycin and amoxicillin) for H. pylori eradication: A phase IV pilot clinical trial. Gastroenterol Hepatol 40: 658–662. 10.1016/j.gastrohep.2017.05.017 [DOI] [PubMed] [Google Scholar]
- 39.Queiroz DM, Dani R, Silva LD, Santos A, Moreira LS, Rocha GA, et al. (2002) Factors associated with treatment failure of Helicobacter pylori infection in a developing country. J Clin Gastroenterol 35: 315–320. 10.1097/00004836-200210000-00007 [DOI] [PubMed] [Google Scholar]
- 40.Lee HJ, Kim JI, Lee JS, Jun EJ, Oh JH, Cheung DY, et al. (2015) Concomitant therapy achieved the best eradication rate for Helicobacter pylori among various treatment strategies. World J Gastroenterol 21: 351–359. 10.3748/wjg.v21.i1.351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Granstrom L, Backman L (1985) Stomach distension in extremely obese and in normal subjects. Acta Chir Scand 151: 367–370. [PubMed] [Google Scholar]
- 42.Maddox A, Horowitz M, Wishart J, Collins P (1989) Gastric and oesophageal emptying in obesity. Scand J Gastroenterol 24: 593–598. 10.3109/00365528909093095 [DOI] [PubMed] [Google Scholar]
- 43.Fish DN, Gotfried MH, Danziger LH, Rodvold KA (1994) Penetration of clarithromycin into lung tissues from patients undergoing lung resection. Antimicrobial agents and chemotherapy 38: 876–878. 10.1128/aac.38.4.876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Honeybourne D, Kees F, Andrews J, Baldwin D, Wise R (1994) The levels of clarithromycin and its 14-hydroxy metabolite in the lung. European Respiratory Journal 7: 1275–1280. 10.1183/09031936.94.07071275 [DOI] [PubMed] [Google Scholar]
- 45.Abdullahi M, Annibale B, Capoccia D, Tari R, Lahner E, Osborn J, et al. (2008) The eradication of Helicobacter pylori is affected by body mass index (BMI). Obes Surg 18: 1450–1454. 10.1007/s11695-008-9477-z [DOI] [PubMed] [Google Scholar]
- 46.Hamrah MS, Hamrah MH, Ishii H, Suzuki S, Hamrah MH, Hamrah AE, et al. (2018) Association between Helicobacter pylori Infection and Cardiovascular Risk Factors among Patients in the Northern Part of Afghanistan: a Cross-Sectional Study in Andkhoy City. Asian Pac J Cancer Prev 19: 1035–1039. 10.22034/APJCP.2018.19.4.1035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pai MP, Bearden DT (2007) Antimicrobial dosing considerations in obese adult patients. Pharmacotherapy 27: 1081–1091. 10.1592/phco.27.8.1081 [DOI] [PubMed] [Google Scholar]
- 48.Spiller RC (1999) Is there any difference in Helicobacter pylori eradication rates in patients with active peptic ulcer, inactive peptic ulcer and functional dyspepsia? Eur J Gastroenterol Hepatol 11 Suppl 2: S25–28; discussion S43-25. [DOI] [PubMed] [Google Scholar]
- 49.DiMatteo MR (2004) Variations in patients' adherence to medical recommendations: a quantitative review of 50 years of research. Med Care 42: 200–209. 10.1097/01.mlr.0000114908.90348.f9 [DOI] [PubMed] [Google Scholar]
- 50.Sousa LAO, Fonteles MMF, Monteiro MP, Mengue SS, Bertoldi AD, Pizzol T, et al. (2018) Prevalence and characteristics of adverse drug events in Brazil. Cad Saude Publica 34: e00040017 10.1590/0102-311X00040017 [DOI] [PubMed] [Google Scholar]
- 51.A J, SN, Mistry M, A G (2015) Evaluation of knowledge and perception toward adverse drug reactions among patients visiting tertiary-care teaching hospital. Natl J Physiol Pharm Pharmacol 5: 280–284. [Google Scholar]
- 52.KA K (2015) Self‐Reporting of Adverse Drug Reactions in Iraqi Hospitals: Patient’s Perspectives. Pharmacology & Pharmacy 6: 556–572. [Google Scholar]
- 53.Sellers EM, Holloway MR (1978) Drug kinetics and alcohol ingestion. Clin Pharmacokinet 3: 440–452. 10.2165/00003088-197803060-00002 [DOI] [PubMed] [Google Scholar]
- 54.Onder G, Landi F, Della Vedova C, Atkinson H, Pedone C, Cesari M, et al. (2002) Moderate alcohol consumption and adverse drug reactions among older adults. Pharmacoepidemiol Drug Saf 11: 385–392. 10.1002/pds.721 [DOI] [PubMed] [Google Scholar]
- 55.Rademaker M (2001) Do women have more adverse drug reactions? Am J Clin Dermatol 2: 349–351. 10.2165/00128071-200102060-00001 [DOI] [PubMed] [Google Scholar]
- 56.Yu Y, Chen J, Li D, Wang L, Wang W, Liu H (2016) Systematic Analysis of Adverse Event Reports for Sex Differences in Adverse Drug Events. Sci Rep 6: 24955 10.1038/srep24955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fennerty MB, Kovacs TO, Krause R, Haber M, Weissfeld A, Siepman N, et al. (1998) A comparison of 10 and 14 days of lansoprazole triple therapy for eradication of Helicobacter pylori. Arch Intern Med 158: 1651–1656. 10.1001/archinte.158.15.1651 [DOI] [PubMed] [Google Scholar]
- 58.Kim SY, Lee SW, Jung SW, Koo JS, Yim HJ, Park JJ, et al. (2008) Comparative study of Helicobacter pylori eradication rates of twice-versus four-times-daily amoxicillin administered with proton pump inhibitor and clarithromycin: a randomized study. Helicobacter 13: 282–287. 10.1111/j.1523-5378.2008.00615.x [DOI] [PubMed] [Google Scholar]
- 59.Liang CM, Chiu CH, Wang HM, Tai WC, Yao CC, Tsai CE, et al. (2017) First-Line Helicobacter pylori Eradication in Patients with Chronic Kidney Diseases in Taiwan. 2017: 3762194. [DOI] [PMC free article] [PubMed] [Google Scholar]