Abstract
Bright red-emitting pyridinium cyanine based styryl probe 2 is synthesized in good yields. Probe 2 demonstrated a large Stokes’ shift (Δλ ≈ 128 nm, 4227 cm−1 in DCM) and excellent fluorescent quantum yield (ϕfl ≈ 0.2 - 0.7) due to strong Intra-molecular charge transfer (ICT). Probe 2 found to exhibit exceptional selectivity for cellular mitochondria in both normal (COS-7) and cancer (A549) cell lines. Probe 2 is readily applicable as a “wash-free” dye to visualize mitochondria as it does not require post-staining washing prior to imaging. Styryl probe 2 also showed an excellent biocompatibility as the calculated LC50 (lethal concentration, 50%) value was > 20 μM. Probe 2 emission did not show any interferences from anionic species or other biological molecules. Probe 2 is readily excitable (λex ∼460 and λem ∼618 nm) with the available laser (454 nm) in commercial microscopes and thus it can be a useful probe for mitochondrial tracking in live cells.
Keywords: Biocompatibility, Intra-molecular charge transfer (ICT), Large Stokes shift, Mitochondria selectivity, Styryl dye, Wash-free application
Graphical Abstract

1. Introduction
Mitochondria are primarily responsible for the production of adenosine triphosphate (ATP) for cellular energy requirement. Mitochondria also play an important role in apoptosis and free radical formation within the cell during oxidative phosphorylation.[1–3] Also, mitochondria produce important precursors for the biosynthesis of macromolecules and for homeostasis of Ca2+ levels within cells.[4,5] Therefore, mitochondria dysfunction can lead to severe disease conditions including neurodegenerative disorders, metabolic disorders, Alzheimer’s disease, Parkinson’s disease and cancers. [6–8] Actual protein composition and the morphology of the mitochondria depends on an organism’s metabolic status.[9–11] Mitochondria in healthy cells appear as an ovoid-shaped dynamic network spread throughout the cell in adherent cultured cell lines.[12] Therefore, visualizing mitochondria to quantify morphological changes plays an important role towards revealing important information related to cellular health and metabolism. ATP production within mitochondria is driven by the electrochemical proton gradient (ΔΨm) generated through lipid bilayer (ΔΨm ≈ 180 -220 mV).[13] Therefore, the membrane potential across the mitochondria lipid bilayer is also considered as a parameter for detecting cellular health. [14] The majority of reported fluorescent mitochondria-targeting probes contain a net positive charge in their structure, which facilitates the mitochondria localization of the probe due to electrostatic interaction with the anionic mitochondrial matrix. Such dyes are called “functional dyes” as the localization of these dyes solely depends on the electrochemical gradient across the mitochondrial membrane (e.g. Rhodamine 123).[4,12] Mitochondria selective probes can also be “structural dyes,” where the mitochondria selectivity of the probe is independant of the electrochemical gradient across the membrane (e.g. MitoTracker® Green FM).
Commercial Mitochondria Probes :
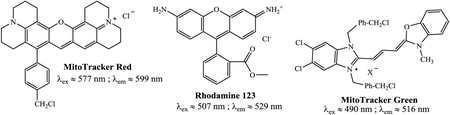
There are several mitochondria selective fluorescent probes that are commercially available, which including MitoTracker® Green FM (λex ∼490 nm, λem ∼516 nm), MitoTracker® Deep Red (λex ∼644 nm, λem ∼665 nm), MitoTracker® Red CMXRos (λex ∼579 nm, λem ∼599 nm) and Rhodamine 123 (λex ∼507 nm, λem ∼529 nm).[15–20] A potential mitochondria probe should demonstrate higher selectivity towards the organelle while minimizing perturbation to cellular activity, so as to minimize potential cytotoxicity. However, most existing commercial mitochondria probes exhibit small Stokes shift (≈ < 30 nm)[21] which significantly reduces the sensitivity and the efficiency of the probe in confocal microscopy imaging. In addition, many commercial mitochondria probes exhibit background interference due to their relatively high fluorescence in aqueous environments. Therefore, it is crucial to develop biocompatible mitochondria selective fluorescent probes with large Stokes shift for mitochondria screening in live cells.
Styryl dyes represent a unique class of chemical probes for biological applications, including mitochondria membrane potential measurements, neuromast staining, plasma membrane staining in prokaryotic cells and cell nucleus staining in eukaryotic cells.[21–23] Their structural simplicity, lipid solubility and fast penetration into cells / tissues makes them useful for biological applications. Although many styryl-based probes exhibit bright red emission with large Stokes shift (Δλ >100 nm), their photophysical properties are sensitive to the solvent polarity. In addition, potential cell toxicity and low photostability also hampers broad use of styryl probes for biological applications. [24–28]
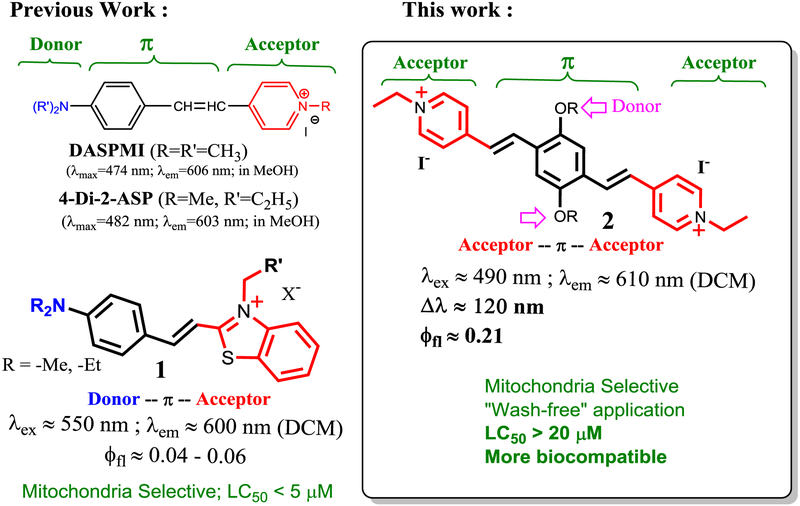
The classical styryl dyes, such as DASPMI and 4-Di-2-ASP (Scheme 1), include a donor-π-acceptor hemicyanine frame, where a pyridinium fragment is used as an acceptor. As a consequence of efficient intramolecular charge transfer (ICT), the styryl dyes exhibit large Stokes but has low fluorescence quantum yield. We are interested in developing styryl dyes with improved characteristics, as shown by recent synthesis of styryl dye 1, in which an amino group is used as a strong donor. The probe 1 is a mitochondria-selective probe, but it exhibits low fluorescence.[29] In a continuous effort to search for a bright styryl dye, we designed an acceptor-π-acceptor system by attaching two pyridinium vinyl units (acceptor) via a para-phenylene bridge (π system), by synthesis of Bp-Cy, 2. In the probe design, two (pyridinium)vinyl units at para-position provide extended π-conjugation, while alkoxy groups on the phenylene bridge are used as moderate electron donors to preserve the large Stokes shift. Interestingly, the probe 2 exhibited bright red fluorescence with large Stokes shift (Δλ ≈ 120 nm, 4227 cm−1 in DCM), and excellent selectivity towards mitochondria in live cells. The new probe also showed a much lower cytotoxicity (LC50 > 20 μM), in comparison with the previously reported hemicyanine based sensor 1 (LC50 < 5 μM) (Scheme 1). In addition, probe 2 was readily excitable with 454 nm laser (λex ∼440 - 490 nm and λem ∼618 nm) in the fluorescence confocal microscope for imaging purposes. Herein we report the synthesis, optical characterization, and its application for cellular mitochondria.
Scheme 1.
Comparison of new probe 2 with styryl probes and previously reported mitochondria probe 1.
2. Materials and Methods
All chemicals for synthesis were purchased from Acros Organics and all chemical were used as they were received without further purification. All molecular biology grade reagents for cell culture and fluorescent confocal microscopy were purchased from Sigma-Aldrich and Fisher Scientific. UV-vis studies were carried out in Hewlett Packard-8453 diode array spectrophotometer at 20°C. Fluorescence studies were carried out in HORIBA Fluoromax-4 spectrofluorometer. Fluorescence confocal microscopy Imaging was performed in Zeiss LSM 710 confocal system with 63x oil objectives, numerical aperture of 1.45 and refractive index of 1.5. Throughout imaging temperature was maintained at 37°C.
2.1. General procedure for synthesis (2).
In a flask, 1 mmol of the corresponding di-aldehyde was dissolved in 20 mL of ethanol. Then, 4-methylpyridinium salt 3 (2.1 mmol) was added and the solution was stirred at room temperature for 15 minutes. Following the addition of pyridine (0.5 mL), the resulting solution was heated up and stirred at 65° C for 48 hours. After completion of the reaction, the reaction mixture was cooled down to room temperature and concentrated under the vacuum. To the resulting dark red crude, ethyl acetate (15 mL) was added, and the product was precipitated out as a red colored solid in the bottom of the flask. After the solution was allowed to settled for 15 minutes, and the resulting solid was collected by vacuum filtration and washed with ethyl acetate (3 × 10 mL) for 3 times. The desired product 2 was collected on the Buchner funnel as a red color solid in 81% yield.
2.2. Characterization of 2.
1H NMR (300 MHz in DMSO) δ 8.94 (d, 4H; J = 3.1 Hz) δ 8.18 (d, 4H; J = 3.6 Hz), δ 8.03 (d, 2H; J = 5.9), δ 7.66 (d, 2H; J = 6.0), δ 7.49 (s, 2H), δ 4.54 (q, 4H; J = 3.15), δ 4.15 (t, 4H; J = 3.2), δ 1.85 (t, 6H; J = 2.85), δ 1.56 (m, 8H; J = 2.80), δ 1.33 (m, 8H; J = 2.80) and δ 0.87 (s, 6H). HRMS (ESI) found (m/z) for [C36H50N2O22+] 271.1921, 271.7012 and 272.2228. For [C36H50IN2O2+ 669.2903, 670.2900 and 671.2999. Calculated (m/z) For [C36H50N2O22+] were 271.1936, 271.6936 and 272.1936. For [C36H50IN2O2+ 669.2917, 670.2917 and 671.2917.
2.3. Fluorescence Quantum yield.
The fluorescence quantum yields (ϕfl) for compounds were calculated by using Rhodamine 6G as the standard. Where ϕfl Rhodamine 6G is 0.95 in ethanol. The following equation was used for calculation quantum yields for compounds 2 at 490 nm. Where A is the absorbance of the sample, I is the integrated fluorescence intensity and η is the refractive index of the solvent.
2.4. Cell Culture and staining.
COS-7 cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) (Invitrogen) containing 10% FBS and 1% Penstrep at 37 °C in a 5% CO2 humidified incubator. A549 cells were maintained in Roswell Park Memorial Institute medium (RPMI) (Invitrogen) containing 10% FBS and 1% Penstrep at 37° C in a 5% CO2 humidified incubator. Probe 2 LysoTracker® Green DND-26 and MitoTracker® Green FM solutions were made in DMSO. For live cell imaging cells were treated with 500 nM probes 2 (final concentration) in cell imaging media for 30 minutes at 37° C. Final concentration of the MitoTracker® Green was maintained at 200 nM for co-localization studies. For lysosome co-localization studies, cells were treated with 70 nM LysoTracker® Green. Final DMSO percentage in cell media was < 0.3% (V/V). For initial cell studies probe 2 treated cells were used for fluorescent confocal imaging without further washing. During colocalization studies with LysoTracker® or MitoTracker® probes, cells were washed 3 times with 1x PBS.
2.5. Fluorescence confocal microscopy.
Cells were imaged by using Zeiss LSM 710 fluorescence confocal microscope with an oil 63 × 1.4 numerical aperture objective. Probe 2 was excited with 454 nm laser and emission was collected from 610 nm to 720 nm range (SYPRO Ruby configuration). MitoTracker® Green and LysoTracker® Green were excited with a 488 nm laser and emission was collected from 500 nm to 580 nm range. Spectra from co-localization experiments were analyzed using the Zeiss LSM software and Mander’s overlap coefficient was calculated by ImageJ (NIH) software. Mander’s overlap coefficient values greater than 0.6 confirms a significant co-localization of the two fluorescent probes.
2.6. Cell viability testing.
For cell viability screening, COS-7 cells were seeded in Corning™ 96 well clear bottom, tissue culture treated opaque microplate. Approximately about 12000 cells were plated in each well (cell density = 1 × 105 cells/ml). 24 hours after seeding cells were treated with increasing concentrations of probe 2 for 1 hour at 37°C. Cell toxicity assays were performed using CellTiter-Glo® Luminescent cell viability assay kit (Promega) according manufactures protocol. Luminescence readings were recorded using Spectramax® 5e microplate reader by using default Spectramx® software installed for CellTiter Glow viability test protocol. LC50 value for probes were calculated by using dose-response formula in Origin8® software.
3. Results and Discussion
3.1. Synthesis.
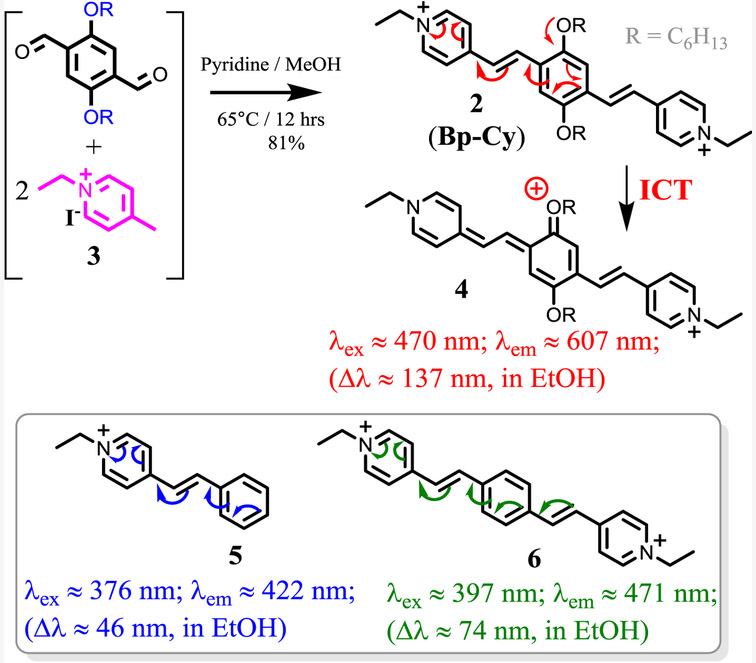
The desired bis(pyridinium vinyl) probe 2 was synthesized by the reaction of 4-methylpyridinium salt 3 with corresponding dialdehyde (Scheme 3), by using a previously reported procedure.[23] The product 2 was conveniently purified by precipitation upon addition of ethyl acetate to the reaction mixture (81% isolation yield). Model compounds 5 and 6 were also synthesized to aid the study. Chemical structure of probe 2 was characterized by 1H NMR, 13C NMR and high-resolution mass spectrometry. (ESI S1-2).
3.2. Spectroscopic Properties.
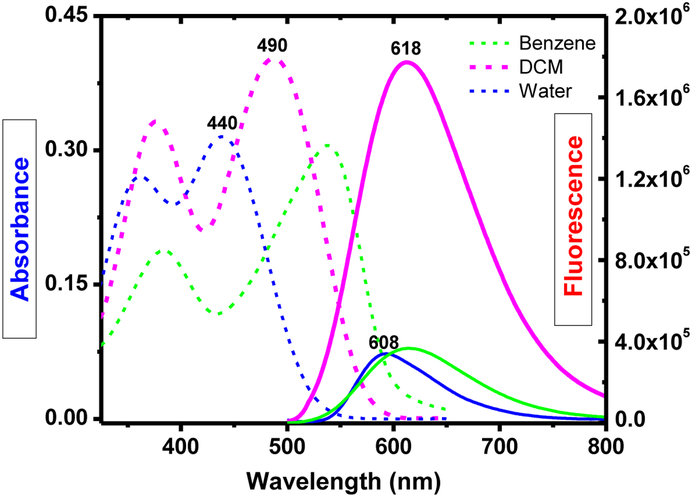
Optical characteristics of the probe 2 was studied and summarized in Table 1. Solvent dependence of 2 was examined in benzene, DCM, DMSO, ethanol and water. The absorption spectra of 2 in water (λmax≈440 nm) observed a hypsochromic shift by ∼100 nm, in comparison with 538 nm in benzene (Figure 1). In contrast, the emission spectra of 2 did not show significant solvatochromic effect as solvent polarity changes (Table 1). Probe 2 exhibit a large Stokes’ shift as a consequence of efficient Intra-molecular charge transfer (ICT) in the probe structure. In addition, observed larger Stokes shift can be attributed partially due to the impact of extended conjugation in the structure (Scheme 3). Large fluorescent quantum yields were observed from 2 in different organic solvents (ϕfl ≈ 0.21 - 0.71), although its fluorescence is relatively weak in highly non-polar (i.e. benzene) and highly polar (i.e. water) solvents. Low fluorescence in benzene could be attributed to potential aggregate formation of the doubly charged 2 in a non-polar environment, which often causes fluorescence quenching.
Table 1.
Optical properties of 2.
| Solvent | Probe 2 | ||||
|---|---|---|---|---|---|
| λabs | λem (QY) |
QY | Δλ (nm) |
Δλ (cm−1) |
|
| Benzene | 538 | 593 | 0.02 | 55 | 1724 |
| DCM | 490 | 618 | 0.21 | 128 | 4227 |
| DMSO | 462 | 616 | 0.71 | 154 | 5411 |
| EtOH | 470 | 607 | 0.51 | 137 | 4802 |
| Water | 440 | 608 | 0.06 | 168 | 6280 |
Figure 1.
Absorbance and fluorescence spectra of 1 (1 × 10−5 M) in different solvents at room temperature.
Comparison between the model compounds 5 and 6 revealed some useful information. The conjugation length of bis(pyridinium) compound 6 was only extended moderately from 5, about 20 nm in λmax, and ∼50 nm in λem wavelength. Therefore, introduction of the second pyridinium vinyl unit only had a limited impact on the conjugation length of 2. However, the alkoxy substituents on 2 played more significant role in extending the absorption and emission to longer wavelength, as λmax, and λem of 2 were red shifted by 70 nm and 135 nm from that of 6, respectively. It was assumed that significant enhancement in fluorescent quantum yield of 2 was due to the presence of alkoxy substituents, which permitted a moderate level of ICT (Scheme 2), which decreased its fluorescence response toward solvent polarity (Table 1).
Scheme 2.
Synthesis and intra-molecular charge transfer process in probe 2.
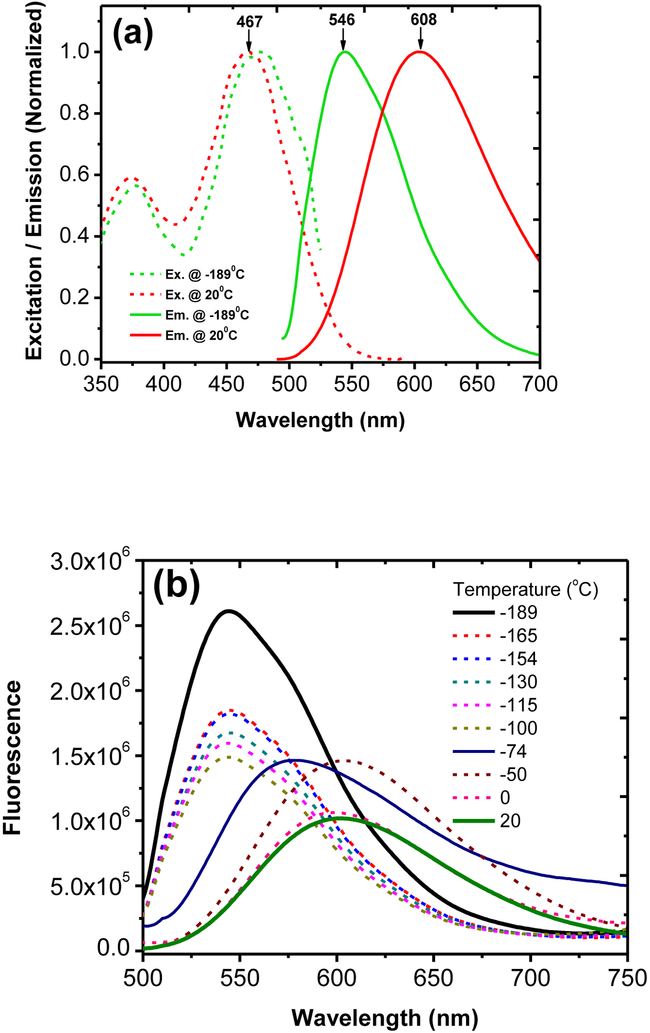
3.3. Low temperature fluorescence.
In order to evaluate the content of ICT effect, we decided to freeze probe 2 (in ethanol) in liquid nitrogen, in an attempt to restrict the molecular motion and bond alteration that are associated with the ICT process. A solution of 2 in a quartz tube was quickly cooled by immersing the sample in liquid nitrogen containing quartz Dewar. The excitation and emission spectra were acquired as the temperature was gradually raised. At the extremely low temperature (at −189°C), the probe 2 was frozen in the solvent matrix, which gave the emission peak at 546 nm (Figure 2). When the temperature was warmed to 20°C, the emission peak of 2 exhibited a notable red-shift to 608 nm. A large spectral shift was recorded (from 546 nm to ∼608 nm, or Δλem ≈ 62 nm) in responding to the temperature rise (Figure 2a). It is important to note that excitation spectrum of 2 at −189°C, was identical to that at 20°C (Figure 2), showing the excitation maxima λex = 468 nm. Therefore, the observed large fluorescence spectral shift at the low temperature is related to the rigid molecular environment in the frozen state, which restrict the ICT process. The fluorescence spectrum at −189°C can be assumed to originate from the locally excited state of 2 (without ICT). It should be noticed that the emission of 2 at −189°C (λem = 546 nm) is at a longer wavelength than 6 (λem=471 nm at 20°C). The low temperature spectroscopic study clearly indicates that the ortho alkoxy groups in probe 2 significantly contribute towards bathochromic shifting, in addition to their influence in ICT.
Figure 2.
Excitation / emission profile (a) and fluorescence emission change of 2 (1 × 10−6 M) in ethanol at different temperature.
3.4. Live cell studies.
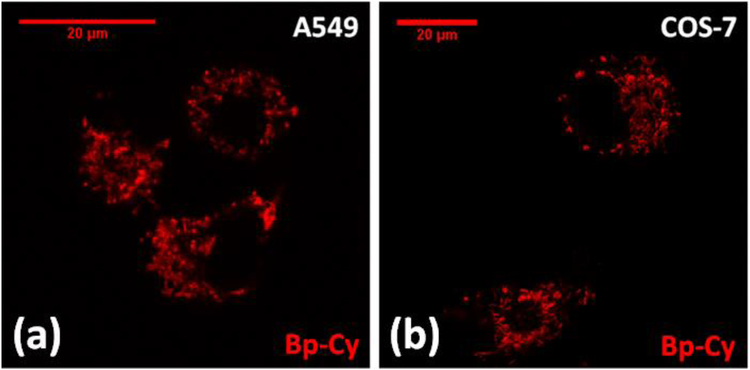
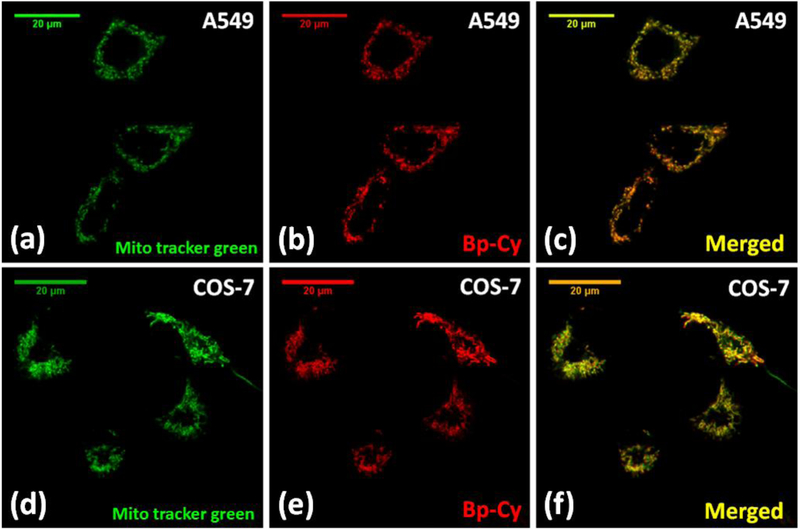
High fluorescence of 2 encouraged us to examine its potential applications in cell imaging. Therefore, probe 2 was studied in both normal (COS-7) and cancer (A549) cell lines. Live cells were stained with 500 nM probe 2 for 30 minutes at 37 °C and studied under fluorescent confocal microscope with 454 nm laser excitation (Figure 3). The observed non-uniform staining pattern led us to hypothesize that probe 2 selectively localized to certain cellular organelle. In order to verify the specific intra-cellular localization of the probe 2, COS-7 cells and A549 cells were co-stained with probe 2 in the presence of commercial MitoTracker® Green-FM (Figure 4). The results showed exceptional co-localization pattern of 2 with MitoTracker® Green, confirming the probe’s selectivity towards cellular mitochondria (Figure 4). Calculated Mendar’s overlap co-efficient for colocalization with commercial MitoTracker® Green found to be 0.81 – 0.83 in both cell lines (calculated sample size n >30), which further verified the selectivity of the probe. Cellular experiments were replicated and the staining procedure by using the probe was found to be reproducible.
Figure 3.
Fluorescent confocal microscopy images of A549 (left) and COS-7 (right) cells (×63 oil) treated with probe 2 (500 nM) for 30 minutes. 454 nm laser was used for excitation and emission was collected from 600 nm to 720 nm (SYPRO Ruby configuration).
Figure 4.
Fluorescent confocal microscopy images of A549 and COS-7 cells (×63 oil) treated with MitoTracker Green-FM (200 nM) and probe 2 (500 nM) for 30 minutes. Figures (a) – (c) represents the colocalization studies in A 549 cells and figures (d) – (f) represents colocalization studies in COS-7 cells. MitoTracker Green was excited with 488 nm lase and emission was collected from 500 nm – 580 nm range. 454 nm laser was used for probe 2 excitation and emission was collected from 610 nm to 720 nm (SYPRO Ruby configuration).
Bright fluorescence images were obtained with 500 nM concentrations of probe 2, which clearly displayed ovoid-shaped mitochondrial networks in fluorescence images. Probe 2 showed lower fluorescent quantum yield in an aqueous medium compared to organic media. Large difference in the fluorescence of probe 2 in aqueous and non-aqueous solutions (Table 1) suggests that staining under “wash-free” conditions might be possible. Therefore 2 was used for cell staining where post staining washing step was not carried out prior to confocal microscopy imaging. Interestingly, probe 2 did not show any noticeable background fluorescence in cell imaging (Figure 3), showing its potential use as a convenient “wash-free” mitochondria probe in live cells. It is important to note that there are only a few “wash-free” mitochondria probes are available. For further confirmation of the selectivity of the probe, COS-7 cells were co-stained with probe 2 in the presence of LysoTracker® Green DND-26 (ESI Figure S6). The resulting fluorescence confocal images did not show any lysosome selectivity of the probe which further confirmed the selectivity of the probes towards mitochondria.
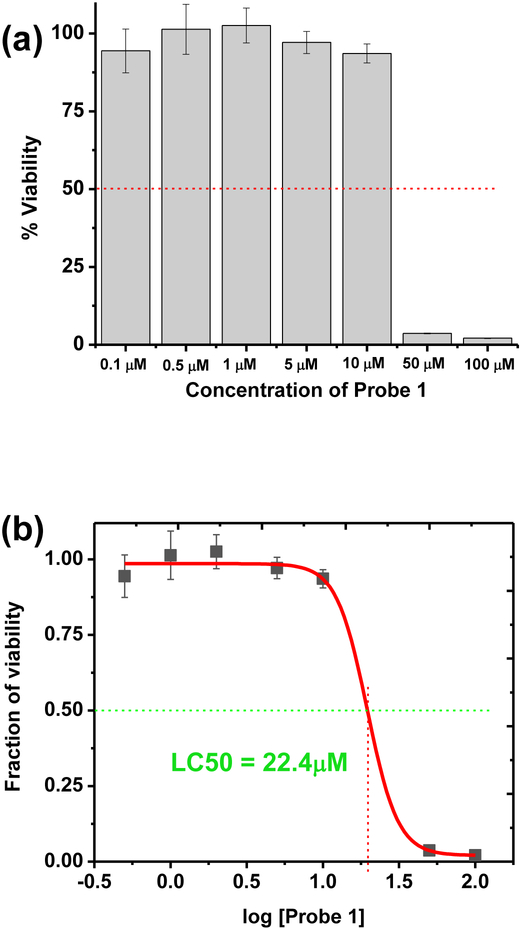
Cell viability evaluation was performed for probe 2 by CellTiter-Glow® Luminescencent cell viability assay in COS-7 cells (Figure 5a). The experimentally calculated LC50 value obtained for 2 was found to be 22.4 μM (Figure 5b). Since the working concentration of 2 (0.5 μM) is significantly lower than the LC50 value, the probe‘s low toxicity could provide a potential advantage for biological cell studies, where biocompatibility and minimal perturbation to the cell activity is desirable. It is known that the toxicity of some commercial MoitoTracker® dyes limits their application.[30] Due to the positively charged nature of the probe 2, it is possible that probe 2 could interact with negatively charged mitochondrial matrix in an electrostatic fashion. Therefore, the localization of the probe 2 in to mitochondria may likely occurs due to its sensitivity towards the electrochemical potential gradient across the membrane. Certain mitochondria probes known as “functional mitochondria probes” (i.e. Rhodamine 123) are accumulated in cellular mitochondria by using above mentioned mechanism. As a supporting experiment we attempted to investigate the ability of probe 2 to visualize mitochondria in fixed cell experiments (ESI Figure S10). However, probe 2 did not show ability to stain mitochondria in fixed cells. Upon fixation, cellular mitochondria are unable to retain membrane potential. This situation resembles “mitochondria dysfunction.” Therefore, it possible to consider probe 2 likely as a “functional mitochondria probe”. It is important to note that the probe 2 exhibited a high biocompatibility (LC50 >10 μM) with comparison to existing mitochondria probes. Therefore, probe 2 can be a very useful tool for visualization mitochondria in live cell studies.
Figure 5.
Cell viability assessment bar-chart (a) and dose-response curve (b) obtained for probe 2 by CellTiter Glow® cell viability assay.
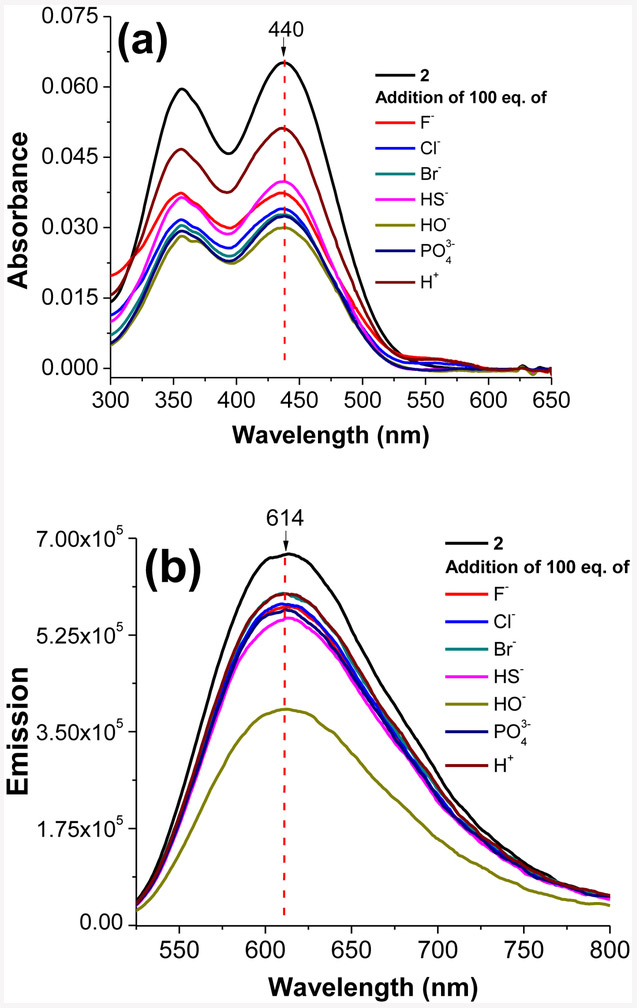
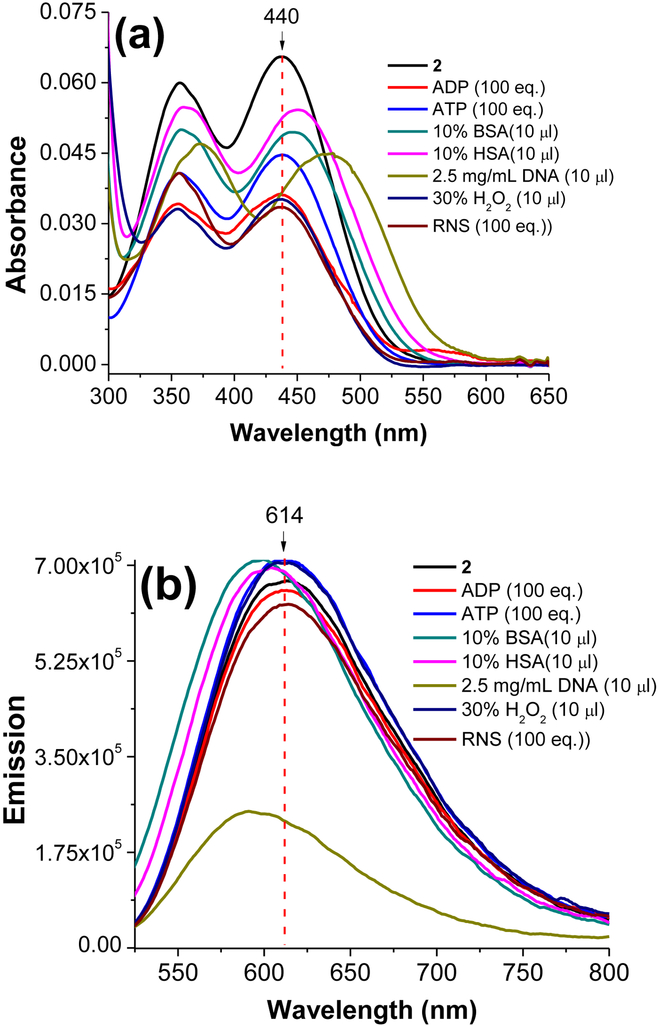
In order to investigate the sensitivity of the probe 2 towards other ionic species, probe 2 (0.5 μM) was tested against different anionic (F−, Cl−, Br−, HS−, OH−, and PO43−) species and H+ in water. Interestingly, probe 2 did not show any noticeable change in the absorption or emission spectra in the presence of different anionic species or protons except for hydroxide ions (Figure 6). Presence of hydroxide ions (i.e., strongly basic conditions) can interrupt the conjugated π system by acting as nucleophiles. It is important to notice that probe 2 exhibited consistent spectral properties (λabs ≈ 440 nm, λem ≈ 614 nm) with the addition of large equivalence of such ionic species which provides a potential advantage in intra-cellular environments to obtained reliable fluorescence response entirely based upon mitochondrial localization. In another experiment, probe 2 (0.5 μM in water) was tested against large quantities (≈ 100 equivalence) of adenosine diphosphate (ADP), adenosine triphosphate (ATP), bovine serum albumin (BSA), human serum albumin (HSA), genomic DNA, reactive oxygen species (O22−), and reactive nitrogen species (NO). Probe 2 exhibited a remarkably stable optical response (λabs ≈ 440 nm, λem ≈ 614 nm) in the presence of all biological species confirming high reliability of the probe in biological environments (Figure 7). In the presence of genomic DNA probe 2 exhibited a slight quenching of fluorescence (Figure 7). However, probe did not show any localization in cellular nucleus upon staining. The characteristic “non-interacting nature” of the probe 2 towards other biological species provide a potential advantage to the probe over many previously reported mitochondria selective fluorescent probes. Also, this evidence further supported the hypothesis that probe 2 may likely use the electrochemical potential gradient as the driving force for mitochondrial selectivity (i.e., functional mitochondrial dyes) due to doubly positive charge nature of the probe. In addition, noninteracting nature of this probe possibly minimizes the background fluorescence that occurs in many other fluorescent probes due to their interactions with biological molecules (i.e., proteins). Therefore, probe 2 could be an ideal candidate for mitochondria screening in live cells.
Figure 6.
Absorbance (a) and emission (b) spectra obtained for probe 2 (5 × 10−7 M) in water upon addition of different ion species at 20 °C. Emission spectra were recorded by exciting probe 2 at 440 nm.
Figure 7.
Absorbance (a) and emission (b) spectra obtained for probe 2 (5 × 10−7 M) in water upon addition of different biological species at 20 °C. Emission spectra were recorded by exciting probe 2 at 440 nm.
4. Conclusions
In summary, a new bis(pyridinium)-based styryl dye 2 has been synthesized in very good yield. Spectroscopic studies of 2, in comparison with the related model compounds, shows that there is strong ICT interaction between the positively charged pyridinium moieties and alkoxy substituents. As a consequence, probe 2 exhibits bright red emission, a large Stokes shift, large fluorescence quantum yield and a large fluorescence response to solvent polarity. The probe has been successfully used for visualizing mitochondria in both healthy and cancer cell lines under lower concentration (i.e., 500 nM). The study reveals that the developed new mitochondria probe has the following advantages: (1) lower toxicity (with LC50 = 22.4 μM); (2) bright red-emission (λem≈610 nm) with large Stokes shift (Δλ≈137 nm); (3) readily excitable with lasers (454 nm) in the fluorescent confocal microscope; and (4) could be used under “wash free conditions” (i.e. without the need of post-staining washing to remove excess fluorescent dyes). Further studies along this direction could lead to high performance and low toxicity probes for visualizing mitochondria. Also probe 2 did not show any optical response towards anionic species and biologically available molecules. Therefore, probe 2 could be a highly reliable fluorescent probe for visualizing mitochondria in live cells without background interferences.
Supplementary Material
Acknowledgements
We thank Nicolas Alexander from University of Akron for acquiring mass spectrometric data. K.J.W. was supported by a NIH CBBI fellowship (T32GM075762). The imaging studies were supported by the Indiana University School of Medicine-South Bend Imaging and Flow Cytometry core (to R.V.S.).
Biography
Chathura Abeywickrama is currently a Ph.D. student in chemistry department at The University of Akron under the supervision of Prof. Pang. His research interest focuses on the synthesis of fluorescent sensors with near infrared emission by coupling the excited state intramolecular proton transfer (ESIPT) mechanism with cyanine fluorophores and developing near infrared fluorescent sensors by coupling intramolecular charge transfer with cyanine fluorophores.
Kaveesha Wiesinghe is currently a postdoctoral research associate at University of Cambridge. She received her Ph.D from The University of Notre Dame under the supervision of Prof. Robert Stahelin. Her research was focused on understanding the lipid protein interactions of the Marburg virus protein VP40.
Robert Stahelin received his Ph.D. in physical chemistry from University of Illinois at Chicago. He is currently a professor in the department of Medicinal Chemistry and Molecular Pharmacology at Purdue Uiversity. His current research interests include investigation of focused on investigating virus assembly and budding from the host cell plasma membrane and discovery of new lipid-binding proteins
Yi Pang received his Ph.D. in organic chemistry from Iowa State University. He is currently professor in the department of chemistry at The University of Akron. His current research interests include synthesis of luminescent polymers and fluorescent molecular probes, and their potential applications for imaging biologically important species.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Hüttemann M, Lee I, Samavati L, Yu H, Doan JW, Regulation of mitochondrial oxidative phosphorylation through cell signaling, Biochim. Biophys. Acta - Mol. Cell Res 1773 (2007) 1701–1720. doi: 10.1016/j.bbamcr.2007.10.001. [DOI] [PubMed] [Google Scholar]
- [2].Nicholls DG, Mitochondrial function and dysfunction in the cell: Its relevance to aging and aging-related disease, Int.J.Biochem.Cell Biol 34(2002)1372–1381. doi: 10.1016/S1357-2725(02)00077-8. [DOI] [PubMed] [Google Scholar]
- [3].Cadenas E, Davies KJ, Mitochondrial free radical generation, oxidative stress, and aging., Free Radic. Biol. Med 29 (2000) 222–230. doi: 10.1016/S0891-5849(00)00317-8. [DOI] [PubMed] [Google Scholar]
- [4].V Johnson L, Walsh ML, Chen LB, Localization of mitochondria in living cells with rhodamine 123, Proc. Natl. Acad. Sci 77 (1980) 990–994. http://www.pnas.Org/content/77/2/990.abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Nicholls D, Åkerman K, Mitochondrial calcium transport, BBA Rev. Bioenerg 683 (1982) 57–88. doi: 10.1016/0304-4173(82)90013-1. [DOI] [PubMed] [Google Scholar]
- [6].Nunnari J, Suomalainen A, Mitochondria: in sickness and in health, Cell. 148 (2012) 1145–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Friedman JR, Nunnari J, Mitochondrial form and function, Nature. 505 (2014) 335–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Novelli A, Reilly JA, Lysko PG, Henneberry RC, Glutamate becomes neurotoxic via the N-methyl-d-aspartate receptor when intracellular energy levels are reduced, Brain Res 451 (1988) 205–212. doi: 10.1016/0006-8993(88)90765-2. [DOI] [PubMed] [Google Scholar]
- [9].Pagliarini DJ, Calvo SE, Chang B, Sheth SA, Vafai SB, Ong S-E, Walford GA, Sugiana C, Boneh A, Chen WK, A mitochondrial protein compendium elucidates complex I disease biology, Cell. 134 (2008) 112–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Sickmann A, Reinders J, Wagner Y, Joppich C, Zahedi R, Meyer HE, Schönfisch B, Perschil I, Chacinska A, Guiard B, The proteome of Saccharomyces cerevisiae mitochondria, Proc. Natl. Acad. Sci 100 (2003) 13207–13212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Neto BAD, Carvalho PHPR, Santos DCBD, Gatto CC, Ramos LM, de Vasconcelos NM, Corrêa JR, Costa MB, de Oliveira HCB, Silva RG, Synthesis, properties and highly selective mitochondria staining with novel, stable and superior benzothiadiazole fluorescent probes, Rsc Adv 2 (2012) 1524–1532. [Google Scholar]
- [12].Cottet-Rousselle C, Ronot X, Leverve X, Mayol J, Cytometric assessment of mitochondria using fluorescent probes, Cytom. Part A. 79 (2011) 405–425. [DOI] [PubMed] [Google Scholar]
- [13].Perry SW, Norman JP, Barbieri J, Brown EB, Gelbard HA, Mitochondrial membrane potential probes and the proton gradient: a practical usage guide, Biotechniques. 50 (2011) 98–115. doi: 10.2144/000113610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Gottlieb E, Armour SM, Harris MH, Thompson CB, Mitochondrial membrane potential regulates matrix configuration and cytochrome c release during apoptosis, Cell Death Differ. 10 (n.d.) 709–717. 10.1038/sj.cdd.4401231. [DOI] [PubMed] [Google Scholar]
- [15].Presley AD, Fuller KM, Arriaga EA, MitoTracker Green labeling of mitochondrial proteins and their subsequent analysis by capillary electrophoresis with laser-induced fluorescence detection, J. Chromatogr. B. 793 (2003) 141–150. [DOI] [PubMed] [Google Scholar]
- [16].Dutta R, McDonough J, Yin X, Peterson J, Chang A, Torres T, Gudz T, Macklin WB, Lewis DA, Fox RJ, Mitochondrial dysfunction as a cause of axonal degeneration in multiple sclerosis patients, Ann. Neurol. 59 (2006) 478–489. [DOI] [PubMed] [Google Scholar]
- [17].Rottenberg H, Wu S, Quantitative assay by flow cytometry of the mitochondrial membrane potential in intact cells, Biochim. Biophys. Acta (BBA)-Molecular Cell Res 1404 (1998) 393–404. [DOI] [PubMed] [Google Scholar]
- [18].Septinus M, Seiffert W, Zimmermann HW, Über hydrophobe Acridinfarbstoffe zur Fluorochromierung von Mitochondrien in lebenden Zellen, Histochemistry. 79 (1983) 443–456. doi: 10.1007/BF00491779. [DOI] [PubMed] [Google Scholar]
- [19].Johnson I, Spence MTZ, The Handbook: A Guide to Fluorescent Probes and Labeling Technologies, (2010). [Google Scholar]
- [20].Salvioli S, Ardizzoni A, Franceschi C, Cossarizza A, JC-1, but not DiOC6 (3) or rhodamine 123, is a reliable fluorescent probe to assess ΔΨ changes in intact cells: implications for studies on mitochondrial functionality during apoptosis, FEBS Lett. 411 (1997) 77–82. [DOI] [PubMed] [Google Scholar]
- [21].Wiederschain GY, The Molecular Probes handbook. A guide to fluorescent probes and labeling technologies, Biochem 76 (2011) 1276–1276. doi: 10.1134/S0006297911110101. [DOI] [Google Scholar]
- [22].Abeywickrama CS, Wijesinghe KJ, Stahelin RV, Pang Y, Bright red-emitting pyrene derivatives with a large Stokes shift for nucleus staining, Chem. Commun. 53 (2017) 5886–5889. doi: 10.1039/C7CC03417B. [DOI] [PubMed] [Google Scholar]
- [23].Dahal D, Ojha KR, Alexander N, Konopka M, Pang Y, An NIR-emitting ESIPT dye with large stokes shift for plasma membrane of prokaryotic (E. coli) cells, Sensors Actuators, B Chem 259 (2018) 44–49. doi: 10.1016/j.snb.2017.12.041. [DOI] [Google Scholar]
- [24].Deligeorgiev T, Vasilev A, Kaloyanova S, Vaquero JJ, Styryl dyes-synthesis and applications during the last 15 years, Color. Technol 126 (2010) 55–80. [Google Scholar]
- [25].Yashchuk VM, V Gusak V, Dmytruk IM, Prokopets VM, Kudrya VY, Losytskyy MY, Tokar VP, Gumenyuk YO, Yarmoluk SM, Kovalska VB, Two-photon excited luminescent styryl dyes as probes for the DNA detection and imaging. Photostability and phototoxic influence on DNA, Mol. Cryst. Liq. Cryst. 467 (2007) 325–338. [Google Scholar]
- [26].Bohländer PR, Wagenknecht H-A, Synthesis and evaluation of cyanine-styryl dyes with enhanced photostability for fluorescent DNA staining, Org. Biomol. Chem. 11 (2013) 7458–7462. [DOI] [PubMed] [Google Scholar]
- [27].Ren J, Wu Z, Zhou Y, Li Y, Xu Z, Colorimetric fluoride sensor based on 1, 8-naphthalimide derivatives, Dye. Pigment. 91 (2011) 442–445. [Google Scholar]
- [28].Li Q, Kim Y, Namm J, Kulkarni A, Rosania GR, Ahn Y-H, Chang Y-T, RNA-selective, live cell imaging probes for studying nuclear structure and function, Chem. Biol 13 (2006) 615–623. [DOI] [PubMed] [Google Scholar]
- [29].Abeywickrama CS, Baumann HJ, Alexander N, Shriver LP, Konopka M, Pang Y, NIR-emitting benzothiazolium cyanines with an enhanced stokes shift for mitochondria imaging in live cells, Org. Biomol. Chem. 16 (2018) 3382–3388. [DOI] [PubMed] [Google Scholar]
- [30].Buckman JF, Hernández H, Kress GJ, Votyakova TV, Pal S, Reynolds IJ, MitoTracker labeling in primary neuronal and astrocytic cultures: Influence of mitochondrial membrane potential and oxidants, J. Neurosci. Methods. 104 (2001) 165–176. doi: 10.1016/S0165-0270(00)00340-X. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.