Abstract
Modified nucleosides in tRNAs are critical for protein translation. N1-methylguanosine-37 and N1-methylinosine-37 in tRNAs, both located at the 3’-adjacent to the anticodon, are formed by Trm5. Here we describe Arabidopsis thaliana AtTRM5 (At3g56120) as a Trm5 ortholog. Attrm5 mutant plants have overall slower growth as observed by slower leaf initiation rate, delayed flowering and reduced primary root length. In Attrm5 mutants, mRNAs of flowering time genes are less abundant and correlated with delayed flowering. We show that AtTRM5 complements the yeast trm5 mutant, and in vitro methylates tRNA guanosine-37 to produce N1-methylguanosine (m1G). We also show in vitro that AtTRM5 methylates tRNA inosine-37 to produce N1-methylinosine (m1I) and in Attrm5 mutant plants, we show a reduction of both N1-methylguanosine and N1-methylinosine. We also show that AtTRM5 is localized to the nucleus in plant cells. Proteomics data showed that photosynthetic protein abundance is affected in Attrm5 mutant plants. Finally, we show tRNA-Ala aminoacylation is not affected in Attrm5 mutants. However the abundance of tRNA-Ala and tRNA-Asp 5’ half cleavage products are deduced. Our findings highlight the bifunctionality of AtTRM5 and the importance of the post-transcriptional tRNA modifications m1G and m1I at tRNA position 37 in general plant growth and development.
Introduction
RNA has over 100 different post-transcriptional modifications that have been identified in organisms across all three domains of life [1–5]. While several RNA modifications have been recently identified on mRNAs in yeast, plants, and animals, tRNAs are still thought to be the most extensively modified cellular RNAs [6–9]. These tRNA modifications are introduced at the post-transcriptional level by specific enzymes. These enzymes recognize polynucleotide substrates and modify individual nucleotide residues at highly specific sites. Some tRNA modifications have been shown to have a clear biological and molecular function [10, 11]. Several tRNA modifications around the anticodon have been demonstrated to have crucial functions in translation, for example, by enhancing decoding [12], influencing the propensity to ribosomal frameshifting or facilitating wobbling [13–15]. Modifications distal to the tRNA anticodon loop can also directly influence the tRNA recognition and/or translation process [16] or can have roles in tRNA folding and stability [1, 17]. However, the precise functions of many tRNA modifications still remain unknown despite often being conserved across species. Often, loss of a tRNA modification does not negatively impair cell growth or cell viability under standard laboratory growth conditions [18]. However, under environmental stress, such mutants display a discernible phenotype [11].
The tRNA anticodon loop position 37 is important to maintain translational fidelity, prevent frameshift errors and translational efficiency [10, 19–21], and almost all tRNAs are modified at this site. There are two prominent modifications at tRNA position 37: N1-methylguanosine (m1G37) and 1-methylinosine (m1I). Trm5 in humans, yeast, and Pyrococcus abyssi has been described as having multifunctionality [22–24]. N1-methylation of guanosine at position 37, m1G37, is performed by TrmD-type enzymes in bacteria, functionally and evolutionarily unrelated Trm5-type proteins in Archaea and Eukaryote, Trm5p in yeast Saccharomyces cerevisiae, and TRMT5 in vivo in humans [21–23, 25–27]. Trm5p complete loss of function mutants in yeast Saccharomyces cerevisiae are lethal whereas mutations in TRMT5 lead to multiple respiratory-chain deficiencies and a reduction in mitochondrial tRNA m1G37 [10, 23, 26]. In humans, TRMT5 (tRNA methyltransferase 5) catalyses the formation of m1G37 in vivo on mitochondrial tRNAPro and tRNALeu [22, 28]. N1-methylguanosine has been described in eukaryotic tRNAs at two positions; at position 37 catalysed by Trm5, and the other at position 9 catalysed by Trm10 [29]. In contrast to bacteria TrmD, which requires a guanosine at position 37, human TRMT5 can also recognise and methylate inosine at position 37 with some limited activity [22]. Similarly,Trm5p has also been shown to catalyse inosine to m1I modification in yeast in a two-step reaction, where the first adenosine-to-inosine modification was mediated by Tad1p [11, 18, 26, 30]. As m1G is an intermediate during the modification of guanosine to wybotusine (yW), tRNAs from trm5 mutants were also devoid of yW [28]. The yeast Trm5p protein has been shown to be localised to the cytoplasm and mitochondria and it is thought that Trm5p protein present in the mitochondria is required to prevent unmodified tRNA affecting translational frameshifting [23, 26]. In the unicellular parasite Trypanosome brucei, Trm5 was located in both the nucleus and mitochondria and reducing Trm5 expression led to reduced mitochondria biogenesis and impaired growth [31]. Interestingly, Trm5 and m1G37 were shown to be essential for mitochondrial protein synthesis but not cytosolic translation [31].
Little is known about tRNA modifying enzymes in plants, especially the plant homolog of this bifunctional methyltransferase. Here, we report the identification and functional analysis of AtTRM5 (At3g56120) from the model plant Arabidopsis thaliana. We demonstrate that Attrm5 mutant plants are slower growing, have reduced shoot and root biomass and display late flowering. Furthermore, we demonstrate that in vitro TRM5 is required for m1G37 and m1I37 methylation at the position 3’ to the anticodon and in vivo tRNAs enriched from Attrm5 plants have reduced m1G and m1I.
Results
Identification of At3g56120 as a TRM5 homolog
In yeast (Saccharomyces cerevisiae), m1G37 nucleoside modification is catalysed by Trm5p/ScTrm5 [26]. We searched for Arabidopsis thaliana homologs by using blastp and HMMER and identified a high confidence candidate, At3g56120, with 49% similarity to ScTrm5 (S2 Fig). Alignment of At3g56120 with yeast, human, Drosophilia, Pyrocococcus, and Methanococcus Trm5 homologs identified three conserved motifs and catalytically required amino acids (R166, D192, E206) present in At3g56120 (S2 Fig). We subsequently will refer to At3g56120 as AtTRM5. In the Arabidopsis genome, AtTrm5 has homology to At4g27340 and At4g04670 and both genes were recently named as TRM5B and TRM5C, respectively (S2 Fig and [32]). We have also identified TRM5 homologs in algae, bryophytes and vascular plants (Fig 1A and S1 Fig). We focussed our experiments on Arabidopsis At3g56120/AtTRM5 as the protein had the highest amino acid similarity to yeast ScTrm5.
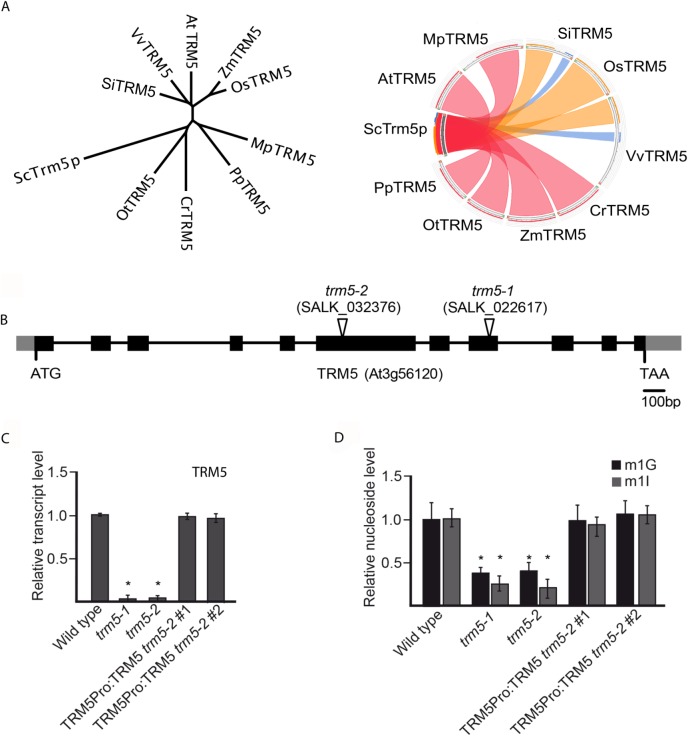
Fig 1. TRM5 is conserved in plants and has dual-functionality in modifying RNA bases.
(A) Unrooted phylogenetic tree and sequence conservation Circos plot of putative TRM5 proteins from yeast (Sc), tomato (Sl), grape (Vv), Arabidopsis (At), maize (Zm), rice (Os), Marchantia (Mp), Physcomitrella (Pp), Chlamydomonas (Cr), and Ostreococcus (Ot). The ribbons were coloured based on sequence identity, with blue < = 25%, green 25–50%, orange 51–75% and red for 76–99%. (B) Exon-intron structure of the putative TRM5 locus (At3g56120) showing the T-DNA insertion sites of the trm5-1 and trm5-2 alleles (as indicated by the open triangles). Black boxes and grey boxes represent coding regions and untranslated regions, respectively. (C) Relative transcript level detected by qPCR in wild type, trm5-1 or trm5-2 seedlings. (D) Relative nucleoside level of modification m1G and m1I detected by HPLC/MS in wild type, trm5-1 or trm5-2 seedlings.
To functionally characterize AtTRM5 we isolated two T-DNA insertions, SALK_022617 and SALK_032376, and identified homozygous mutant plants for each insertion (Fig 1B). SALK_022617 and SALK_032376 were named trm5-1 and trm5-2, respectively. Next, we measured AtTRM5 mRNA abundance in both mutants and detected almost no transcripts in both mutants (Fig 1C). We generated a genomic construct of AtTRM5 that contained the endogenous promoter, coding region, and UTRs, transformed the construct into trm5-2 and demonstrated that the AtTRM5 mRNA levels were similar in two complemented lines when compared to wild type plants. Subsequently, the extracted tRNAs from wild type and the trm5 mutants were purified, digested and modified nucleosides measured by mass spectrometry (Fig 1D). In both trm5 mutant alleles, nucleoside m1G levels were reduced to about 30% of the wild type and m1G levels were restored to wild type levels in both complemented lines (Fig 1D). Nucleoside m1G is present at tRNA positions 9 and 37 [26], therefore the residual m1G levels in trm5 mutants may be the result of tRNA m1G at position 9. In saying this, we cannot exclude an alternative explanation that the residual m1G levels in trm5 plants are the result of activity of either TRM5B or TRM5C.
In yeast, Trm5 has also been reported to also catalyse m1I on tRNAs [11, 26]. We therefore measured m1I levels in purified tRNAs from both Arabidopsis trm5 mutants and wild type control plants. In both trm5-1 and trm5-2 mutant alleles, nucleoside m1I levels were reduced to about 10% of wild type levels and were restored to wild type levels in plants of both complemented lines (Fig 1D). It is possible that the residual m1I in trm5 plants may be m1I at position 57 [4, 33, 34]. In summary, we identified At3g56120 as a TRM5 homolog in Arabidopsis thaliana, identified two AtTRM5 mutant alleles, trm5-1 and trm5-2, and both mutants showed a significant reduction in m1G and m1I.
AtTRM5 is involved in leaf and root development and flowering time regulation
Before undertaking growth measurements, we grew wild-type Columbia, trm5-1, two complemented lines, and two overexpression lines together under long-day conditions, harvested and dried the seeds to minimise any maternal or environmental effects. To observe the early growth stages of seedlings, we grew the six lines (wild type, trm5-1, two complemented lines and two overexpression lines) on plates for 10 days (Fig 2A). The trm5-1 seedlings were noticeably smaller than the wild type. In contrast, no clear differences were evident between wild type, the complemented and overexpression lines. To rule out the possibility that the reduced growth in trm5-1 seedlings was due to slower germination, we measured the germination of trm5-1 and wild type and no difference was observed (S3 Fig). Reduced growth of trm5-1 roots was also evident on plate-grown plants (Fig 2B). Interestingly, trm5-1 primary, lateral and total (primary + lateral) root lengths were reduced in trm5-1 when compared to wild-type plants (S4 Fig). We also measured the lateral root number and found that trm5-1 plants had reduced numbers when compared to the wild type (S4 Fig). In contrast, no differences in the root growth were evident upon comparison of the wild type and the complemented lines. In TRM5 overexpression lines, primary and lateral root lengths were slightly longer than in the wild type (Fig 2B and S4 Fig).
Fig 2. Phenotype analysis of trm5, complemented lines (35S:TRM5 trm5-1) and TRM5 overexpression lines (35S:TRM5).
(A) Seedlings were sown on ½ MS media plates and grown for 7 days and photographed. (B) Seedlings of wild type, trm5, complemented lines (35S:TRM5 trm5-1), TRM5 overexpression lines (35S:TRM5) were vertically grown on ½ MS medium for 10 days and then photographed. (C) Plants were grown on soil under long day photoperiods and photographed 15 days after germination. (D) Wild type, trm5-1, two complementing (35S:TRM5 trm5-1) and two overexpressing lines (35S:TRM5) were grown under long days and representative plants photographed at flowering.
At inflorescence emergence in wild-type plants grown under long days, we observed reduced rosette leaf numbers, smaller leaves and reduced fresh weight in trm5-1 plants (Fig 2C and 2D; S3 Fig). Sectioning of the shoot apical meristems of wild type and trm5-1 plants at wild-type floral transition, confirmed that trm5 plants were later flowering (S3 Fig). We measured the flowering time of wild type, trm5-1, complemented, and overexpression lines under both short and long days and observed that mutants produced more rosette leaves and flowered later than the wild type (Fig 3A; S3 Fig). Plants overexpressing TRM5 flowered slightly earlier than wild type under both long and short-day conditions (Fig 2D; S3 Fig).
Fig 3. More leaves, slower flowering and impacted photosynthetic genes in trm5 mutant of Arabidopsis thaliana.
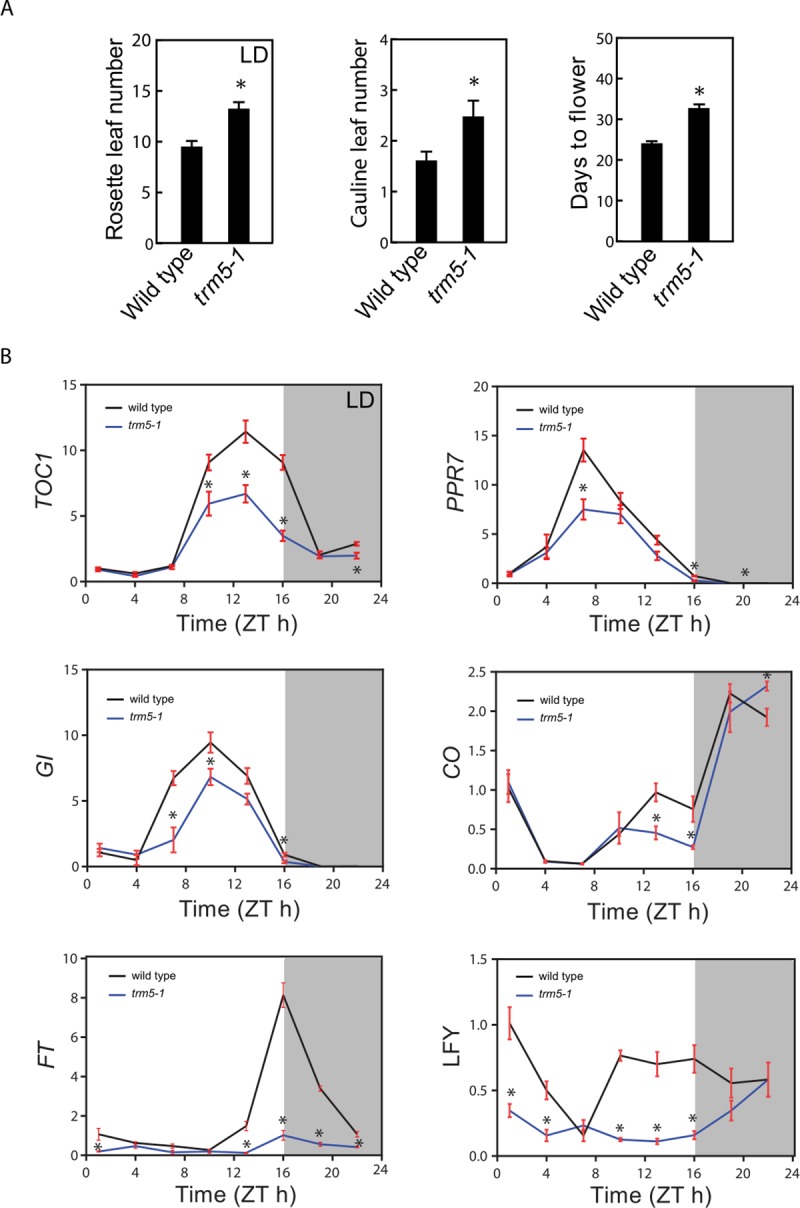
(A) Rosette leaf number (long-day conditions), cauline leaf number and days to flower (short day conditions) of wild type and trm5 mutant. (B) The mRNA abundance of circadian clock-related genes over a 24-hour period. 17-day-old seedlings of wild type, trm5-1, complemented lines (35S:TRM5 trm5-1), TRM5 overexpression lines (35S:TRM5) were grown on ½ MS medium for 10 days and then harvested every 3 hours. The expression levels of TOC1, PRR7, GI, CO, FT, and LFY were measured and normalized relative to EF-1-α. Data presented are means. Error bars are ± SE, n = 3 biological replicates. An asterisk indicates a statistical difference (P<0.05) as determined by Student’s t-test. 0 hours is lights on and 16 hours is light off. Shaded area indicates night, whereas Zeitgeber time is abbreviated as ZT.
AtTRM5 is involved in circadian clock and flowering time gene expression
To explore the molecular basis of delayed flowering in trm5-1, we measured the mRNA abundance of circadian clock and flowering time related genes by quantitative RT-PCR over a 24-hour period (Fig 3). In trm5-1 plants, lower abundance of the clock genes TIMING OF CAB EXPRESSION 1 (TOC1), and PSEUDO RESPONSE REGULATOR 7 (PPR7), and the flowering time regulator genes GIGANTEA (GI), CONSTANS (CO) and FLOWERING LOCUS T (FT) were observed at ZT10 to ZT22. The reduced abundance of the flowering time regulators GI, CO, and FT in trm5-1 mutants correlates with delayed flowering. As expected, the downstream floral meristem identity gene LEAFY (LFY) had lower abundance at almost all tested time points in the trm5 mutant when compared to wild type (Fig 3B). The downregulation of circadian-clock related genes were also detected in the RNA-seq data (S2 Table). Together these results support a role for AtTRM5 in plant growth, development, and flowering time regulation.
AtTRM5 m1G methyltransferase activity
To test AtTRM5 m1G methyltransferase activity in vivo, a Δtrm5 mutant strain in yeast (Saccharomyces cerevisiae, Sc) that is defective for the tRNA m1G37 modification was used for genetic complementation. The mutant not only has defective tRNA m1G37 but also a slow growth phenotype when compared to wild type or a congenic strain (Fig 4A). Full-length ScTrm5 and AtTRM5 were cloned into yeast expression vectors. From the AtTRM5 expression vector, a catalytically inactive mutant Attrm5 R166D was generated by site-directed mutagenesis. After the three vectors had been transformed into the yeast Δtrm5 mutant, cell growth and m1G nucleoside levels were observed (Fig 4). Not only were the slow growth and nucleoside levels rescued when expressing ScTrm5 but they were also rescued when expressing AtTRM5 (Fig 4A and 4B). However, the catalytically inactive Attrm5 R166D did not rescue either the slow growth or m1G nucleoside levels (Fig 4A and 4B).
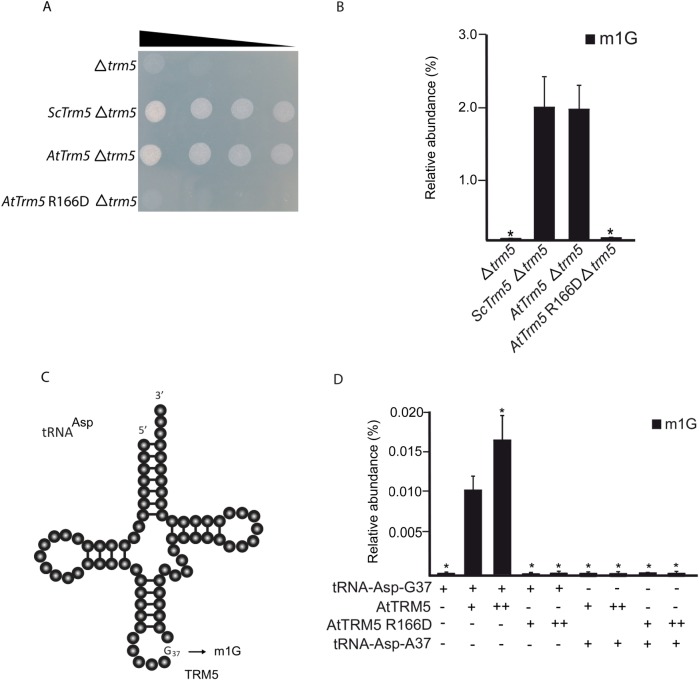
Fig 4. TRM5 modifies tRNA37 guanosine (G) to m1G in yeast.
(A) Serial-dilution growth assay. Complementation experiment of TRM5 from yeast (Sc), Arabidopsis thaliana (At) or a catalytically inactive mutant AtTrm5 R166D in the yeast trm5 mutant. (B) Relative nucleoside level of modification m1G quantified by HPLC/MS of each complemented yeast strains with either ScTRM5 or AtTRM5. (C) Proposed model of TRM5-mediated m1G modification of yeast tRNA-Asp. (D) Relative nucleoside level of modification m1G with varying conditions of tRNA-Asp-G37, AtTRM5, AtTRM5 catalytic mutant, and tRNA-Asp-A37. + indicates presence, ++ indicates two-fold increase,—indicates absence.
To test the m1G methyltransferase activity of AtTRM5 in vitro, we incubated purified recombinant proteins with tRNA substrates and measured the m1G levels. We expressed AtTRM5 as a GST fusion protein and purified the recombinant GST-AtTRM5 protein. We also generated a catalytically inactive GST-AtTRM5 recombinant protein by using site directed mutagenesis, expressed and purified the GST-AtTRM5 recombinant fusion protein. In yeast, ScTrm5 methylates tRNA-His-GUG, tRNA-Leu-UAA, tRNA-Asp-GUC and other tRNA isoacceptors [11, 26, 32]. Yeast tRNA-Asp-GUC RNA transcripts were generated in vitro by using T7 RNA polymerase, the tRNA transcripts incubated with the recombinant fusion proteins in the presence of AdoMet and m1G nucleoside levels were measured (Fig 4D). m1G was detected only when AtTRM5 was provided (Fig 4D) and in a dosage dependent manner. No m1G was detected when the catalytically inactive mutant AtTRM5 was provided (Fig 4D). To test the specificity of the methyltransferase activity on tRNA-Asp guanine at position 37, the guanine nucleotide was mutated to an adenine nucleotide (tRNA-Asp-A37) and the m1G methyltransferase activity was measured. No m1G was detected after incubation with the fusion proteins (Fig 4D). The overall results of the yeast complementation experiments suggest that guanosine methylation occurred at position 37 of tRNA.
AtTRM5 tRNA m1I methyltransferase activity
Previously in plants, TAD1 was demonstrated to oxidatively deaminate adenosine at position 37 of tRNA-Ala-(UGC) to inosine, and subsequently methylated by an unknown enzyme to N1-methylinosine (m1I; Fig 5A). Human TRM5 has been reported to methylate tRNA I37 but with limited activity [22]. Given our observation that Attrm5 mutant plants had reduced m1I (Fig 1D), we asked the question whether AtTRM5 has methyltransferase activity on tRNA I37. We developed a two-step approach, whereby purified AtTAD1 was first incubated with the substrate tRNA-Ala-A37 to produce tRNA-Ala-I37 and then the inosine methyltransferase activity of AtTRM5 was measured by incubating AtTRM5 with the tRNA-Ala-I37 substrate. Previously, in yeast ScTAD1 was demonstrated to deaminate tRNA-Ala-A37 to tRNA-Ala-I37 in vitro [26] and Arabidopsis thaliana tad1 mutants were reported to have reduced tRNA-Ala-I37 [11]. We expressed AtTAD1 as a GST fusion protein and purified the recombinant GST-AtTAD1 protein. We also generated a catalytically inactive GST-AtTAD1 mutant recombinant protein by using site-directed mutagenesis and expressed and purified the GST-AtTAD1 mutant fusion protein. In a two-step assay, yeast tRNA-Ala-UGC RNA transcripts were generated by in vitro transcription using T7 RNA polymerase, the tRNA transcripts were then incubated with the recombinant fusion proteins in the presence of Mg2+ and methyl donor S-adenosyl-methionine (AdoMet), and the m1I nucleoside levels measured (Fig 5B). m1I was detected only when GST-AtTAD1 and GST-AtTRM5 were provided, and its production occurred in a dosage-dependent manner (Fig 5B). No m1I was detected when the catalytically inactive mutants AtTAD1 E76S or AtTRM5 R166D were provided (Fig 5B). To test the specificity of the methyltransferase activity on tRNA-Ala alanine at position 37, the alanine nucleotide was mutated to a cytosine nucleotide (tRNA-Ala-C37) and the m1I methyltransferase activity was measured. No m1I was detected after incubation with the fusion proteins (Fig 5B). Collectively, these findings interestingly suggest that inosine methylation also occurs at position 37, in addition to guanosine methylation.
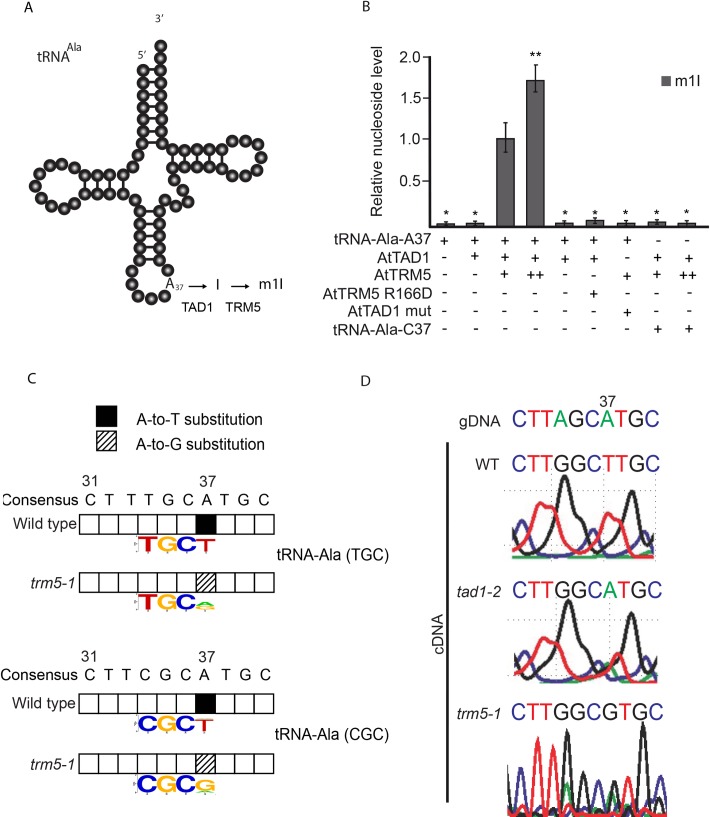
Fig 5. TRM5-mediated m1I modifications in two-step reaction.
(A) Proposed two-step modification model of TRM5-mediated m1G modification of tRNA-Ala. (B) Relative nucleoside level of modification m1I with varying conditions of tRNA-Asp-A37, AtTAD1, AtTRM5 mutant, AtTAD1 mutant, and tRNA-Ala-C37. + indicates presence, ++ indicates two-fold increase,—indicates absence. (C) Qualitative analysis of tRNA-Ala(TGC) and tRNA-Ala(CGC) modifications. tRNAs were enriched, deep sequenced, aligned using segemehl to tRNA references and the modifications present were inferred from observed base substitutions between wild type and trm5-1. Position 37 in the gDNA, labelled as consensus, is an adenine. Sequence logo shows the proportion of nucleotides and hence inferred modifications at positon 37. Anticodons TGC and CGC are shown in the sequence logos for Ala(TGC) and tRNA-Ala(CGC), respectively. Base substitutions observed from 3 biological replicates are shown. (D) Qualitative analysis of tRNA-Ala(AGC) modifications by Sanger sequencing. tRNA-Ala(AGC) was PCR amplified from wild type, tad1-2 and trm5-1 and Sanger sequenced. Position 37 in the gDNA is an adenine. In wild-type cDNA, a thymine was detected, in tad1-2 cDNA an adenine was observed and in trm5-1 a guanine was detected.
To test the AtTRM5 inosine methyltransferase activity in vivo, we measured tRNA position modifications by cDNA sequencing from either mutant or wild type plants (Fig 5C and 5D). In the sequencing assay, modification events at position 37 of tRNAs can be directly detected by sequencing of amplified cDNA obtained by reverse transcription and comparison to the DNA reference sequence as inosine is read as guanine (G) and m1I is read as thymine (T) by the reverse transcriptase [10, 35]. As expected, we detected substitutions of A in the reference to T at position 37 for tRNA-Ala-(TGC) and tRNA-Ala-(CGC) by using Illumina sequencing in wild-type plants which is consistent with the presence of m1I (Fig 5C). m1I modifications have previously been described in tRNA-Ala at position 37 in eukaryotes [11, 30, 33]. In the trm5-1 mutant, no T’s were detected at position 37 (Fig 5C) which is consistent with AtTRM5 acting as a tRNA m1I methyltransferase at position 37. Based on our in vitro assay results, AtTAD1 first deaminates tRNA-Ala-A37 to tRNA-Ala-I37, and then AtTRM5 methylates tRNA-Ala-I37 to tRNA-Ala-m1I37. We confirmed this pathway in tad1 and trm5 mutant plants by Sanger sequencing of tRNA-Ala-(AGC) (Fig 5D). As expected, at position 37, A was substituted to T in the wild type, whereas in trm5 mutants a G and in tad1 mutants an A were observed (Fig 5D). These sequencing results are consistent with AtTAD1 first deaminating A37 to I37 as previously reported by Zhou, Karcher (11), and AtTRM5 then methylating I37 to m1I. We also attempted to detect the putative loss of m1G in the tRNA-sequencing data, as it has been reported that m1G is prone to be called as a T in sequencing [35]. However, this was not observed in our datasets. Together, our in vitro and in vivo data provide support for AtTRM5 possessing tRNA m1I methyltransferase activity.
AtTRM5 is localized to the nucleus
In yeast, ScTRM5 is localized to both the nucleus and mitochondria [23, 36]. Localisation to mitochondria is thought to be important as yeast strains with only nuclear-localized ScTRM5 exhibited a significantly lower rate of oxygen consumption [23]. In order to determine to which subcellular compartment(s) AtTRM5 is localized in Nicotiana benthamiana, we fused TRM5 to the Green Fluorescent Protein (GFP) reporter, transiently infiltrated the construct into leaves and performed laser-scanning confocal microscopy to detect GFP fluorescence. To unambiguously identify the nucleus, we stained the cells with DAPI. When we imaged the cells (n = 100), we observed distinct DAPI fluorescence in a single large circular structure per cell, as expected for the nucleus (Fig 6). Next, we imaged the same cells for GFP fluorescence (Fig 6C) and overlayed the DAPI and GFP fluorescence. The two fluorescence signals showed perfect overlap (Fig 6D). We then searched for nuclear localisation signals (NLS) using the LOCALIZER (http://localizer.csiro.au/), LocSigDB (http://genome.unmc.edu/LocSigDB/), and cNLS mapper (http://nls-mapper.iab.keio.ac.jp/cgi-bin/NLS_Mapper_form.cgi) programs [37–39]. While LOCALIZER and LocSigDB did not predict any canonical or bipartite NLS, cNLS mapper predicted with high confidence a 29 amino acid importin α-dependent NLS, QKGCFVYANDLNPDSVRYLKINAKFNKVD, that starts at amino acid 236. Previous finding in yeast suggested that no common canonical or bipartite NLS was detected in ScTrm5 [26], which explains the outcome from LOCALIZER and LocSigDB. Multiple sequence alignment of ScTrm5 and AtTRM5 showed that only a few amino acids were conserved at the region which the importin α-dependent NLS is detected for AtTRM5 (S2 Fig). In summary, we conclude that, unlike in yeast, AtTRM5 in Arabidopsis is only localized to the nucleus and may be imported from the cytoplasm into the nucleus by the importin α-dependent pathway.
Fig 6. Subcellular localisation of TRM5 translational reporter protein in Nicotiana benthamiana leaves.
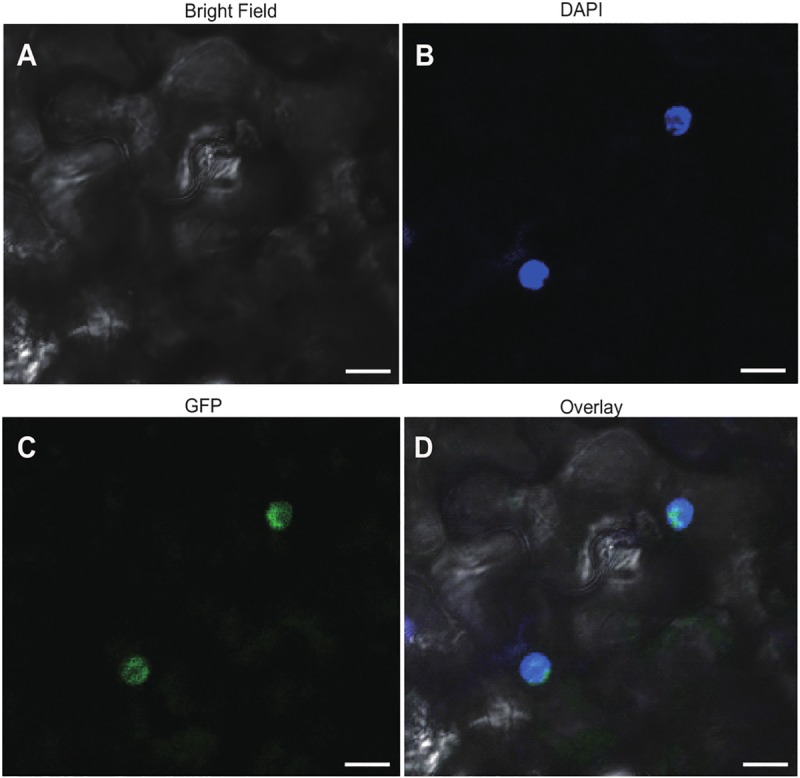
TRM5 was fused to Green Fluorescent Protein (GFP) to yield a TRM5-GFP translational fusion recombinant protein. The construct was transiently expressed in N. benthamiana leaves and subcellular localisation was determined by confocal laser-scanning microscopy. (A) Bright field, (B) DAPI stained, (C) GFP fluorescence, (D) overlay of DAPI and GFP imagines. Scale bars = 20 μm.
Proteins involved in photosynthesis are affected in trm5 mutant plants
Next, we performed a proteomic analysis to identify proteins that differentially accumulate in trm5-1 plants when compared to the wild type by using the Tandem Mass Tag (TMT) method (Fig 7). A total of 61571 peptide-spectrum match (PSM) were recorded, corresponding to 29011 peptides and 23055 unique peptides, respectively. 5242 protein groups were identified by blastp searches against the TAIR_pep database. Proteins with fold changes ≥1.20 or ≤0.83 and a significance level of P ≤0.05 were considered to be differentially expressed. In this way, a total of 263 proteins were identified (S3 Table). 102 proteins were upregulated, and 151 proteins were downregulated in trm5 (Fig 7A). GO annotation of these differentially accumulating proteins revealed enrichment of the GO terms thylakoid, chloroplast, and photosystem I (Fig 7B). KEGG annotation revealed enrichment of proteins involved in photosynthesis, and photosynthetic proteins, with most of these proteins encoded by genes found in the nuclear and chloroplast genome. (Fig 7C). A further inspection on these GO terms with our proteomics data showed that the photosynthesis-related proteins were downregulated in trm5 mutant. In the future, the abundance of these photosynthesis-related proteins could be validation by quantitative western blotting analysis. It is worthwhile to state that the abundance of these photosynthesis-related proteins may be directly or indirectly affected by the loss of TRM5 in our experiment. Taken together, the GO and KEGG analysis demonstrated that most differentially accumulating proteins are involved in processes related to photosynthesis.
Fig 7. Proteomic analysis of wild type and trm5-1.
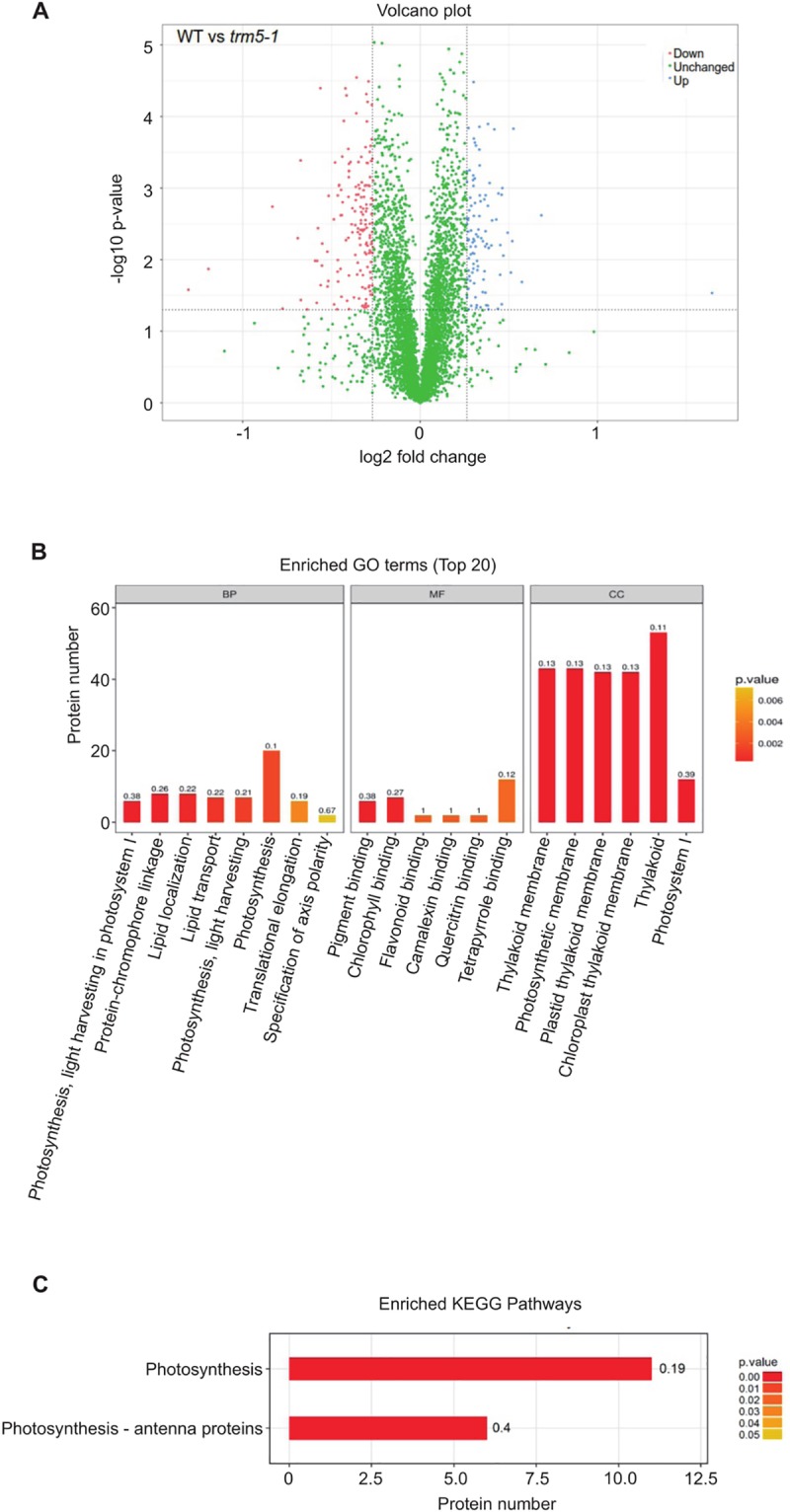
(A) Volcano plot of differentially abundant proteins between wild type and trm5-1. In trm5-1, proteins that were increased in abundance are represented as blue dots, proteins decreased in abundance as red dots, with threshold fold change > 1.2 or < 0.83 (increased or decreased) and P-value < 0.05. There were 102 proteins increased and 161 proteins decreased in trm5-1. (B) GO and (C) KEGG terms enrichment analysis. Each differentially abundant protein was first annotated in the GO or KEGG databases, enrichment analysis was performed based on annotated differentially expressed proteins in wild type and trm5-1. The top 20 enriched GO terms from Biological Processes (BP), Molecular function (MF), and Cellular Component (CC) are reported.
Defects in tRNA m1G methylation can be expected to affect mRNA translation, particularly of proteins that have high numbers of affected codons. Therefore, we were interested in identifying genes that showed reduced expression at the protein level in our proteomics analysis of trm5 plants, but no detectable reduction in mRNA abundance. To identify such mRNAs, we performed RNA-seq on wild type and trm5 plants. We identified 1186 transcripts that were reduced in abundance in trm5 and 580 transcripts that were increased in abundance by at least 2-fold and hierarchically clustered these transcripts (S2 Table and S5 Fig). Comparison of the RNA-seq and proteomics datasets identified 133 proteins with reduced abundance, but with no detectable reduction in mRNA abundance (S5 Fig). We further inspected the data by selecting four candidate proteins with the highest fold change reported in the proteomics data (Table 1). From the selected candidate proteins, we discovered that three of the differentially expressed proteins reported in the proteomics data were not differentially expressed in the RNA-seq data, indicating that there is no change in transcripts levels leading to the fluctuation of their corresponding proteins. Only one protein candidate (AT2G45180.1) showed decreased fold change of mRNA, which correlates with the decrease of its corresponding protein reported in the proteomics data.
Table 1. Comparison between RNA-seq data and proteomics data of selected protein candidates.
| Gene ID | Protein | log2FC | Status | ||
|---|---|---|---|---|---|
| RNA-seq | Proteomics | RNA-seq | Proteomics | ||
| AT1G03540.1 | Pentatricopeptide repeat (PPR-like) superfamily protein | N/A | 3.12815 | non-differentially expressed | upregulated |
| AT2G45180.1 | Bifunctional inhibitor/lipid-transfer protein/seed storage 2S albumin superfamily protein | 2.012 | 0.56136 | downregulated | downregulated |
| AT2G39480.1 | P-glycoprotein 6 | N/A | 0.43734 | non-differentially expressed | downregulated |
| AT5G50160.1 | ferric reduction oxidase 8 | N/A | 0.40435 | non-differentially expressed | downregulated |
Four protein candidates with the highest fold change (p-value < 0.05) reported in the proteomics data were selected for further comparison.
Finally, we performed a codon usage frequency analysis for this class of genes and detected no codon bias towards triplets read by both m1I and m1G modified tRNAs (S5 Fig).
Modifications at position 37 affects tRNA-halve abundance
Since our previous results showed no bias towards codon usage in Attrm5 mutants when compared to wild type, we then asked if depleting m1G37 and m1I37 affects tRNA abundance. To test this, we performed a northern blot analysis on the AtTRM5 substrates tRNA-Ala-(AGC) and tRNA-Asp-(GTC), respectively (Fig 8A). We did not observe any substantial change in the full-length tRNA abundances for both tRNA-Ala-(AGC) and tRNA-Asp-(GTC). However, we unexpectedly observed significant decreases in the 5’ half abundance of both tRNA-Ala-(AGC) and tRNA-Asp-(GTC) (Fig 8A), suggesting that the loss of m1G37 and m1I37 affects tRNA halve steady state abundance.
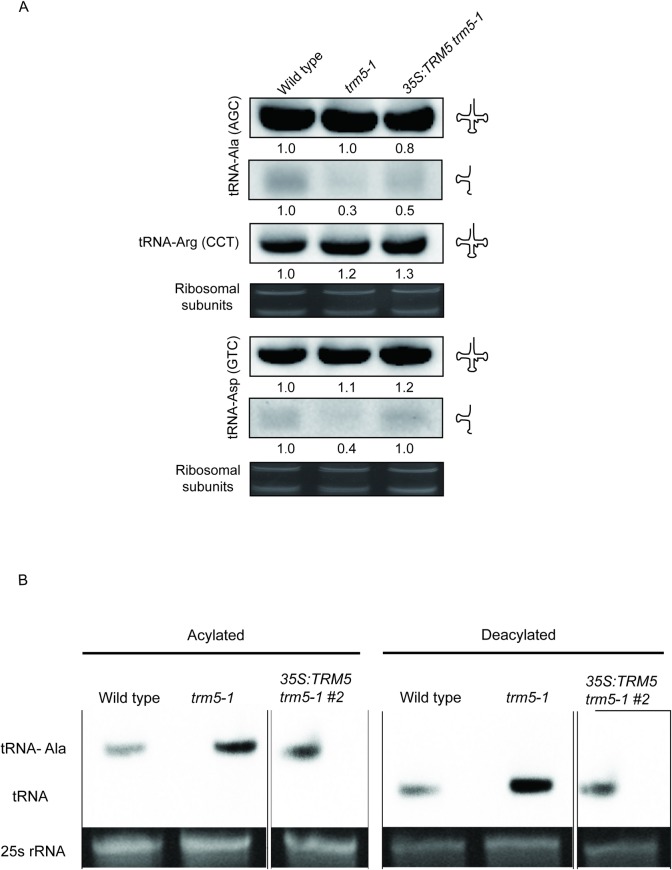
Fig 8. Loss of function of AtTRM5 affects tRNA halve abundance.
(A) Northern blot analysis of RNA from wild type, trm5-1 and 35S:TRM5 trm5-1. tRNA-Ala (AGC) was inspected for changes in m1G37 and tRNA-Asp (GTC) and m1I37. tRNA-Arg (CCT) and rRNA were used as loading controls. The blots were probed with either tRNA-Arg (CCT), tRNA-Asp (GTC), or tRNA-Ala (AGC). (B) Accumulation and aminoacylation of tRNA-Ala (AGC) in wild-type plants, trm5-1 mutant plants and 35S:TRM5 trm5-1. The aminoacylated tRNA migrates slower than its corresponding deacylated species. To visualize the difference in electrophoretic mobility, aliquots of the same samples were deacylated in vitro. 10 μg of RNA was separated by electrophoresis, blotted and hybridized to a tRNA-Ala (AGC)-specific probe. The 25S rRNA band of the ethidium bromide-stained gel prior to blotting is shown as a loading control.
The anticodon region of tRNAs are known to impact tRNA aminoacylation as it interacts with tRNA aminoacyl synthetase [40] and that tRNA position 37 at the 3’ anticodon region has a significant role in facilitating tRNA structure fidelity and anticodon interactions [20]. Therefore, we investigated if the tRNA position 37 which is proximal to the 3’ anticodon could affect tRNA aminoacylation for tRNA-Ala-(AGC). We performed a tRNA aminoacylation experiment to determine if the loss of m1G37 modification at the anticodon region of tRNA-Ala-(AGC) affects tRNA charging (Fig 8B) and our results show that the loss of function of AtTRM5 does not affect acylation of tRNA-Ala (Fig 8B). One explanation for this observation can be attributed to the nature of alanyl-tRNA synthetases that recognizes G3:U70 of tRNA acceptor stem for tRNA charging instead of the anticodon region [41]. This showed that the loss of m1G37 modification does not inhibit aminoacylation.
Discussion
The discovery of m1G and m1I at position 37 in tRNAs of a wide range of eukaryotic and prokaryotic organisms underscores its importance as a key regulator of tRNA function and, presumably, translation [10, 21, 23]. Previous studies in bacteria have shown that m1G37 is required for translational fidelity [21, 42, 43] and that mutations in the enzymes catalysing m1G37 severely impact growth or cause lethal phenotypes [10, 23, 31]. The presence of m1G37 modification on tRNA-Asp prevents erroneous amino-acylation by arginyl-tRNA synthetase as both tRNA-Asp and tRNA-Arg have highly similar structures [44].
In eukaryotes, m1G37 modification requires the methyltransferase TRM5. Here we report that in plants, trm5 mutants have a 60% reduction in m1G and m1I levels, display severely reduced growth and delayed transition to flowering. This is somewhat similar to human patients that are heterozygous for one mutant and one functional allele of Trm5. They show childhood failure to thrive and exercise intolerance symptoms [10].
With the 60% reduction of m1G and 75% reduction of m1I in trm5 plants, it is reasonable to anticipate a significant impact on protein translation due to the reduced stacking effect of m1G37 on base-pairing between position 36 and the first nucleoside in translated mRNA codons. We observed a reduction in abundance of proteins involved in ribosome biogenesis and photosynthesis, and this is likely to account for the observed reduced growth of the mutant plants. A similar study by Jin et al. (2019) showed higher levels of polysomes in attrm5a mutants compared to wild type and decreased levels of ribosomal subunit, which is anticipated to reduce translation rate [45]. In the future, it would be interesting to test if translational errors, such as ribosome stalling or frame shifting, occur in trm5 plants which would be similar to what has been previously reported in bacteria with reduced tRNA m1G [26].
We localized Arabidopsis TRM5 to the nucleus which is in contrast to the cytoplasmic and mitochondrial localisation in other eukaryotes: Trypanosoma brucei, Homo sapiens (HeLa cells) and Saccharomyces cerevisiae [10, 23, 31], although in one study S. cerevisiae Trm5 has also been localized exclusively to the nucleus [36]. Interestingly, in yeast, Trm5 acts in the nucleus to form m1G on retrograde imported tRNAPhe after initial export from the nucleus and subsequent splicing at the mitochondrial outer membrane. As retrograde tRNA import is conserved from yeast to vertebrates [46, 47], it is tempting to speculate that TRM5-mediated m1G formation occurs in retrograde imported tRNAs in Arabidopsis.
Direct comparison of RNA-seq and proteomics data has been challenging as variation in protein abundance in proteomics datasets can be confounded by multiple factors. Several factors can cause fluctuation in protein levels with no change of mRNA abundance: mRNA transcript abundance, translation rate, and translation resources availability (as reviewed in Liu, Beyer (48)). This may explain the results of the initial comparison between our RNA-seq and proteomics data, and the seeming discrepancies in the derived sets of differentially expressed genes (S2 and S3 Tables). In our RNA-seq data, microtubule-related genes appeared to be significantly downregulated (AT1G21810, AT1G52410, AT2G28620, AT2G44190, AT3G23670, AT3G60840, AT3G63670, AT5G67270, AT4G14150, AT5G27000). However, we could not detect any differential expression of these proteins in our proteomics dataset. This is not unexpected, as it has been reported that protein and transcript abundances only rarely correlate in data analysis [48]. However, it has been shown that transcript abundance can still be used to infer protein abundance [49]. Among the selected four candidate proteins (Table 1), we could observe only one event of protein downregulation due to decreased transcript level: the lipid transfer protein (AT2G45180) which is involved in lipid transport in chloroplasts. Lipid transport is crucial in the formation of photosynthetic membranes in plants [50]. As several lipids are important functional components of thylakoidal protein complexes involved in photosynthesis, the impacted lipid transport and lipid synthesis revealed by the GO term analysis may contribute to the observed dysregulation of photosynthesis-related genes. This finding is consistent with Jin et al. (2019) [45]. The remaining three candidate genes displayed no changes in transcript levels, but one candidate gene showed upregulation and the other two downregulation at the protein level. The exact function of pentatricopeptide repeat (PPR-like) superfamily protein (AT1G03540.1) is unknown, but the protein was suggested to play a significant role in post-transcriptional modification of tRNAs [51]. The P-glycoprotein 6 (AT2G39480) belongs to the large P-glycoprotein family and is known to play a role in mediating auxin transport, and disturbed auxin transport is known to affect plant growth [52]. Our proteomics data suggested that the auxin transport is affected (AT5G35735, AT3G07390, AT4G12980, AT5G35735) in the trm5 mutant. This can be inferred to the reduced auxin levels in attrm5a as reported by Jin et al. (2019) [45]. Ferric reduction oxidase 8 (AT5G50160/AtFRO8) has been shown to participate in iron reduction and was implicated in leaf vein transport [53]. We proposed that disturbed photosynthesis in trm5 mutant plants is a secondary effect of dysregulated transport processes.
Our findings also show that modified bases at tRNA-Ala at position 37 does not affect tRNA aminoacylation but does affect tRNA-Ala-derived 5’ half steady state abundance. Both tRNA aminoacylation and tRNA-derived halves were shown to effect translational fidelity [40, 54]. In yeast, the introduction of tRNA-derived halves, both 5’ and 3’ fragments, affects tRNA aminoacylation, suggesting that tRNA-derived fragments (tRFs) has a regulatory role in translation [54]. It was also demonstrated that these tRFs regulate tRNA-aminoacylation via interacting with ribosome. The decreased amount of tRNA-Ala 5’ halves may explain the polysome profile changes reported by Jin et al. (2019) [45].
The role of post-transcriptional RNA modifications in tRNA and mRNA metabolism and their impact on plant growth and development in plants are only beginning to be elucidated. Here, we have described the AtTRM5-mediated m1G and m1I methylation in tRNAs and identified crucial links between this modification, photosynthesis, plant growth, and protein translation. It appears likely that the many other tRNA modifications in plant tRNAs also play important roles in translation and/or translational regulation which remain to be discovered.
Materials and methods
Plant material and root growth experiments
Arabidopsis thaliana (Columbia accession) wild type and mutant plants were grown in Phoenix Biosystems growth under metal halide lights as previously described [55]. For plate experiments, seeds were first surface sterilized, plated on ½ MS medium supplemented with 1% sucrose and sealed as previously described [1, 56]. All plants were grown under either long-day photoperiod conditions of 16 h light and 8 h darkness or short-day photoperiods of 10 h light and 14 h darkness.
Characterization of the mutant alleles, trm5-1 (SALK_022617) and trm5-2 (SALK_032376) are as described previously [57]. The tad1-2 mutant was used as described previously [11]. Nucleotide sequence data for the following genes are available from The Arabidopsis Information Resource (TAIR) database under the following accession numbers: TRM5 (At3g56120), At4g27340, At4g04670, and TAD1 (At1g01760).
Analysis of root phenotypes was carried out on 11-day-old seedlings grown on ½ MS agar plates. A flatbed scanner (Epson) was used to non-destructively acquire images of seedling roots grown on the agar surface. Once captured, the images were analysed by software package RootNav [58, 59].
Plasmid construction and generation of transgenic plants
For the 35SCaMV:TRM5 construct, the full-length genomic region of At3g56120 including the 5’UTR and 3’UTR was amplified from Col-0 genomic DNA template with primers provided in (S1 Table) and cloned into Gateway entry vector pENTRTM/SD/D-TOPO (Invitrogen). The insert was sequenced and then cloned into the binary destination vector pGWB5 by an LR recombination reaction, using the Gateway cloning system following the manufacturers protocol (Invitrogen), resulting in the 35S:TRM5 construct. For the TRM5Pro::TRM5 construct, the full-length genomic region of At3g56120 that included the promoter, 5’UTR and 3’UTR was amplified from Col-0 genomic DNA template with primers provided in (S1 Table) and cloned into Gateway entry vector PCR8 TOPO-TA (Invitrogen). The insert was sequenced and then cloned into the a modified (35S promoter removed) destination pMDC32 vector, using the Gateway cloning system [60] following the manufacturers protocol (Invitrogen), resulting in TRM5Pro::TRM5. The 35S:TRM5 construct was transformed into A. thaliana wild type Col-0 plants or trm5-1 mutant plants by Agrobacterium-mediated floral dip method respectively [61]. The TRM5Pro::TRM5 construct was transformed into trm5-2 mutant plants by Agrobacterium-mediated floral dip method. Transgenic plants were selected on ½ MS media supplemented with 50 μg ml-1 kanamycin. TRM5 transcript abundance was assessed in at least five independent T1 plants using qRT-PCR and two lines showing the highest TRM5 transcript levels were carried through to homozygous T3 generation for phenotypic analysis.
Sub-cellular localization of TRM5
For analysis of subcellular localization of TRM5, a 35S:TRM5:GFP construct was produced by PCR amplification of the TRM5 coding sequence from Arabidopsis seedling cDNA by using the primers described in the supplementary data (S1 Table). The TRM5 cDNA was recombined cloned into pCR8, sequenced and then recombined in frame into pMDC83 to produce 35S:TRM5:GFP. The 35S:TRM5:GFP construct was introduced into A. tumefaciens strain GV3101 and transiently expressed in 5-week-old Nicotiana benthamiana leaves. Fluorescence was analysed using a confocal laser-scanning microscope (Zeiss microscope, LSM700) and excited with 488-nm line of an argon ion laser. GFP fluorescence was detected via a 505- to 530-nm band-pass filter. The cut leaves were immersed in 10 μM 4ˊ, 6-diamidino-2-phenylindole (DAPI) at room temperature for 45 min, and then washed with PBS for 3 times (5 min each). The blue fluorescence of DAPI was imaged using 404-nm line for excitation and a 435- to 485-nm band pass filter for emission.
Shoot apical meristem sections
14, 18, or 22-day-old seedlings were fixed for 1 day in FAA containing 50% ethanol, 5% acetic acid and 3.7% formaldehyde. The samples were then dehydrated through an ethanol series of five one-hour steps (50, 60, 70, 85, 95% ethanol) ending in absolute ethanol (100%). The ethanol was gradually replaced with Histoclear containing safranine to stain the tissue, as following: incubated in a Histoclear series (75:25, 50:50, 25:75 Ethanol: Histoclear) for 30 min and followed by twice one-hour incubation in 100% Histoclear. The paraffin was polymerised by baking overnight at 60°C, and the samples were embedded in paraffin. Sections were cut, attached to slides and dried on a slide warmer overnight at 42°C to allow complete fixation. The shoot apical meristem was observed using light microscopy.
Quantitative real-time PCR (qPCR)
For the transcription profiling of flowering-related genes and circadian clock-related genes, 17-day-old seedlings were sampled from Zeitgeber time (ZT) 1 and collected every 3h during the day and night cycles, respectively. Total RNA was extracted the leaf samples using Trizol reagent (Invitrogen). The relative expression levels of AtTRM5 were determined using quantitative real-time PCR (qPCR) with gene-specific primers (S1 Table). The qPCR was performed using the StepOnePlus real-time PCR system (Applied Biosystems) using Absolute SYBR Green ROX mix (Applied Biosystems) for quantification. Three biological replicates were carried out for each sample set. The relative expression was corrected using a reference gene EF1alpha (At5g60390) and calculated using the 2–ΔΔCq method as described previously [11].
mRNA-sequencing
Total RNA, 1 ug, was extracted from 20-day-old Arabidopsis leaf samples using Trizol reagent (Invitrogen) and purified using the RNAeasy Mini RNA kit (Qiagen). One hundred nanograms of RNA were used for RNA-seq library construction according the manufacturer’s recommendations (Illumina). First-strand cDNA was synthesized using SuperScript II Reverse Transcriptase (Invitrogen). After second strand cDNA synthesis and adaptor ligation, cDNA fragments were enriched, purified and then sequenced on the Illumina Hiseq X Ten. Three biological replicates were used for RNA-seq experiments.
tRNA purification and tRNA-sequencing
Total RNA was isolated from wild type and trm5 10-day-old Arabidopsis seedlings using the Spectrum Plant total RNA kit (SIGMA-ALDRICH) and contaminating DNA removed using DNase I (SIGMA-ALDRICH). To enrich for tRNAs, 10μg of total RNA was separated on a 10% polyacrylamide gel, the region containing 65–85 nts was removed and RNA was purified as previously described [56]. Purified tRNAs were used for library construction using NEB Ultradirectional RNA library kit. Given the short sequences of tRNAs, the fragmentation step of the library preparation was omitted, and samples were quickly processed for first-strand cDNA synthesis after the addition of the fragmentation buffer. The remaining steps of library construction were performed as per the manufacturer’s instructions. Illumina sequencing was performed on a MiSeq platform at The Australian Cancer Research Foundation (ACRF) Cancer Genomics Facility, Adelaide.
Yeast complementation
AtTrm5 (At3g56120) and ScTrm5 (YHR070W) was PCR amplified from cDNA and cloned into pYE19 using a Gibson assembly reaction (NEB). Mutant AtTrm5 (R166D) was generated by synthesising gene blocks (IDT) with nucleotides that mutated the translated proteins at R166 and the gene block was cloned into pYE19 using a Gibson assembly reaction (NEB). Yeast △trm5 (Mat a, hisD1, leu2D1, met15D0, trm5:KanMX) was previously described [62]. Recombinant plasmids were transformed into △trm5 mutant strain and the resulting strain was analysed for growth phenotypes and m1G nucleoside levels.
AtTrm5, AtTAD1, ScTrm5 protein expression and purification and tRNA methylation
Full length AtTrm5, mutant AtTrm5 (R166D) and AtTAD1 (At1g01760) cDNAs were cloned into pGEX resulting in GST-AtTrm5, GST-AtTRM5-mutant, GST-TAD1, respectively. Mutant AtTAD1 (E76S) was generated by synthesising a gene block (IDT) with mutated nucleotides and the gene block was cloned into pGEX using a Gibson assembly reaction (NEB) resulting in GST-TAD1 mutant. IPTG (0.5 μM) was used to induce expression of the proteins and the recombinant proteins were purified on a GST resin column (ThermoFisher Scientific). tRNA-Asp-GUC or tRNA-Ala-AGC were transcribed in vitro with T7 RNA polymerase (Promega). Methylation reactions were performed in 100 mM Tris-HCl, 5 mM MgCl2, 100 mM KCl, 2 mM DTT, 50 mM EDTA, 0.03 mg/mL BSA and 25 μM AdoMet. Substrate tRNA was provided in a final concentration of 1–5 μM, AtTrm5 or AtTrm5 mutant proteins from 6.0 to 12 μM and AtTAD1 or AtTAD1 mutant proteins at 5.0 μM.
mRNA-seq and tRNA-seq bioinformatics analysis
Global Mapping: Reads were first adapter trimmed using Trim galore v0.4.2 (http://www.bioinformatics.babraham.ac.uk/projects/trim_galore/) with default parameter. The quality of the trimmed reads was checked using Fastqc (https://www.bioinformatics.babraham.ac.uk/projects/fastqc/) and ngsReports (https://github.com/UofABioinformaticsHub/ngsReports). Reads were then globally mapped to Arabidopsis reference genomes (TAIR10) [63] using STAR v2.5.3 [64]. Mapped reads were counted using featureCounts [65]. The raw read counts were normalized using sample size factor for sequence depth and differential expression analysis between wild type and trm5-1 mutant samples was performed using DESeq2 v1.18.1 [66]. The tRNA-enriched reads were mapped to tDNA reference acquired from GtRNAdb (generated from tRNA-Scan SE v2.0) of Arabidopsis thaliana derived from TAIR10 reference genome [63, 67–69] using segemehl v0.2.0 [68]. The mapped reads were then processed for variant calling using GATK v3.7 [70]. Haplotype Caller in GATK v3.7 was implied to call for variants, with the variant filtered using hard filtering as recommended.
Proportion estimation: SNPs were identified in the wild type and trm5-1 samples were then compared. SNPs at position 37 were extracted and analysed using vcftools [71]. Changes in base pair modifications were indicated by base substitution due to the property of next generation sequencing as mentioned in [10, 35]. The ratio of the expected A-to-T conversion in wild type samples and both the ratio of A-to-T (indicating no change in comparison to wild type) and A-to-G in trm5-1 were analyzed as an indication of m1I depletion.
Sanger sequence analysis of tRNA editing
tRNA purification and tRNA editing analysis were performed as described previously [11]. Cytosolic tRNA-Ala (AGC) were amplified by reverse transcriptase (RT)-PCR with specific primers (tRNA-Ala-f and tRNA-Ala-r, S1 Table), and purified PCR products were directly Sanger sequenced.
TMT-based proteome determination and data analysis
Total protein was extracted from 20-day-old Arabidopsis leaf samples and purified according to a method described by [72]. Protein digestion was performed according to FASP procedure described previously [73], and 100 ųg peptide mixture of each sample was labelled using TMT reagent according to the manufacturer’s instructions (Thermo Fisher Scientific). LC-MS/MS analysis was performed on a Q Exactive mass spectrometer (Thermo Fisher Scientific) that was coupled to Easy nLC (Thermo Fisher Scientific). MS data was acquired using a data-dependent top20 method dynamically choosing the most abundant precursor ions from the survey scan (200–1800 m/z) for HCD fragmentation (Shanghai Applied Protein Technology Co., Ltd). Determination of the target value is based on predictive Automatic Gain Control (pAGC). SEQUEST HT search engine configured with Proteome Discoverer 1.4 workflow (Thermo Fisher Scientific) was used for mass spectrometer data analyses. The latest Arabidopsis protein databases (2018) was downloaded from http://www.arabidopsis.org/download/index-auto.jsp?dir=/download_files/Proteins and configured with SEQUEST HT for searching the datasets. The following screening criteria was deemed as differentially expression: fold-changes >1.2 or <0.83 (up- or down-regulation) and the Benjamini-Hochberg corrected p-value, P < 0.05 were considered significant.
tRNA nucleoside analysis
tRNAs were purified as previously described [56]. Twenty-five μg of tRNAs were digested with P1 nuclease (Sigma- Aldrich) and 1.75 units of calf intestine alkaline phosphatase in 20 mM Hepes–KOH (pH 7.0) at 37°C for 3 h. The mixture was diluted to a concentration of 15 ng/uL and samples were injected into an API 4000 Q-Trap (ThermoFisher Scientific) mass spectrometer coupled with LC-20A HPLC system (Shimadzu). All RNA samples analyzed were from three biological replicates. A diode array UV detector monitored the LC signals from the nucleosides, whereas in counts were recorded in positive mode (190−400 nm). The abundance of each modified nucleoside was represented by its unique ion peak area and normalized to the sum of the four canonical nucleosides (A, C, G and U nucleosides). Since m1G, m2G and m7G have the same Q1 and Q3 mass, they were discriminated by retention time, in the order of m7G, m1G and m2G, respectively. The identity of m7G peak was confirmed with external standard (TriLink Biotechnologies) and the identity of m1G versus m2G was indirectly confirmed by results from yeast trm5 mutant
Northern blot for tRNA detection
Total RNA was isolated from the wild type, trm5-1 and 35S:TRM5 trm5-1 Arabidopsis seedlings by using the TRIZOL Reagent (ThermoFisher Scientific). 20μg of total RNA was on 15% urea-polyacrylamide gel and RNA was transferred to Amersham Hybond-N+membrane. After cross-linking, prehybridization was performed for 3 hours at 68°C in 20μl of DIG easy Hyb Granules (Roche). 60 pmol of 3-end Digoxin labelled oligonucleotides was added to hybridization buffer (S1 Table) and hybridize for overnight at 40°C with gentle agitation. The membrane was washed at low-stringency (2X SSC, 0.1% SDS) twice and high-stringency (0.1X SSC, 0.1% SDS) twice. The probe-target hybrid was localised with the anti-DIG-alkaline phosphatase antibody (Roche), and the membrane was put into washing and blocking and detection buffer to visualize using the CDP-Star, ready to use (Roche).
tRNA aminoacylation assay
Total RNA of wild type, trm5-1 and 35S:TRM5 trm5-1 Arabidopsis seedlings were isolated and analysed using the tRNA aminoacylation protocol as described in Zhou, Karcher (11), using a specific probe for tRNA-Ala (AGC) (S1 Table).
Supporting information
(A) Circos plot of sequence conservation of TRM5 orthologues in yeast (Sc), tomato (Sl), grape (Vv), Arabidopsis (At), maize (Zm), rice (Os), Marchantia (Mp), Physcomitrella (Pp), Chlamydomonas (Cr),Ostreococcus (Ot), humans (HsTrm5), Drosophila melanogaster (DmTrm5), Pyrococcus horikoshii (PhTYW2), and Methanococcus jannaschii (MjTYW2). The ribbons were coloured based on sequence identity, with blue < = 25%, green 25–50%, orange 51–75% and red for 76–99%. (B) Unrooted phylogenetic tree of the same TRM5 orthologues used to for sequence conservation analysis.
(TIF)
(A) Multiple sequence alignment of TRM5 proteins from Arabidopsis thaliana (At), yeast (ScTrm5), humans (HsTrm5), Drosophila melanogaster (DmTrm5), Pyrococcus horikoshii (PhTYW2), and Methanococcus jannaschii (MjTYW2). Black shaded boxes are identical across all species. Light shaded boxes are similar and nearly conserved residues. Asterisk indicates catalytic important amino acids. The predicted 29 aa importin α-dependent NLS is boxed in red. (B) Multiple sequence alignment of yeast Trm5p, Arabidopsis TRM5 (At3g56120) and the two closest related proteins from Arabidopsis. Black shaded boxes are conserved in at least 2 sequences. Met 10+ like domain and S-adenosyl Methyltransferase Motif (SAM) are detected in Arabidopsis TRM5 using NCBI Conserved Domains Search (https://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi). The position of importin α-dependent NLS is also indicated.
(TIF)
(A) Seeds (n = 100) of wild type and trm5-1 were sown on ½ MS plates, stratified at 4 oC and then grown at 21 oC under long day conditions for 32 hours. Germination was measured at 8, 16, 24 and 32 hours after shifting to 21 oC. (B) Sections of the shoot apical meristems of wild type and trm5-1 plants grown under long days for 14, 18 and 22 days. (C) The average fresh plant weight of long day grown plants. (D) The average rosette leaf number at flowering; (E) The average days to flowering under long days. (F) The rosette leaf number under short days. Data presented are means. Error bars are ± SE (n = 16). NF = did not flower. An asterisk indicates a statistical difference (P<0.05) as determined by Student’s t-test.
(TIF)
Seedlings of wild type, trm5, complemented lines (35S:TRM5 trm5-1), TRM5 overexpression lines (35S:TRM5) were vertically grown on ½ MS medium for 10 days and then measured. (A) Total root length, (B) Primary root, (PR) length, (C) average lateral root (LR) length and (D) LR number were measured 10 days after germination. Data presented are means. Error bars are ± SE (n = 10 plants).
(TIF)
(A) RNA was purified from 10-day-old seedlings of wild type (wt) and trm5-1 (n = 3). RNA-seq analysis was performed and differentially abundant transcripts were hierarchically clustered. (B) An upset plot showing positive overlapping mRNAs and proteins identified by RNA-seq and proteomics analysis. (C) Codon bias analysis of codons in the up and down regulated proteins identified by proteomics analysis.
(TIF)
(DOCX)
(XLSX)
(XLSX)
Acknowledgments
We thank the staff at The Australian Cancer Research Foundation (ACRF) Cancer Genomics Facility, Adelaide for their technical expertise in sequencing. We would also like to thank the staff members of the University of Adelaide Bioinformatics Hub for bioinformatics consultation.
Data Availability
The data used in this paper is submitted into NCBI with the GEO accession number GSE114898. Proteomics raw data has been deposited on iProX, with under accession number IPX0001222000 and is available at https://www.iprox.org//page/project.html?id=IPX0001222000.
Funding Statement
The research was partially supported by ARC grant FT130100525, partially funded an Australia-China Science and Research Fund grant ACSRF48187 awarded to I.S. and an APA awarded to PQ.N and J.L. This research was also partially supported by the National Natural Science Foundation (31570234) awarded to W.Z., W.Z. was supported by the Innovation Program of Chinese Academy of Agricultural Sciences and the Elite Youth Program of the Chinese Academy of Agricultural Science.
References
- 1.Burgess A, David R, Searle IR. Deciphering the epitranscriptome: A green perspective. Journal of integrative plant biology. 2016;58(10):822–35. 10.1111/jipb.12483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Edelheit S, Schwartz S, Mumbach MR, Wurtzel O, Sorek R. Transcriptome-wide mapping of 5-methylcytidine RNA modifications in bacteria, archaea, and yeast reveals m5C within archaeal mRNAs. PLoS Genetics. 2013;9(6):e1003602 10.1371/journal.pgen.1003602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jackman JE, Alfonzo JD. Transfer RNA modifications: nature's combinatorial chemistry playground. Wiley interdisciplinary reviews RNA. 2013;4(1):35–48. 10.1002/wrna.1144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Machnicka MA, Milanowska K, Osman Oglou O, Purta E, Kurkowska M, Olchowik A, et al. MODOMICS: a database of RNA modification pathways—2013 update. Nucleic Acids Research. 2013;41(Database issue):D262–7. 10.1093/nar/gks1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Motorin Y, Lyko F, Helm M. 5-methylcytosine in RNA: detection, enzymatic formation and biological functions. Nucleic Acids Research. 2010;38(5):1415–30. 10.1093/nar/gkp1117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dominissini D, Moshitch-Moshkovitz S, Schwartz S, Salmon-Divon M, Ungar L, Osenberg S, et al. Topology of the human and mouse m6A RNA methylomes revealed by m6A-seq. Nature. 2012;485(7397):201–6. 10.1038/nature11112 [DOI] [PubMed] [Google Scholar]
- 7.Dominissini D, Nachtergaele S, Moshitch-Moshkovitz S, Peer E, Kol N, Ben-Haim MS, et al. The dynamic N(1)-methyladenosine methylome in eukaryotic messenger RNA. Nature. 2016;530(7591):441–6. 10.1038/nature16998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meyer KD, Saletore Y, Zumbo P, Elemento O, Mason CE, Jaffrey SR. Comprehensive analysis of mRNA methylation reveals enrichment in 3' UTRs and near stop codons. Cell. 2012;149(7):1635–46. 10.1016/j.cell.2012.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schwartz S, Agarwala SD, Mumbach MR, Jovanovic M, Mertins P, Shishkin A, et al. High-resolution mapping reveals a conserved, widespread, dynamic mRNA methylation program in yeast meiosis. Cell. 2013;155(6):1409–21. 10.1016/j.cell.2013.10.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Powell CA, Kopajtich R, D'Souza AR, Rorbach J, Kremer LS, Husain RA, et al. TRMT5 Mutations Cause a Defect in Post-transcriptional Modification of Mitochondrial tRNA Associated with Multiple Respiratory-Chain Deficiencies. American Journal of Human Genetics. 2015;97(2):319–28. 10.1016/j.ajhg.2015.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou W, Karcher D, Bock R. Importance of adenosine-to-inosine editing adjacent to the anticodon in an Arabidopsis alanine tRNA under environmental stress. Nucleic Acids Research. 2013;41(5):3362–72. 10.1093/nar/gkt013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alfonzo JD, Blanc V, Estévez AM, Rubio MA, Simpson L. C to U editing of the anticodon of imported mitochondrial tRNA(Trp) allows decoding of the UGA stop codon in Leishmania tarentolae. The EMBO Journal. 1999;18(24):7056–62. 10.1093/emboj/18.24.7056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Agris PF, Vendeix FAP, Graham WD. tRNA's wobble decoding of the genome: 40 years of modification. Journal of Molecular Biology. 2007;366(1):1–13. 10.1016/j.jmb.2006.11.046 [DOI] [PubMed] [Google Scholar]
- 14.Allnér O, Nilsson L. Nucleotide modifications and tRNA anticodon-mRNA codon interactions on the ribosome. RNA (New York). 2011;17(12):2177–88. 10.1261/rna.029231.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nasvall SJ, Chen P, Bjork GR. The modified wobble nucleoside uridine-5-oxyacetic acid in tRNAPro(cmo5UGG) promotes reading of all four proline codons in vivo. RNA (New York). 2004;10(10):1662–73. 10.1261/rna.7106404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aström SU, Byström AS. Rit1, a tRNA backbone-modifying enzyme that mediates initiator and elongator tRNA discrimination. Cell. 1994;79(3):535–46. 10.1016/0092-8674(94)90262-3 [DOI] [PubMed] [Google Scholar]
- 17.Phizicky EM, Alfonzo JD. Do all modifications benefit all tRNAs? FEBS Letters. 2010;584(2):265–71. 10.1016/j.febslet.2009.11.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gerber A, Grosjean H, Melcher T, Keller W. Tad1p, a yeast tRNA-specific adenosine deaminase, is related to the mammalian pre-mRNA editing enzymes ADAR1 and ADAR2. The EMBO Journal. 1998;17(16):4780–9. 10.1093/emboj/17.16.4780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gamper HB, Masuda I, Frenkel-Morgenstern M, Hou Y-M. Maintenance of protein synthesis reading frame by EF-P and m(1)G37-tRNA. Nature Communications. 2015;6:7226 10.1038/ncomms8226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Helm M, Alfonzo JD. Posttranscriptional RNA Modifications: playing metabolic games in a cell's chemical Legoland. Chemistry & Biology. 2014;21(2):174–85. 10.1016/j.chembiol.2013.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Björk GR, Wikström PM, Byström AS. Prevention of translational frameshifting by the modified nucleoside 1-methylguanosine. Science. 1989;244(4907):986–9. 10.1126/science.2471265 [DOI] [PubMed] [Google Scholar]
- 22.Brulé H, Elliott M, Redlak M, Zehner ZE, Holmes WM. Isolation and characterization of the human tRNA-(N1G37) methyltransferase (TRM5) and comparison to the Escherichia coli TrmD protein. Biochemistry. 2004;43(28):9243–55. 10.1021/bi049671q [DOI] [PubMed] [Google Scholar]
- 23.Lee C, Kramer G, Graham DE, Appling DR. Yeast mitochondrial initiator tRNA is methylated at guanosine 37 by the Trm5-encoded tRNA (guanine-N1-)-methyltransferase. The Journal of Biological Chemistry. 2007;282(38):27744–53. 10.1074/jbc.M704572200 [DOI] [PubMed] [Google Scholar]
- 24.Wang C, Jia Q, Zeng J, Chen R, Xie W. Structural insight into the methyltransfer mechanism of the bifunctional Trm5. Science advances. 2017;3(12):e1700195 10.1126/sciadv.1700195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Christian T, Hou Y-M. Distinct determinants of tRNA recognition by the TrmD and Trm5 methyl transferases. Journal of Molecular Biology. 2007;373(3):623–32. 10.1016/j.jmb.2007.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Björk GR, Jacobsson K, Nilsson K, Johansson MJ, Byström AS, Persson OP. A primordial tRNA modification required for the evolution of life? The EMBO Journal. 2001;20(1–2):231–9. 10.1093/emboj/20.1.231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hori H. Transfer RNA methyltransferases with a SpoU-TrmD (SPOUT) fold and their modified nucleosides in tRNA. Biomolecules. 2017;7(1). Epub 2017/03/08. 10.3390/biom7010023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Noma A, Kirino Y, Ikeuchi Y, Suzuki T. Biosynthesis of wybutosine, a hyper-modified nucleoside in eukaryotic phenylalanine tRNA. The EMBO Journal. 2006;25(10):2142–54. 10.1038/sj.emboj.7601105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jackman JE, Montange RK, Malik HS, Phizicky EM. Identification of the yeast gene encoding the tRNA m1G methyltransferase responsible for modification at position 9. RNA (New York). 2003;9(5):574–85. 10.1261/rna.5070303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grosjean H, Auxilien S, Constantinesco F, Simon C, Corda Y, Becker HF, et al. Enzymatic conversion of adenosine to inosine and to N1-methylinosine in transfer RNAs: a review. Biochimie. 1996;78(6):488–501. 10.1016/0300-9084(96)84755-9 [DOI] [PubMed] [Google Scholar]
- 31.Paris Z, Horáková E, Rubio MAT, Sample P, Fleming IMC, Armocida S, et al. The T. brucei TRM5 methyltransferase plays an essential role in mitochondrial protein synthesis and function. RNA (New York). 2013;19(5):649–58. 10.1261/rna.036665.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Y, Pang C, Li X, Hu Z, Lv Z, Zheng B, et al. Identification of tRNA nucleoside modification genes critical for stress response and development in rice and Arabidopsis. BMC Plant Biology. 2017;17(1):261 10.1186/s12870-017-1206-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Torres AG, Piñeyro D, Filonava L, Stracker TH, Batlle E, Ribas de Pouplana L. A-to-I editing on tRNAs: biochemical, biological and evolutionary implications. FEBS Letters. 2014;588(23):4279–86. 10.1016/j.febslet.2014.09.025 [DOI] [PubMed] [Google Scholar]
- 34.Juhling F, Morl M, Hartmann RK, Sprinzl M, Stadler PF, Putz J. tRNAdb 2009: compilation of tRNA sequences and tRNA genes. Nucleic Acids Res. 2009;37(Database issue):D159–62. Epub 2008/10/30. 10.1093/nar/gkn772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ryvkin P, Leung YY, Silverman IM, Childress M, Valladares O, Dragomir I, et al. HAMR: high-throughput annotation of modified ribonucleotides. Rna. 2013;19(12):1684–92. 10.1261/rna.036806.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ohira T, Suzuki T. Retrograde nuclear import of tRNA precursors is required for modified base biogenesis in yeast. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(26):10502–7. 10.1073/pnas.1105645108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kosugi S, Hasebe M, Tomita M, Yanagawa H. Systematic identification of cell cycle-dependent yeast nucleocytoplasmic shuttling proteins by prediction of composite motifs. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(25):10171–6. 10.1073/pnas.0900604106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Negi S, Pandey S, Srinivasan SM, Mohammed A, Guda C. LocSigDB: a database of protein localization signals. Database: the Journal of Biological Databases and Curation. 2015;2015(0). 10.1093/database/bav003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sperschneider J, Catanzariti A-M, DeBoer K, Petre B, Gardiner DM, Singh KB, et al. LOCALIZER: subcellular localization prediction of both plant and effector proteins in the plant cell. Scientific reports. 2017;7:44598 10.1038/srep44598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Giegé R, Sissler M, Florentz C. Universal rules and idiosyncratic features in tRNA identity. Nucleic Acids Res. 1998;26(22):5017–35. 10.1093/nar/26.22.5017 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chong YE, Guo M, Yang XL, Kuhle B, Naganuma M, Sekine SI, et al. Distinct ways of G:U recognition by conserved tRNA binding motifs. Proc Natl Acad Sci U S A. 2018;115(29):7527–32. Epub 2018/07/04. 10.1073/pnas.1807109115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hagervall TG, Tuohy TM, Atkins JF, Björk GR. Deficiency of 1-methylguanosine in tRNA from Salmonella typhimurium induces frameshifting by quadruplet translocation. Journal of Molecular Biology. 1993;232(3):756–65. 10.1006/jmbi.1993.1429 [DOI] [PubMed] [Google Scholar]
- 43.Li JN, Björk GR. 1-Methylguanosine deficiency of tRNA influences cognate codon interaction and metabolism in Salmonella typhimurium. Journal of Bacteriology. 1995;177(22):6593–600. 10.1128/jb.177.22.6593-6600.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Veronique Perret AG, Henri Grosjean, Jean.Pierre Ebel, Catherine Florentz & Richard Giege. Relaxation of a transfer RNA specificity by removal of modified nucleotides. Nature. 1990;344:787–9. 10.1038/344787a0 [DOI] [PubMed] [Google Scholar]
- 45.Jin X, Lv Z, Gao J, Zhang R, Zheng T, Yin P, et al. AtTrm5a catalyses 1-methylguanosine and 1-methylinosine formation on tRNAs and is important for vegetative and reproductive growth in Arabidopsis thaliana. Nucleic acids research. 2019;47(2):883–98. 10.1093/nar/gky1205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shaheen HH, Horetsky RL, Kimball SR, Murthi A, Jefferson LS, Hopper AK. Retrograde nuclear accumulation of cytoplasmic tRNA in rat hepatoma cells in response to amino acid deprivation. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(21):8845–50. 10.1073/pnas.0700765104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Takano A, Endo T, Yoshihisa T. tRNA actively shuttles between the nucleus and cytosol in yeast. Science. 2005;309(5731):140–2. 10.1126/science.1113346 [DOI] [PubMed] [Google Scholar]
- 48.Liu Y, Beyer A, Aebersold R. On the Dependency of Cellular Protein Levels on mRNA Abundance. Cell. 2016;165(3):535–50. 10.1016/j.cell.2016.03.014 [DOI] [PubMed] [Google Scholar]
- 49.Li JJ, Bickel PJ, Biggin MD. System wide analyses have underestimated protein abundances and the importance of transcription in mammals. PeerJ. 2014;2:e270 10.7717/peerj.270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.LaBrant E, Barnes AC, Roston RL. Lipid transport required to make lipids of photosynthetic membranes. Photosynthesis research. 2018:1–16. [DOI] [PubMed] [Google Scholar]
- 51.Manna S. An overview of pentatricopeptide repeat proteins and their applications. Biochimie. 2015;113:93–9. 10.1016/j.biochi.2015.04.004 [DOI] [PubMed] [Google Scholar]
- 52.Geisler M, Murphy AS. The ABC of auxin transport: the role of p-glycoproteins in plant development. FEBS Letters. 2006;580(4):1094–102. 10.1016/j.febslet.2005.11.054 [DOI] [PubMed] [Google Scholar]
- 53.Wu H, Li L, Du J, Yuan Y, Cheng X, Ling H-Q. Molecular and biochemical characterization of the Fe(III) chelate reductase gene family in Arabidopsis thaliana. Plant & Cell Physiology. 2005;46(9):1505–14. 10.1093/pcp/pci163 [DOI] [PubMed] [Google Scholar]
- 54.Mleczko AM, Celichowski P, Bąkowska-Żywicka K. Transfer RNA-derived fragments target and regulate ribosome-associated aminoacyl-transfer RNA synthetases. Biochimica et Biophysica Acta (BBA)—Gene Regulatory Mechanisms. 2018;1861(7):647–56. doi: 10.1016/j.bbagrm.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 55.David R, Burgess A, Parker B, Li J, Pulsford K, Sibbritt T, et al. Transcriptome-Wide Mapping of RNA 5-Methylcytosine in Arabidopsis mRNAs and Noncoding RNAs. The Plant Cell. 2017;29(3):445–60. 10.1105/tpc.16.00751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Burgess AL, David R, Searle IR. Conservation of tRNA and rRNA 5-methylcytosine in the kingdom Plantae. BMC Plant Biology. 2015;15:199 10.1186/s12870-015-0580-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Alonso JM, Stepanova AN, Leisse TJ, Kim CJ, Chen H, Shinn P, et al. Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science. 2003;301(5633):653–7. 10.1126/science.1086391 [DOI] [PubMed] [Google Scholar]
- 58.Guo Q, Love J, Roche J, Song J, Turnbull MH, Jameson PE. A RootNav analysis of morphological changes in Brassica napus L. roots in response to different nitrogen forms. Plant growth regulation. 2017;83(1):83–92. 10.1007/s10725-017-0285-0 [DOI] [Google Scholar]
- 59.Pound MP, French AP, Atkinson JA, Wells DM, Bennett MJ, Pridmore T. RootNav: navigating images of complex root architectures. Plant Physiology. 2013;162(4):1802–14. 10.1104/pp.113.221531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Curtis MD, Grossniklaus U. A gateway cloning vector set for high-throughput functional analysis of genes in planta. Plant Physiology. 2003;133(2):462–9. 10.1104/pp.103.027979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Davis AM, Hall A, Millar AJ, Darrah C, Davis SJ. Protocol: Streamlined sub-protocols for floral-dip transformation and selection of transformants in Arabidopsis thaliana. Plant Methods. 2009;5:3 10.1186/1746-4811-5-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Urbonavicius J, Stahl G, Durand JMB, Ben Salem SN, Qian Q, Farabaugh PJ, et al. Transfer RNA modifications that alter +1 frameshifting in general fail to affect -1 frameshifting. RNA (New York). 2003;9(6):760–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lamesch P, Berardini TZ, Li D, Swarbreck D, Wilks C, Sasidharan R, et al. The Arabidopsis Information Resource (TAIR): improved gene annotation and new tools. Nucleic Acids Research. 2012;40(Database issue):D1202–10. 10.1093/nar/gkr1090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dobin A, Gingeras TR. Mapping RNA‐seq reads with STAR. Current protocols in bioinformatics. 2015;51(1):11.4. 1–.4.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liao Y, Smyth GK, Shi W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics. 2014;30(7):923–30. 10.1093/bioinformatics/btt656 [DOI] [PubMed] [Google Scholar]
- 66.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biology. 2014;15(12):550 10.1186/s13059-014-0550-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chan PP, Lowe TM. GtRNAdb 2.0: an expanded database of transfer RNA genes identified in complete and draft genomes. Nucleic Acids Research. 2016;44(D1):D184–9. 10.1093/nar/gkv1309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hoffmann S, Otto C, Kurtz S, Sharma CM, Khaitovich P, Vogel J, et al. Fast mapping of short sequences with mismatches, insertions and deletions using index structures. PLoS Computational Biology. 2009;5(9):e1000502 10.1371/journal.pcbi.1000502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lowe TM, Chan PP. tRNAscan-SE On-line: integrating search and context for analysis of transfer RNA genes. Nucleic Acids Research. 2016;44(W1):W54–7. 10.1093/nar/gkw413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.DePristo MA, Banks E, Poplin R, Garimella KV, Maguire JR, Hartl C, et al. A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nature Genetics. 2011;43(5):491–8. 10.1038/ng.806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Danecek P, Auton A, Abecasis G, Albers CA, Banks E, DePristo MA, et al. The variant call format and VCFtools. Bioinformatics. 2011;27(15):2156–8. 10.1093/bioinformatics/btr330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lan P, Li W, Wen T-N, Shiau J-Y, Wu Y-C, Lin W, et al. iTRAQ protein profile analysis of Arabidopsis roots reveals new aspects critical for iron homeostasis. Plant Physiology. 2011;155(2):821–34. 10.1104/pp.110.169508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wiśniewski JR, Zougman A, Nagaraj N, Mann M. Universal sample preparation method for proteome analysis. Nature Methods. 2009;6(5):359–62. 10.1038/nmeth.1322 [DOI] [PubMed] [Google Scholar]