INTRODUCTION
Inflammatory myofibroblastic tumor (IMT) is a rare, indolent spindle cell tumor that typically affects children and young adults.1,2 IMTs occur predominantly in visceral soft tissue and are categorized as an intermediate malignancy because they have a potential for local recurrence but rarely progress to distant metastases.2,3 Approximately 50% of IMTs harbor ALK rearrangements, although gene fusions involving ROS1, NTRK3, and PDGFRβ have also been identified.2,4-6 Previously, the presence of ALK gene fusions was typically identified using immunohistochemistry7; however, newer techniques such as hybridization capture-based next-generation sequencing (NGS) and NGS-based anchored multiplex polymerase chain reaction (PCR) testing can now be used to detect gene fusions.8-10 Identifying patients with actionable gene fusions is important, given the availability of promising therapeutic strategies.2,4
Surgical resection is the standard of care for patients with a solitary IMT,11 but patients with unresectable or advanced disease have limited treatment options. The current National Comprehensive Cancer Network guidelines for soft tissue sarcoma recommend the use of ALK inhibitors for patients with ALK gene fusions12; however, there are no targeted agents approved specifically for use in fusion-positive IMTs. Entrectinib is an investigational, CNS-active, potent, and selective inhibitor of TRK, ROS1, and ALK.8,13,14 In phase I trials, entrectinib demonstrated clinical efficacy in patients with different tumor types harboring NTRK, ROS1, or ALK fusions, regardless of the fusion partner gene.8 A majority of the responses to entrectinib occurred in a rapid and durable manner, and some patients remained on study because they received clinical benefit, even after experiencing disease progression.8
Here, we present two patients with fusion-positive IMTs (DCTN1-ALK and TFG-ROS1) who were enrolled in the ongoing STARTRK-NG phase I/IB trial (NCT02650401: Study of RXDX-101 in Children With Recurrent or Refractory Solid Tumors and Primary CNS Tumors, With or Without TRK, ROS1, or ALK Fusions) and treated with entrectinib. These patients were identified as having targetable gene fusions using two diagnostic testing methods, a hybrid capture-based targeted exome sequencing assay and anchored multiplex PCR,15,16 at different stages of a diagnostic testing workflow.
CASE REPORTS
Patient 1 is a 16-year-old girl who presented with a lump on the right side of her face that gradually progressed over a 3-month period. On imaging, a soft tissue mass was noted arising from the parotid gland. After an inconclusive needle biopsy, she underwent superficial parotidectomy resulting in gross tumor resection; however, the margins were positive. Pathology revealed an IMT, and fluorescence in situ hybridization testing suggested ALK rearrangement. The tumor grew rapidly to presurgical measurements within 3 months. A complete resection was not feasible because facial nerve branches were involved. Further molecular testing using a hybrid capture-based targeted exome sequencing assay15 revealed a DCTN1-ALK fusion (Fig 1A), which determined eligibility to enroll in the STARTRK-NG trial. The study was approved by the local institutional review board and is being conducted in accordance with the Declaration of Helsinki and Good Clinical Practice.
Fig 1.
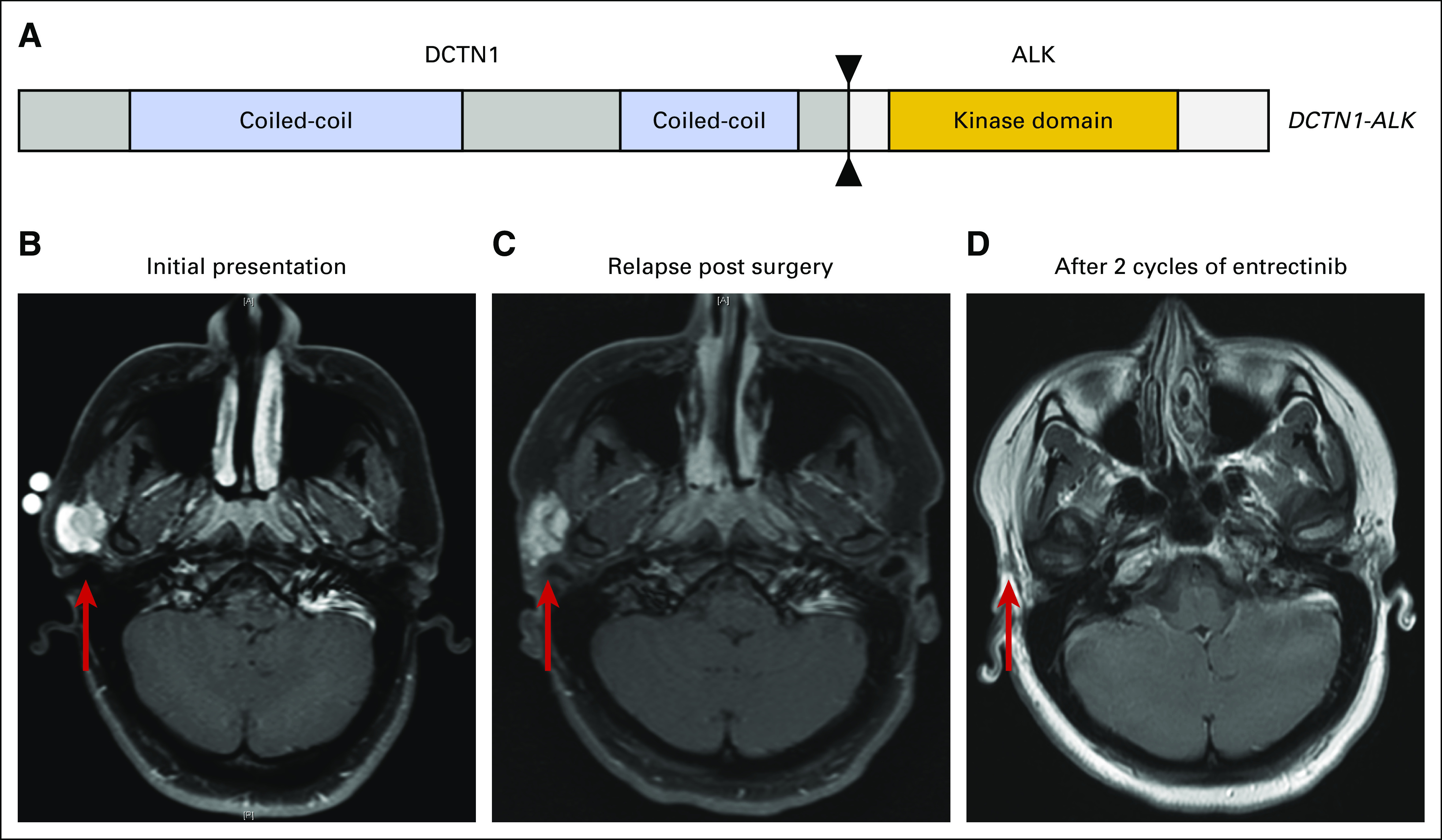
(A) The DCTN1-ALK rearrangement identified in patient 1 is a deletion that results in the fusion of DCTN1 exons 1-26 with ALK exons 20-29. The fusion is predicted to be in frame and includes the kinase domain of ALK. (B) Axial T1 fat saturated (T1FS) postcontrast image of patient 1 showing the extent of the inflammatory myofibroblastic tumor arising in the right parotid before the initial surgery (initial presentation measured 2.1 × 2.1 cm; red arrow). (C) Axial T1FS postcontrast image shows regrowth of tumor within 3 months of superficial parotidectomy (relapse postsurgery measurement: 2.9 × 2.2 cm; red arrow). (D) Axial T1 postcontrast image shows complete response of the tumor to entrectinib treatment (no measurable tumor present, red arrow).
The patient provided informed consent, and entrectinib was started in May 2017 at a dose of 550 mg/m2 taken orally once per day (1 cycle was 28 days). The tumor became less conspicuous by the end of cycle 1 and was not palpable by mid cycle 2. Follow-up scans at the end of cycle 2 revealed complete response with no evidence of parotid mass (Fig 1B-1D). During entrectinib treatment, the patient experienced altered taste during cycle 1 that resolved completely without any dose modification. She also had weight gain (9%) that peaked at cycle 3 and remained stable with dietary modifications and physical activity. At the time of this report, she continues daily entrectinib and has no limitations in academic or athletic activities.
Patient 2 is a 10-year-old girl who presented with daily high-grade fevers, cough, weight loss, and changes in physical activity over a 2-month period. A rheumatology consultation and computed tomography scan of the chest revealed a large mass. The patient was referred for surgical consultation, a biopsy confirmed a diagnosis of IMT, and the patient was advised that pneumonectomy would be required for complete resection. Hybrid capture-based targeted exome sequencing and anchored multiplex PCR as described above15,16 identified a TFG-ROS1 gene fusion (Fig 2A), and she was subsequently enrolled in the STARTRK-NG trial.
Fig 2.
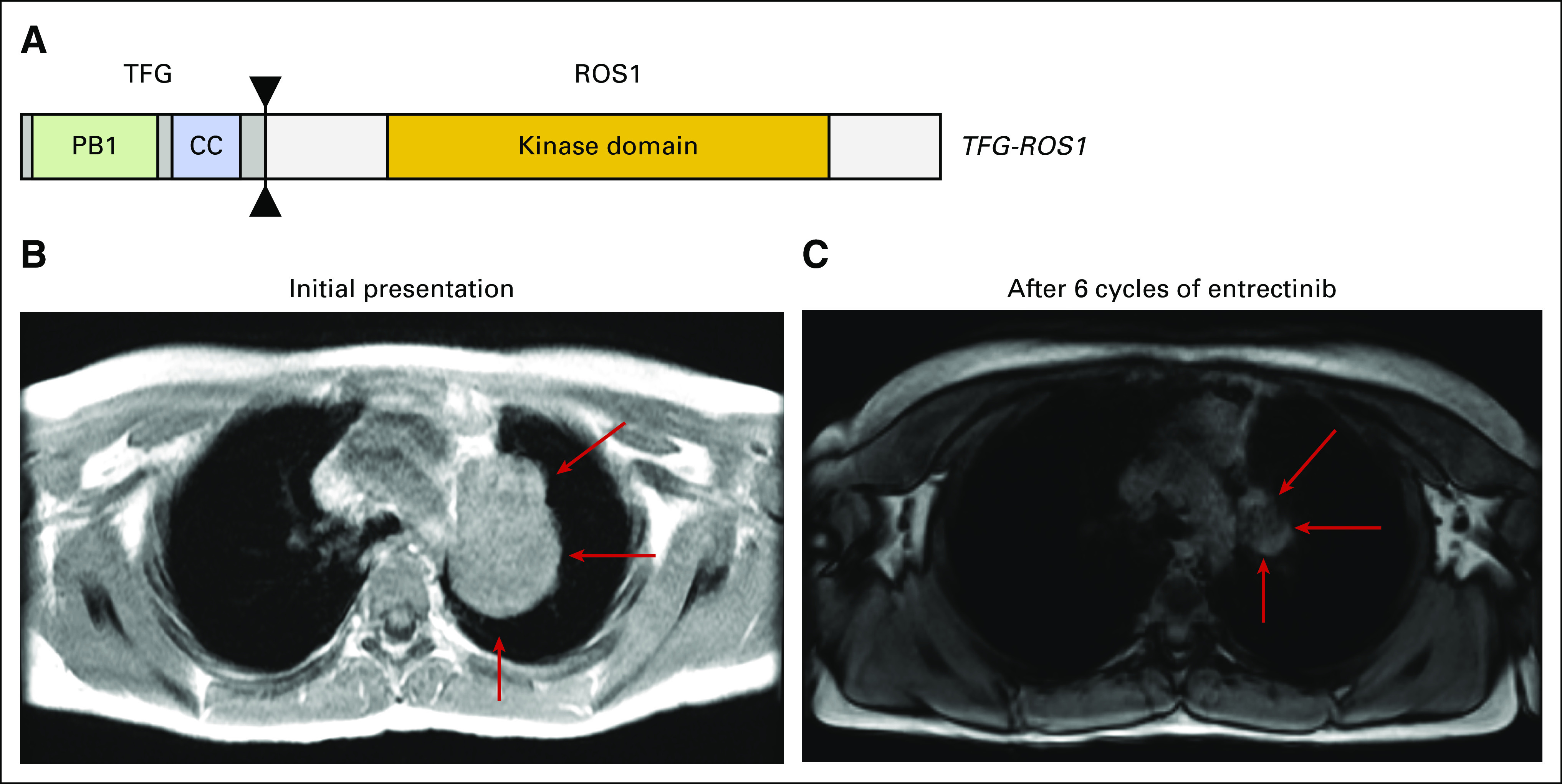
(A) TFG-ROS1 rearrangement, resulting in an in-frame fusion between TFG exon 4 and ROS1 exon 35. Contrast-enhancing magnetic resonance imaging scan of the chest of patient 2 taken from axial 3D DualEcho sequences (B) at baseline (measurements: 5.8 × 4.2 cm; red arrow) and (C) after six cycles of treatment with entrectinib, demonstrating significant reduction in pulmonary mass (measurements: 3.1 × 1.8 cm; red arrow). CC, coiled-coil.
The patient provided informed consent and entrectinib was started in May 2017 at a dose of 550 mg/m2 orally once per day. She experienced a > 50% response (confirmed partial response) as determined by Response Evaluation Criteria in Solid Tumors v1.1 (RECIST v1.1), had resolution of fever and cough, and experienced normalization of inflammatory markers that has been sustained through 13 treatment cycles. In addition, by month 4 of treatment, the residual mass was primarily nonenhancing on contrast-enhancing magnetic resonance imaging, suggesting relative lack of activity (Fig 2B-2C). A positron emission tomography scan obtained at the completion of cycle 13 revealed no hypermetabolic uptake in the residual mass. While on treatment, she experienced constipation, mild bloating, nausea, and weight gain. She also experienced nighttime urinary incontinence that was potentially related to treatment and improved with simple changes in her nighttime routine. Just before this report was submitted for publication, this patient electively discontinued entrectinib in favor of surveillance imaging only and will be closely monitored via serial magnetic resonance imaging scans.
DISCUSSION
We have presented two cases of pediatric patients with IMTs harboring ALK or ROS1 gene fusions. Both patients exhibited clinically significant responses to the investigational agent entrectinib, which was well tolerated, and the observed adverse effects were consistent with the reported safety profile of entrectinib.8
These cases are in agreement with previous reports demonstrating the clinical efficacy and tolerability of entrectinib in patients with solid tumors harboring NTRK, ROS1, or ALK gene fusions, regardless of fusion partner.8,17-20 Entrectinib seems to be well tolerated, as demonstrated in 203 patients treated at the recommended phase II dose of 600 mg once per day.21 The most common treatment-related adverse events (TRAEs) of any grade were dysgeusia (38%), fatigue (29%), constipation (23%), and dizziness (23%). The majority of TRAEs were grade ≤ 2, and all TRAEs were reversible upon dose modification.21
This report also adds to recent data on entrectinib treatment in pediatric cancer. In preclinical studies, entrectinib was a potent inhibitor of TRK-driven neuroblastoma,22 and it has demonstrated clinical activity in a 20-month-old patient with infantile fibrosarcoma harboring an NTRK fusion.23 The benefit observed in the two patients reported here along with published data on the benefit of entrectinib in TRK fusion-positive infantile fibrosarcoma highlight the potential of entrectinib in pediatric tumors harboring TRK, ROS1, or ALK fusions. The STARTRK-NG trial is currently enrolling pediatric patients with cancer, including primary CNS tumors, neuroblastoma, and other non-neuroblastoma extracranial solid tumors harboring gene fusions in NTRK, ROS1, or ALK, or harboring ALK molecular alterations.23
These cases add to the growing body of evidence that demonstrates the diversity of gene fusion drivers in the formation of IMT and underscore the importance of screening patients with IMTs for NTRK, ROS1, and ALK gene fusions by using the best available diagnostic techniques. The gene fusions present in these patients were identified by using a workflow that sequentially uses two different diagnostic platforms: a hybrid capture-based targeted exome sequencing assay (MSK-IMPACT) and anchored multiplex PCR (Archer FusionPlex). MSK-IMPACT is an NGS assay for targeted deep sequencing of more than 450 key cancer genes.15,24,25 The Archer FusionPlex system is also based on NGS, but it uses RNA as its substrate along with anchored multiplex PCR, which allows for the identification of gene fusion partners without prior knowledge of the partner.16,26 Although the specific attributes of these assays warrant a separate discussion, the most important clinical implication is that these patients were identified with actionable gene fusions and therefore were able to receive targeted investigational treatment.
These cases also highlight the importance of treating patients with IMTs who harbor TRK, ROS1, or ALK fusions with drugs, such as entrectinib, that bind and inhibit the kinase domains of these fusion proteins. IMT is often characterized by the presence of ALK rearrangements; however, additional gene fusions have also been identified in this rare cancer, including ROS1, NTRK3, and PDGFRβ,2,4,5 including a patient with ETV6-NTRK3 fusion-positive IMT who is being considered for treatment with entrectinib.27 In patients with ALK fusion-positive IMT, there have been varying efficacy results reported when patients are treated with ALK inhibitors, including crizotinib, ceritinib, and alectinib.28-32 In addition, a recent report noted that a patient with IMT harboring a TFG-ROS1 fusion exhibited a poor response to crizotinib.6 There remains an unmet need in this patient population for safe and effective therapies that can treat patients across a wide range of gene fusion drivers. These results demonstrate the potential benefit of entrectinib in that it inhibits select targeted kinases with high potency, allowing for the treatment of multiple distinct patient populations with one therapy.
We have demonstrated clinically significant improvement in two patients with IMTs who harbor actionable gene fusions treated with entrectinib in the STARTRK-NG trial. Entrectinib was well tolerated, and the observed responses continued beyond 12 months of treatment at the time of this report. These encouraging results demonstrate the value of molecular testing for targetable gene fusions in this patient population, and the results of the STARTRK-NG trial will provide valuable information on the tolerability and efficacy of entrectinib in pediatric patients.
ACKNOWLEDGMENTS
The authors thank the patients who granted us consent to present their cases for the benefit of the scientific, medical, and patient communities worldwide and Nick Cianciola, of The Lockwood Group, for providing medical writing support funded by Ignyta.
Supported by Ignyta; funded by National Institutes of Health National Cancer Institute Grant No. P30 CA008748.
S.R.A. and E.K.S. contributed equally to the work.
AUTHOR CONTRIBUTIONS
Conception and design: All authors
Provision of study materials or patients: Srikanth R. Ambati, Emily K. Slotkin, Ellen M. Basu
Collection and assembly of data: All authors
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/po/author-center.
Srikanth R. Ambati
No relationship to disclose
Emily K. Slotkin
No relationship to disclose
Edna Chow Maneval
Employment: Ignyta
Stock and Other Ownership Interests: Ignyta
Ellen M. Basu
No relationship to disclose
REFERENCES
- 1.Surabhi VR, Chua S, Patel RP, et al. : Inflammatory myofibroblastic tumors: Current update. Radiol Clin North Am 54:553-563 2016 [DOI] [PubMed] [Google Scholar]
- 2.Yamamoto H, Yoshida A, Taguchi K, et al. : ALK, ROS1 and NTRK3 gene rearrangements in inflammatory myofibroblastic tumours. Histopathology 69:72-83 2016 [DOI] [PubMed] [Google Scholar]
- 3.Lai LM, McCarville MB, Kirby P, et al. : Shedding light on inflammatory pseudotumor in children: Spotlight on inflammatory myofibroblastic tumor. Pediatr Radiol 45:1738-1752 2015 [DOI] [PubMed] [Google Scholar]
- 4.Antonescu CR, Suurmeijer AJ, Zhang L, et al. : Molecular characterization of inflammatory myofibroblastic tumors with frequent ALK and ROS1 gene fusions and rare novel RET rearrangement. Am J Surg Pathol 39:957-967 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lovly CM, Gupta A, Lipson D, et al. : Inflammatory myofibroblastic tumors harbor multiple potentially actionable kinase fusions. Cancer Discov 4:889-895 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Daniel S, Gay LM, Vergilio J-A, et al. : A clinical and genomic profile of inflammatory myofibroblastic tumors. J Clin Oncol 35 2017. (suppl; abstr 1538) [Google Scholar]
- 7.Pavlick D, Schrock AB, Malicki D, et al. : Identification of NTRK fusions in pediatric mesenchymal tumors. Pediatr Blood Cancer 64(8), 2017 [DOI] [PubMed] [Google Scholar]
- 8.Drilon A, Siena S, Ou SI, et al. : Safety and antitumor activity of the multitargeted pan-TRK, ROS1, and ALK inhibitor entrectinib: Combined results from two phase I trials (ALKA-372-001 and STARTRK-1). Cancer Discov 7:400-409 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin JJ, Shaw AT: Recent advances in targeting ROS1 in lung cancer. J Thorac Oncol 12:1611-1625 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Loke BN, Lee VKM, Sudhanshi J, et al. : Novel exon-exon breakpoint in CIC-DUX4 fusion sarcoma identified by anchored multiplex PCR (Archer FusionPlex Sarcoma Panel). J Clin Pathol 70:697-701 2017 [DOI] [PubMed] [Google Scholar]
- 11.PDQ Treatment Editorial Board : Childhood Soft Tissue Sarcoma Treatment (PDQ): Health Professional Version, PDQ Cancer Information Summaries. National Cancer Institute; Bethesda, MD. 2017 [Google Scholar]
- 12.von Mehren M, Randall L, Benjamin RS, et al. : Soft Tissue Sarcoma, Version 2.2018, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 16:536-563 2018 [DOI] [PubMed] [Google Scholar]
- 13.Menichincheri M, Ardini E, Magnaghi P, et al. : Discovery of entrectinib: A new 3-aminoindazole as a potent anaplastic lymphoma kinase (ALK), c-ros oncogene 1 kinase (ROS1), and pan-tropomyosin receptor kinases (pan-TRKs) inhibitor. J Med Chem 59:3392-3408 2016 [DOI] [PubMed] [Google Scholar]
- 14.Ardini E, Menichincheri M, Banfi P, et al. : Entrectinib, a pan-TRK, ROS1, and ALK inhibitor with activity in multiple molecularly defined cancer indications. Mol Cancer Ther 15:628-639 2016 [DOI] [PubMed] [Google Scholar]
- 15.Cheng DT, Mitchell TN, Zehir A, et al. : Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT): A hybridization capture-based next-generation sequencing clinical assay for solid tumor molecular oncology. J Mol Diagn 17:251-264 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Green DC, Deharvengt SJ, de Abreu FB, et al. : Use of anchored multiplex PCR enrichment for detection of gene fusions in solid tumors by next generation sequencing. FASEB J 31 2017. (abst 807.20) [Google Scholar]
- 17.Alvarez-Breckenridge C, Miller JJ, Nayyar N, et al. : Clinical and radiographic response following targeting of BCAN-NTRK1 fusion in glioneuronal tumor. NPJ Precis Oncol 1:5 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Farago AF, Le LP, Zheng Z, et al. : Durable clinical response to entrectinib in NTRK1-rearranged non-small cell lung cancer. J Thorac Oncol 10:1670-1674 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Drilon A, Li G, Dogan S, et al. : What hides behind the MASC: Clinical response and acquired resistance to entrectinib after ETV6-NTRK3 identification in a mammary analogue secretory carcinoma (MASC). Ann Oncol 27:920-926 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sartore-Bianchi A, Ardini E, Bosotti R, et al. : Sensitivity to entrectinib associated with a novel LMNA-NTRK1 gene fusion in metastatic colorectal cancer. J Natl Cancer Inst 108 (1), 2015. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=26563355&dopt=Abstract [DOI] [PMC free article] [PubMed]
- 21.Ahn MJ, Cho BC, Siena S, et al. : Entrectinib in patients with locally advanced or metastatic ROS1 fusion-positive non-small cell lung cancer (NSCLC). J Thorac Oncol 12 2017 (abstr OA 14.06) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iyer R, Wehrmann L, Golden RL, et al. : Entrectinib is a potent inhibitor of Trk-driven neuroblastomas in a xenograft mouse model. Cancer Lett 372:179-186 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rangaraju S, Li G, Christiansen J, et al. : Pediatric phase 1/1b study of entrectinib in patients with primary brain tumors, neuroblastoma, and NTRK, ROS1, or ALK fusions. Neuro-Oncol 19 2017. (abstr Trth-10) [Google Scholar]
- 24.Zehir A, Benayed R, Shah RH, et al. : Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nat Med 23:703-713 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.U.S. Food and Drug Administration : FDA unveils a streamlined path for the authorization of tumor profiling tests alongside it latest product action. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm585347.htm
- 26.Vendrell JA, Taviaux S, Béganton B, et al. : Detection of known and novel ALK fusion transcripts in lung cancer patients using next-generation sequencing approaches. Sci Rep 7:12510 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Data on file, Ignyta. San Diego, CA, 2017
- 28.Butrynski JE, D’Adamo DR, Hornick JL, et al. : Crizotinib in ALK-rearranged inflammatory myofibroblastic tumor. N Engl J Med 363:1727-1733 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Subbiah V, McMahon C, Patel S, et al. : STUMP un“stumped”: Anti-tumor response to anaplastic lymphoma kinase (ALK) inhibitor based targeted therapy in uterine inflammatory myofibroblastic tumor with myxoid features harboring DCTN1-ALK fusion. J Hematol Oncol 8:66 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ono A, Murakami H, Serizawa M, et al. : Drastic initial response and subsequent response to two ALK inhibitors in a patient with a highly aggressive ALK-rearranged inflammatory myofibroblastic tumor arising in the pleural cavity. Lung Cancer 99:151-154 2016 [DOI] [PubMed] [Google Scholar]
- 31.Mansfield AS, Murphy SJ, Harris FR, et al. : Chromoplectic TPM3-ALK rearrangement in a patient with inflammatory myofibroblastic tumor who responded to ceritinib after progression on crizotinib. Ann Oncol 27:2111-2117 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saiki M, Ohyanagi F, Ariyasu R, et al. : Dramatic response to alectinib in inflammatory myofibroblastic tumor with anaplastic lymphoma kinase fusion gene. Jpn J Clin Oncol 47:1189-1192 2017 [DOI] [PubMed] [Google Scholar]


