Abstract
Over the last two decades, there have been no significant changes in patient outcomes in relation to the treatment of osteosarcoma, an aggressive malignant neoplasm. It is known that vascular endothelial growth factor-A (VEGF-A) plays a crucial role in angiogenesis and in osteosarcoma. Moreover, VEGF-A expression correlates with clinical stages of osteosarcoma. The adipokine resistin exhibits proinflammatory, proangiogenic and metastatic properties, and evidence suggests that resistin may serve as a prognostic biomarker linking obesity and inflammation to cancer. However, whether resistin has a role in osteosarcoma angiogenesis is unclear. This investigation shows that resistin promotes VEGF-A expression in human osteosarcoma cells and activates the extracellular signal-regulated kinase (ERK), c-Jun N-terminal kinase (JNK) and p38 signaling pathways, while ERK, JNK, and p38 inhibitors or their small interfering RNAs (siRNAs) inhibit resistin-induced VEGF-A expression as well as endothelial progenitor cell (EPC) migration and tube formation. We also found that resistin upregulates VEGF-A expression by enhancing activation of the transcription factor nuclear factor-kappa B (NF-κB). Finally, resistin promotes angiogenesis in the chick chorioallantoic membrane (CAM) model. Resistin appears to be a promising target for human osteosarcoma.
Keywords: resistin, VEGF-A, MAPK, angiogenesis, osteosarcoma
INTRODUCTION
Osteosarcoma accounts for 30–80% of primary skeletal sarcomas and is well-recognized for its high metastatic rates, which are believed to underline the poor survival rates in this disease. Lung metastases are found in approximately 20% of patients at initial diagnosis; around 40% of patients develop metastases at a later stage [1]. Following the development of metastases, osteosarcoma has a 5-year survival rate of 20% [2]. Angiogenesis is key to the development of osteosarcoma, its subsequent progression and metastasis [3, 4]. Results from using differential co-expression network, finding angiogenesis is closely related to osteosarcoma progression and metastasis [5]. Thus, antiangiogenic therapy might be feasible for osteosarcoma patients [6].
Vascular endothelial growth factor A (VEGF-A; also referred to as VEGF) is a major promoter of angiogenesis [7]. Antiangiogenic inhibitors targeting the VEGF-A pathway are currently used in antitumor therapy [8]. We have previously reported correlations between levels of VEGF-A expression and tumor stages in human osteosarcoma tissue specimens [9, 10]. More clarification is needed as to the effects of VEGF-A in angiogenesis pathways and its underlying mechanisms in human osteosarcoma.
Resistin, an adipocyte-derived cytokine, has been investigated for its role in inflammation and obesity-related cancers [11]. Increasing serum resistin levels correlate with the progression of breast, colon and endometrial cancers [12]. However, the role of resistin in osteosarcoma is unclear. In this study, we examined how resistin affects VEGF-A expression and tumor angiogenesis. We then investigated the signaling pathways underlying resistin-induced VEGF-A-dependent angiogenesis in human osteosarcoma cells. We consider that our findings will be helpful in the development of new treatment strategies for osteosarcoma.
RESULTS
Clinical significance of resistin and VEGF-A expression in human osteosarcoma tissue specimens
Previous studies have reported that resistin stimulates the growth of many tumors by promoting the secretion of VEGF-A, which promotes tumor invasion [13–16]. However, there are few studies of resistin in relation to osteosarcoma. When we examined resistin expression profiles in human osteosarcoma tissue specimens, we found that higher resistin expression significantly correlated with increasing tumor stage (Figure 1A). It has been reported that circulating VEGF-A levels are associated with the development of lung metastasis and with the clinical outcomes of osteosarcoma [4]. We therefore compared resistin IHC scores with the VEGF-A profiles we had previously obtained from clinical osteosarcoma tissue samples [9, 10] and found a highly positive relationship between resistin and VEGF-A expression (Figure 1B). These results implicate the involvement of resistin in the levels of VEGF-A expression and tumor progression in osteosarcoma patients.
Figure 1.
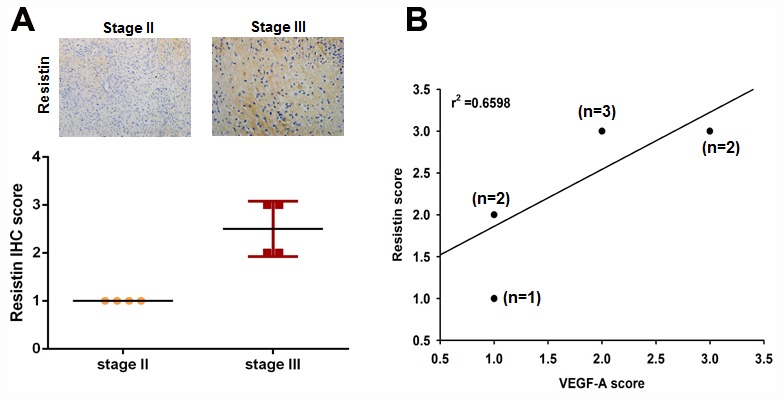
Clinical significance of resistin expression in tissue specimens from patients with osteosarcoma. (A) Tumor specimens were subjected to immunohistochemistry (IHC) evaluations with anti-resistin monoclonal antibody and the staining intensity was graded as 0 (no positive cell staining), 1 (1–24%, weakly positive), 2 (25–49%, moderately positive), or 3 (50–100%, strongly positive). (B) Positive correlations were observed between levels of resistin and VEGF-A expression.
Resistin regulates angiogenesis by upregulating VEGF-A expression in osteosarcoma cells
As our IHC results indicated that levels of resistin expression correlate with VEGF-A expression in human osteosarcoma specimens, we then sought to determine whether resistin promotes VEGF-A expression in human osteosarcoma. Our results showed that resistin increased levels of VEGF-A expression and secretion in osteosarcoma cells (Figure 2A and 2B). It has been reported that EPCs contribute to neovascularization in tumors [17]. In this study, CM from resistin-treated osteosarcoma cells enhanced EPC migration and tube formation (VEGF-A served as the positive control) (Figure 2C and 2D), indicating that EPCs may be recruited to the tumor microenvironment and form new blood vessels via resistin-promoted VEGF-A expression. This suggests that resistin induces angiogenesis in a VEGF-A-dependent manner.
Figure 2.
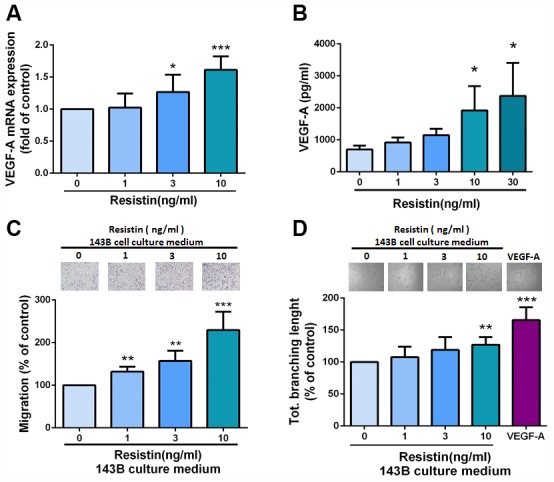
Resistin mediates angiogenesis by inducing increases in VEGF-A expression in osteosarcoma cells. (A–B) After incubating osteosarcoma 143B cells with resistin (0–10 ng/ml) for 24 h, VEGF-A expression was measured by qPCR and ELISA. (C–D) Osteosarcoma 143B cells were pretreated with resistin (1–10 ng/ml) for 24 h. CM was collected and applied to EPCs for 24 h. Cell migration and capillary-like structure formation in EPCs were examined by Transwell and tube formation assays, respectively. The results were obtained from three independent experiments. * p < 0.05; ** p < 0.01; *** p < 0.001 compared with controls.
The MAPK signaling pathway is involved in resistin-promoted VEGF-A expression and contributes to angiogenesis
Previous research has reported that intracellular signaling of resistin converges in the activation of the mitogen-activated protein kinase (MAPK) signaling pathway [18], which consists of the ERK, JNK and p38 families; their activation is also involved in the regulation of VEGF-A expression [10, 19]. To investigate whether the MAPK pathway affects resistin-induced VEGF-A expression, we incubated cells with resistin and observed subsequent increases in ERK, JNK and p38 phosphorylation (Figure 3A). Pretreatment with inhibitors of ERK, JNK and p38 or their siRNAs reversed resistin-induced increases in VEGF-A expression (Figure 3B). Furthermore, EPC migration and tube formation, which were induced by treatment with the indicated inhibitors or siRNA-treated CM, was also abolished (Figure 3C and 3D). These results indicate that resistin promotes VEGF-A expression in osteosarcoma cells and contributes to angiogenesis by activating the ERK, JNK and p38 pathways.
Figure 3.
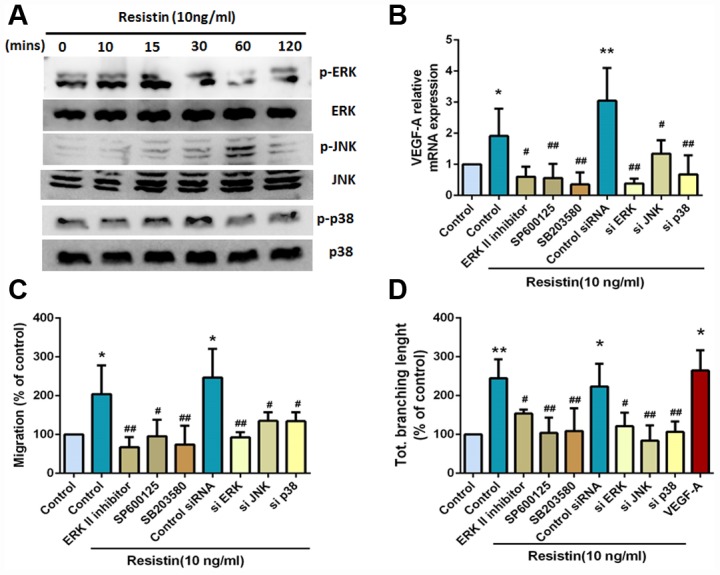
The MAPK signaling pathway is involved in resistin-promoted VEGF-A expression and contributes to angiogenesis. (A) Osteosarcoma 143B cells were incubated with resistin (10 ng/ml) for the indicated times, and ERK, JNK and p38 phosphorylation was determined by Western blot analysis. (B) Osteosarcoma 143B cells were pretreated with an ERKII inhibitor (10 μM), a JNK inhibitor (SP600125; 10 μM) and a p38 inhibitor (SB203580; 10 μM) for 30 min or transfected with ERK, JNK, and p38 siRNAs for 24 h, followed by resistin (10 ng/ml) stimulation for 24 h. VEGF-A expression was examined by qPCR. (C–D) Osteosarcoma 143B cells were pretreated with an ERKII inhibitor (10 μM), a JNK inhibitor (SP600125; 10 μM) and a p38 inhibitor (SB203580; 10 μM) for 30 min, or transfected with ERK, JNK and p38 siRNAs for 24 h, then stimulated with resistin (10 ng/ml) for 24 h. CM was collected and then applied to EPCs for 24 h. Cell migration and capillary-like structure formation in EPCs were examined by Transwell and tube formation assays, respectively. The results were obtained from three independent experiments. * p < 0.05; ** p < 0.01; *** p < 0.001 compared with controls. # p < 0.05; ## p < 0.01 compared with resistin-treated control groups.
Resistin promotes VEGF-A expression in osteosarcoma and contributes to angiogenesis through the NF-κB signaling pathway
The transcription factor nuclear factor kappa-B (NF-κB) is involved in VEGF-A expression [20, 21]. In this study, we observed that resistin treatment time- dependently increased NF-κB p65 protein phosphorylation (Figure 4A) (Supplementary Figure 1). Moreover, pretreatment with NF-κB inhibitors PDTC and TPCK reversed resistin-induced increases in VEGF-A expression, EPC migration and tube formation (Figure 4B–4D). In addition, co-transfecting the κB-luciferase plasmid with ERK, JNK and p38 siRNAs also reduced resistin-induced κB-luciferase activity (Figure 4E). These data suggest that resistin regulates VEGF-A expression and promotes angiogenesis via NF-kB transcription factor activation.
Figure 4.
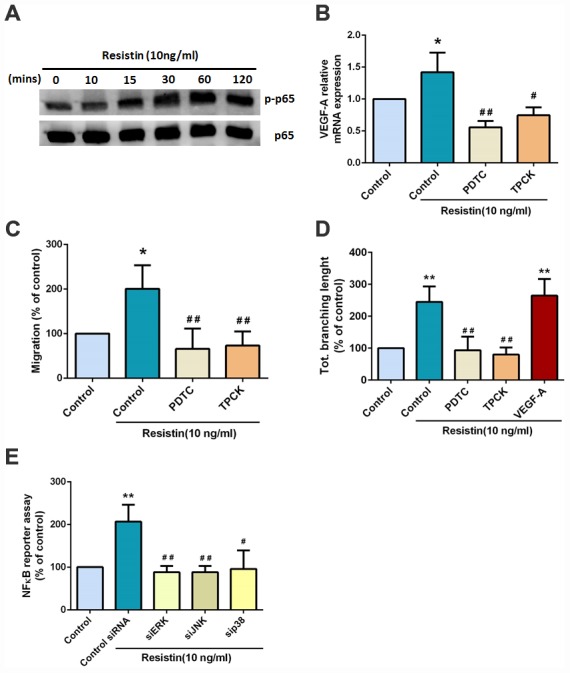
Resistin promotes VEGF-A expression in osteosarcoma and contributes to angiogenesis through the NF-κB signaling pathway. (A) Osteosarcoma 143B cells were incubated with resistin (10 ng/ml) for the indicated times and p65 phosphorylation was determined by Western blot analysis. (B) Osteosarcoma 143B cells were pretreated with NF-κB inhibitors (PDTC 10 μM; TPCK 3 μM) for 30 min then stimulated with resistin (10 ng/ml) for 24 h. VEGF-A expression was examined by qPCR. (C–D) Osteosarcoma 143B cells were pretreated with NF-κB inhibitors (PDTC 10 μM; TPCK 3 μM) for 30 min then stimulated with resistin (10 ng/ml) for 24 h. CM was collected and applied to EPCs for 24 h. Cell migration and capillary-like structure formation in EPCs were examined by Transwell and tube formation assays, respectively. (E) Cells were transfected with indicated siRNA, the luciferase activity was examined. The results were obtained from three independent experiments. * p < 0.05; ** p < 0.01; *** p < 0.001 compared with controls. # p < 0.05; ## p < 0.01 compared with resistin-treated control groups.
Effects of resistin on VEGF-A-induced angiogenesis in the CAM model
Finally, we used the in vivo CAM model to confirm the effects of resistin on VEGF-A-induced angiogenesis. As expected, CM collected from resistin-treated cells enhanced CAM angiogenesis (VEGF-A served as the positive control) (Figure 5). These results indicate that resistin promotes angiogenesis in vivo.
Figure 5.
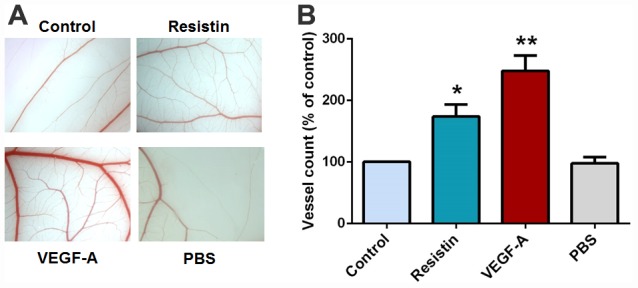
Effects of resistin on VEGF-A-induced angiogenesis in the CAM model. (A) PBS, VEGF-A (50 ng/ml), control osteosarcoma 143B cell CM and resistin-treated osteosarcoma 143B CM were mixed in Matrigel and subjected to the CAM assay, then photographed with a stereomicroscope on developmental day 12. (B) Angiogenesis was quantified by counting the number of blood vessel branches. * p < 0.05; ** p < 0.01; *** p < 0.001 compared with controls.
DISCUSSION
For many tumors, the ability to progress to a state of malignancy depends on the microenvironment. Blood capillaries are usually static under physiological conditions but are induced to proliferate by direct exposure to angiogenic factors, such as VEGF-A [6], which support tumor growth and metastasis [22]. Increasing evidence shows that adipocytes and adipokines are involved in primary inflammatory processes and disease [23]. Resistin, an adipocyte- derived cytokine, has been studied extensively for its role in inflammation and obesity-related cancers [11]. Serum levels of resistin are known to increase in breast, colon, and endometrial cancer during tumor progression [12], although few studies have investigated the role of resistin in bone cancers, especially in osteosarcoma [24, 25]. Evidence has linked increasing resistin expression with poor prognosis and the development of lung adenocarcinoma [26]. Similarly, in our study, IHC results from clinical osteosarcoma specimens showed that resistin and VEGF-A expression levels were positively correlated with increasing tumor stage.
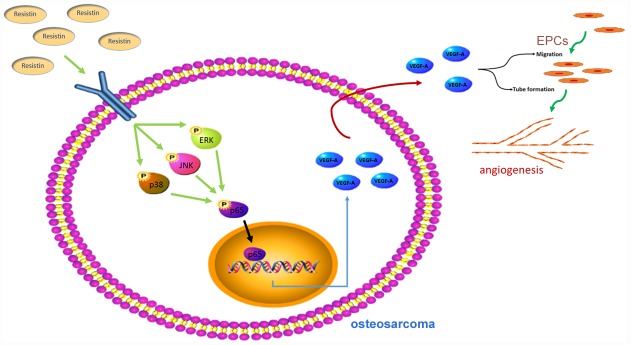
Previous research has reported that intracellular signaling of resistin converges in the activation of the MAPK signaling pathway [17]. The MAPK pathway plays an important role in the regulation of VEGF-A expression levels [9, 24]. Our analyses revealed that resistin time-dependently stimulates VEGF-A production in human osteosarcoma cells via the ERK, JNK and p38 pathway, which subsequently increased EPC migration, tube formation and tumor angiogenesis (Figure 6). Pretreatment of cells with ERK, JNK, or p38 inhibitors prevented resistin-induced increases in VEGF-A expression, EPC migration and tube formation. Our results clearly indicate that the ERK, JNK and p38 pathway enhances resistin-induced stimulation of angiogenic functions by upregulating VEGF-A expression.
Figure 6.
Schematic presentation of the signaling pathways involved in resistin-induced angiogenesis in human osteosarcoma cells.
In addition to intracellular signaling, levels of oxidative stress are critical in osteosarcoma pathology [27]. Whereas low levels of oxidative stress promote survival and proliferation of cancer cells, higher levels induce apoptosis and cell cycle arrest [28]. Mitochondrial defects have also been reported in many different cancers, including osteosarcoma, and have been considered as a prognostic biomarker [29]. Apoptosis appears to be linked to higher mitochondrial oxidative stress [30]. However, this study failed to detect levels of oxidative stress and subunits of oxidative phosphorylation (mitochondrial markers Complexes I–V). These should be investigated in future research.
In conclusion, resistin is expressed at high levels in osteosarcoma and correlates with clinical stages of this disease. Resistin promotes VEGF-A expression by activating the MAPK signaling pathway, which subsequently promotes angiogenesis. Thus, resistin may represent a new molecular therapeutic target in osteosarcoma angiogenesis and metastasis.
MATERIALS AND METHODS
Materials
Human recombinant resistin and VEGF-A protein were purchased from PeproTech (Rocky Hill, NJ, USA). VEGF-A antibody was purchased from Abcam (Cambridge, MA, USA). Antibodies for resistin, ERK, JNK, p38 and p65 (all mouse monoclonal IgG antibodies) and for pERK, pJNK, pp38 and pp65 (all rabbit monoclonal IgG antibodies) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). SP600125, SB203580, PDTC and TPCK were purchased from Calbiochem (San Diego, CA, USA). ON-TARGETplus small interfering RNAs (siRNAs) of JNK, ERK and p38 were purchased from Dharmacon Research (Lafayette, CO, USA). Lipofectamine 2000 was purchased from Invitrogen (Carlsbad, CA, USA). Reporter lysis buffer was purchased from Promega (Madison, WI, USA) and human osteosarcoma tissue arrays were purchased from Biomax (Rockville, MD, USA). All other chemicals were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Cell culture
The human osteosarcoma cell line 143B was purchased from the Bioresource Collection and Research Center (BCRC) (Hsinchu, Taiwan). Cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 20 mM HEPES and 10% heat-inactivated fetal bovine serum (FBS), 2 mM glutamine, penicillin (100 U/ml) and streptomycin (100 μg/ml) at 37°C with 5% CO2.
Endothelial progenitor cell (EPC) was purchased from Lonza (Walkersville, MD, USA) and was cultured in MV2 medium that contained MV2 basal medium and growth supplement (PromoCell, Heidelberg, Germany) with the addition of 20% FBS (HyClone, Logan, UT, USA). Cultures were seeded onto 1% gelatin-coated plasticware and maintained at 37°C in a humidified 5% CO2 atmosphere.
Immunohistochemical staining
An osteosarcoma tissue array was deparaffinized with xylene and rehydrated with ethanol. Endogenous peroxidase activity was blocked with 3% hydrogen peroxide in methanol for 10 min. Heat-induced antigen retrieval was carried out for all sections in 0.01 M sodium citrate buffer (pH 6) at 95°C for 25 min. Human resistin antibody was applied at a dilution of 1:250 and incubated at 4°C overnight. The antibody-binding signal was detected using the NovoLink Polymer Detection System (Leica Microsystems) and reaction products were visualized using diaminobenzidine. The sections were counterstained with hematoxylin. Immunohistochemistry (IHC) results were scored by accounting for the percentage of positive detection and intensity of the staining in calculations using MacBiophotonics Image J software. Staining intensity was graded as 0 (no positive cell staining), 1 (1–24%, weakly positive), 2 (25–49%, moderately positive), or 3 (50–100%, strongly positive).
Reverse-transcribed quantitative real-time PCR
Total RNA was extracted from osteosarcoma cells using a TRIzol kit (MDBio Inc., Taipei, Taiwan). Total RNA (2 μg) was reverse-transcribed (RT) into complementary DNA (cDNA) using an oligo(dT) primer. Quantitative real-time polymerase chain reaction (q-PCR) analysis was carried out using TaqMan® one-step PCR Master Mix (Applied Biosystems, Foster City, CA, USA). Total cDNA (100 ng/25μl reaction) was mixed with sequence-specific primers and TaqMan® probes according to the manufacturer’s instructions. All target gene primers and probes were purchased commercially (GAPDH was used as the internal control) (Applied Biosystems, Foster City, CA, USA). RT-qPCR assays were carried out in triplicate using a StepOnePlus sequence detection system. The cycling conditions were 10 min of polymerase activation at 95°C, followed by 40 cycles at 95°C for 15 s and 60°C for 60 s.
ELISA assay
Human osteosarcoma cells were cultured in 6-well plates. After reaching confluence, cells were changed to serum-free medium. The cells were then treated with resistin for 24 h. After treatment, the medium was removed and stored at −80°C. The amount of VEGF-A in the medium was determined using a VEGF-A ELISA kit (PeproTech, Rocky Hill, NJ, USA), according to the manufacturer's protocol.
EPC migration assay
A cell migration assay was performed using 6.5 mm Transwell chambers with 8.0 μm pore polycarbonate membrane sizes (Corning Incorporated, Corning, NY, USA). EPCs (1 × 104 cells/well) were seeded onto the upper chambers with MV2 medium, then incubated in the lower chambers with 50% MV2 medium and 50% osteosarcoma cell culture medium (CM). The plates were incubated for 24 h at 37°C in 5% CO2, then the cells were fixed in 4% formaldehyde solution for 15 min and stained with 0.05% crystal violet in phosphate-buffered saline (PBS) for 15 min. Cells on the upper sides of the filters were removed with cotton-tipped swabs and the filters were washed with PBS. Cell migration was quantified by counting the number of stained cells in 10 random fields using an inverted phase contrast microscope and then photographed.
Tube formation assay
Matrigel (BD Biosciences, Bedford, MA, USA) was dissolved at 4°C overnight and 48-well plates were prepared with 150 μl Matrigel in each well, then incubated at 37°C for 30 min. After gel formation, EPCs (1×104 cells) were seeded into each well onto a layer of polymerized Matrigel in CM containing 50% MV2 complete medium and 50% osteosarcoma cell CM, then incubated for 4 h at 37°C. EPC tube formation was examined using an inverted phase contrast microscope. The number of tube branches and total tube length were calculated using the MacBiophotonics Image J software.
Western blot analysis
Total protein concentration was determined using the Thermo Scientific Pierce BCA Protein Assay Kit (Thermo Fisher Scientific Inc., Rockford, IL, USA). Proteins were resolved with SDS-PAGE and transferred to Immobilon® polyvinylidene difluoride (PVDF) membranes. The blots were blocked with 5% BSA for 1 h at room temperature then incubated with primary antibodies for 1 h at room temperature. After 3 washes in Tris-buffered saline with 0.05% Tween 20 (TBS-Tween), the blots were subsequently incubated with a donkey anti-rabbit or anti-mouse peroxidase-conjugated secondary antibody for 1 h at room temperature. The blots were visualized by enhanced chemiluminescence using Kodak X-OMAT LS film (Eastman Kodak, Rochester, NY, USA).
Transfection and reporter gene assay
Osteosarcoma 143B cells were co-transfected with 1 mg κB-luciferase plasmid and 0.5 mg β-galactosidase expression vector, then were grown to 80% confluence in 24-well plates and transfected on the following day with Lipofectamine® 2000 (LF2000; Invitrogen, Carlsbad, CA, USA). DNA and LF2000 were premixed for 20 min before being applied to cells. After 24 h of transfection, the cells were incubated with the indicated agents. After a further 24 h of incubation, the media were removed and the cells were washed once with cold PBS. To prepare lysates, 100 ml reporter lysis buffer (Promega, Madison, WI, USA) was added to each well and the cells were scraped from the dishes. The supernatant was collected after centrifugation at 13,000 rpm for 5 min. Aliquots of cell lysates (20 ml) containing equal amounts of protein (20–30 mg) were placed into wells of black opaque 96-well microplate. An equal volume of luciferase substrate was added to all samples, and luminescence was measured in a microplate luminometer. The value of luciferase activity was normalized using the co-transfected β-galactosidase expression vector as the internal control.
Chick chorioallantoic membrane (CAM) assay
The influence of resistin on blood vessel formation was performed by a CAM assay using fertilized chicken eggs. Eggs were incubated at 38°C in an 80–90% humidified atmosphere. On day 7, an ostiole of approximately 1 cm2 was opened in the air sac of each egg, before adding 100 μl of CM collected from different conditions. CAM results were analyzed on the fourth day. Chorioallantoic membranes were collected for microscopy and photographic documentation. Angiogenesis was quantified by counting the number of blood vessel branches; at least 10 viable embryos were tested for each treatment. All animal investigations were performed in accordance with a protocol approved by China Medical University’s Institutional Animal Care and Use Committee (Taichung, Taiwan).
Statistics
Statistical data were analyzed using SPSS Statistics version 20.0 (SPSS, Chicago, USA). All data are expressed as the mean ± standard error of the mean. Statistical comparisons between two samples were performed using the Student's t-test, and a one-way analysis of variance (ANOVA) with post hoc Bonferroni correction was used to compare multiple groups. A value of p < 0.05 was considered significant.
Supplementary Material
ACKNOWLEDGMENTS
We would like to thank Iona J. MacDonald for her English language revision of this manuscript.
Footnotes
CONFLICTS OF INTEREST: The authors have no financial or personal relationships that could inappropriately influence this research.
FUNDING: This study was supported by grants from the Taiwan’s Ministry of Science and Technology (MOST 108-2314-B-039-034-MY3) and China Medical University (CMU108-ASIA-09).
REFERENCES
- 1.Taran SJ, Taran R, Malipatil NB. Pediatric Osteosarcoma: An Updated Review. Indian J Med Paediatr Oncol. 2017; 38:33–43. 10.4103/0971-5851.203513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lindsey BA, Markel JE, Kleinerman ES. Osteosarcoma Overview. Rheumatol Ther. 2017; 4:25–43. 10.1007/s40744-016-0050-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perut F, Roncuzzi L, Zini N, Massa A, Baldini N. Extracellular Nanovesicles Secreted by Human Osteosarcoma Cells Promote Angiogenesis. Cancers (Basel). 2019; 11:E779. 10.3390/cancers11060779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xie L, Ji T, Guo W. Anti-angiogenesis target therapy for advanced osteosarcoma (Review). Oncol Rep. 2017; 38:625–36. 10.3892/or.2017.5735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Q. Identification of biomarkers for metastatic osteosarcoma based on DNA microarray data. Neoplasma. 2015; 62:365–71. 10.4149/neo_2015_044 [DOI] [PubMed] [Google Scholar]
- 6.Chen X. Prediction of optimal gene functions for osteosarcoma using network-based- guilt by association method based on gene oncology and microarray profile. J Bone Oncol. 2017; 7:18–22. 10.1016/j.jbo.2017.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Claesson-Welsh L, Welsh M. VEGFA and tumour angiogenesis. J Intern Med. 2013; 273:114–27. 10.1111/joim.12019 [DOI] [PubMed] [Google Scholar]
- 8.Zhang Q, Lu S, Li T, Yu L, Zhang Y, Zeng H, Qian X, Bi J, Lin Y. ACE2 inhibits breast cancer angiogenesis via suppressing the VEGFa/VEGFR2/ERK pathway. J Exp Clin Cancer Res. 2019; 38:173. 10.1186/s13046-019-1156-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tzeng HE, Tsai CH, Chang ZL, Su CM, Wang SW, Hwang WL, Tang CH. Interleukin-6 induces vascular endothelial growth factor expression and promotes angiogenesis through apoptosis signal-regulating kinase 1 in human osteosarcoma. Biochem Pharmacol. 2013; 85:531–40. 10.1016/j.bcp.2012.11.021 [DOI] [PubMed] [Google Scholar]
- 10.Liao YY, Tsai HC, Chou PY, Wang SW, Chen HT, Lin YM, Chiang IP, Chang TM, Hsu SK, Chou MC, Tang CH, Fong YC. CCL3 promotes angiogenesis by dysregulation of miR-374b/ VEGF-A axis in human osteosarcoma cells. Oncotarget. 2016; 7:4310–25. 10.18632/oncotarget.6708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deshmukh SK, Srivastava SK, Zubair H, Bhardwaj A, Tyagi N, Al-Ghadhban A, Singh AP, Dyess DL, Carter JE, Singh S. Resistin potentiates chemoresistance and stemness of breast cancer cells: implications for racially disparate therapeutic outcomes. Cancer Lett. 2017; 396:21–29. 10.1016/j.canlet.2017.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singh S, Chouhan S, Mohammad N, Bhat MK. Resistin causes G1 arrest in colon cancer cells through upregulation of SOCS3. FEBS Lett. 2017; 591:1371–82. 10.1002/1873-3468.12655 [DOI] [PubMed] [Google Scholar]
- 13.Morris EV, Edwards CM. Adipokines, adiposity, and bone marrow adipocytes: dangerous accomplices in multiple myeloma. J Cell Physiol. 2018; 233:9159–66. 10.1002/jcp.26884 [DOI] [PubMed] [Google Scholar]
- 14.Yang G, Fan W, Luo B, Xu Z, Wang P, Tang S, Xu P, Yu M. Circulating Resistin Levels and Risk of Colorectal Cancer: A Meta-Analysis. Biomed Res Int. 2016; 2016:7367485. 10.1155/2016/7367485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li J, Han X. Adipocytokines and breast cancer. Curr Probl Cancer. 2018; 42:208–14. 10.1016/j.currproblcancer.2018.01.004 [DOI] [PubMed] [Google Scholar]
- 16.Chen SS, Tang CH, Chie MJ, Tsai CH, Fong YC, Lu YC, Chen WC, Lai CT, Wei CY, Tai HC, Chou WY, Wang SW. Resistin facilitates VEGF-A-dependent angiogenesis by inhibiting miR-16-5p in human chondrosarcoma cells. Cell Death Dis. 2019; 10:31. 10.1038/s41419-018-1241-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.An R, Schmid R, Klausing A, Robering JW, Weber M, Bäuerle T, Detsch R, Boccaccini AR, Horch RE, Boos AM, Weigand A. Proangiogenic effects of tumor cells on endothelial progenitor cells vary with tumor type in an in vitro and in vivo rat model. FASEB J. 2018; 32:5587–601. 10.1096/fj.201800135RR [DOI] [PubMed] [Google Scholar]
- 18.Codoñer-Franch P, Alonso-Iglesias E. Resistin: insulin resistance to malignancy. Clin Chim Acta. 2015; 438:46–54. 10.1016/j.cca.2014.07.043 [DOI] [PubMed] [Google Scholar]
- 19.Song M, Finley SD. Mechanistic insight into activation of MAPK signaling by pro-angiogenic factors. BMC Syst Biol. 2018; 12:145. 10.1186/s12918-018-0668-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jia M, Guo Y, Zhu D, Zhang N, Li L, Jiang J, Dong Y, Xu Q, Zhang X, Wang M, Yu H, Wang F, Tian K, et al. Pro-metastatic activity of AGR2 interrupts angiogenesis target bevacizumab efficiency via direct interaction with VEGFA and activation of NF-κB pathway. Biochim Biophys Acta Mol Basis Dis. 2018; 1864:1622–33. 10.1016/j.bbadis.2018.01.021 [DOI] [PubMed] [Google Scholar]
- 21.Chen YJ, Chan DC, Chiang CK, Wang CC, Yang TH, Lan KC, Chao SC, Tsai KS, Yang RS, Liu SH. Advanced glycation end-products induced VEGF production and inflammatory responses in human synoviocytes via RAGE-NF-κB pathway activation. J Orthop Res. 2016; 34:791–800. 10.1002/jor.23083 [DOI] [PubMed] [Google Scholar]
- 22.Evola FR, Costarella L, Pavone V, Caff G, Cannavò L, Sessa A, Avondo S, Sessa G. Biomarkers of Osteosarcoma, Chondrosarcoma, and Ewing Sarcoma. Front Pharmacol. 2017; 8:150. 10.3389/fphar.2017.00150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Svestak M, Sporova L, Hejduk P, Lacnak B, Stejskal D. Collagenous repeat-containing sequence of 26 kDa protein - a newly discovered adipokine - sensu lato - A minireview. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2010; 154:199–202. 10.5507/bp.2010.030 [DOI] [PubMed] [Google Scholar]
- 24.Tsai CH, Tsai HC, Huang HN, Hung CH, Hsu CJ, Fong YC, Hsu HC, Huang YL, Tang CH. Resistin promotes tumor metastasis by down-regulation of miR-519d through the AMPK/p38 signaling pathway in human chondrosarcoma cells. Oncotarget. 2015; 6:258–70. 10.18632/oncotarget.2724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Su CM, Tang CH, Chi MJ, Lin CY, Fong YC, Liu YC, Chen WC, Wang SW. Resistin facilitates VEGF-C-associated lymphangiogenesis by inhibiting miR-186 in human chondrosarcoma cells. Biochem Pharmacol. 2018; 154:234–42. 10.1016/j.bcp.2018.05.001 [DOI] [PubMed] [Google Scholar]
- 26.Zhao CC, Chen J, Niu RF, Liu Y, Zhang CG. Increased resistin suggests poor prognosis and promotes development of lung adenocarcinoma. Oncol Rep. 2018; 40:3392–404. 10.3892/or.2018.6736 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 27.Liu JF, Hou CH, Lin FL, Tsao YT, Hou SM. Nimbolide Induces ROS-Regulated Apoptosis and Inhibits Cell Migration in Osteosarcoma. Int J Mol Sci. 2015; 16:23405–24. 10.3390/ijms161023405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liou GY, Storz P. Reactive oxygen species in cancer. Free Radic Res. 2010; 44:479–96. 10.3109/10715761003667554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jackson M, Serada N, Sheehan M, Srinivasan S, Mason N, Guha M, Avadhani N. Mitochondrial genome and functional defects in osteosarcoma are associated with their aggressive phenotype. PLoS One. 2018; 13:e0209489. 10.1371/journal.pone.0209489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schüll S, Günther SD, Brodesser S, Seeger JM, Tosetti B, Wiegmann K, Pongratz C, Diaz F, Witt A, Andree M, Brinkmann K, Krönke M, Wiesner RJ, Kashkar H. Cytochrome c oxidase deficiency accelerates mitochondrial apoptosis by activating ceramide synthase 6. Cell Death Dis. 2015; 6:e1691. 10.1038/cddis.2015.62 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.