Abstract
Several studies have documented that length gain often lags behind weight gain during infancy and early childhood, suggesting that linear growth is partly regulated by initial body mass or fatness. To investigate this hypothesis, we analysed data from four longitudinal studies on growth of infants in the first 12 months: (1) U.S. breast‐fed and formula‐fed infants (n = 89); (2) breast‐fed infants in Ghana (n = 190); (3) normal birthweight, breast‐fed infants in Honduras (n = 108); and (4) term, low‐birthweight breast‐fed infants in Honduras (n = 119). The dependent variable was length gain during each 3‐month interval (1– 4, 2–5, 3–6, 4–7, 5–8, 6–9, 7–10, 8–11 and 9–12 months). Three main independent variables were examined: initial weight‐for‐length z‐score (W/L), weight change during the prior 3 months, and initial skinfold thickness. Controlling for maternal height, infant sex, and initial length‐for‐age z‐score, length gain was positively correlated with initial W/L and prior weight change during all age intervals and with initial skinfold thickness at 3 and 4 months (r = 0.15–0.36; P < 0.01). There was no evidence of a threshold effect. These associations were evident in all four populations, in both boys and girls, and in breast‐fed and formula‐fed infants. The consistency of this relationship across studies supports the hypothesis that linear growth is partly regulated by initial body mass or fatness in infants.
Keywords: breast‐feeding, infant growth, fatness, body composition
Introduction
In infants and children, growth in weight does not always occur in synchrony with growth in length. Whereas weight deficits can be rapidly reversed with proper nutrition, length deficits develop more slowly, and can cause permanent stunting if not restored (Costello 1989; Waterlow 1994).
Several studies have examined the relationship between weight gain and length gain in infants, suggesting that during catch‐up growth, weight gain takes precedence over length gain (Brown et al. 1982; Nabarro et al. 1988; Waterlow 1994). For example, in a longitudinal study in Bangladesh, Brown et al. (1982) examined seasonal fluctuations in food supplies and seasonal growth patterns in children. They found that during periods of food shortage, the first sign of growth deficit was impaired weight gain. As nutritional status improved, weight gain occurred first, followed by a lag period of 3–4 months before length gain was detectable. Similar findings were reported for Nepal by Nabarro et al. (1988). More recently, Xu et al. (2001) also found seasonal growth patterns among infants in China, with a time lag of about 3 months between maximal weight velocity and maximal length velocity in the aggregated data. However, at the individual level, the correlation between weight gain in one age interval and length gain in the next interval was close to zero. It is possible that body composition, rather than the rate of prior weight gain, is a better predictor of subsequent linear growth at the individual level. In a retrospective study in Jamaica, Walker & Golden (1988) analysed the records of 369 children recovering from malnutrition and found that the children generally needed to attain at least 85% of expected weight‐for‐length (W/L) before linear growth was initiated. In Nepal, Costello (1989) measured child growth over a period of 6 months and found that weight gain and length gain were related in opposite ways to initial weight‐for‐length. There was very little height gain but maximal weight gain in children who were initially low in W/L, whereas height gain was greatest in children who were initially high in W/L (Costello 1989; Waterlow 1994). These and other results may provide a clue to the regulation of linear growth in children.
Currently there is limited knowledge on the mechanisms governing linear growth in infants. The aim of the present study was to examine if there is a consistent association between initial W/L or fatness and subsequent change in length, and if there is a lagged association between weight gain and subsequent change in length in infants aged 0–12 months. If there is an association between these variables, we also wanted to examine whether this relationship is site related, gender specific, or related to feeding mode. Furthermore, we wanted to determine whether the relationship between W/L and length gain is linear or non‐linear (e.g. a minimum threshold W/L is necessary before length gain is observed).
Methods
Subjects
Data from four previous longitudinal studies were used for this study: the DARLING study conducted in the U.S. (Davis Area Research on Lactation, Infant Nutrition and Growth), which included both breast‐fed (n = 50) and formula‐fed infants (n = 39), a study of breast‐fed infants in Ghana (n = 190) described elsewhere (Dewey 1998), and two studies of breast‐fed infants conducted in Honduras, one of normal birthweight infants (n = 108, Honduras‐NBW), and one of low birthweight, full term infants (n = 119, Honduras‐LBW).
(1) The U.S. study
The selection criteria for the U.S. study were as follows: all infants were healthy, born at term with a birthweight of > 2500 g, and did not receive solid food until after 4 months of age. Stratified matching procedures were used to ensure that the feeding groups would be similar in parental socioeconomic status, education, ethnic group and anthropometric indexes, and infant age and birthweight. The criterion for the breast‐fed group was that no other milk source would be used during the infant's first year except for an occasional bottle containing < 120 mL d−1. For the formula‐fed group, the sample consisted of infants who were breast‐fed for < 3 months and who received an iron‐fortified, cow milk formula until 12 months of age (Dewey 1998). In most of the analyses presented herein, only the breast‐fed cohort was included; the formula‐fed cohort was included when data were examined by feeding mode.
(2) The Ghana study
Healthy infants with birthweight > 2000 g were recruited for this study at 1 month of age. Breast milk was the predominant source of nutrients for ≥ 3 months (mothers were advised to breast‐feed exclusively for ≈ 6 months, but most introduced small amounts of other foods before then). From 6 to 12 months, the infants were randomly assigned to four intervention groups, which received various complementary food mixtures (Dewey 1998; Lartey et al. 2000).
(3) The Honduras studies
For the normal birthweight infants, the selection criteria were that women were primiparous, willing to breast‐feed exclusively for 26 weeks, not employed outside the home before 6 months postpartum, low income (household earnings of less than $150/month), and at least 16 years old and healthy (not taking medication on a regular basis), and the infants were healthy, term and weighed at least 2500 g at birth (Cohen et al. 1995). The length of the infants was not measured during the first 4 months. For the low birthweight infants, the selection criteria were similar, except that there were no income or parity criteria, women ≥ 15 years were included, and birth‐weight was 1500–2500 g (Dewey et al. 1999). In both studies the infants were exclusively breast‐fed for 4 months, and then were randomly assigned to continue exclusive breast‐feeding until 6 months, or to receive commercially prepared, nutritionally enhanced solid foods in addition to breast milk between 4 and 6 months. All infants were given home‐prepared complementary foods after 6 months. At 12 months a majority of the infants in both groups (>93%) were still breast‐fed.
Anthropometry
The anthropometric measurements included maternal height, and monthly assessment of infant weight, length, and for the U.S. and Ghana, mid‐upper‐arm circumference and triceps and subscapular skinfolds. Weight‐for‐age (W/A), length‐for‐age (L/A), and weight‐for‐length (W/L) Z‐scores (SD values) were calculated using U.S. National Center for Health Statistics reference data (NCHS 1977).
Data analysis
Data analysis was carried out with the SAS System for Windows Release 6 (SAS Institute, Inc., Cory, NC, USA). The dependent variable was change in length during each of the following 3‐month intervals: 1– 4, 2–5, 3–6, 4–7, 5–8, 6–9, 7–10, 8–11, and 9–12 months. Initially, analyses were performed using length gain intervals of both 2 months and 3 months duration. Because little difference in the results was observed, we decided to perform all of the subsequent analyses using the 3 months outcomes.
The three main independent variables were: (1) W/L at the beginning of each 3‐month interval (‘initial W/L’); (2) skinfold thickness (triceps + subscapular (U.S. and Ghana studies only)) at the beginning of each 3‐month interval (‘initial skinfold thickness’); and (3) weight change during the 3 months prior to each of the length gain intervals (‘prior weight change’). Other independent variables, depending on the model, included study, maternal height, infant sex, length‐for‐age z‐score at the beginning of each 3‐month interval (‘initial L/A’), concurrent weight change (i.e. during the same 3‐month interval) and feeding mode (breast‐feeding (BF) or formula feeding (FF) (U.S. only)).
After pooling data for the breast‐fed infants in all four studies, correlation analyses were used to determine the strength of the associations among the three main independent variables (initial W/L, skinfold thickness and prior weight change). To examine the relationships between the three main independent variables and subsequent change in length during each interval, partial correlations were calculated, controlling for study, infant sex, maternal height and initial L/A. The partial correlations with prior weight change were examined both with and without controlling for concurrent weight change. In addition, the partial correlations with initial W/L were examined while controlling for both prior and concurrent weight change, and the partial correlations with prior weight change were examined while controlling for initial W/L and concurrent weight change (in addition to the other covariates listed above).
Analysis of covariance (SAS GLM procedure), including a square term for initial W/L, was used to determine if the relationship between change in length and initial W/L was non‐linear (i.e. to examine whether a minimum W/L is necessary before length gain occurs), again controlling for the covariates listed above. The square term was significant in only one period (1– 4 months), indicating that the relationship was linear at nearly all the age intervals examined. Thus, the square term was omitted from all subsequent analyses.
We then examined whether the correlations between initial W/L or prior weight change and subsequent length gain were (1) site‐ or study‐related, or (2) sex‐specific, or (3) related to feeding mode. For the first set of analyses, the ANCOVA procedure included the interaction term between study and initial W/L or prior weight change. Other independent variables included in the models were study, infant sex, maternal height and initial L/A. For the second set of analyses, the same procedures were used, but the models included the interaction term between infant sex and initial W/L or prior weight change. For the third set of analyses, only data from the U.S. were used, with the models including the interaction between feeding mode (breast‐fed or formula‐fed) and initial W/L or prior weight change, as well as infant sex, maternal height and initial L/A.
P‐values of < 0.05 for main effects and < 0.10 for interaction effects were considered significant.
Results
Selected characteristics of subjects in the four populations are shown in Table 1. There were large differences across populations in mean birthweight, maternal height, and infant length, initial W/L and skinfold thickness. Although there was a wide range in W/L Z‐scores at 1 and 6 months, relatively few infants were < −2 SD (2 infants at 1 month, both in Ghana; 7 infants at 6 months, of whom 5 were in Ghana and 1 each were in the two Honduran cohorts). By 12 months, there were 25 infants with W/L < −2 SD: 1 from the U.S. study, 13 from Ghana, 2 from the Honduras‐NBW study, and 9 from the Honduras‐LBW study. Figure 1 shows the mean length gain of the infants by study and during each age interval. Mean length gain was initially lower in infants from the U.S. study but this trend reversed after 6 months of age when the length gain of the U.S. infants exceeded that of the other three cohorts.
Table 1.
Subject characteristics in the four cohorts of breast‐fed infants
| n | U.S. 50* | Ghana 190 | Honduras NBW 108 | Honduras LBW 119 |
|---|---|---|---|---|
| Birthweight (g) | ||||
| Mean | 3605 | 3210 | 3065 | 2330 |
| SD | 496 | 437 | 405 | 166 |
| Maternal height (cm) | ||||
| Mean | 165 | 158 | 154 | 150 |
| SD | 6.4 | 5.8 | 5.4 | 6.5 |
| Infant length (cm) | ||||
| 1 month | ||||
| Mean | 55.5 | 53.5 | NA † | 50.6 |
| SD | 2.4 | 2.1 | 1.7 | |
| 6 months | ||||
| Mean | 67.1 | 66.4 | 66.1 | 63.7 |
| SD | 2.5 | 2.2 | 2.3 | 2.2 |
| 12 months | ||||
| Mean | 75.1 | 73.4 | 73.1 | 70.7 |
| SD | 2.7 | 2.5 | 2.7 | 2.8 |
| Initial W/L (z‐score) | ||||
| 1 month | ||||
| Mean | 0.17 | 0.17 | −0.24 | |
| SD | 0.65 | 0.86 | 0.65 | |
| 6 months | ||||
| Mean | 0.09 | −0.15 | 0.51 | 0.08 |
| SD | 0.88 | 0.86 | 0.95 | 0.98 |
| 12 months | ||||
| Mean | −0.45 | −0.84 | −0.35 | −0.73 |
| SD | 0.95 | 0.88 | 0.85 | 0.94 |
| Skinfold thickness ‡ (mm) | ||||
| 1 month | ||||
| Mean | 14.8 | 14.7 | ||
| SD | 2.8 | 2.5 | ||
| 6 months | ||||
| Mean | 17.8 | 15.0 | ||
| SD | 3.5 | 2.4 | ||
| 12 months | ||||
| Mean | 15.9 | 14.0 | ||
| SD | 3.7 | 2.5 | ||
NBW, normal birthweight; LBW, low birthweight; *Breast‐fed cohort only; †Length not measured during the first 4 months; ‡Sum of triceps and subscapular skinfolds.
Figure 1.
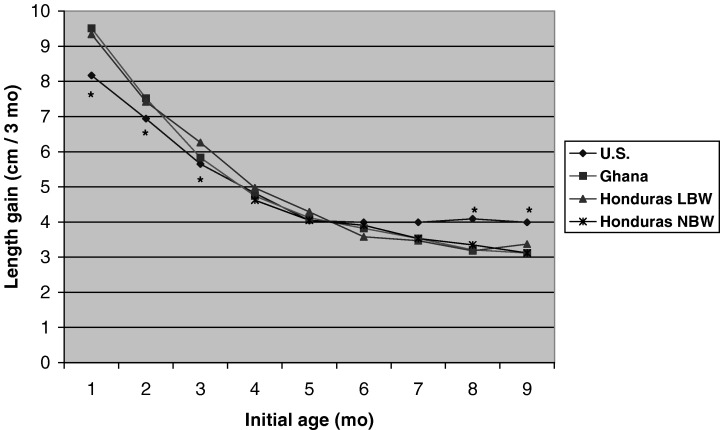
Mean length gain (cm per 3‐month interval) during each age interval, by study. Compared to length gain of infants in the U.S., length gain of infants in the other sites was significantly greater at 1–4, 2–5 and 3–6 months and significantly lower at 8–11 and 9–12 months.
Table 2 shows that the correlation coefficients among the 3 main independent variables were all statistically significant. The correlations between initial W/L and prior weight change generally decreased with age, from about 0.50–0.60 at 3 months to 0.25 at 12 months. The correlations between initial W/L and skinfold thickness increased with infant age, from 0.51 at 1 month to 0.70 or greater at 10–12 months.
Table 2.
Correlations among independent variables (initial W/L, prior weight change, and sum of skinfold thickness) for breast‐fed infants in the four cohorts combined
| Age (months) of infant at beginning of interval | W/L and prior weight change | W/L and SF | Prior weight change and SF | |||
|---|---|---|---|---|---|---|
| r | n | r | n | r | n | |
| 1 | 0.51* | 231 | ||||
| 2 | 0.59* | 237 | ||||
| 3 | 0.53* | 337 | 0.60* | 238 | 0.62* | 205 |
| 4 | 0.56* | 475 | 0.59* | 239 | 0.52* | 235 |
| 5 | 0.60* | 468 | 0.60* | 240 | 0.44* | 238 |
| 6 | 0.60* | 465 | 0.61* | 240 | 0.46* | 238 |
| 7 | 0.54* | 458 | 0.62* | 238 | 0.43* | 237 |
| 8 | 0.47* | 458 | 0.66* | 239 | 0.38* | 239 |
| 9 | 0.37* | 459 | 0.69* | 240 | 0.30* | 240 |
| 10 | 0.31* | 447 | 0.72* | 236 | 0.33* | 235 |
| 11 | 0.26* | 440 | 0.72* | 235 | 0.27** | 234 |
| 12 | 0.25* | 437 | 0.70* | 236 | 0.25** | 236 |
SF = Skinfold thickness, measured only in the U.S. and Ghana studies; *P < 0.0001; **P ≤ 0.0005.
Table 3 shows that the partial correlations between initial W/L and subsequent length gain were statistically significant at all age intervals. The partial correlations between skinfold thickness and subsequent change in length were significant only at 3 and 4 months. The partial correlations between prior weight change and subsequent length gain were significant at all age intervals and showed little change when controlling for concurrent weight change. Table 4 indicates that the partial correlations with initial W/L were attenuated but generally still significant when controlling for prior and concurrent weight change, and that the partial correlations with prior weight gain were also attenuated but generally still significant when controlling for initial W/L and concurrent weight change.
Table 3.
Partial correlations of subsequent 3‐month length gain with initial W/L, skinfold thickness and prior weight change † , for breast‐fed infants in the four cohorts combined
| Age (months) of infant at beginning of interval | Initial W/L | Skinfold thickness‡ | Prior weight change | Prior weight change controlling for concurrent weight change | ||||
|---|---|---|---|---|---|---|---|---|
| r | n | r | n | r | n | r | n | |
| 1 | 0.19** | 339 | 0.06 | 235 | ||||
| 2 | 0.22* | 358 | 0.11 | 236 | ||||
| 3 | 0.27* | 356 | 0.22** | 238 | 0.26* | 323 | 0.19** | 323 |
| 4 | 0.30* | 456 | 0.23** | 237 | 0.34* | 453 | 0.27* | 453 |
| 5 | 0.33* | 458 | 0.08 | 238 | 0.36* | 456 | 0.32* | 456 |
| 6 | 0.26* | 459 | −0.06 | 240 | 0.27* | 447 | 0.27* | 447 |
| 7 | 0.23* | 446 | 0.00 | 236 | 0.25* | 445 | 0.25* | 445 |
| 8 | 0.19* | 440 | 0.07 | 234 | 0.29* | 440 | 0.32* | 440 |
| 9 | 0.15** | 437 | 0.02 | 234 | 0.15** | 437 | 0.16** | 437 |
P < 0.0001;
P < 0.01;
Controlling for study, maternal height, infant sex and initial length‐for‐age z‐score;
‡ Data from the U.S. and Ghana only.
Table 4.
Partial correlations of subsequent 3‐month length gain with initial W/L (controlling for concurrent and prior weight change) and with prior weight change (controlling for W/L and concurrent weight change) † , for breast‐fed infants in the four cohorts combined
| Age (months) of infant at beginning of interval | Initial W/L ‡ | Prior weight gain § r | |
|---|---|---|---|
| n | r | ||
| 3 | 323 | 0.08 | 0.05 |
| 4 | 453 | 0.10* | 0.13** |
| 5 | 456 | 0.15** | 0.13** |
| 6 | 457 | 0.15** | 0.11* |
| 7 | 445 | 0.18** | 0.11* |
| 8 | 440 | 0.13** | 0.23** |
| 9 | 437 | 0.14** | 0.10* |
P < 0.05;
P < 0.01;
† Controlling for study, maternal height, infant sex and initial length‐for‐age z‐score;
Controlling for concurrent and prior weight change;
Controlling for initial W/L and concurrent weight change.
In nearly all the age intervals, the relationship between initial W/L and length gain was linear. Only at 1– 4 months did we see a significant non‐linear relationship (P = 0.016); at that age, length gain did not continue to increase with higher W/L, but there was no obvious inflection point.
Figure 2 shows that the slope of the relationship between W/L and subsequent length gain was similar in boys and girls except at 1 month, when the slope was significantly higher in boys than in girls. After 3 months, the slopes tended to decline with age (more so in girls than in boys). The slope of the relationship between prior weight change and length gain was significantly higher in girls than in boys at 4 months, but otherwise there were no significant differences by infant sex (data not shown).
Figure 2.
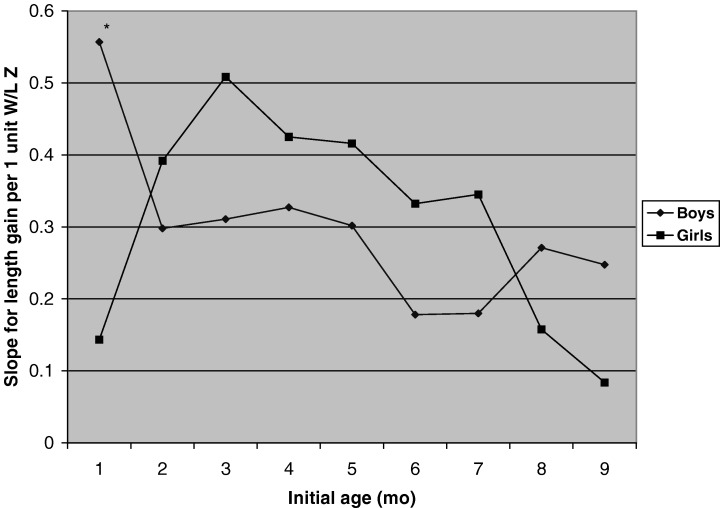
Slopes of the relationship between initial W/L and subsequent length gain during each 3‐month interval (cm/3 months per 1 unit Z‐score in W/L), by infant sex, controlling for study, maternal height and initial L /A. The main effect of initial W/L was significant at all age intervals. The interaction between sex and initial W/L was significant at 1– 4 months (P = 0.019), but not at any other age. At 1– 4 months, initial W/L was significantly associated with subsequent length gain in boys (P < 0.0001) but not in girls (P = 0.22), and the slopes for boys and girls differed significantly (P < 0.05).
Figure 3 shows that the slopes of the relationship between initial W/L and subsequent change in length were similar across the studies except at 1, 5 and 6 months (at 1 month, the slope was higher in Ghana than in the U.S.; at 5 months, the slope was lower in Ghana than in the U.S. or Honduras‐LBW studies; and at 6 months, the slope was lower in Ghana than in the Honduras studies). Similar results were found when prior weight change was used instead of initial W/L (data not shown: at 4 and 5 months the slope for the Honduras‐LBW study was greater than for the other studies).
Figure 3.
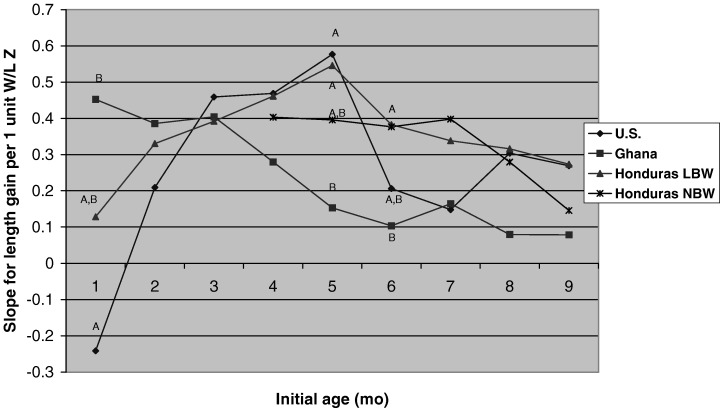
Slopes of the relationship between initial W/L and subsequent length gain during each 3‐month interval (cm/3 months per 1 unit Z‐score in W/L), by study, controlling for infant sex, maternal height and initial L /A. Slopes with different superscripts are significantly different between studies (P < 0.05). The main effect of initial W/L was significant (P < 0.001) at all age intervals. The interaction between study and initial W/L was significant at 1– 4 months (P = 0.04), 5–8 months (P = 0.0007) and 6–9 months (P = 0.0034), but not at 2–5, 3–6, 4–7, 7–10, 8–11 or 9–12 months. At 1– 4 months, initial W/L was significantly associated with subsequent length gain in Ghana (P < 0.0001), but not in the U.S. or Honduras‐LBW studies. At 5–8 months, initial W/L was significantly associated with subsequent length gain in all sites (P ≤ 0.001) except Ghana (P = 0.08). At 6–9 months, the relationship was significant in the two Honduras studies (P < 0.001), but not in Ghana or the U.S. (though no pairs of slopes differed significantly from each other).
Figure 4 shows the slopes of the relationship between W/L and subsequent change in length by feeding mode (U.S. data only). The only statistically significant difference was at 2 months, when the slope for the formula‐fed infants was greater than the slope for the breast‐fed infants. When prior weight change was used instead of initial W/L, there was a statistically significant difference in slopes only at 5 months (lower in breast‐fed than in formula‐fed infants (data not shown)).
Figure 4.
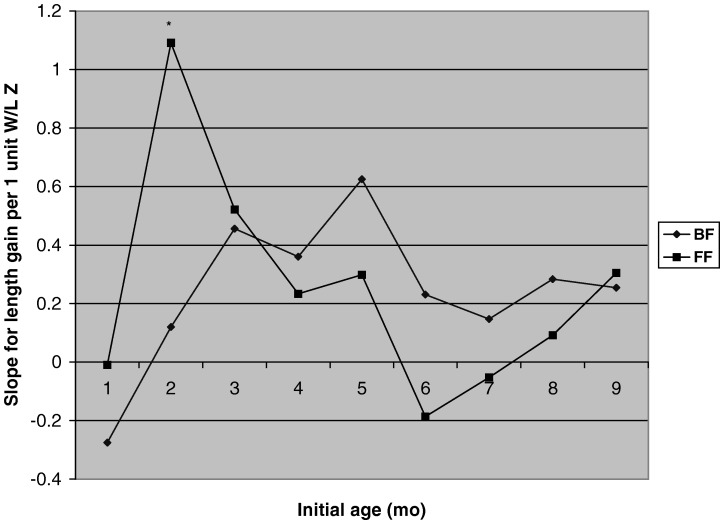
Slopes of the relationship between initial W/L and subsequent length gain during each 3‐month interval (cm/3 months per 1 unit Z‐score in W/L) in the U.S., by feeding mode, controlling for infant sex, maternal height and initial L/A. The main effect of initial W/L was significant (P < 0.05) at 2–5, 3–6, 4–7, 5–8 and 9–12 months, but not at 1–4, 6–9, 7–10 or 8–11 months. The interaction between feeding mode and initial W/L was significant at 2–5 months (P = 0.013), when the relationship of initial W/L and subsequent length gain was significant in formula‐fed infants (P = 0.0009) but not in breast‐fed infants (P = 0.61), and the slopes differed significantly (P < 0.05) between feeding groups.
Discussion
There is limited knowledge regarding the mechanisms governing growth in infants. We found that length gain was positively correlated with initial W/L and prior weight change during all age intervals. These associations were evident in all four populations, in both girls and boys, and in breast‐fed and formula‐fed infants. There were relatively few differences between subgroups in the slopes of the relationship between length gain and initial W/L or prior weight change during the age intervals examined, and those that were significant could have been due to chance, given the number of comparisons made. The consistency of this relationship across studies supports the hypothesis that linear growth is partly regulated by initial body mass or fatness in infants. The relationship between length gain and skinfold thickness was significant only at 3–7 months of age, however. Skinfold thickness is difficult to measure accurately and may not adequately reflect total body fatness during infancy, which could account for the relative weakness of this relationship. Alternatively, it may be that accrual of lean body mass rather than fat mass best explains the association between initial W/L and subsequent length gain. One could argue that the association with W/L is simply a result of shorter infants (who would tend to have a higher W/L) regressing to the population mean, but this would not explain our results because we controlled for initial length‐for–age and the associations were still significant.
In Bangladesh and Nepal, catch‐up growth in weight was followed by a lag period of 3–4 months before catch‐up growth in length gain occurred in infants and children (Brown et al. 1982; Nabarro et al. 1988). By contrast, Xu et al. (2001) found little evidence for a lagged response in length gain among infants in China born between 1980 and 1990 (Xu et al. 2001). Our results indicated that length gain was correlated with prior weight change even when controlling for concurrent weight change and initial W/L. This supports the concept of a lagged response in linear growth. However, it should be noted that the partial correlation coefficients were relatively low (generally 0.1–0.2). The results suggest that both initial W/L and prior weight change have a stimulatory effect on linear growth.
The study in Jamaica by Walker & Golden (1988) suggested that malnourished children need to attain a minimum threshold in W/L before linear growth is resumed. In our study, we did not find any evidence for a threshold effect between initial W/L and length gain. However, because all the infants in our study were breast‐fed and fed complementary foods according to recommendations, few were below −2 SD for W/L in the first year of life (2 infants were below −2 SD for initial W/L at 1 month, and 25 infants were below this cutoff by 12 months of age). This made it difficult for us to examine the relationship between W/L and length gain at the lower end of the range in W/L. Apart from this limitation, the difference between our findings and those of Walker and Golden may also be caused by other factors, such as infant micronutrient status. Zinc, for example, is an important micronutrient for infant growth (Brown et al. 2002). Infants recovering from malnutrition may require time for their micronutrient status to normalize as they gain weight before linear growth is triggered.
Little is known about the potential mechanisms underlying the relationship between body mass or fatness and linear growth. One possibility is that hormones associated with fatness, such as leptin, promote linear growth. Several studies indicate that leptin, which is synthesized by adipocytes and positively correlated with bodyweight and percentage of body fat in both children and adults, is a regulator of food intake and energy metabolism (Havel 1998; Lönnerdal & Havel 2000). Evidence is accumulating that leptin is also involved in bone metabolism during human development (Whipple et al. 2002), with higher levels of leptin having a stimulatory effect on skeletal growth, possibly in conjunction with growth hormone. Serum leptin concentration has been positively correlated with serum insulin‐like growth factor‐I (IGF‐I) in healthy infants (Palacio et al. 2002) and children (Kratzsch et al. 2002), and IGF‐I concentration has been associated with subsequent linear growth (Juul et al. 1994). Thus, there appears to be a link between leptin and the IGF‐I‐growth hormone axis with regard to the regulation of body composition, which could explain our findings. Another possibility is that infants who have a higher food intake and put on more body mass simply take in more nutrients that stimulate IGF‐I and linear growth, without body mass necessarily playing a mediating role.
In conclusion, the results of this and other studies suggest that linear growth is regulated in part by initial body mass or fatness in infants. The public health implication of this finding is that in populations where malnutrition is highly prevalent, weight gain is likely to be the first sign of nutritional improvement, not necessarily length gain. Thus, even when the major long‐term goal is to prevent stunting in children, strategies to optimize weight‐for‐length may play a critical role.
Acknowledgements
We gratefully acknowledge all those who were involved with data collection for the four studies, particularly MJ Heinig, LA Nommsen‐Rivers and B Lonnerdal (the U.S. study), L Landa Rivera and J Canahuati (the Honduras studies), and A Manu (the Ghana study).
References
- Brown K.H., Black R.E. & Becker S. (1982) Seasonal changes in nutritional status and the prevalence of malnutrition in a longitudinal study of young children in rural Bangladesh. American Journal of Clinical Nutrition, 36, 303–313. [PubMed] [Google Scholar]
- Brown K.H., Peerson J.M., Rivera J. & Allen L.H. (2002) Effect of supplemental zinc on the growth and serum zinc concentrations of prepubertal children: a meta‐analysis of randomized controlled trials. American Journal of Clinical Nutrition, 75, 1062–1071. [DOI] [PubMed] [Google Scholar]
- Cohen R.J., Brown K.H., Canahuati J., Landa Rivera L. & Dewey K.G. (1995) Determinants of growth from birth to 12 months among breast‐fed Honduran infants in relation to age of introduction of complementary foods. Pediatrics, 96, 504–510. [PubMed] [Google Scholar]
- Costello A.M. (1989) Growth velocity and stunting in rural Nepal. Archives of Disease in Childhood, 64, 1478–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewey K.G. (1998) Cross‐cultural patterns of growth and nutritional status of breast‐fed infants. American Journal of Clinical Nutrition, 67, 10–17. [DOI] [PubMed] [Google Scholar]
- Dewey K.G., Cohen R.J., Brown K.H. & Landa Rivera L. (1999) Age of introduction of complementary foods and growth of term, low‐birth‐weight, breast‐fed infants: a randomized intervention study in Honduras. American Journal of Clinical Nutrition, 69, 679–686. [DOI] [PubMed] [Google Scholar]
- Havel P.J. (1998) Leptin production and action: relevance to energy balance in humans. American Journal of Clinical Nutrition, 67, 355–356. [DOI] [PubMed] [Google Scholar]
- Juul A., Bang P., Hertel N.T., Main K., Dalgaard P., Jorgensen K. et al. (1994) Serum insulin‐like growth factor‐I in 1030 healthy children, adolescents, and adults: relation to age, sex, stage of puberty, testicular size, and body mass index. The Journal of Clinical Endocrinology and Metabolism, 78, 744–752. [DOI] [PubMed] [Google Scholar]
- Kratzsch J., Lammert A., Bottner A., Seidel B., Mueller G., Thiery J. et al. (2002) Circulating soluble leptin receptor and free leptin index during childhood, puberty, and adolescence. The Journal of Clinical Endocrinology and Metabolism, 87, 4587–4594. [DOI] [PubMed] [Google Scholar]
- Lartey A., Manu A., Brown K.H., Peerson J.M. & Dewey K.G. (2000) Predictors of growth from 1 to 18 months among breast‐fed Ghanaian infants. European Journal of Clinical Nutrition, 54, 41–49. [DOI] [PubMed] [Google Scholar]
- Lönnerdal B. & Havel P.J. (2000) Serum leptin concentrations in infants: effects of diet, sex, and adiposity. American Journal of Clinical Nutrition, 72, 484–489. [DOI] [PubMed] [Google Scholar]
- Nabarro D., Howard P., Cassels C., Pant M., Wijga A. & Padfield N. (1988) The importance of infections and environmental factors as possible determinants of growth retardation in children In: Linear Growth Retardation in Less‐Developed Countries (ed. Waterlow JC.), pp 165–184. Raven Press: New York, NJ. [Google Scholar]
- National Center for Health Statistics (NCHS) (1977) NCHS Growth Curves for Children: Birth‐ 18 Years. US Department of Health, Education, and Welfare: Washington, DC. [Google Scholar]
- Palacio A.P., Perez‐Bravo F., Santos J.L., Schlesinger L. & Monckeberg F. (2002) Leptin levels and IgF‐binding proteins in malnourished children: effect of weight gain. Nutrition, 18, 17–19. [DOI] [PubMed] [Google Scholar]
- Walker S.P. & Golden M.H.N. (1988) Growth in length of children recovering from severe malnutrition. European Journal of Clinical Nutrition, 42, 395–404. [PubMed] [Google Scholar]
- Waterlow J.C. (1994) Relationship of gain in height to gain in weight. European Journal of Clinical Nutrition, 48(Suppl. 1), S72–S74. [PubMed] [Google Scholar]
- Whipple T., Sharkey N., Demers L. & Williams N. (2002) Leptin and the skeleton. Clinical Endocrinology, 57, 701–711. [DOI] [PubMed] [Google Scholar]
- Xu X., Wang W.P., Guo Z.P., Cheung Y.B. & Karlberg J. (2001) Seasonality of growth in Shanghai infants (n= 4128) born in 11 consecutive years. European Journal of Clinical Nutrition, 55, 714–725. [DOI] [PubMed] [Google Scholar]


