Abstract
Objective:
The present study was designed to determine whether healthy older adults with age-related vestibular loss have deficits in spatial navigation.
Methods:
154 adults participating in the Baltimore Longitudinal Study of Aging were tested for semicircular canal, saccular, and utricular function and spatial navigation ability using the blindfolded Triangle Completion Test (TCT). Multiple linear regression was used to investigate the relationships between each measure of vestibular function and performance on the TCT (angular error, end point error, and distance walked) while controlling for age and sex.
Results:
Individuals with abnormal saccular function made larger angular errors (β = 4.2 degrees, p < 0.05) and larger end point errors (β = 13.6 cm, p < 0.05). Independent of vestibular function, older age was associated with larger angular (β’s = 2.2 - 2.8 degrees, p’s < 0.005) and end point errors (β’s = 7.5 - 9.0 cm, p’s < 0.005) for each decade increment in age.
Conclusions:
Saccular function appears to play a prominent role in accurate spatial navigation during a blindfolded navigation task.
Significance:
We hypothesize that gravitational cues detected by the saccule may be integrated into estimation of place as well as heading direction.
Keywords: vestibular function, aging, spatial orientation, gait, posture
1. Introduction
Spatial navigation is an essential cognitive motor skill that humans utilize on a daily basis for survival (Foo et al. 2005). Navigation strategies can be allocentric or egocentric. Allocentric navigation depends on the memory of certain landmarks and the ability to orient oneself with respect to a previously known object or aspect of a scene (Foo et al. 2005). Egocentric navigation, however, relies on path integration, the continuous updating of both the distance and direction traveled from an initial starting point using oneself as a frame of reference (Foo et al. 2005; Koutakis et al. 2013; Xie et al. 2017).
Path integration allows humans to monitor their positions in space based on multiple self-motion cues (Adamo et al. 2012). These cues include inputs from the visual, vestibular, and proprioceptive systems. The visual system processes visual information stemming from physical movement of oneself or the environment which results in optic flow (Gibson 1958; Adamo et al. 2012). This visual information is used for obstacle avoidance (Patla and Vickers 2003) and is combined with vestibular signals to identify where objects are represented in space (Dokka et al. 2015; Vélez-Fort et al. 2018). The somatosensory and proprioceptive systems provide sensory feedback from skin, muscles, joints, and tendons specifically regarding touch or pressure contact and limb segmental motion (Zehr and Stein 1999). The proprioceptive system only provides spatial cues for aspects of the environment directly contacting the individual, but likely contributes to spatial orientation during self-motion by mapping limb speed to an internal model of the body (Vazquez et al. 2015). The vestibular system senses head motion and differentiates between linear (saccule and utricle) and angular (semicircular canals) accelerations respectively (Fernandez and Goldberg 1971; Goldberg and Fernández 1975). Vestibular signals contribute to both heading direction and object location via inputs to head direction cells and place cells respectively (Smith et al. 2015).
The triangle completion task (TCT) has been used as a clinical test of path integration in several previous studies (Loomis et al. 1993; Klatzky 1999; Marlinsky 1999; Adamo et al. 2012). The vestibular system plays a critical role in updating spatial information derived from sensing changes in the speed of linear translation (distance-specific) and rotation (direction-specific relative to global spatial coordinates) (Adamo et al. 2012). Individuals with vestibulopathy struggle with estimating and reproducing a simple or complex path or direction of a previously observed target (Glasauer et al. 1994, 2002, Borel et al. 2004, 2008). Individuals with vestibular loss also have greater difficulty with virtual navigation compared to individuals with a healthy vestibular system (Kremmyda et al. 2016). Consequently, the vestibular system provides significant input to path integration. In the absence of vision, vestibular inputs provide the sole cues regarding the earth fixed frame of reference which serves as the basis for egocentric navigation (Borel et al. 2008).
Recently, we demonstrated that older adults with vestibulopathy had worse performance on a dynamic spatial navigation task compared to both healthy young and healthy older adults (Xie et al. 2017). Vestibular function declines with age (Paige 1992; Yang et al. 2015; Zalewski 2015); however, it is not known whether age related changes in vestibular function contribute to impairments in spatial navigation. The TCT is a test of spatial navigation that allows for distinct identification of end point errors and heading or angular errors, which may reflect the sensitivity of the vestibular system to both linear and rotational motion respectively. Therefore, it may be feasible to provide mechanistic insight regarding vestibular contributions to human spatial navigation using the TCT. In this study, we investigated the association between distance walked, angular errors, and end point errors measured during performance on the TCT with vestibular function in a cohort of healthy older adults.
2. METHODS
2.1. Study Participants
The Baltimore Longitudinal Study of Aging is a prospective cohort study started in 1958 that is conducted by the Intramural Research Program of the National Institute on Aging. Community-dwelling adults ages 20 and older participate in three days of standardized tests every 1-4 years at the Clinical Research Unit of the IRP-NIA in Baltimore, MD. A cross-sectional sample of 154 participants who underwent TCT testing and vestibular function testing from January 2016 to March 2017 were evaluated for this study. All participants provided written informed consent prior to testing. The National Institute of Environmental Health Sciences Institutional Review Board approved the study protocol.
2.2. Vestibular Function Tests
The vestibular system consists of semicircular canals which detect head rotations and otolith organs (saccule and utricle) which sense linear head movements and gravitational acceleration. Baltimore Longitudinal Study of Aging participants who consented to vestibular testing underwent tests for both otolith function and semicircular canal function, briefly described below. Detailed methods to measure otolith and semicircular canal function in this population have been published previously (Li et al. 2015a, 2015b).
2.2.1. Video Head Impulse Testing
Participants wore the GN Otometrics video-oculography system (GN Otometrics, Schaumberg, IL USA), while sitting and viewed a wall mounted visual fixation target approximately 1.25 meters away. Trained examiners performed 10-15 small amplitude (5°-15°) left and right head impulses, with peak velocities typically between 150°-250°/second. The ratio of the area under the curve for eye velocity divided by head velocity was used to calculate the horizontal vestibulo-ocular reflex (VOR) gain (MacDougall et al. 2009). Individuals with restriction/pain during neck rotation or previous cervical spine surgery were excluded from head impulse testing.
2.2.2. Vestibular evoked myogenic potential (VEMP) testing
The air-conduction evoked cervical VEMP (cVEMP) evaluates mainly saccular function, while the ocular VEMP (oVEMP) evaluates mainly utricular function. Electromyographic (EMG) signals were recorded with pregelled, disposable, self-adhesive, Ag/AgCl electrodes from GN Otometrics (Schaumburg, IL, USA) using a commercial EMG system (Carefusion Synergy, version 14.1, Dublin, OH, USA). EMG signal processing included 2500x amplification and band-pass filtering between 3-500 Hz for ocular VEMPs and between 20–2000 Hz for cervical VEMPs (Nguyen et al., 2010).
2.2.2.1. Ocular VEMPs
Participants were reclined at 30 degrees from horizontal while maintaining a 20 degree upward gaze at a target on the ceiling. Fifty midline head taps delivered at Fz with an Aesculap model ACO12C reflex hammer. An inertial microswitch triggered the EMG recording. The oVEMP waveform is characterized by a negative peak (n10, approximately 10 ms after the head tap), followed by a positive peak (p16, approximately 16 ms after the head tap). An absent oVEMP response was defined as EMG recordings lacking definable n10 waves. Participants unable to see the oVEMP fixation target due to visual impairments such as blindness, or who declined to participate were excluded.
2.2.2.2. Cervical VEMPs
While reclined at 30 degrees from horizontal, participants lifted and turned their heads to the right and left to provide tonic background sternocleidomastoid (SCM) activity during cVEMP testing. Air-conducted positive polarity tone bursts at 500 Hz and 125 dB SPL (2 ms plateau and 1 ms ramps presented at a frequency of 5Hz) were delivered monaurally through an Audiocups noise-excluding headset (Amplivox, Eden Prairie, MN). The cVEMP waveform consists of a positive peak (p13, approximately 13 ms after each tone burst), followed by a negative peak (n23, approximately 23 ms after each tone burst). Peak to peak cVEMP amplitudes were normalized to tonic background SCM activity. An absent cVEMP response was defined as EMG recordings lacking definable p13 waves. Participants were excluded from the cVEMP test if they had conductive hearing loss or if they had pain when turning their head to the right or left.
For both cVEMP and oVEMP tests, normal vestibular function was defined as the presence of a vestibular evoked myogenic potential in both ears. Abnormal vestibular function was defined as either unilaterally or bilaterally absent function.
2.3. Triangle Completion Test
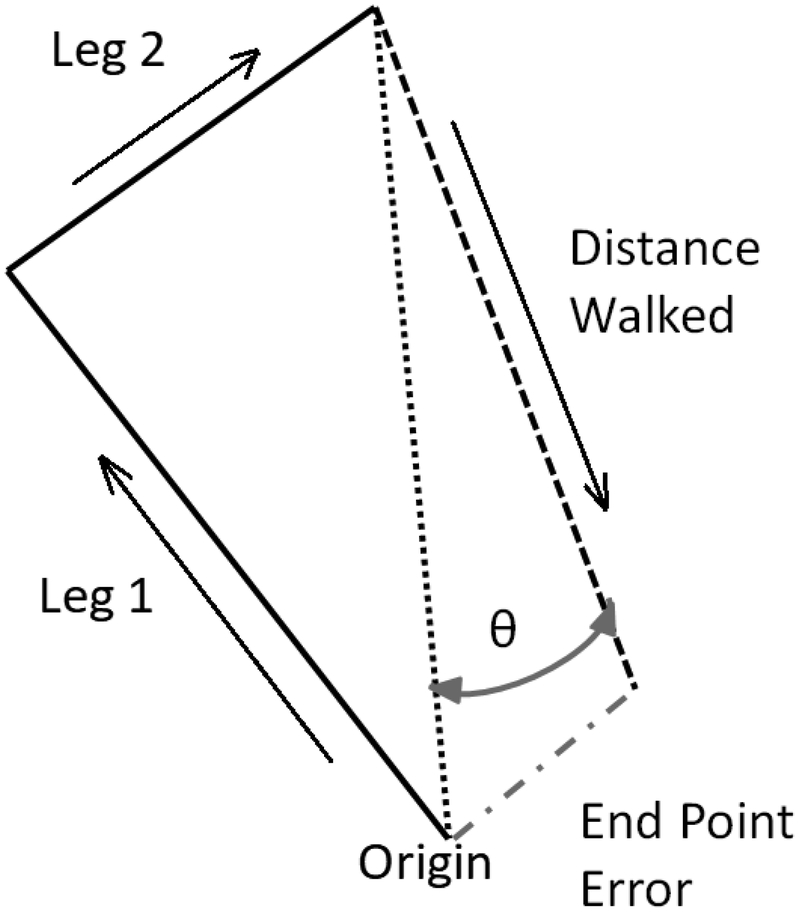
The corners of four triangles were distinctly marked with colored tape on the floor. Each triangle was a right triangle, in which the sides were of lengths 1 meter, 2 meters, and 2.23 meters respectively, as shown in Figure 1 (Klatzky 1998). Participants were instructed to visualize each triangle before donning a blindfold and noise-attenuating headphones. They were passively guided along one leg (either 1 or 2 meters), a 90 degree turn, and the next leg (either 2 or 1 meters). Participants then were instructed to “turn and complete the triangle” and they attempted to independently return to the origin. Participants always had to walk the hypotenuse leg of the triangle. Each triangle was only attempted once. The distance walked for the third leg of the triangle (distance walked), the linear distance (in cm) between where the participant stopped and the end point (end point error) and the absolute value of the angular error (degrees) with respect to the ideal rotation angle (angle of deviation) were recorded and averaged across all completed triangles. No data were recorded for trials that required intervention from the tester to prevent the participant from walking into a wall or falling.
Figure 1.
Example layout of a triangle. Blindfolded participants are guided along Leg 1 and Leg 2 in the directions of the arrows and asked to “turn and complete the triangle.” An accurate performance is exemplified by the dotted black line connecting the end of “Leg 2” to the “Origin”. An inaccurate performance is demonstrated by the dashed black line, with the arrow indicating the direction of walking. Θ represents the angular error (degrees), end point error represents the linear error (cm), and distance walked is the linear distance the participant walked (cm).
2.4. Data Analysis
Separate multiple linear regression models adjusting for age and sex were developed to explore the association between VOR gain, oVEMP, and cVEMP function and distance walked, angle of deviation, and end point error on the TCT. cVEMP and oVEMP function were coded as 0 normal and 1 for abnormal. Statistical significance was determined based on p-values of less than 0.05 for each analysis. STATA version 14 (College Station, TX, USA) was used for all statistical analyses.
3. Results
The study population consisted of 154 participants with a mean age of 69.8 +/− 13.6 years (Table 1). A majority of participants (54.5%) were female. One participant with a VOR > 1.6 was excluded as an outlier from the VOR analysis due to high leverage based on regression diagnostics. VOR gain was less than 0.8 for 13 participants. VOR gain and VEMP function is described in Table 1.
Table 1:
Demographic Characteristics of Study Population, Baltimore Longitudinal Study of Aging (N = 154)
| Mean (SD) | N (%) | |
|---|---|---|
| Sex | ||
| Male | 70 (45.5%) | |
| Female | 84 (54.5%) | |
| Mean age (SD) [Range] | 69.8 (13.6) [24-93] | |
| Mean VOR gain a | 0.98 (0.11) | |
| VOR Hypofunction (< 0.8) | 13 (10%) | |
| cVEMP b | ||
| Normal Function | 92 (61.3%) | |
| Mean Normalized Amplitude | 2.00 (1.05) | |
| Mean p13 Latency (ms) | 13.88 (0.86) | |
| Mean n23 Latency (ms) | 21.13 (1.59) | |
| Abnormal Function | 58 (38.7%) | |
| Unilateral Absent | 32 (21.3%) | |
| Mean Normalized Amplitude | 1.12 (0.88) | |
| Mean p13 Latency (ms) | 13.78 (1.06) | |
| Mean n23 Latency (ms) | 20.09 (1.48) | |
| Bilateral Absent | 26 (17.3%) | |
| oVEMP c | ||
| Normal Function | 113 (74.3%) | |
| Mean Amplitude (microvolts) | 12.94 (8.28) | |
| Mean n10 Latency (ms) | 7.79 (0.78) | |
| Mean p16 Latency (ms) | 12.36 (1.08) | |
| Abnormal Function | 39 (25.7%) | |
| Unilateral Absent | 20 (13.2%) | |
| Mean Amplitude (microvolts) | 6.42 (3.68) | |
| Mean n10 Latency (ms) | 7.97 (1.37) | |
| Mean p16 Latency (ms) | 11.69 (1.04) | |
| Bilateral Absent | 19 (12.5%) | |
130 participants completed VOR testing
150 participants completed cVEMP testing
152 participants completed oVEMP testing
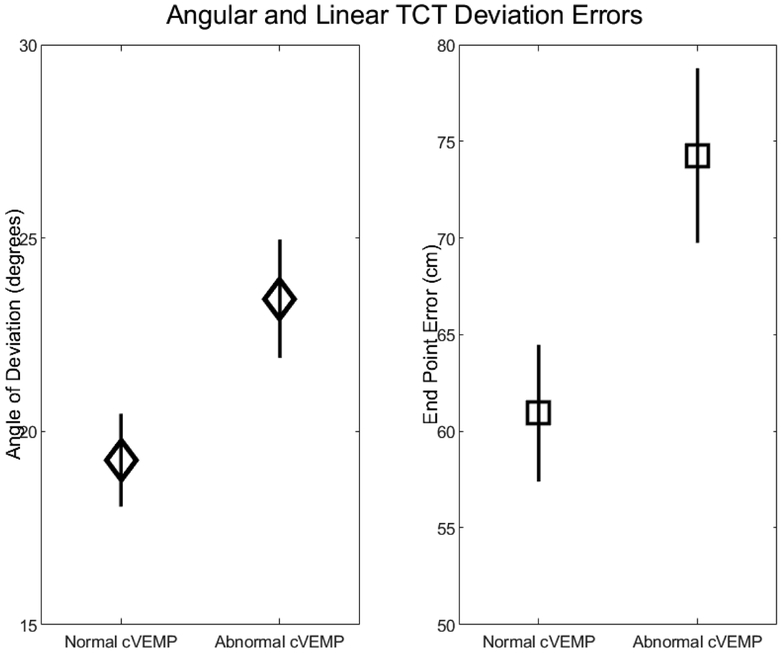
After controlling for age and sex, individuals with abnormal cVEMPs demonstrated significantly larger angular errors on the TCT (β 4.2 degrees, 95% CI 0.27, 8.1), see Figure 2. VOR gain (β 11.8, 95% CI −5.6, 29.1) and abnormal oVEMPs (β 2.67, 95% CI −1.61, 6.95) were not significantly associated with angular error on the TCT, see Table 2. Similarly, individuals with abnormal cVEMPs also had larger end point errors on the TCT (β 13.3 cm, 95% CI 1.8, 24.9), see Figure 2. VOR gain (β 26.8, 95% CI −26.3, 79.8) and abnormal oVEMPs (β 7.4, 95% CI −5.32, 20.01) were not significantly associated with distance of deviation on the TCT, see Table 3. No measure of vestibular function was associated with the distance walked, see Table 4.
Figure 2.
Angular deviation and end point errors increase when saccular function is abnormal (unilaterally or bilaterally absent) compared to normal (bilaterally present). Diamonds and squares represent the marginal means for angular deviation (degrees) and end point error (cm) respectively, controlling for age and sex. Error bars represent standard errors.
Table 2.
Association between semicircular canal and otolith function (present/absent) with angular rotational error (degrees) on the TCT while controlling for age and sex.
| Predictors | β | S.E. | 95% CI |
|---|---|---|---|
| VOR gaina | 11.8 | 8.8 | [−5.6 – 29.1] |
| Age (years) | 0.28 degrees/year * | 0.07 | [0.14 – 0.42] |
| Sex | |||
| Female | Ref | Ref | Ref |
| Male | 3.04 | 2.03 | [−0.97 – 7.05] |
| cVEMP functionb | 4.2 degrees * | 1.98 | [0.27 – 8.1] |
| Age (years) | 0.22 degrees/year * | 0.07 | [0.08 – 0.37] |
| Sex | |||
| Female | Ref | Ref | Ref |
| Male | 0.73 | 1.9 | [−2.95 – 4.41] |
| oVEMP functionc | 2.67 | 2.17 | [−1.61 – 6.95] |
| Age (years) | 0.25 degrees/year * | 0.07 | [0.11 – 0.39] |
| Sex | |||
| Female | Ref | Ref | Ref |
| Male | 0.63 | 1.9 | [−3.06 – 4.31] |
n = 130
n = 150
n = 152
indicates significance with p < 0.05, units provided only for significant relationships.
Table 3.
Association between semicircular canal and otolith function (present/absent) with end point error (cm) on the TCT while controlling for age and sex.
| Predictors | β | S.E. | 95% CI |
|---|---|---|---|
| VOR gaina | 26.8 | 26.8 | [−26.3 – 79.8] |
| Age (years) | 0.90 cm/year * | 0.22 | [0.47 – 1.32] |
| Sex | |||
| Female | Ref | Ref | Ref |
| Male | 2.22 | 6.21 | [−10.07 – 14.5] |
| cVEMP functionb | 13.3 cm * | 5.85 | [1.77 – 24.91] |
| Age (years) | 0.75 cm/year * | 0.21 | [0.33 – 1.17] |
| Sex | |||
| Female | Ref | Ref | Ref |
| Male | −1.8 | 5.5 | [−12.7 – 9.06] |
| oVEMP functionc | 7.35 | 6.41 | [−5.32 – 20.01] |
| Age (years) | 0.82 cm/year * | 0.21 | [0.41 – 1.24] |
| Sex | |||
| Female | Ref | Ref | Ref |
| Male | −2.63 | 5.5 | [−13.53 – 8.28] |
n = 130
n = 150
n = 152
indicates significance with p < 0.05, units provided only for significant relationships.
Table 4.
Association between semicircular canal and otolith function (present/absent) with distance walked (cm) on the TCT while controlling for age and sex.
| Predictors | β | S.E. | 95% CI |
|---|---|---|---|
| VOR gaina | −11.5 | 21.1 | [−53.3 – 30.3] |
| Age (years) | 0.08 | 0.17 | [−0.41 – 0.26] |
| Sex | |||
| Female | Ref | Ref | Ref |
| Male | −8.19 | 4.89 | [−17.9 – 1.5] |
| cVEMP functionb | 3.68 | 4.6 | [−5.4 – 12.76] |
| Age (years) | −0.15 | 0.17 | [−0.48 – 0.18] |
| Sex | |||
| Female | Ref | Ref | Ref |
| Male | −7.57 | 4.3 | [−16.1 – 0.97] |
| oVEMP functionc | 7.67 | 4.96 | [−2.13 – 17.46] |
| Age (years) | −0.17 | 0.16 | [−0.49 – 0.15] |
| Sex | |||
| Female | Ref | Ref | Ref |
| Male | −7.35 | 4.3 | [−15.78 – 1.08] |
n = 130
n = 150
n = 152
indicates significance with p < 0.05, units provided only for significant relationships.
Independent of vestibular function, age was significantly associated with an increased angle of deviation and end point error on the TCT. Angular deviation increased linearly with age independent of VOR gain (β = 0.28 degrees, 95% CI [0.14, 0.42]), saccular function (β = 0.22, 95% CI [0.08, 0.37]), and utricular function (β = 0.26, 95% CI [0.12, 0.4]). This corresponds to an increase of 2.8, 2.2, and 2.6 degrees of rotational error per decade respectively. End point error increased linearly with age independent of VOR gain (β = 0.90 cm, 95% CI [0.47, 1.32]), saccular function (β = 0.75, 95% CI [0.33, 1.17]), and utricular function (β = 0.82, 95% CI [0.41, 1.24]). This corresponds to an increase of 9.0, 7.5, and 8.2 cm of end point error per decade respectively. Sex was not significantly associated with end point error, angle of deviation, or distance walked on the TCT in any of the regression models.
To address concerns that these effects may be driven by saccular or utricular asymmetry, we conducted a sensitivity analysis as follows (data not shown). We explored whether there was a relationship between cVEMP and oVEMP asymmetry and TCT measures (angular deviation, end-point error, distance walked) in this sub-set in 3 ways. First we examined whether there was a relationship between the cVEMP and oVEMP asymmetry ratios (asymmetry = minimum/maximum, when responses were present bilaterally) and TCT outcome measures. There were no significant relationships between the continuous asymmetry ratios for either cVEMP (n = 92) or oVEMPS (n = 113) and any TCT outcome variable. Second we dichotomized the cVEMP asymmetry ratios using 30% difference and the oVEMP asymmetry ratios using 35% difference. There were no significant relationships between the dichotomized cVEMP (n=92) or oVEMP (n=113) asymmetry variables and any TCT outcome variables. We then added participants with unilateral loss as an extreme case of asymmetry. There were no significant relationships between the dichotomized cVEMP (n=124) or oVEMP (n=133) asymmetry variables and any TCT outcome variables.
4. Discussion
In a cohort of healthy adults, spatial navigation accuracy was degraded when saccular function was impaired. Both unilateral and bilateral loss of saccular function were associated with errors in directional heading and distance to target errors. This is consistent with prior work demonstrating a relationship between saccular function and tests of visuo-spatial ability (Bigelow et al. 2015). These present results suggest a mechanistic influence of gravitational cues on spatial orientation in the context of a blindfolded navigational task. Interestingly, these results do not appear to be driven by asymmetrical saccular function. Taken together, this suggests that in the context of age related decline in otolith function, asymmetrical but bilaterally present saccular function provides sufficient gravitational cues to facilitate spatial orientation required for the TCT task. It is not clear from these results whether impaired saccular function adversely impacts the gravitational anchor of the starting point, or interferes with estimates of self-motion through space. Animal studies have demonstrated that otolith dysfunction can have a negative impact on the functioning of head direction cells (Yoder and Taube 2009). This suggests that abnormal saccular function would negatively impact perception of heading, contributing to the increased errors observed in individuals with impaired saccular function. Place cells are also influenced by vestibular input and are known to change their firing pattern with bilateral loss of vestibular function (Smith et al. 2015; Harvey et al. 2018). Therefore, loss of saccular function may also impair the accurate spatial encoding of the starting location of the triangle.
Secondary descending neurons from the saccule pass primarily through the lateral vestibular spinal tract (LSVT) and are thought to contribute to postural control (Uchino and Kushiro 2011). Unilateral loss of otolith function results in impaired perception of whether the ground is horizontal when standing, likely due to asymmetrical LSVT input (Beule and Allum 2006). The abnormal saccular function may negatively impact gait trajectory due to reduced vestibulo-spinal reflex modulation. This is particularly relevant to the blindfolded TCT task as vestibulo-spinal reflex modulation is increased at slower walking speeds (Dakin et al. 2013), and overall gait speed is slower when walking without vision (Collins and Kuo 2013; Schniepp et al. 2014). Although we did not measure time to complete each participant’s performance on the TCT, we observed an unquantified slower walking pattern. Thus, abnormal vestibulo-spinal modulation during the gait cycle may also have contributed to the TCT deviations associated with saccular dysfunction.
Somewhat surprisingly, semicircular canal function was not associated with heading errors as measured by angle of deviation. Semicircular canal function has been shown to contribute to rotational spatial orientation and thus may have contributed to the accuracy of the initial self-directed rotation, but it appears that only profound bilateral loss of function results in impaired rotational spatial orientation (Jáuregui-Renaud et al. 2008; Cohen et al. 2017). The estimation of angular deviation was taken from the end point, not the actual rotation during the first turn, which may contribute to the lack of relationship between VOR gain and angular error. Anecdotally, participants did not always walk in a straight line and occasionally made heading adjustments, not necessarily in the correct direction, prior to stopping. However, our result is in agreement with results indicating that passive VOR gain is not associated with rotational spatial orientation accuracy except in cases of severe bilateral vestibular loss (Cohen et al. 2017). The participants in this cohort do not demonstrate severe bilateral vestibular loss. Semicircular canal inputs also are known to contribute to the function of head direction cells (Muir et al. 2009), but it appears that in this cohort semicircular canal function alone did not account for spatial navigation performance. Additionally, there may be insufficient variability in VOR gain as a measure of semicircular canal function in this healthy aging cohort (Li et al. 2015b), particularly for individuals under 80 years of age to demonstrate a significant relationship between angular error and VOR gain.
In the current study, there was no relationship between vestibular function and distance walked back to the starting point. Similarly in a previous study, individuals with unilateral vestibular hypofunction did not differ in verbal estimation or self-directed blindfolded walking to a remembered target (Arthur et al. 2012). In fact, only during the acute phase of recovery after unilateral vestibular neurectomy do individuals have difficulty walking the appropriate distance to a remembered target (Borel et al. 2004), which was attributed to loss of balance and not underestimation of distance. Despite the correct distance estimation, a consistent lateral deviation remained at 1 month. Our results indicating angular and end point errors but not distance walked were associated with saccular function supports previous work and provides some insight into which vestibular organs may be responsible for these errors in spatial navigation. Cognitive strategies such as counting steps is another possible explanation for the lack of relationship between distance walked and vestibular function. Unlike Arthur et al. (Arthur et al. 2012) there was no cognitive task to prevent the participants from counting steps. Anecdotally, some participants volunteered that they employed a step counting strategy during the testing. As our triangles had “short” and “long” sides, a counting strategy could have been used to more accurately estimate the necessary distance to walk when returning to the origin.
The spatial navigation task presented here allowed participants to hold on to a visual representation of the ideal triangle path since they were instructed to visualize each triangle prior to donning the blindfold (Glasauer et al. 2002). It is unclear from the current analysis whether the relationship between saccular dysfunction and spatial navigation errors is mediated by impaired visuo-spatial cognitive abilities (Bigelow et al. 2015; Semenov et al. 2016). It is possible that saccular dysfunction contributed to an inaccurate visuo-spatial representation of the triangle path yet to be walked during the visualization, in addition to aberrant sensation of self-motion during the walking. Normally, visual and vestibular input regarding self-motion are optimally fused in the dorsal medial superior temporal area (Gu et al. 2012). Saccular dysfunction may lead to sub-optimal multisensory perception of extra-personal space and self-motion. The mapping of objects in extra-personal space identified when standing still (like the corners of the triangles marked on the floor) may shift or compress during guided walking in the presence of vestibular dysfunction (Borel et al. 2014). Any shifting of the perceived location of the triangle due to reduced vestibular afference would impact the egocentric representation of the individual within space. Future work should investigate the influence of active self-motion on perception of extra-personal space and spatial orientation.
Fall prone older adults made larger end point and angular errors in a similar triangle walking test under a reduced vision condition (Barrett et al. 2013). In that study the authors suggested an impaired ability to integrate interoceptive spatial cues (vestibular and proprioception) resulted in the increased errors. However, vestibular diagnostic tests were not performed in that study. Vestibular loss is known to contribute to falls (Liston et al. 2014), and it is possible that some of their fall prone subjects had undocumented vestibular loss (Agrawal et al. 2012).
5. Future Directions
Future studies should investigate whether sequence memorization such as viewing the entire spatial layout prior to the walk impacts performance on a spatial navigation task. Virtual reality could be used to present only a single walking target at a time, minimizing any effect of step-counting relative to the length of the sides. Adding a continuous non-numeric counting task (i.e., listing alphabet letters) could prevent step counting as a strategy for distance walked.
6. Limitations
These data are cross-sectional and do not prove causality between spatial navigation and vestibular function. However, these results highlight saccular function as a potential mechanism important for spatial navigation ability. Vertical semicircular canal VOR gain may have a stronger relationship with distance walked as there is significant head pitch during walking (Grossman et al. 1988; King et al. 1992). VOR gain was not measured for the vertical semicircular canals, and the VOR gain results may differ if based on vertical semicircular canal VOR gain. This population consisted of healthy adults and the results presented here may differ for individuals with more severe vestibular or balance problems.
6. Conclusions
Saccular dysfunction and increasing age independently contribute to deficits in spatial orientation during a walking navigation task. Gravitational cues detected by the saccule likely contribute to estimation of place, heading direction or both. Despite associations with path integration errors, vestibular function was not associated with overall distance walked.
Highlights.
Gravitational cues detected by the saccule contribute to estimation of place and heading direction.
Spatial orientation errors increase with both aging and reduced saccular function.
Vestibular function was not associated with distance walked.
Acknowledgments
Funding
This work was supported in part by the National Institutes of Health [NIDCD K23 DC013056, NIDCD T32 DC000023].
Conflict of Interest
This work was supported in part by the National Institutes of Health [NIDCD K23 DC013056 (YA), NIDCD T32 DC000023 (EA)]. All other authors report no conflict of interest.
References
- Adamo DE, Briceño EM, Sindone JA, Alexander NB, Moffat SD. Age differences in virtual environment and real world path integration. Front Aging Neurosci. 2012;4:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal Y, Zuniga MG, Davalos-Bichara M, Schubert MC, Walston JD, Hughes J, et al. Decline in semicircular canal and otolith function with age. Otol Neurotol. 2012;33(5):832–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur JC, Kortte KB, Shelhamer M, Schubert MC. Linear Path Integration Deficits in Patients with Abnormal Vestibular Afference. Seeing Perceiving. 2012;25(2):155–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett MM, Doheny EP, Setti A, Maguinness C, Foran TG, Kenny RA, et al. Reduced vision selectively impairs spatial updating in fall-prone older adults. Multisens Res. 2013;26(1–2):69–94. [DOI] [PubMed] [Google Scholar]
- Beule AG, Allum JHJ. Otolith Function Assessed with the Subjective Postural Horizontal and Standardised Stance and Gait Tasks. Audiol Neurotol. 2006;11(3):172–82. [DOI] [PubMed] [Google Scholar]
- Bigelow RT, Semenov YR, Trevino C, Ferrucci L, Resnick SM, Simonsick EM, et al. Association between visuospatial ability and vestibular function in the Baltimore Longitudinal Study of Aging. J Am Geriatr Soc. 2015;63(9):1837–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borel L, Harlay F, Lopez C, Magnan J, Chays A, Lacour M. Walking performance of vestibular-defective patients before and after unilateral vestibular neurotomy. Behav Brain Res. 2004;150(1–2):191–200. [DOI] [PubMed] [Google Scholar]
- Borel L, Lopez C, Péruch P, Lacour M. Vestibular syndrome: A change in internal spatial representation. Clin Neurophysiol. 2008;38(6):375–89. [DOI] [PubMed] [Google Scholar]
- Borel L, Redon-Zouiteni C, Cauvin P, Dumitrescu M, Devèze A, Magnan J, et al. Unilateral vestibular loss impairs external space representation. PLoS One. 2014;9(2):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen BS, Provasi J, Leboucher P, Israël I. Effects of vestibular disorders on vestibular reflex and imagery. Exp Brain Res. 2017;235(7):2181–8. [DOI] [PubMed] [Google Scholar]
- Collins SH, Kuo AD. Two Independent Contributions to Step Variability during Over-Ground Human Walking. PLoS One. 2013;8(8):e73597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dakin CJ, Inglis JT, Chua R, Blouin J-S. Muscle-specific modulation of vestibular reflexes with increased locomotor velocity and cadence. J Neurophysiol. 2013;110(1):86–94. [DOI] [PubMed] [Google Scholar]
- Dokka K, DeAngelis GC, Angelaki DE. Multisensory Integration of Visual and Vestibular Signals Improves Heading Discrimination in the Presence of a Moving Object. J Neurosci. 2015;35(40):13599–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez C, Goldberg JM. Physiology of peripheral neurons innervating semicircular canals of the squirrel monkey. II. Response to sinusoidal stimulation and dynamics of peripheral vestibular system. J Neurophysiol. 1971;34(4):661–75. [DOI] [PubMed] [Google Scholar]
- Foo P, Warren WH, Duchon A, Tarr M. Do Humans Integrate Routes Into A Cognitive Map? Map-versus Landmark-Based Navigation of Novel Shortcuts. Exp Psychol. 2005;31(2):195–215. [DOI] [PubMed] [Google Scholar]
- Gibson JJ. Visually controlled locomotion and visual orientation in animals. Br J Psychol. 1958;49(3):182–94. [DOI] [PubMed] [Google Scholar]
- Glasauer S, Amorim M-A, Viaud-Delmon I, Berthoz A. Differential effects of labyrinthine dysfunction on distance and direction during blindfolded walking of a triangular path. Exp Brain Res. 2002;145(4):489–97. [DOI] [PubMed] [Google Scholar]
- Glasauer S, Amorim M-A, Vitte E, Berthoz A. Goal-directed linear locomotion in normal and labyrinthine-defective subjects. Exp Brain Res. 1994;98(2):323–35. [DOI] [PubMed] [Google Scholar]
- Goldberg JM, Fernández C. Responses of peripheral vestibular neurons to angular and linear accelerations in the squirrel monkey. Acta Otolaryngol. 1975;80(1–2):101–10. [DOI] [PubMed] [Google Scholar]
- Grossman GE, Leigh RJ, Abel LA, Lanska DJ, Thurston SE. Frequency and velocity of rotational head perturbations during locomotion. Exp brain Res. 1988;70(3):470–6. [DOI] [PubMed] [Google Scholar]
- Gu Y, DeAngelis GC, Angelaki DE. Causal Links between Dorsal Medial Superior Temporal Area Neurons and Multisensory Heading Perception. J Neurosci. 2012;32(7):2299–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey RE, Rutan SA, Willey GR, Siegel JJ, Clark BJ, Yoder RM. Linear Self-Motion Cues Support the Spatial Distribution and Stability of Hippocampal Place Cells. Curr Biol. 2018; [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jáuregui-Renaud K, Sang FYP, Gresty MA, Green DA, Bronstein AM. Depersonalisation/derealisation symptoms and updating orientation in patients with vestibular disease. J Neurol Neurosurg Psychiatry. 2008;79(3):276–83. [DOI] [PubMed] [Google Scholar]
- King OS, Seidman SH, Leigh RJ. Control of head stability and gaze during locomotion in normal subjects and patients with deficient vestibular function In: Berthoz A, Graf W, Vidal P-P, editors. The Head-Neck Sensory Motor System. New York: Oxford University Press; 1992. p. 568–70. [Google Scholar]
- Klatzky RL. Allocentric and Egocentric Spatial Representations: Definitions, Distinctions, and Interconnections In: Freksa C, Habel C, Wender KF, editors. Spatial Cognition: An Interdisciplinary Approach to Representing and Processing Spatial Knowledge. Berlin, Heidelberg: Springer Berlin Heidelberg; 1998. p. 1–17. [Google Scholar]
- Klatzky RL. Path completion after haptic exploration without vision: implications for haptic spatial representations. Percept Psychophys. 1999;61(2):220–35. [DOI] [PubMed] [Google Scholar]
- Koutakis P, Mukherjee M, Vallabhajosula S, Blanke DJ, Stergiou N. Path integration: Effect of curved path complexity and sensory system on blindfolded walking. Gait Posture. 2013;37(2):154–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremmyda O, Hüfner K, Flanagin VL, Hamilton DA, Linn J, Strupp M, et al. Beyond Dizziness: Virtual Navigation, Spatial Anxiety and Hippocampal Volume in Bilateral Vestibulopathy. Front Hum Neurosci. 2016;10:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Layman AJ, Carey JP, Agrawal Y. Epidemiology of vestibular evoked myogenic potentials: data from the Baltimore Longitudinal Study of Aging. Clin Neurophysiol. 2015a;126(11):2207–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Layman AJ, Geary R, Anson E, Carey JP, Ferrucci L, et al. Epidemiology of vestibulo-ocular reflex function: data from the Baltimore Longitudinal Study of Aging. Otol Neurotol. 2015b;36(2):267–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liston MB, Bamiou D-E, Martin F, Hopper A, Koohi N, Luxon L, et al. Peripheral vestibular dysfunction is prevalent in older adults experiencing multiple non-syncopal falls versus age-matched non-fallers: a pilot study. Age Ageing. 2014;43(1):38–43. [DOI] [PubMed] [Google Scholar]
- Loomis JM, Klatzky RL, Golledge RG, Cicinelli JG, Pellegrino JW, Fry PA. Nonvisual Navigation by Blind and Sighted : Assessment of Path Integration Ability. J Exp Psychol Gen. 1993; 122(1):73–91. [DOI] [PubMed] [Google Scholar]
- MacDougall HG, Weber KP, McGarvie LA. The video head impulse test Diagnostic accuracy in peripheral vestibulopathy. Neurology. 2009;73:1134–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlinsky V V Vestibular and vestibulo-proprioceptive perception of motion in the horizontal plane in blindfolded man--II. Estimations of rotations about the earth-vertical axis. Neuroscience. 1999;90(2):395–401. [DOI] [PubMed] [Google Scholar]
- Muir GM, Brown JE, Carey JP, Hirvonen TP, Della Santina CC, Minor LB, et al. Disruption of the head direction cell signal after occlusion of the semicircular canals in the freely moving chinchilla. J Neurosci. 2009;29(46):14521–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paige GD. Senescence of human visual-vestibular interactions. 1. Vestibulo-ocular reflex and adaptive plasticity with aging. J Vestib Res. 1992;2(2):133–51. [PubMed] [Google Scholar]
- Patla AE, Vickers JN. How far ahead do we look when required to step on specific locations in the travel path during locomotion? Exp brain Res. 2003; 148(1):133–8. [DOI] [PubMed] [Google Scholar]
- Schniepp R, Kugler Gã, Wuehr M, Eckl M, Huppert D, Huth S, et al. Quantification of gait changes in subjects with visual height intolerance when exposed to heights. Front Hum Neurosci. 2014;8:963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenov YR, Bigelow RT, Xue Q-L, Lac S du, Agrawal Y. Association between vestibular and cognitive function in U.S. adults: data from the National Health and Nutrition Examination Survey. J Gerontol A Biol Sci Med Sci. 2016;71(2):243–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PF, Darlington CL, Zheng Y. The Effects of Complete Vestibular Deafferentation on Spatial Memory and the Hippocampus in the Rat: The Dunedin Experience. Multisens Res. 2015;28(5–6):461–85. [DOI] [PubMed] [Google Scholar]
- Uchino Y, Kushiro K. Differences between otolith- and semicircular canal-activated neural circuitry in the vestibular system. Neurosci Res. 2011;71(4):315–27. [DOI] [PubMed] [Google Scholar]
- Vazquez A, Statton MA, Busgang SA, Bastian AJ. Split-belt walking adaptation recalibrates sensorimotor estimates of leg speed but not position or force. J Neurophysiol. 2015;114(6):3255–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vélez-Fort M, Bracey EF, Keshavarzi S, Rousseau C V, Cossell L, Lenzi SC, et al. A Circuit for Integration of Head- and Visual-Motion Signals in Layer 6 of Mouse Primary Visual Cortex. Neuron. 2018;98(1):179–191.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y, Bigelow RT, Frankenthaler SF, Studenski SA, Moffat SD, Agrawal Y. Vestibular loss in older adults is associated with impaired spatial navigation: Data from the triangle completion task. Front Neurol. 2017;8:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Pu F, Lv X, Li S, Li J, Li D, et al. Comparison of postural responses to galvanic vestibular stimulation between pilots and the general populace. Biomed Res Int. 2015;2015:567690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder RM, Taube JS. Head Direction Cell Activity in Mice: Robust Directional Signal Depends on Intact Otolith Organs. J Neurosci. 2009;29(4):1061–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalewski CK. Aging of the Human Vestibular System. Semin Hear. 2015;36(3):175–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehr EP, Stein RB. What Functions Do Reflexes Serve During Human Locomotion? Prog Neurobiol. 1999;58(2):185–205. [DOI] [PubMed] [Google Scholar]