Abstract
Methylation aberrations play an important role in many metabolic disorders including cancer. Methylated metabolites are direct indicators of metabolic aberrations, and currently, there is no Liquid chromatography - Mass spectrometry (LC-MS) based method available to cover all classes of methylated metabolites at low detection limits. In this study, we have developed a method for the detection of methylated metabolites, and it’s biological application. In this approach, we used a HILIC based HPLC with MS to measure methylated organic acids, amino acids, and nucleotides. These metabolites were separated from each other by their hydrophobic interactions and analyzed by targeted metabolomics of single reaction monitoring by positive and negative mode of electrospray ionization. These metabolites were quantified, and the interday reproducibility was <10% relative standard deviation. Furthermore, by applying this method, we identified high levels of methylated metabolites in bladder cancer cell lines compared to benign cells. In vitro treatment of cancer cells with methylation inhibitor, 5- aza-2’-deoxycytidine showed a decrease in these methylated metabolites. This data indicates that HPLC analysis using this HILIC based method could be a powerful tool for measuring methylated metabolites in biological specimens. This method is rapid, sensitive, selective, and precise to measure methylated metabolites.
Introduction
Methylation represents the addition of a methyl group on a substrate or the replacement of an atom or group of atoms by a methyl group. In biological systems, the epigenetics describes heritable changes in the phenotype of the cell which are independent of alterations occurring in the DNA sequence [1]. Until today, there are at least four different DNA modifications [2, 3] and 16 types of histone modifications [4, 5] which are altered by chromatin modifying enzymes in a highly regulated manner. One of these modifications is methylation of nucleotides such as cytosine, adenine and amino acids present on histones particularly lysine and arginine [6]. Methylation can regulate gene expression, RNA processing, DNA repair, and protein function. Abnormal methylation is one of the hallmarks of cancer development [7].
DNA methylation at CpG islands is catalyzed by DNA methyl transferases resulting in the generation of 5-methylcytosine (5mC). It plays an important role in promoter hypermethylation of tumor-suppressor genes, which is a hallmark of human cancer [8]. The methylations at CpG are erased by α-ketoglutarate-dependent ten- eleven translocation (TET) dioxygenases, through sequential oxidation of 5mC to 5- hydroxymethylcytosine (5hmC) and finally converted to unmethylated cytosine [9–11]. The quantitative levels 5mC and 5hmC reveal the DNA methylation status, which serves as epigenetic markers for cancer and various other diseases [12–14]. The methylation of amino acid arginine is known to play a major role in signal transduction, nuclear transport or direct modulation of nucleic acid interactions [15]. Methylated organic acids can act as diagnostic markers for vitamin B-12 deficiency in humans, which can lead to excess production of methylmalonic acid [16, 17]. Despite the biological significance of the methylated metabolites, there are very few methods available to identify and quantify the methylated metabolites [18–20]. However, due to low sensitivity and inability to cover multiple methylated metabolites of current methods, there is a need to develop new methods with high sensitivity to understand their biological role.
HPLC coupled to MS provides high sensitivity and selectivity, making these assays superior to the conventional detection technologies. Hydrophilic interaction liquid chromatography (HILIC) in conjunction with MS has become a powerful tool for the analysis of a wide variety of analytes. It separates compounds by eluting with strong organic mobile phase against a hydrophilic stationary phase where elution is driven by increasing the water content in the mobile phase. It has been reported that the ionization responses for basic and acidic polar compounds were enhanced by 5–8 fold in the positive ionization mode and up to 20-fold in the negative ionization mode by the HILIC LC-MS/MS methods as compared to the reverse phase LC-MS/MS methods[21]. An application of this technique has increased dramatically over the past decade, especially for the analysis of polar analytes like methylated metabolites where reverse phase chromatography is unsuitable. The major advantage of HILIC is that even with the small volume of samples methylated metabolites are analyzed with high resolution and high sensitivity. The LC-ESI-MS based methods are extremely selective and sensitive allowing it to be utilized in applications with limited material such as clinical samples.
In this study, we have developed a new HILIC based LC/MS method for the measurement of multiple methylated organic acids, nucleotides and amino acids. This method allows the quantitative determination of these metabolites up to picogram level. We have validated this method in bladder benign, cancer cells and found that cancer cells have high levels of methylated metabolites. We further observed low levels of these methylated metabolites upon 5-aza-2’-deoxycytidine (AZA) treatment in bladder cancer (BLCA) cells by the same method.
Methods
Reagents and internal standards
HPLC grade ammonium acetate, acetonitrile, methanol, chloroform, ethanol, hexane, and water were procured from Burdick & Jackson (Morristown, NJ). MS grade formic acid, standard compounds and isotope labeled standards, including N-acetyl aspartic acid-d3, tryptophan-15N2, sarcosine-d3, glutamic acid-d5, thymine-d4, gibberellic acid, trans-zeatin, jasmonic acid, 15N anthranilic acid, and testosterone-d3, were purchased from Sigma-Aldrich (St. Louis, MO).
Preparation of a standard calibration curve
All standard compounds were dissolved as per the vendor’s instructions, and 1mg/ml stock solutions were prepared. A standard mixture stock solution was prepared so that the final concentration of each standard was 1000 ng/ml. The standard curve dilutions were made with (1:1) methanol: water. The concentration of the lowest dilution is 0.2 pg/ml, and the injection volume is 5μL.
Cell culture and AZA treatments
Cells were maintained using standard cell culture procedures in an incubator at 37°C with 5% CO2 and atmospheric oxygen. Cells were cultured in respective media, SVHUC-1 (F-12K), RT4, T24 (McCoy’s 5A), 5637, J82, TCCSUP, UMUC3 (RPMI) supplemented with 10% fetal bovine serum and 1% pen-strep. AZA was resuspended in DMSO, and 5μΜ final concentration was added to the culture media for the 72 hours. After the time point cells were harvested and equal number of cells were collected from control and treatment conditions. The cell pellets were flash frozen and stored at −80°C until metabolites were extracted.
Sample preparation for mass spectrometry and metabolomics analysis
Metabolites were extracted from benign and BLCA cell lines. Mouse liver pool was used as quality controls and followed the extraction procedure as described previously [22–26] We used 5 million cells for the extraction and to each sample 750 μL of ice-cold methanol: water (4:1) containing 20 μL spiked internal standard was added and homogenized. Ice-cold chloroform and water were added in a 3:1 ratio for a final proportion of 4:3:2 methanol:chloroform: water. Both the organic and aqueous layers were transferred into a new tube, dried and resuspended in 50:50 methanol: water. The resuspended samples were deproteinized by using a 3kDa molecular filter (Amicon ultracel-3K Membrane; Millipore Corporation, Billerica, MA) and the filtrate was dried under vacuum (Genevac EZ-2plus, NY). Prior to MS, the dried extracts were resuspended in 50:50 methanol: water and were subjected into LC-MS.
Data acquisition through LC/MS Analysis
The MS experiments were performed on a 6495 QQQ mass spectrometer (Agilent Technologies, Santa Clara, CA). The sample was introduced into the ion source using 1290 HPLC with a degasser, binary pump, thermostatted auto sampler and column oven. MS source parameters used were as follows: gas temperature 250 °C; gas flow14 l/min; nebulizer gas pressure is 20psi; sheath gas temperature is 350 °C; sheath gas flow is 12 l/min; capillary voltage 3000 V positive and 3000 V negative; nozzle voltage 1500 V positive and 1500 V negative. Approximately 8–11 data points were acquired per detected metabolite. All the standards were acquired in the scan mode mass range 50– 1200 Da using electrospray ionization (ESI) positive mode and negative mode. The collision-induced dissociation mass spectra (CID) for all methylated compounds were recorded by mass selecting the precursor ions using quadrupole 1, with collision energy and allowing collision gas of nitrogen (>99.9% purity) in the collision cell quadrupole 2, the resulting ions were analyzed by scanning quadrupole 3. These experiments were performed by the Agilent automated mass hunter optimizer and most abundant fragment ions are used as quantifiers. All the metabolites were measured by selected reactions monitoring (SRM) mode. The developed method was validated concerning specificity, sensitivity, linearity, precision, accuracy, matrix effect.
Separation of Metabolites
We used two methods to separate the methylated metabolites.
Method A:
In ESI positive mode the HPLC column was waters X-bridge amide 3.5 μm, x 100 mm (PN: 186004868, Waters Milford, MA). Mobile phase A and B were 0.1% formic acid in water and acetonitrile respectively. Gradient flow: 0–3 min 85 % B; 3–12 min 30 % B, 12–15 min 2 % B, 16 min 95%B, followed by re-equilibration till the end of the gradient 23 min to the initial starting condition of 85% B. Flow rate of the solvents used for the analysis is 0.3 ml/min.
Method B :
In ESI negative mode the HPLC column was waters X-bridge amide 3.5 μm, 4.6 × 100 mm (PN: 186004868, Waters, Milford, MA). Mobile phase A and B were 20mM ammonium acetate in water with pH 9.0 and 100% acetonitrile respectively. Gradient flow: 0–3 min 85% B, 3–12 min 30% B, 12–15 min 2%B, 15–16 min 85% B followed by re-equilibration till the end of the gradient 23 min to the initial starting condition of 85% B. Flow rate of the solvents used for analysis is 0.3 ml/min.
Statistical analysis
Limit of Detection (LOD) and Limit of Quantification (LOQ) were calculated by the calibration curve method using mean slope and standard deviation (SD) of intercept. Agilent massHunter workstation software quantitative analysis was used for manual review of chromatograms, and peak area was integrated based on the retention time. The normalization of each metabolite peak area was done by the peak area of the spiked internal standard (zeatin), and then the data were log2 transformed. For every metabolite in the normalized dataset, two sample t-tests were conducted to compare the levels of methylated metabolites between normal and BLCA cell lines. Differential metabolites were identified by adjusting the p-values for multiple testing at a false discovery rate (FDR) threshold of <0.25 for generating the heat maps and box plots.
Results and Discussion
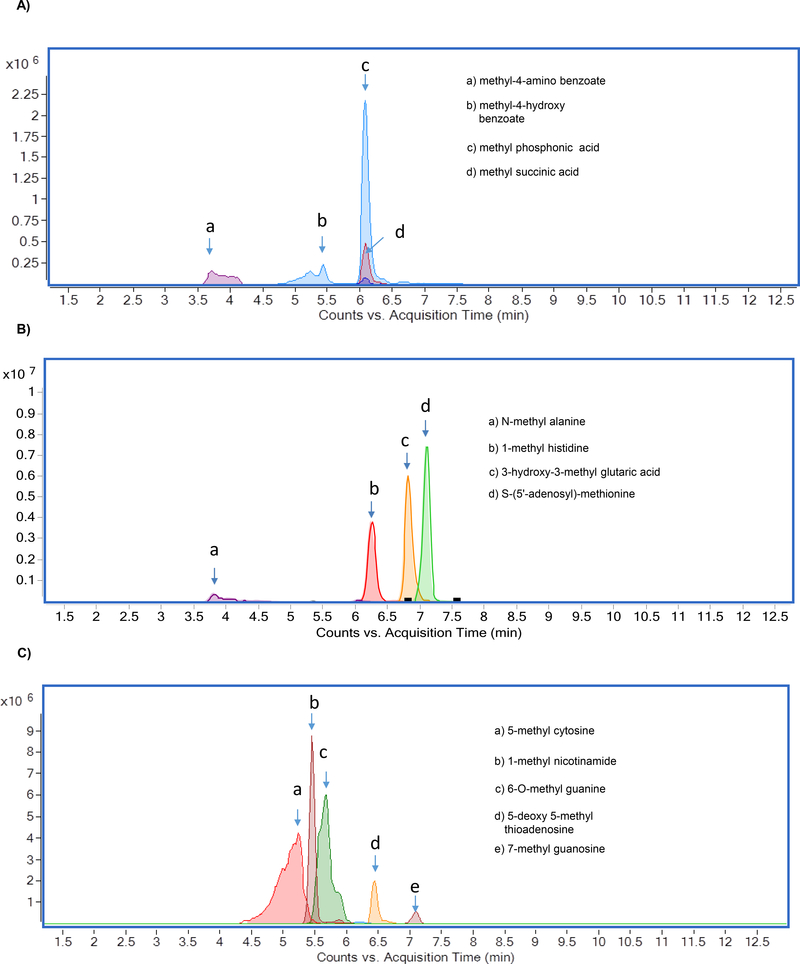
The chromatographic separation of methylated metabolites measured in this study is shown in Figure 1. The list of metabolites and SRM transitions are shown in Table 1. The individual methylated metabolites and its chromatograms are shown in Figure 2.
Figure 1.
Overlaid chromatograms of methylated metabolites A) methylated organic acids, B) methylated amino acids, and C) methylated nucleotides
Table 1:
Methylated metabolites and their SRM transitions
| Class | Metabolite | Precursor ion (m/z) | SRM (m/z) | Polarity | RT (min) |
|---|---|---|---|---|---|
| Methylated acids | Methyl chloroformate | 95 | 58 | Positive | 5.4 |
| Methyl phosphonic acid | 97 | 79 | 6 | ||
| Methyl-4-amino benzoate | 152 | 120/93 | 3.7 | ||
| Methyl-4-hydroxy benzoate | 153 | 121/59 | 5.4 | ||
| Methyl malonic acid | 117 | 73/55 | Negative | 3.1 | |
| Methyl succinic acid | 131 | 87/113 | 6 | ||
| Methylated amino acids | N-methyl-L-alanine | 104 | 58 | Positive | 6.3 |
| 1-methy-L-histidine | 170 | 124/83 | 6.8 | ||
| 1-methyl-D-tryptophan | 219 | 173/160 | 6.6 | ||
| S-(5'-adenosyl)-L-methionine | 399 | 136/250 | 7 | ||
| 2-methyl glutaric acid | 145 | 101 | Negative | 6 | |
| 3-hydroxy-3-methyl glutaric acid | 161 | 57/99 | 6 | ||
| 5-methoxy-3-indole acetic acid | 204 | 160/145 | 5.3 | ||
| Methylated nucleotides | 5-methylcytosine | 126 | 109 | Positive | 5.4 |
| 1-methylnicotinamide | 138 | 79/93 | 6.3 | ||
| 6-O-methyl gauanine | 166 | 149/134 | 5.3 | ||
| 2-amino-6-methyl mercaptopurine | 182 | 134/107 | 5.1 | ||
| 5-methyl cytidine | 258 | 126/109 | 6.4 | ||
| 7-methyl guanosine | 299 | 167 | 5.4 | ||
| 6-methyl -2-thiouracil | 141 | 58 | Negative | 5.4 | |
| 5-deoxy-5-methyl thio adenosine | 296 | 134 | 5.3 |
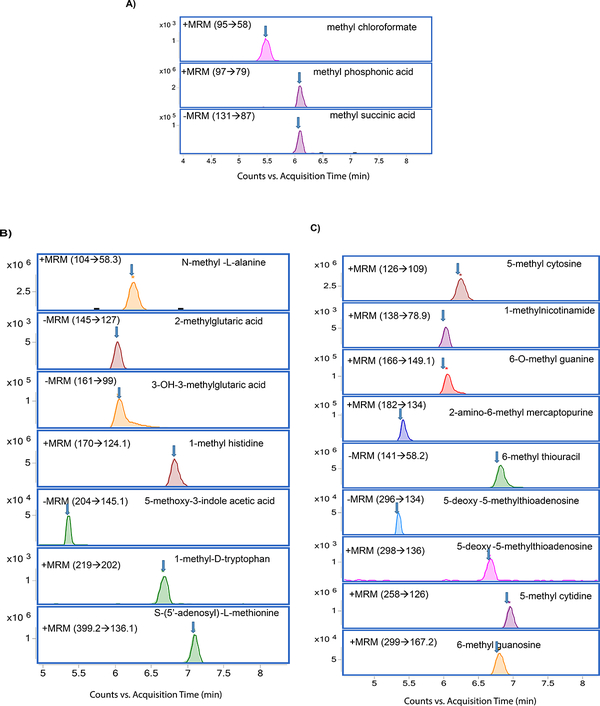
Figure 2.
Chromatograms of methylated metabolites A) methylated organic acids, B) methylated amino acids, and C) methylated nucleotides
Separation of methylated metabolites
Methylated organic acids, amino acids, and nucleotides were separated by using HILIC. (Figure 1). Selection of HILIC enabled the detection of metabolites in picogram level (Table 2), and clear separation of methylated organic acids, amino acids, and nucleotides were observed at different retention times (Figure 2). Using gradient, we were able to achieve narrow, clear, and reproducible retention times with high sensitivity. Re-equilibration on the column after injection can be useful for effective removal of any contaminants. All methylated metabolites are eluted within four minutes range. On the basis of chromatographic results, 0.1% formic acid in acetonitrile, 0.1% formic acid in water and 20mM ammonium acetate in water by gradient elution at 0.3 ml/min was chosen as mobile phases.
Table 2.
Methylated metabolites LOD’s, LOQ’s, R2, and reproducibility
| Metabolite | LOD (pg/ml) | LOQ (pg/ml) | R2 | CV% | Ionization |
|---|---|---|---|---|---|
| Methyl chloroformate | 421 | 606 | 0.999 | 5.6 | Positive |
| Methyl phosphonic acid | 25 | 100 | 0.999 | 7.8 | Positive |
| Methyl-4-hydroxy benzoate | 159 | 390 | 0.999 | 6.3 | Positive |
| 1-methyl histdine | 1.0 | 5.0 | 0.999 | 8.9 | Positive |
| 5-methyl cytosine | 50 | 152 | 0.997 | 8.7 | Positive |
| 1-methyl nicotinamide | 32 | 56 | 0.993 | 9.3 | Positive |
| 2-amino-6-methyl mercaptopurine | 2.0 | 6.0 | 0.991 | 5.8 | Positive |
| 5-methyl cytidine | 6.0 | 16 | 0.999 | 9.5 | Positive |
| 5-deoxy-5-methyl thioadenosine | 2.0 | 6.0 | 0.999 | 7.5 | Positive |
| 5-methoxy-3-indole acetic acid | 66 | 119 | 0.999 | 5.3 | Negative |
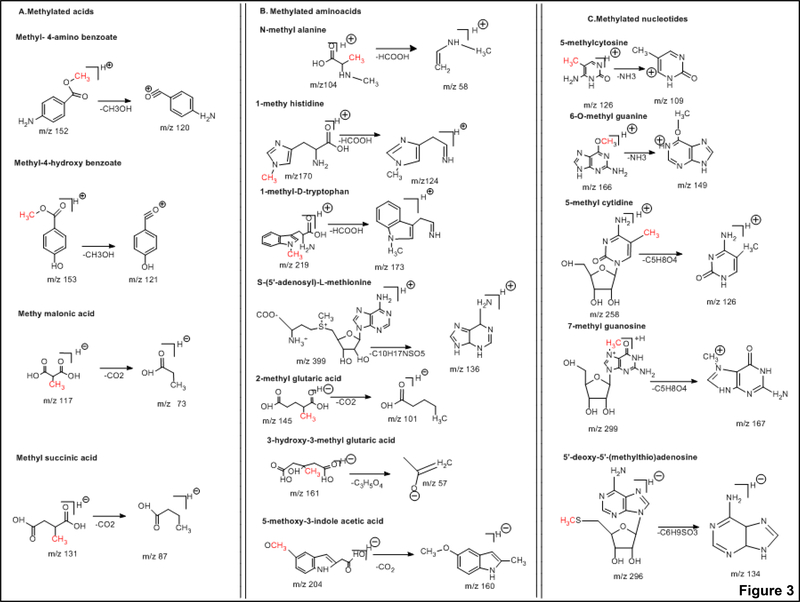
Fragmentation pathways of the methylated metabolites and their degradation products
The collision-induced dissociation (CID) spectra of methylated organic acids
The CID of methyl chloroformate [M+H]+ ion (m/z 95), resulted in the major fragment ion of m/z 58 corresponding to loss of HCl. The CID of methyl phosphonic acid [M+H]+ ion (m/z 97), yielded the major fragment ion of m/z 79 corresponding to loss of H2O. The CID of methyl-4-amino benzoate [M+H]+ ion (m/z 152), resulted in the major fragment ion of m/z 93 corresponding to loss of CH3O. The CID of methyl-4-hydroxy benzoate [M+H]+ ion (m/z 153), resulted in the major fragment ion of m/z of 120 which resulted in the loss of CH3O. The CID of methyl malonic acid [M-H]− ion (m/z 117), produced the major fragment ion of m/z 73 corresponding to loss of CO2. The CID of methyl succinic acid [M-H]− ion (m/z 131), resulted in the major fragment ion of m/z 87corresponding to loss of CO2. The methylated organic acids precursors and fragment ion structures are represented in Figure 3A and CID spectra are represented in Supplementary Figure 1.
Figure 3.
Precursor and product ion structures of A) methylated organic acids B) methylated amino acids, and C) methylated nucleotides
The CID spectra of methylated amino acids
The CID of N-methyl alanine [M+H] + ion (m/z 104), produced the major fragment ion of m/z 58 corresponding to loss of HCOOH. The CID of 1-methyl histidine [M+H]+ ion (m/z 170), resulted in the major fragment ion of m/z 124 corresponding to loss of HCOOH. The CID of 1-methyl-D-tryptophan (m/z 219), [M+H]+ ion produced the major fragment ion of m/z 173 corresponding to loss of HCOOH. The CID of S-(5’-adenosyl)- L-methionine [M+H]+ ion (m/z 399), produced the major fragment ion of m/z 136 corresponding to loss of C10H17NSO5. The CID of 2-methyl glutaric acid (m/z 145), [M-H]− ion yielded a major fragment ion of m/z 101 corresponding to loss of CO2. The CID of 3-hydroxy-3-methyl-glutaric acid (m/z 160), [M-H]- ion produced the major fragment ion of m/z 57 corresponding to loss of C3H5O4. The CID of 5-methoxy-3-indole acetic acid (m/z 204), [M-H]− ion produced the major fragment ion of m/z 160 corresponding to loss of CO2. The methylated amino acids precursors and fragment ion structures are shown in Figure 3B and CID spectra are represented in Supplementary Figure 2.
The CID spectra of methylated nucleotides
The CID of 5-methyl cytosine [M+H]+ ion (m/z 125), produced the major fragment ion of (m/z 109) corresponding to loss of NH3. The CID of 1-methyl nicotinamide [M+H] + ion (m/z 138), yielded the major fragment ion of m/z 78 corresponding to loss of CH3COOH. The CID of 6-O-methyl guanine [M+H]+ (m/z 166), produced the major fragment ion of m/z 149 with the loss of NH3. The CID of 2-amino-6-methyl mercaptopurine [M+H]+ ion (m/z 182), produced the major fragment ion of m/z 134 corresponding to loss of CH3SH. The CID spectra of 5-methyl cytidine [M+H]+ ion (m/z 258), resulted in the major fragment ion of m/z 126 corresponding to loss of C5H8O4. The CID of 7-methylguanosine [M+H]+ ion (m/z 299), yielded the major fragment ions of m/z 167 corresponding to loss of C5H8O4. The CID of 6-methyl-2-thiouracil [M-H]− ion (m/z 141), produced the major fragment ion of m/z 58 corresponding to loss of NCS. The CID of 5’-deoxy-5’-methyl thioadenosine [M-H]− ion (m/z 296), produced the major fragment ion of m/z 134 corresponding to loss of C6H9SO3. The methylated nucleotides fragment ion structures are shown in Figure 3C. CID spectra are represented in Supplementary Figure 3.
Method validation, limit of detection, limit of quantitation and an accurate calibration curve
The most vital criterion of a quantitative analytical method is the accuracy of detection and sensitivity to quantitation of the results produced. The parameters obtained by LC- MS/MS analysis of methylated metabolites are coefficient of linearity (R2), LOD, LOQ, and repeatability (CV %) are represented in Table 2. The LOD of methyl chloroformate is 421 ±2 pg/ml, methyl phosphonic acid is 25±3 pg/ml,methyl-4-hydroxy benzoate is 159±1 pg/ml,1-methyl histidine is 1± 0.5 pg/ml, 5-methyl cytosine is 50±2 pg/ml,1- methyl nicotinamide is 32±0.4 pg/ml, 2-amino-6-methyl mercaptopurine is 2±0.7 pg/ml, 5-methyl cytidine is 6 ±0.7 pg/ml, 5-deoxy-5-methyl thioadenosine is 2±0.6 pg/ml,and 5- methoxy-3-indole acetic acid is 66 ±0.8 pg/ml. The LOQ of methylated organic acids, amino acids and nucleotides linear range was found to be 25 − 1× 106 pg/ml, 5 − 1× 106 pg/ml, 5 − 1× 106 pg/ml, respectively.
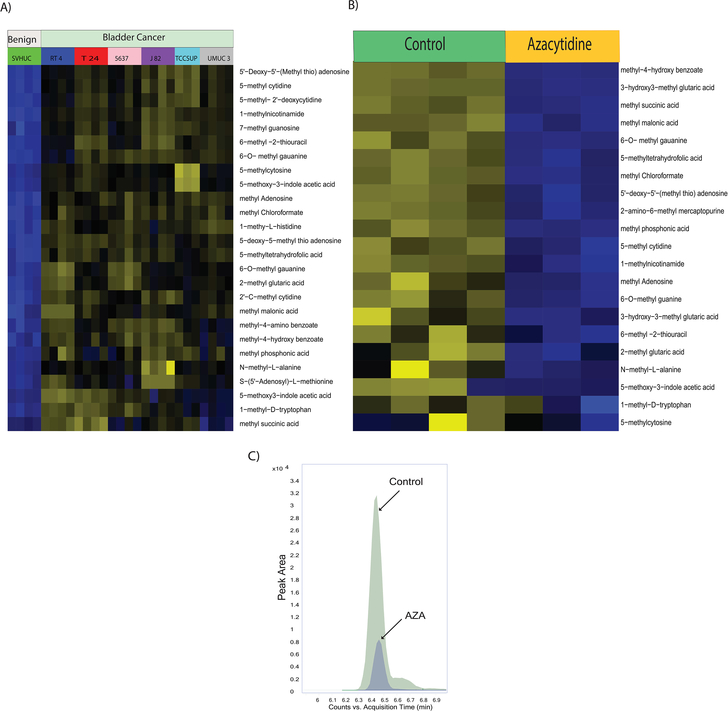
Measurement of methylated metabolites in BLCA cell lines
Epigenetic alterations such as DNA and histone methylations influence the chromatin states and impact gene expression during cancer progression [27]. Methylated nucleotides and amino acids are intermediates of these methylation reactions. We further investigated the developed method in bladder benign and cancer cells and found that methylated organic acids, methylated amino acids, and methylated nucleotides in BLCA cells (RT4, 5637, T24, UMUC3, TCCSUP) were upregulated compared to benign cells (SVHUC-1) (Figure 4A, Supplementary Figure 4). Interestingly, central methyl donor s-adenosyl methionine (SAM) is upregulated in BLCA cells, suggesting their role in providing methyl groups for epigenetic modifications. DNA methyl transferases execute genomic methylation process that plays a crucial role in epigenetic modifications at the promoter regions of specific genes which in turn contributes to cancer development. EZH2 and nicotinamide N-methyltransferase (NNMT), is overexpressed in many cancers, alters protein (histone) methylation profiles and thereby promotes carcinogenesis [28–30]. Drug development focused on inhibition of methylation has been shown to be promising agents to target advanced cancers.
Figure 4.
A) Heat map of hierarchical clustering of differential metabolites in benign and BLCA cells. B) Heat map of hierarchical clustering of differential metabolites in BLCA cells upon AZA treatment. C) Overlaid chromatogram of 1-methyl nicotinamide in control and AZA treated bladder cancer cells.
AZA treatments and expression of methylated metabolites in BLCA cell lines
Drugs that inhibit methylation, most notably the DNA methyltransferase inhibitor AZA is used to treat cancer [31]. We treated the UMUC3 BLCA cells with AZA which is a known inhibitor for methylation. We found significant reduction of methylated metabolites such as methyl succinic acid, methyl malonic acid, methyl chloroformate, methyl phosphonic acid, methylated amino acids such as, 2-methyl glutaric acid, 1-methyl-D-tryptophan and methylated nucleotides such as 6-O-methyl guanine, 5’-deoxy-5’-(Methyl thio) adenosine, 5-methyl cytidine, methyl adenosine, 6-O-methyl guanine, N-methyl-L- alanine upon AZA treatment supporting its therapeutic potential for BLCA (Figure 4B). 1-methyl nicotinamide is downregulated upon AZA treatment which was represented in Figure 4C.
Conclusion
In this study, for the first time, we developed a very robust, accurate, and precise LC- MS-based method to measure 24 methylated metabolites including organic acids, amino acids, and nucleotides. This method can be applied to solid tissues, blood and urine samples with a sensitivity of picogram levels. We have validated this method in bladder benign and cancer cells and found the significant elevation of methylated metabolites in cancer compared to benign cells. Also, treatment of BLCA cells with methylation inhibitor AZA resulted in down-regulation of these methylated metabolites. This LC-MS method proves to be useful for qualitative and quantitative analysis of the methylated organic acids, amino acids, and nucleotides which are deregulated in any disease conditions.
Supplementary Material
Supplementary Figure 1. Product ion spectrums of methylated organic acids: methyl chloroformate, methyl phosphonic acid, methyl-4-amino benzoate, methyl-4-hydroxy benzoate, methyl malonic acid, and methyl succinic acid
Supplementary Figure 2. Product ion spectrums of methylated amino acids: N-methyl-L-alanine, 1-methyl-L-histidine, 1-methyl-D-tryptophan, S-(5’-adenosyl)-L-methionine, 2- methyl glutaric acid, 3-hydroxy-3-methyl glutaric acid, and 5-methoxy-3-indole acetic acid.
Supplementary Figure 3. Product ion spectrums of methylated nucleotides: 5-methyl cytosine, 1-methylnicotinamide, 6-O-methyl guanine, 2-amino-6-methyl mercaptopurine, 5-methyl cytidine, 7-methyl guanosine, 6-methyl −2-thiouracil, and 5-deoxy-5-methyl thio adenosine
Supplementary Figure 4. Box plots of differential metabolites in benign and BLCA cells.
Acknowledgments
This research was supported by CPRIT Proteomics and Metabolomics Core Facility (N.P), (RP170005), P30CA125123 Metabolomics Shared Resources, and Dan L. Duncan Cancer Center and partially supported by the following grants American Cancer Society (ACS) Award 127430-RSG-15-105-01-CNE (N.P.), NIH R01CA220297 (N.P) NIH R01CA216426 (N.P), U01CA179674-01A1 (A.S.K) and NIH/NCI U01 CA214263 (NP). This project was also supported by the Agilent Technologies Center of Excellence in Mass Spectrometry, Prostate Cancer Foundation (A.S.K), Brockman Foundation at Baylor College of Medicine, Metabolomics Core.
Footnotes
Conflict of interest statement: Authors do not have any conflict of interest
References
- 1.Berger SL, et al. , An operational definition of epigenetics. Genes Dev, 2009. 23(7): p. 781–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baylin SB and Jones PA, A decade of exploring the cancer epigenome - biological and translational implications. Nat Rev Cancer, 2011. 11(10): p. 726–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu H and Zhang Y, Mechanisms and functions of Tet protein-mediated 5- methylcytosine oxidation. Genes Dev, 2011. 25(23): p. 2436–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kouzarides T, Chromatin modifications and their function. Cell, 2007. 128(4): p. 693705. [DOI] [PubMed] [Google Scholar]
- 5.Tan M, et al. , Identification of 67 histone marks and histone lysine crotonylation as a new type of histone modification. Cell, 2011. 146(6): p. 1016–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dawson MA and Kouzarides T, Cancer epigenetics: from mechanism to therapy. Cell, 2012. 150(1): p. 12–27. [DOI] [PubMed] [Google Scholar]
- 7.Esteller M, et al. , DNA methylation patterns in hereditary human cancers mimic sporadic tumorigenesis. Hum Mol Genet, 2001. 10(26): p. 3001–7. [DOI] [PubMed] [Google Scholar]
- 8.Robertson KD, DNA methylation and human disease. Nat Rev Genet, 2005. 6(8): p. 597–610. [DOI] [PubMed] [Google Scholar]
- 9.Ito S, et al. , Tet proteins can convert 5-methylcytosine to 5-formylcytosine and 5- carboxylcytosine. Science, 2011. 333(6047): p. 1300–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tahiliani M, et al. , Conversion of 5-methylcytosine to 5-hydroxymethylcytosine in mammalian DNA by MLLpartner TET1. Science, 2009. 324(5929): p. 930–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang H, et al. , Tumor development is associated with decrease of TET gene expression and 5-methylcytosine hydroxylation. Oncogene, 2013. 32(5): p. 663–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chowdhury B, et al. , Quantification of 5-methylcytosine, 5-hydroxymethylcytosine and 5-carboxylcytosine from the blood of cancer patients by an enzyme-based immunoassay. Anal Chim Acta, 2014. 852: p. 212–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen ML, et al. , Quantification of 5-methylcytosine and 5-hydroxymethylcytosine in genomic DNA from hepatocellular carcinoma tissues by capillary hydrophilic-interaction liquid chromatography/quadrupole TOF mass spectrometry. Clin Chem, 2013. 59(5): p. 824–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheng Y, et al. , 5-Hydroxymethylcytosine: A new player in brain disorders? Exp Neurol, 2015. 268: p. 3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gary JD and Clarke S, RNA and protein interactions modulated by protein arginine methylation. Prog Nucleic Acid Res Mol Biol, 1998. 61: p. 65–131. [DOI] [PubMed] [Google Scholar]
- 16.Sobczynska-Malefora A, et al. , An audit of holotranscobalamin (“Active” B12) and methylmalonic acid assays for the assessment of vitamin B12 status: application in a mixed patient population. Clin Biochem, 2014. 47(1–2): p. 82–6. [DOI] [PubMed] [Google Scholar]
- 17.Harrington DJ, Laboratory assessment of vitamin B12 status. J Clin Pathol, 2017. 70(2): p. 168–173. [DOI] [PubMed] [Google Scholar]
- 18.Martens-Lobenhoffer J and Bode-Boger SM, Chromatographic-mass spectrometric methods for the quantification of L-arginine and its methylated metabolites in biological fluids. J Chromatogr B Analyt Technol Biomed Life Sci, 2007. 851(1–2): p. 30–41. [DOI] [PubMed] [Google Scholar]
- 19.Hu CW, et al. , Optimization of global DNA methylation measurement by LC-MS/MS and its application in lung cancer patients. Anal Bioanal Chem, 2013. 405(27): p. 885969. [DOI] [PubMed] [Google Scholar]
- 20.Wang H and Wang Y, LC-MS/MS coupled with stable isotope dilution method for the quantification of 6-thioguanine and S(6)-methylthioguanine in genomic DNA of human cancer cells treated with 6-thioguanine. Anal Chem, 2010. 82(13): p. 5797–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang L, et al. , Simultaneous determination of global DNA methylation and hydroxymethylation levels by hydrophilic interaction liquid chromatography-tandem mass spectrometry. J Biomol Screen, 2012. 17(7): p. 877–84. [DOI] [PubMed] [Google Scholar]
- 22.Vantaku V, et al. , Expression of ganglioside GD2, reprogram the lipid metabolism and EMT phenotype in bladder cancer. Oncotarget, 2017. 8(56): p. 95620–95631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jin F, et al. , Tobacco-Specific Carcinogens Induce Hypermethylation, DNA Adducts, and DNA Damage in Bladder Cancer. Cancer Prev Res (Phila), 2017. 10(10): p. 588–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Piyarathna DWB, et al. , Distinct Lipidomic Landscapes Associated with Clinical Stages of Urothelial Cancer of the Bladder. Eur Urol Focus, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.von Rundstedt FC, et al. , Integrative Pathway Analysis of Metabolic Signature in Bladder Cancer: A Linkage to The Cancer Genome Atlas Project and Prediction of Survival. J Urol, 2016. 195(6): p. 1911–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Putluri N, et al. , Metabolomic profiling reveals potential markers and bioprocesses altered in bladder cancer progression. Cancer Res, 2011. 71(24): p. 7376–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park YJ, et al. , Genome-wide epigenetic modifications in cancer. Prog Drug Res, 2011. 67: p. 25–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim KH and Roberts CW, Targeting EZH2 in cancer. Nat Med, 2016. 22(2): p. 12834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yamagishi M and Uchimaru K, Targeting EZH2 in cancer therapy. Curr Opin Oncol, 2017. 29(5): p. 375–381. [DOI] [PubMed] [Google Scholar]
- 30.Shlomi T and Rabinowitz JD, Metabolism: Cancer mistunes methylation. Nat Chem Biol, 2013. 9(5): p. 293–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sharma S, Kelly TK, and Jones PA, Epigenetics in cancer. Carcinogenesis, 2010. 31(1): p. 27–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Product ion spectrums of methylated organic acids: methyl chloroformate, methyl phosphonic acid, methyl-4-amino benzoate, methyl-4-hydroxy benzoate, methyl malonic acid, and methyl succinic acid
Supplementary Figure 2. Product ion spectrums of methylated amino acids: N-methyl-L-alanine, 1-methyl-L-histidine, 1-methyl-D-tryptophan, S-(5’-adenosyl)-L-methionine, 2- methyl glutaric acid, 3-hydroxy-3-methyl glutaric acid, and 5-methoxy-3-indole acetic acid.
Supplementary Figure 3. Product ion spectrums of methylated nucleotides: 5-methyl cytosine, 1-methylnicotinamide, 6-O-methyl guanine, 2-amino-6-methyl mercaptopurine, 5-methyl cytidine, 7-methyl guanosine, 6-methyl −2-thiouracil, and 5-deoxy-5-methyl thio adenosine
Supplementary Figure 4. Box plots of differential metabolites in benign and BLCA cells.