Abstract
Complex in vitro models of the tissue microenvironment, termed microphysiological systems, have enormous potential to transform the process of discovering drugs and disease mechanisms. Such a paradigm shift is urgently needed in acute respiratory distress syndrome (ARDS), an acute lung condition with no successful therapies and a 40% mortality rate. Here, we consider how microphysiological systems could improve understanding of biological mechanisms driving ARDS and ultimately improve the success of therapies in clinical trials. We first discuss how microphysiological systems could explain the biological mechanisms underlying the segregation of ARDS patients into two clinically distinct phenotypes. Then, we contend that ARDS-mimetic microphysiological systems should recapitulate three critical aspects of the distal airway microenvironment, namely, mechanical force, inflammation, and fibrosis, and we review models that incorporate each of these aspects. Finally, we recognize the substantial challenges associated with combining inflammation, fibrosis, and/or mechanical force in microphysiological systems. Nevertheless, complex in vitro models are a novel paradigm for studying ARDS, and they could ultimately improve patient care.
I. PATHOPHYSIOLOGY AND ENDOTYPES OF ARDS
A. Background
Acute Respiratory Distress Syndrome (ARDS) is a life-threatening acute lung condition characterized by the sudden onset of severe pulmonary inflammation and edema resulting in secondary hypoxemia and pulmonary fibroproliferation. ARDS can be triggered by various insults, whether direct (e.g., aspiration, pneumonia, and mechanical ventilation) or indirect (e.g., sepsis, trauma, and blood transfusion).2,8,84 Following such insults, most ARDS patients must be placed on positive pressure mechanical ventilation that can cause ventilator-associated lung injury, which exacerbates the initial tissue injury.101 Despite over 50 years of intense study and attempts at pharmacological treatment, the mortality rate in ARDS patients hovers at 35%–45% and the condition afflicts an estimated 190 000 patients per year84 in the United States. It is also responsible for up to 10% of intensive care admissions globally.11,35,46 Only modest improvements in survival have been made due to mechanical ventilation strategies that minimize ventilator associated lung injury.118 So far, no pharmacological therapies have reduced ARDS mortality, including those aimed at attenuating inflammation, preventing or suppressing fibrosis, addressing infection, or surfactant replacement to reduce fluid mechanical stress.46,64,67
B. ARDS pathophysiology
Pathophysiology of ARDS occurs in 3 chronological phases. In the exudative phase, severe inflammation causes diffuse alveolar injury and increased epithelial and microvascular permeability. Proteinaceous vascular fluid leaks into the alveolar lumen, and large amounts of alveolar epithelial cells die. Surfactant production is compromised as type II pneumocytes are lost so that the fluid flooding the lumen has an abnormally high surface tension.28 Leukocytes, especially neutrophils, are aggressively recruited and release proinflammatory cytokines, proteases, and neutrophil extracellular traps. Apoptotic epithelial cells and neutrophils accumulate in the alveolar lumen and begin to form hyaline membranes composed of immunoglobulin, complement, dead cell debris, and fibrin. Fibroblasts infiltrate into this environment to repair the tissue damage sustained by the initial injury. In the proliferative phase, type II alveolar epithelial cells and fibroblasts proliferate and cover sites of denudation in the alveoli. Fibroblasts become activated and deposit fibronectin to re-establish a basement membrane, and type II alveolar epithelial cells differentiate to type I epithelium and restore gas exchange and barrier function to sites of denudation. Immune cells are continuously recruited to mediate tissue repair. Hyaline membranes are a characteristic histological finding during this phase.19 The fibroproliferative phase is characterized by myofibroblast invasion, fibroblast proliferation, and collagen production. Surviving patients often experience a permanent decline in lung function.22 For detailed pathophysiology that is outside the scope of this review, readers are referred to recent comprehensive reviews by Matthay et al.84 and Sapru et al.108
C. Injury-inflammation-repair in ARDS
Tissue repair, especially restoration of barrier function, is coordinated through inflammatory and fibrotic processes that are influenced by the mechanical and biochemical microenvironment [Fig. 1 (Refs. 2, 7, 15, 27–29, 53, 69, 88, 89, 101, 121, and 123)]. The initial injury is caused primarily by neutrophil-dependent and platelet-dependent damage to the endothelial and epithelial barriers of the small airways and alveoli. Studies in large animals showed that alveolar edema occurs only when there is damage to both the endothelium and epithelium.138 In healthy repair, inflammation and fibrosis restore barrier and gas exchange function to the epithelium and endothelium and subsequently resolve.4,7,12,27 While the endothelium's morphology appears unaffected aside from its compromised barrier function, the epithelium experiences significant denudation and apoptosis in addition to loss of barrier function, which prevents removal of alveolar edema fluid and deprives the lung of adequate quantities of surfactant.84
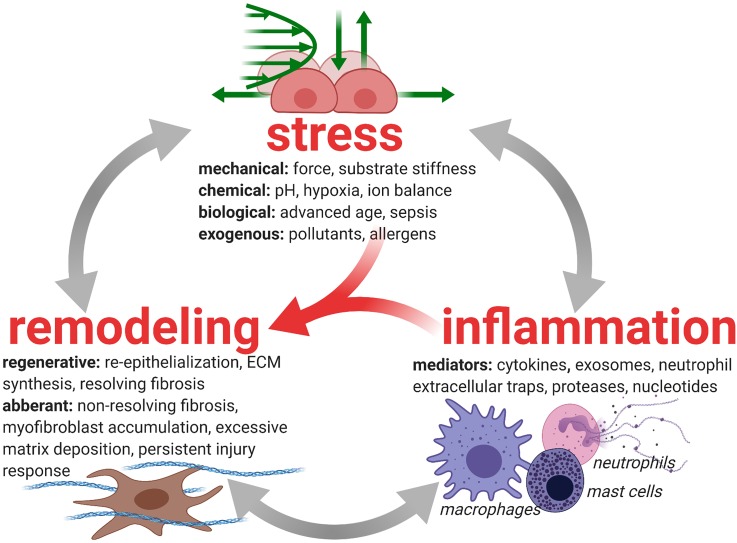
FIG. 1.
Injury-inflammation-repair in lung disease. Inflammation and fibrosis (resolving or nonresolving) cooperate to remodel lung tissue after injury.7,27 Both processes are heavily influenced by stressors in the tissue microenvironment. In ARDS, mechanical forces arising from surfactant dysfunction28 and mechanical ventilation101 interfere with the tissue repair process by reinjuring the tissue,2,29,53,69 thereby inducing inflammation123 and promoting fibrosis.15,121,131 The interactions between tissue stress, inflammation, and remodeling can direct the tissue toward successful tissue repair or toward aberrant remodeling that consists of progressive fibrosis and systemically dysregulated immunity.7,15,88,89,131
Epithelial repair in ARDS is often dysregulated. Blood neutrophils are recruited massively to the lumen, where they extend their lifespan in the lung tissue and perpetuate inflammation.48 Meanwhile, activated fibroblasts deposit excess collagen that impairs gas exchange.86 Most patients require mechanical ventilation and experience surfactant dysfunction, exacerbating epithelial injury during the repair process and imparting sublethal stresses on the epithelium. Compounding the impact of ventilation is the fact that the epithelium, immune cells, and fibroblasts sense and respond to mechanical forces14,31,60,85,109,121,137,144 in the lung microenvironment. Therefore, models of inflammation and fibrosis during mechanical ventilation are critical to understanding how epithelial repair impacts ARDS endotype development and consequently the patient's chance of survival.
D. ARDS endotypes
ARDS is a clinically heterogeneous condition. Approximately 10% of ARDS patients recover rapidly and in the acute phase (<3 days),110 and these patients may not require intervention aside from ventilation. Meanwhile, a larger subset of patients experiences progressive fibrosis80,81,89 and/or systemically dysregulated inflammation,17,90,104 both of which are associated with a higher risk of mortality.17,21,65,81,87,118 These nonresolving patients might benefit from pharmacologic intervention, especially if their risk of developing to this stage could be identified prior to the onset of symptoms. Additionally, ARDS clinical trials74,106,128 have reported inconsistent therapeutic responses. This failure could be explained by the syndrome's heterogeneity. Therefore, there is great interest in predicting (a) which patients will not recover rapidly to determine when intensive treatment and/or trial enrollment is most beneficial38 and (b) which nonresolving patients might respond to which therapeutics to enrich clinical trial cohorts with potential responders.
To address these urgent questions, clinicians have developed prognostic markers that correlate ARDS outcomes with epidemiology, genomics, clinical features, physiology, and biomarkers.26,116 The ladder classifier is advantageous because it stratifies patients based on indicators of the underlyling biology of their disease. This powerful connection to biology might enable clinicians to predict therapeutic responses using these biomarker-based classificiations when the pathophysiology driving each biomarker profile is better understood. For example, Calfee et al.17 used latent class analysis to show that ARDS patients cluster into two clinical endotypes based on biomarkers: hyper- and hypoinflammatory. The former experienced higher rates of shock and metabolic acidosis, had significantly worse outcomes, and had higher mortality in response to mechanical ventilation with low positive end expiratory pressure. These findings were later verified using cluster analysis,13 and a second retrospective trial analysis showed endotype-associated responses to simvastatin.18
In these retrospective studies, Calfee's classifiers have demonstrated the potential to transform patient care by treating patients based on the biology driving their disease. However, biomarker-based endotyping cannot fulfill its promise of predicting endotype-specific responses to drugs until the biological mechanisms behind each endotype are understood. Only then will endotyping be a convincing determinant of patient enrollment in clinical trials. Sinha and Calfee116 provide a more extensive review of ARDS endotyping and the need for biological mechanisms.
II. MODELING ARDS ENDOTYPES
A. Traditional models
To understand the biological mechanisms that drive ARDS endotypes, preclinical models of ARDS pathophysiology are essential. The ideal preclinical model of ARDS pathophysiology should recapitulate only the critical aspects of the complex disease microenvironment, focusing on a specific etiology and patient endotype. Current models are limited in their ability to represent human pathophysiology for the study of disease and drug mechanisms.
2D monoculture of the airway epithelium in vitro cannot capture intricacies of inflammatory networks and cross talk between processes of injury, inflammation, and remodeling. This culture method typically also neglects tissue-level stresses such as mechanical force and does not account for fluid stresses that are dominant in ARDS due to surfactant depletion. Finally, cell lines are limited in their relevance to pathophysiology. However, primary human cells are becoming more accessible. For example, the Marisco Lung Institute's CF Center Tissue Procurement and Cell Culture Core has pioneered isolation and culture techniques for primary human lung cells.43
Animal models, notably mouse models, capture complex interactions between injury, inflammation, and tissue repair in ARDS, making them more suitable than current in vitro studies for drug testing. For pathophysiology studies, however, species-specific differences in lung physiology could interfere with attempts to correlate biomarkers with pathophysiological mechanisms. There is conflicting evidence regarding whether murine gene expression profiles in response to lung injury correlate well with those in humans. Sweeney et al. argue for significant similarity between murine and human inflammation after lung injury,122 although limitations to this study include the small human sample size (n = 3) and the number of genes evaluated (n = 432). Further, this study compared human samples from patients with non-ARDS lung injury. Seok et al., in contrast, assert that when comparing almost 5000 human and murine genes altered by the same inflammatory stressors (i.e., burn, trauma, and hypoxemia), mouse models of inflammation show a close to random (R2 between 0 and 0.1) association to human gene counterparts.112 Inflammation in ARDS involves thousands of responsive genes,104 and a comprehensive determination has not been reached about the relevance of murine gene expression to human ARDS. Therefore, there is interest in studying human cells to complement animal studies.
Inherent limitations hinder the study of primary human samples. It is impossible to control the cell types (e.g., immune cells, epithelium, and fibroblasts) and mediators (e.g., cytokines, chemokines, and extracellular matrix components) present in a patient's lung microenvironment, limiting the ability to interrogate individual components' contribution to pathophysiology. This limitation can result in studies that are descriptive rather than mechanistic. Biopsy samples are acquired primarily from deceased patients because biopsies are a high-risk procedure for living patients. Because of this, human lung samples are biased toward severe disease and provide little opportunity to study the evolution of the disease microenvironment from the early to late stage. Bronchoalveolar lavage fluid (BALF) provides a snapshot of the distal lung's cytokine, immune cell, and mucus content, but cellular-level mechanisms cannot be positively inferred without corroborating in vitro data.
Whole blood is readily available but provides limited information about the lung microenvironment. Particularly, peripheral neutrophils are often studied in ARDS, but their relevance to the lung microenvironment is unclear because lung neutrophils appear to acquire novel phenotypes upon recruitment to the airways. In vitro, transepithelial migration of primary peripheral neutrophils into pediatric ARDS patient airway fluid activates neutrophils toward a proinflammatory phenotype with paradoxically decreased bacterial killing potency.51 Additionally, primary neutrophils isolated from adult ARDS BALF display impaired bacterial killing and superoxide production compared to blood and local arterial neutrophils.83 Overall, human samples are a vital component of ARDS pathophysiology research but corresponding in vitro data from more sophisticated models are needed to study mechanisms. For a more in-depth review of preclinical ARDS models, see the excellent analysis by Laffey and Kavanagh.72
B. Modeling ARDS in microphysiological systems (MPSs)
Microphysiological systems (MPSs) are in vitro cell culture systems incorporating 3-D culture, coculture, physical forces, or other tissue-level phenomena that aim to create a tissue-mimetic microenvironment. They capture complex tissue-level physiology and disease phenomena in vitro using primary human cells and fluids from patients,68 lending them the potential to bridge the gap between animal models and human pathophysiology. MPSs supplement human and animal models and provide a third paradigm for the study of complex disease processes and drug mechanisms. MPS modeling different ARDS endotypes could explain the different responses to simvastatin and protective ventilation that were observed between hyperinflammatory and hypoinflammatory cohorts in clinical trials.
Of course, the challenge of recreating a pathophysiology that is poorly understood, for the purpose of advancing its understanding, cannot be overstated. ARDS endotypes are not correlated strongly with disease etiology or epidemiology, and as a result, there are currently no preclinical models of endotype-specific ARDS. There are several excellent etiology-specific models: Hecker and colleagues recently developed a “two-hit” murine model of ARDS combined with aging that resembles human ARDS more closely than traditional mouse models and may prove more clinically relevant for therapeutic efficacy evaluation.99 Nemzek et al. have studied a two-hit aspiration-induced ARDS model.96 However, these models do not capture endotype-specific ARDS.
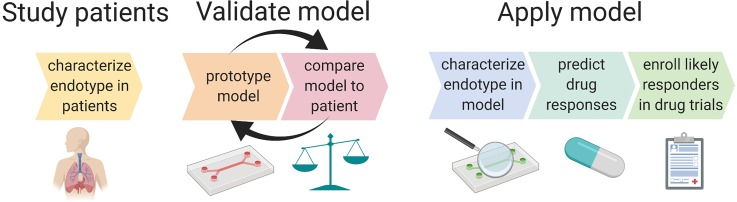
MPSs are uniquely suited to become the first preclinical ARDS endotype models. MPSs can undergo iterative prototyping until a desired pathophysiological feature is adequately captured. This strategy is illustrated in Fig. 2. MPS designers compare their prototype to the human phenotype using metrics such as biomarkers, immune cell phenotypes, and responses to stimuli (e.g., strain, hypoxia, and infection). The prototype is then adjusted to better reflect in vivo metrics through the precise control of microenvironmental factors such as cell types, inflammatory and fibrotic mediators, and type/degree of mechanical force. MPSs capture disease processes to the extent necessary to produce endotype-specific biomarkers but remain simple enough to obtain a high signal-to-noise ratio, which is a desirable feature of in vitro models.
FIG. 2.
Iterative model design with validation against patient phenotype will lead to an endotype-specific model of ARDS that can be used for predictive enrichment of ARDS clinical trials. Endotype-specific metrics such as cytokine ratios, immune cell functions (e.g., bacterial killing, metabolism, and NETosis), and degree of surfactant production can be compared between patients and in vitro models. Iterative adjustments to model parameters, such as genetics (e.g., MUC5A upregulated epithelium), physical forces, degree of initial injury, degree of fibrosis, and the type and ratio of inflammatory mediators, could enable the development of a model that produces biomarkers or functional characteristics (e.g., response to therapeutics, response to mechanical strain, and immune cell phenotype changes such as enhanced NETosis), mimicking those of a specific endotype. The model should also be validated by testing functional outputs such as barrier function of the epithelium and tissue healing (scratch wound assay). The final model provides the opportunity for pathophysiological mechanisms of disease to be clarified and for drug candidates to be tested in vitro. Both pathophysiology and drug testing will help predict whether a certain endotype is likely to respond to novel treatments.
This review focuses on the bioengineering challenges of constructing an ARDS microphysiological system that could address clinical problems, especially the need to understand endotype pathophysiology. Critical aspects of the alveolar microenvironment during ARDS should be identified and included in experimental models of ARDS. This review highlights mechanical forces, inflammation, and fibrosis because they are central to ARDS pathophysiology and tissue repair, difficult to capture in vitro with traditional methods, and readily adaptable to existing MPS technology.
III. CAPTURING MECHANICAL FORCES IN MPS
A. Physiological forces in the distal airways
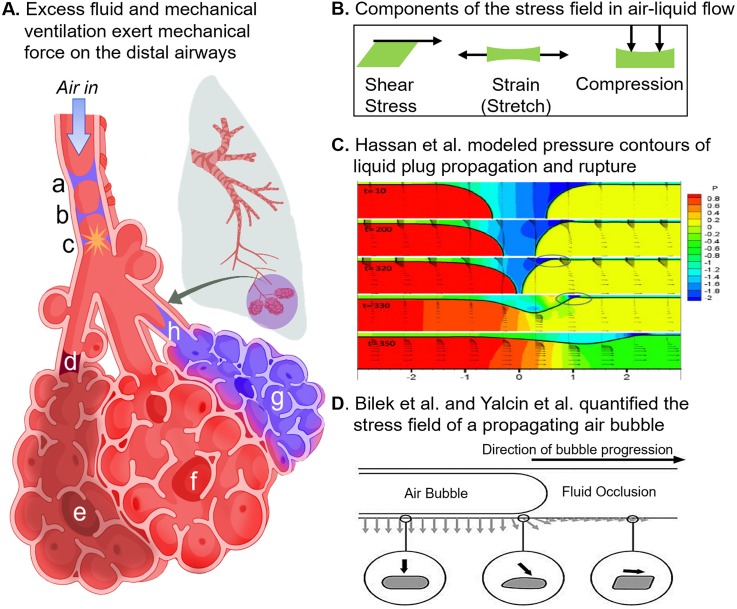
Because a majority of ARDS patients are mechanically ventilated, it is important to understand how the forces inflicted by positive pressure ventilation and mechanical stress from excess fluid in the bronchoalveolar region impact the tissue repair process. After ARDS onset, surfactant function is compromised due to the death of type II alveolar epithelial cells and flooding of distal airways with proteinaceous fluid.8 This increases the surface tension of the liquid covering the surface of the alveolar and small airway epithelia, leading to abnormally high levels of fluid stresses. These are exacerbated by alveolar collapse and reopening (atelectasis), overdistension of the alveoli (volutrauma), and/or high peak inspiratory or driving pressures (barotrauma) during mechanical ventilation.45,97,98 Figure 3(A) illustrates forces experienced by the small airways and alveoli during ARDS. Of note, surfactant trials to alleviate fluid stress have not succeeded. Studies suggest they may have failed as a result of inadequate delivery to the alveoli due to low instilled dose volume, as indicated by computational modeling.39,50
FIG. 3.
Physiologic mechanical forces in the bronchoalveolar region and their computational models. (A) The acinus consists of alveoli sacs that branch off of common terminal bronchiole (d) or (h); sacs (e), (f), and (g) are depicted in this figure. Sac (e) is cut off from air flow by the stagnant plug at (d); sac (f) is overinflated, and sac (g) is flooded with proteinaceous fluid. (B) Shear, strain, and compression are the main components of force present in the lungs, either independently or in concert. In the above depiction, strain results from overinflation of sac (f) due to obstruction of sac (d) and collapse of sac (g). Compression of adjacent sac results from the overinflation of (f). Shear stress is a component of the stress field produced during airway reopening at (h).10,142 Interfacial flow damages the small airways when liquid plugs propagate and rupture during inspiration61,126 (a)–(c) Transient liquid plugs form when the small airways collapse slightly and liquid on either side of the airways meets, forming the plug depicted in (a). Upon inspiration, the plug is pushed by pressure-driven flow, becoming thinner and thinner (b) until it loses integrity and pops (c), creating the crackle sounds that are observed upon auscultation of the lungs. (C) Hassan et al.54 modeled liquid plug propagation and rupture and found that the leading edge of the plug creates a narrow capillary wave (circled). The wave's extreme pressure gradient imparts severe stress on the airway wall. (D) The first in vitro model of airway reopening was introduced by Bilek et al.10 Using this model, Yalcin et al. found that smaller airway diameters experience greater stress.142 Reproduced with permission from Hassan et al., Int. J. Numer. Methods Fluids 67, 1373 (2011). Copyright 2011, John Wiley and Sons.54 Reproduced with permission from Bilek et al. J. Appl. Physiol. 94, 770 (2003). Copyright 2003, American Physiological Society.10
B. Existing mechanical force MPS
Lung mechanical forces can be categorized broadly as compressive stress, shear stress, and stretch [Fig. 3(B)].140 Shear stress is the force per area that acts parallel to a plane, often considered a “slipping” force. Strain (stretch) is the change in the length of a plane divided by the initial length. Compressive stress is the force per area applied perpendicular to a plane; it includes pressure and normal force. The effects of mechanical forces on pulmonary epithelia have been studied in vitro for several decades.76,137 Most models incorporate membranes that allow for strain or compressive stress to be applied to well-differentiated cell lines or primary airway cells in air-liquid interface (ALI) culture (Table I).
TABLE I.
Representative sample of in vitro platforms that replicate mechanical forces in the lungs.
| References | Mechanical Stimuli | Disease | Cell type(s) | Model type | Force application method | Key metrics in response to force |
|---|---|---|---|---|---|---|
| Ressler et al.105 | Compression | Asthma | Rat tracheal epithelial | Transwell | Air pressurization above culture | RNA coding for Egr-1, endothelin-1, TGF-β |
| Swartz et al.121 | Compression | Asthma | Normal human bronchial epithelial and normal human lung fibroblast (CCL-186) | Transwell epithelium culture over fibroblasts | Air pressurization above culture | Quantitative production of collagen, fibronectin |
| Tschumperlin et al.132 | Compression | Asthma | Normal human bronchial epithelial | Transwell | Air pressurization above culture | MAP kinase and herparin-binding epidermal growth factor (HB-EGF) |
| Bilek et al.10 | Stress field, pressure gradient | VILI | Fetal rat pulmonary epithelial (CCL-149) | Parallel plate flow chamber | Air bubble propagation | Cell death (live/dead stain) |
| Chu et al.24 | Compression | Asthma | Normal human bronchial epithelial | Transwell | Air pressurization above culture | Quantitative expression of epidermal growth factor receptor ligands HB-EGF, epiregulin, amphiregulin, TGF-β |
| Tarran et al.125 | Shear stress | Cystic fibrosis | Primary human epithelial | Transwell | Phasic motion of culture | Adenosine triphosphate (ATP) release, periciliary layer thickness |
| Choe et al.23 | Compression | Asthma | Human fetal lung fibroblast (CCL-186) | Tissue-engineered human airway wall | Dynamic lateral compressive strain | Deposition of types III and IV collagen, MMPs-2 and-9 |
| Huh et al.61 | Shear stress, pressure gradient | Pulmonary edema | Primary human small airway epithelial | Microfluidic chip | Liquid plug propagation and rupture | Cell death (live/dead stain) |
| Yalcin et al.142 | Shear stress, pressure gradient | ARDS | Fetal rat pulmonary epithelial (CCL-149) | Height adjustable parallel plate flow chamber | Air bubble propagation | Cell death (live/dead stain) |
| Sidhaye et al.114 | Shear stress | General | Normal human bronchial epithelial | Cell culture insert | Laminar fluid flow | Paracellular permeability |
| Fronius et al.41 | Shear stress | Cystic fibrosis | Xenopus oocyte | Xenopus oocyte | Fluid stream | Epithelial sodium channel activation |
| Huh et al.63 | Strain | General | Human pulmonary microvascular endothelial and alveolar epithelial, peripheral neutrophil (H441, A549, E10) | Microfluidic chip | Stretching porous membrane | ICAM-1 (endothelium), ROS generation (epithelium), nanoparticle translocation |
| Douville et al.32 | Shear stress, strain | ARDS | Human alveolar basal epithelial (A549) and primary murine alveolar epithelial | Microfluidic chip with flexible membrane | Membrane stretch and air-liquid interface oscillation | Cell death (live/dead stain) |
| Jacob and Gaver69 | Stress field, pressure gradient | ARDS | Human pulmonary epithelial (H441) | Parallel plate flow chamber | Air bubble propagation | Paracellular permeability; Distribution of tight junction proteins ZO-1 and claudin 4 |
| Stucki et al.119 | Strain | General | Bronchial epithelial (16HBE14o−), primary human pulmonary alveolar epithelial, HUVEC | Microfluidic chip | Microdiaphragm stretching | Proliferation, IL-8 secretion |
| Manuyakorn et al.79 | Strain | Asthma | Primary bronchial fibroblasts | Flexible silastic membranes | Cyclic strain | Proteoglycans, αSMA, collagens I and III, MMPs 2 & 8, IL-8 |
| Song et al.117 | Shear stress, pressure gradient | Obstructive lung diseases | Primary human small airway epithelial (CC-2547) | Microfluidic chip | Liquid plug propagation | Cell viability, NF-κB, stress fibers |
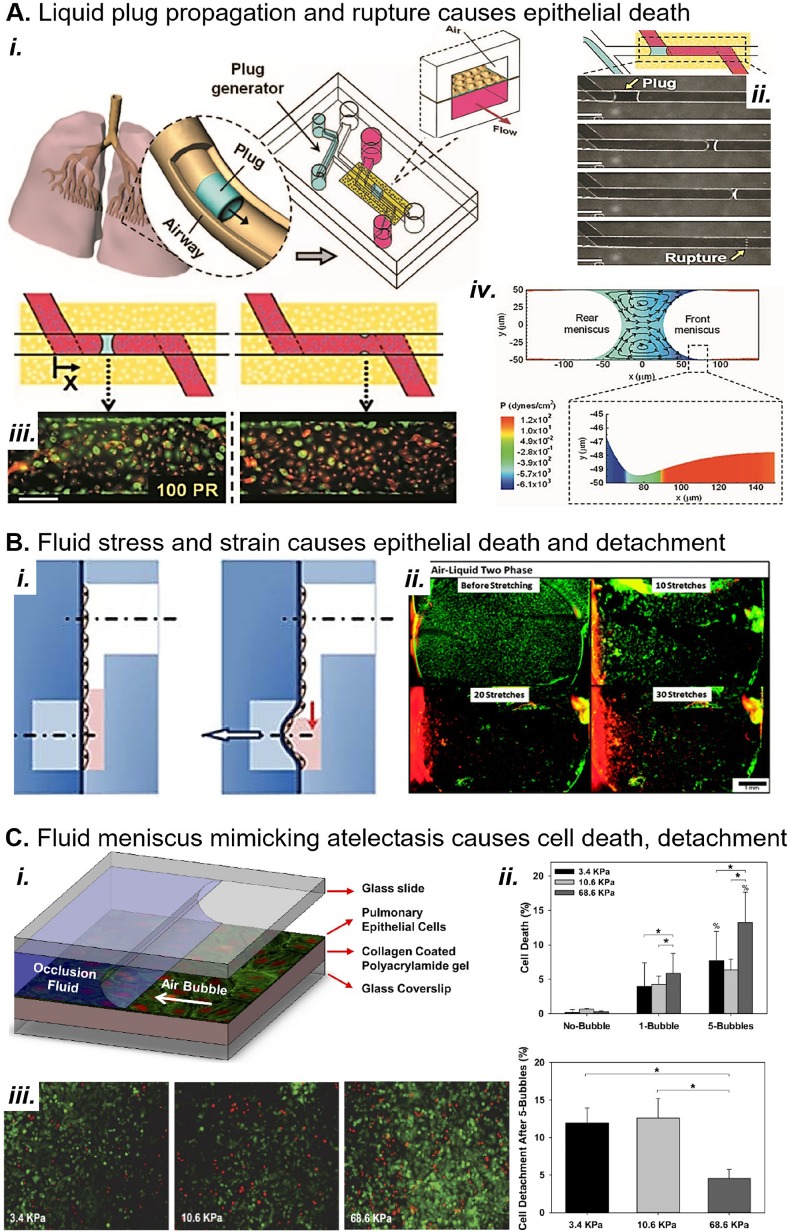
Complex fluid stresses also contribute significantly to lung injury.2,49,101,107 Investigators have modeled the fluid stresses imparted by liquid plug propagation and rupture, small airway collapse and reopening, and alveolar collapse and reopening. The Gaver group was the first to report an in vitro system for modeling the stress field associated with alveolar recruitment using a moving air bubble [Fig. 3(D) (Refs. 10 and 142)]. They showed that slower bubble speeds increase cell death, despite a milder shear gradient, because the pressure gradient is significantly increased.70 In the same moving air bubble model, Higuita-Castro et al. showed that increasing the substrate stiffness caused greater cell death after 1 and 5 bubbles57 [Fig. 4(c)].
FIG. 4.
MPS models of mechanical force in lung disease. (a) (i) A microfluidic device replicates the generation of crackle sounds frequently heard in the distal airways of patients with pulmonary edema. (ii) The device generates liquid plugs that propagate and rupture in channels over alveolar type I pneumocytes. (iii) Plugs result in epithelial cell death (red) during propagation (left) and especially at the rupture site (right). Scale bar, 150 μM. (iv) Fluid dynamic simulations show that the leading edge of the plug applies severe shear stress to the epithelium.42,54 Reproduced with permission from Huh et al. Proc. Natl. Acad. Sci. U. S. A. 104(48), 18886–18891 (2007). Copyright 2007 National Academy of Sciences.61 (b) (i) Douville et al. report a device that applies simultaneous fluid shear stress and mechanical strain to alveolar epithelium; vacuum stretches the membrane and simultaneously lowers the fluid level. (ii) Fluid stress and strain result in death (red cells) and detachment of alveolar epithelium in the device. Scale bar, 1 mm. Reproduced with permission from Lab on a Chip 11, 609–619 (2011). Copyright 2011 Royal Society of Chemistry.32 (c) (i) Higuita-Castro et al. mimic small airway or alveolar reopening by propagating an air bubble over the epithelium.57 The device design, first conceived by Bilek et al.,10 has been extensively used to characterize the damage of liquid stress during atelectasis and airway reopening.57,58,69,70,142 (ii) and (iii) Higuita-Castro et al. show that the fluid meniscus causes increasing cell death and detachment with increasing substrate stiffness. Reproduced with permission from Higuita-Castro et al., J. Appl. Physiol. 117, 1231 (2014). Copyright 2014 American Physiological Society.57
Additionally, Takayama and colleagues modeled liquid plug propagation and rupture in vitro61,126 [Fig. 4(a) (Refs. 42, 54, and 61)]. They found that liquid plug propagation caused cell death even without plug rupture. To explain this observation, Fujioka et al. showed that the front meniscus of a moving liquid plug imparts large stresses on the airway wall due to a narrow capillary wave that appears ahead of the plug's leading edge [Fig. 3(C), capillary wave circled].42,54 Recently, Muradoglu et al. also showed that surfactant reduces the mechanical stress imparted by the liquid plug.95 In an alveolar closure/reopening model, Douville et al. showed that repeat strain combined with fluid stress caused cell death and detachment, suggesting a mechanism for how atelectasis affects lung function32 [Fig. 4(b)]. The work of the preceding investigators has brought attention to the major role of fluid stresses in promoting lung injury. Additionally, we provide a representative history of all types of pulmonary epithelial force models in Table I.10,23,24,32,41,61,63,69,79,105,114,117,119,121,125,132,142
C. Limitations of mechanical force MPS
Few pulmonary force models consider the physical properties of the extracellular matrix, such as stiffness and substrate ligands. It is well established that substrate ligands affect epithelial and endothelial properties and that focal adhesions mediate mechanosensing.1,73 Of relevance to mechanical force models, aligned collagen fibers in the substrate amplify cell-cell mechanotransduction across distances greater than several cell diameters.78 In the moving air-finger model [Fig. 4(c) (Refs. 10, 57, 58, 69, 70, and 142)], Higuita-Castro et al. showed that increased substrate compliance leads to greater cell detachment and less necrosis.57 More investigation is needed to determine the effects of substrate properties on physiology in mechanical force models.
Mechanical force models rarely contain multiple stress types, despite indications that stressors work synergistically to promote injury. For example, simultaneous surfactant loss and overstretching of the alveoli cooperatively promote secondary lung injury during mechanical ventilation.97,98 Even models that do capture complex force combinations or stress fields often fall short of describing the force's downstream effects on the tissue. Huh et al. and Douville et al., shown in Figs. 4(a) and 4(b), capture complex forces but characterize only cell death and detachment. Ghadiali and colleagues, in contrast, have characterized mechanotransduction in the moving air-finger model [Fig. 4(c)].57,58,69 Still, both models lack immune and fibroblast coculture.
Because tissue repair is an essential component of recovery from ARDS, it is critical to capture the interaction between tissue repair and mechanical forces. This dynamic relationship remains poorly understood despite two decades of in vitro studies on the effects of mechanical forces on isolated epithelial, fibroblast, and immune cells.85,127,137,144 In the following, we discuss the challenges and opportunities for modeling this aspect of ARDS in MPS.
IV. CAPTURING INFLAMMATION AND FIBROSIS IN MPS
A. Inflammation and fibrosis in ARDS
Inflammation in ARDS is characterized by early and persistent neutrophilia, mast cell recruitment and activation,135 and recruitment of monocytes and macrophages in the distal airways and alveoli.84 Neutrophil activity is believed to damage tissue in ARDS.48,146 Neutrophils secrete proteases, such as neutrophil elastase and matrix metalloproteases (MMPs), which degrade the extracellular matrix, and neutrophils promote hypoxia through rapid oxygen consumption and the excessive production of reactive oxygen species (ROSs).25,48,146 Neutrophils are also affected by mechanical stressors in vitro and in vivo. Force induces the production of chemotactic factors, including TNF-α,60,102,103,129 IL-8,102,131,136 and IL-6.103,123,129,141 IL-6 has long been considered a biomarker of ventilator-induced lung injury, and TNF-α and IL-8 are master regulators of inflammation.
Concurrently, fibroproliferation and tissue repair proceed in response to both the primary tissue injury and the damage caused by recruited neutrophils. In the terminal bronchioles and alveoli, type II pneumocytes proliferate and fibroblasts become activated. Proliferating fibroblasts deposit provisional extracellular matrix (ECM) consisting of fibronectin, and later collagen, to repair ECM damage and restore epithelial integrity.84,89 Dysregulated fibroproliferative repair can develop into nonresolving fibrosis that has a poor prognosis in ARDS and is refractory to treatment.80,81,87 Aside from the introduction of protective ventilation strategies that limit ventilator-associated lung injury, attempts to prevent the occurrence of nonresolving fibrosis and dysregulated immunity, or to slow progression, have not yielded success.
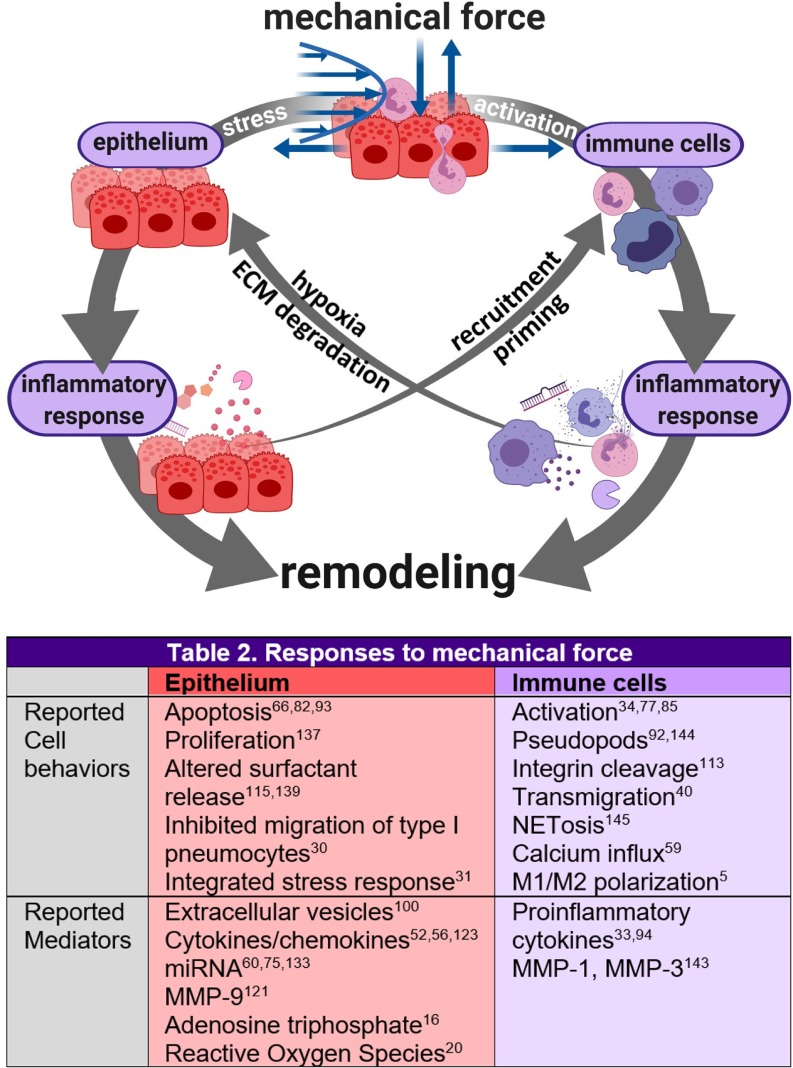
Crosstalk between the epithelium and immune system in response to mechanical force plays a significant role in mediating tissue repair. This process is poorly characterized due to its complexity and heterogeneity, but general mechanisms of mechanical force-induced epithelial-immune cross talk that have been reported individually are presented in Fig. 5 (Refs. 5, 12, 16, 20, 27, 30, 31, 33, 34, 40, 44, 48, 52, 56, 59, 60, 66, 75, 77, 82, 85, 92–94, 100, 107, 113, 115, 117, 120, 121, 123, 124, 130, 133, 137, 139, and 143–146).
FIG. 5.
Reported mechanical stress-induced cell behaviors and inflammatory mediators that enable cross talk between pulmonary epithelium and immune cells. Mechanical stresses due to surfactant dysfunction, edema, and mechanical ventilation cause the epithelium to produce inflammatory and fibrotic mediators107,130 and may induce the integrated stress response, a key mediator of mechanical stress-induced epithelial injury.31 Mechanical force-induced cell behaviors and mediators of cross talk between the epithelium and immune cells are listed in Fig. 5. Such cross talk drives remodeling pathways; for example, hyperactivated neutrophils deplete oxygen in their microenvironment through the excessive production of reactive oxygen species (ROSs). This hypoxia stresses the epithelium and leads to apoptosis or inflammatory signaling that promotes fibroblast activation and epithelial proliferation. Neutrophils also contribute directly to remodeling during epithelial transmigration;12 massive neutrophil influx compromises tight junction integrity and stimulates repair of the lamina propria.146 Mechanically activated neutrophils and alveolar macrophages also secrete proteases that degrade the extracellular matrix (ECM), such as neutrophil elastase, and promote continued ECM destruction and activation of fibroblasts to repair the ECM damage.27,44,124 Because neutrophils are significant drivers of this destructive remodeling cycle, they are a target of therapeutic research.120
B. Challenges of modeling inflammation and fibrosis in MPS
The choice of cell types, source, and degree of activation or polarization can greatly affect cell phenotype and responses to stimuli. Tissue repair in vivo involves the migration, activation, and cross-contact of multiple cell types including fibroblasts, neutrophils, monocytes, and lymphocytes. The ideal model would capture a high degree of in vivo cell functionality including fibroblast proliferation, immune transmigration and in situ activation, degranulation, neutrophil extracellular trap (NET) formation, and elevated phagocytosis. However, many of these behaviors are difficult to control and modulate in complex model systems with multiple cell types. For example, TGF-β activates fibroblasts but may have concurrent effects on the epithelium and immune cells. MPSs should capture the minimal activation necessary to recapitulate the disease mechanism of interest.
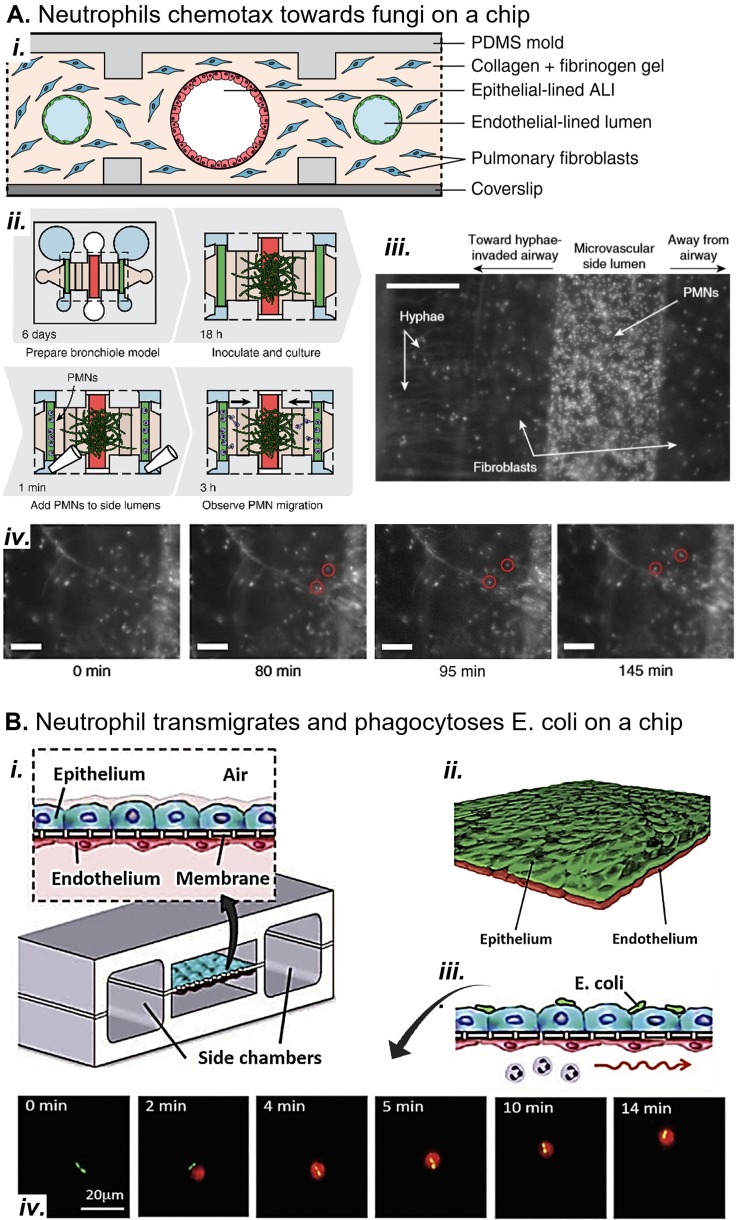
Barkal et al., for example, focused on neutrophil activation in their small airway-on-a-chip model of fungal infection. Neutrophils migrated toward volatile fungal chemoattractants through the endothelium and epithelium [Fig. 6(a)].6 In a model of invasive aspergillosis, the device recapitulated the well-characterized inflammatory cytokine response and increased recruitment of neutrophils observed in murine and zebrafish models. In another example, Choe et al. focused on activation of fibroblasts.23 They applied strain to a coculture of human bronchial epithelial cells atop a fibroblast-seeded collagen gel. They found that strain induced myofibroblast differentiation and type III and IV collagen deposition. Both myofibroblasts and type III collagen were concentrated close to the basal side of the epithelium, suggesting that the epithelium is a source of profibrotic mediators that promote matrix remodeling. Their in vitro model included the minimum cell types and activating stimuli to capture remodeling events. Furthermore, the model showed that the pathway is not mediated by immune cells because they were not present. The rational choice of the required cell types and activating stimuli enables MPSs to remain simple enough for analysis but sophisticated enough to capture inflammation and remodeling in vitro.
FIG. 6.
Models of pulmonary inflammation in vitro. (a) (i) A microfluidic small airway-on-a-chip replicates the endothelium, interstitial fibroblasts, and epithelium. (ii) Fungal infection is simulated by inoculating the epithelium with wild type Aspergillus fumigatus, a model fungal pathogen. (iii) Neutrophils are added to the endothelial channels after the hyphae have extended into the interstitial space. Scale bar, 200 μM. (iv) Neutrophils chemotax from the endothelium through the interstitium toward fungal hyphae, attracted by volatile compounds produced by the fungus. Scale bars, 100 μM. Reproduced with permission from Barkal et al. Nat. Commun. 8, 1770 (2017). Copyright 2017 Authors, licensed under a CC BY 4.0 (https://creativecommons.org/licenses/by/4.0/).6 (b) (i) and (ii) A microfluidic alveolus-on-a-chip incorporates strain and neutrophil transmigration in a bilayer epithelium-endothelium coculture model. (iii) E. coli on the epithelium attracts neutrophils to transmigrate from the basal channel through endothelium and epithelium. (iv) Two E. coli bacteria (green) on the epithelium are chased and phagocytosed by a neutrophil (red) on the epithelium of the device. Reproduced with permission from Huh et al., Science 328, 1662 (2010). Copyright 2010 AAAS.63
Immune cell and fibroblast cocultures greatly improve the physiological relevance of ARDS MPSs but present significant design challenges. First, very few MPSs have studied the impact of mechanical strain on fibrosis. Swartz et al.121 and Choe et al.23 showed that strain-induced fibrosis can be mediated by the epithelium, but such mechanical force pathways that induce fibrosis are likely complex and multifactorial. As such, they should be further explored in ARDS MPSs that can incorporate physiological forces in coculture systems. Additionally, the presence of multiple cell types obfuscates the source of inflammatory and fibrotic mediators (e.g., cytokines, proteases, miRNA, reactive oxygen species, and TGF-β). To overcome this hurdle, MPS data are sometimes analyzed with systems biology techniques similar to those used to parse out in vivo signaling pathways.9,134
Additionally, airway epithelia, especially from primary cells, require many days or weeks to polarize. Media optimization may not be adequate to maintain the health and desired phenotype of all cell types present in coculture in the long term. Sellgren et al. reported a triple coculture of primary airway epithelium, fibroblasts, and endothelium but noted that an airwaylike phenotype (cobblestone morphology, mucus production, and cilia) was difficult to maintain in coculture conditions.111 MPS designers should consider if long-term coculture can be avoided, and if not, what media formulations can maintain cocultured cells in their desired phenotypes.
Substrate properties and mechanical forces also affect immune and fibroblast cell phenotypes. MPSs should have physiologically relevant physical forces and substrate properties so that immune and fibroblast phenotype mimic those in vivo. While models have independently considered immune cell and fibroblast mechanobiology in response to single stresses such as substrate stiffness or mechanical force, few combine multiple stress types in the same microenvironment. Although Huh et al. (2010) [Fig. 6(b)] incorporated interstitial flow, strain, and transmigration into their alveolus on-a-chip, they did not study how these forces affected the neutrophils in the model.
V. GENERAL CHALLENGES OF MODELING ARDS IN MPS
A. Complexity
A major challenge of designing MPSs is determining the level of model complexity. An overly complex model will produce noisy data, but an overly simplistic one is not useful. One option is to utilize functional readouts that are already familiar to the biology community, such as phenotypic assays (e.g., assays for bacterial phagocytosis and killing by neutrophils), to reduce the dimensionality of the data while keeping the model relatively complex. MPSs are, however, limited in how complex they can become before losing physiological relevance. A model that is too complicated could create conditions that induce nonphysiological cell behaviors. Additionally, elaborate models are difficult to fabricate, which limits their throughput and accessibility to the greater research community. Designers must consider what aspect of pathophysiology they desire to model and carefully consider what features are necessary to capture the phenomenon while minimizing the components of the system.
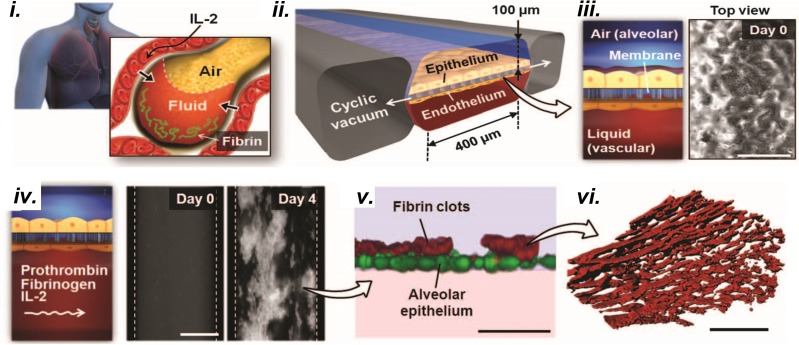
ARDS pathophysiology is complex and involves multiple stages with different characteristics. The designer must consider what aspects of disease progression to model. For example, Huh et al. captured pulmonary edema, fibrin deposition, and impaired gas exchange in response to toxic levels of IL-2 in a lung-on-a-chip microdevice including only the epithelium and endothelium (Fig. 7). They discovered that immune cells and fibroblasts were not necessary to produce these tissue-level functions, but strain was necessary, indicating that strain is a significant initiator of early pulmonary drug toxicity responses in vivo.
FIG. 7.
In vitro models of the lung microenvironment could be applied to study fibroproliferative disease in ARDS. (i) A lung-on-a-chip that replicates vascular leakage, leading to pulmonary edema and fibrin clotting.62 (ii) Strain is applied, by pulling vacuum on either side of the chamber, to a membrane (iii) with alveolar epithelium on the apical side and endothelium on the basal side. Scale bar, 200 μM. (iv) IL-2 induces endothelial and epithelial permeability allowing basal media loaded with prothrombin and fibrin to pass through the membrane and flood the apical channel, simulating pulmonary edema. Scale bar, 200 μM. (v) and (vi) Fibrin clots form on the apical channel after it becomes flooded with basal media containing fibrin and prothrombin. Scale bar (v), 50 μM. Scale bar (vi), 5 μM. Reproduced with permission from Huh et al. Sci. Transl. Med. 4, 159ra147 (2012). Copyright 2012 AAAS.62
Conversely, designers must consider whether even the most complex MPS is comprehensive enough to replicate the phenomenon of interest. For example, a single MPS could not capture multiple organ failure. Systemic dysregulated immunity that is observed in sepsis is likewise unlikely to be captured in a single MPS. Many MPSs also lack an immune component, a challenge that has not been addressed sufficiently. However, the simplicity of MPSs compared to in vivo models is often a benefit because it allows the isolation of confounding factors from the system, such as in Choe et al.23 and Huh et al.62
B. Heterogeneity
Because ARDS is a heterogeneous syndrome, it is impossible to construct a unifying model that incorporates every possible phenotype. MPSs could, however, be used to generate high throughput microenvironments mimicking the phenotypes to better understand divergent biological pathways driving phenotypic differences. For cell culture, primary human cell heterogeneity is also a significant challenge. Quality control of primary-cell-sourced cultures is difficult, especially in microfluidic culture with very small cell populations, due to variability across patients and even among cells from a single source. Conversely, models constructed with cell lines typically lack adequate cell heterogeneity. For example, models of the small airways that use H441 club cell lines lack the small populations of goblet cells, basal cells, and macrophages also present in this microenvironment. Most MPSs mimicking the alveoli only include alveolar type I pneumocytes and neglect type II pneumocytes, macrophages, and fibroblasts. Mertz et al.91 provide a discussion of the considerations of cell heterogeneity in MPSs.
C. Data collection in microfluidic systems
Traditional assays are difficult to adapt to microfluidic MPSs. Epithelial barrier permeability measurement is usually absent from microfluidic devices, especially real time permeability.55 This measure of epithelial response to stress and recovery from injury would greatly increase the information provided by microfluidic MPSs. Similarly, the scratch wound assay is a common metric of epithelial repair and recovery from injury that has only been adapted to microfluidics by Felder et al.36,37 in a custom device. Cytokine levels produced by very small cell numbers could fall below the detection limit of Luminex or ELISA assays. The MPS designer who considers microfluidics should determine whether their study will be sensitive to these limitations.
D. Clinical relevance
Finally, for MPSs to move from proof-of-concept to clinical applications, close cooperation with clinicians and the medical research community is essential. Clinicians connect researchers with urgent medical needs of patients and help researchers design their models in the context of a specific motivating question. Researchers in disease-specific fields provide essential information from studies of primary samples and basic science experiments that direct the design of more complex systems. An accurate model will be validated against clinical data and will recapitulate relevant aspects of ARDS pathophysiology or treatment.
VI. OUTLOOK
Despite the challenges of using MPSs for ARDS research, opportunities abound. These models could elucidate mechanisms that drive tissue repair toward regenerative or maladaptive responses to injury in ARDS. Additionally, MPSs can be applied to study pulmonary drug delivery for surfactant replacement or other therapies.47 MPSs are also applicable to other lung diseases: asthma and bronchiectasis endotypes have been described recently, and similar to ARDS, little is known about the biological mechanisms behind them.3,71 However, both diseases also involve inflammation, remodeling, and mechanical force in the lungs.
In conclusion, ARDS is a heterogeneous syndrome with high mortality and few effective treatment options. In-depth analyses have identified subgroups of patients that respond differently to supportive interventions and have different morbidity and mortality rates, but the biological mechanisms driving these differences in outcome are unclear, hindering the translation of these phenotyping methods to patients. MPSs have transformed in vitro cell culture and opened the door to complex in vitro analysis that could uncover these biological mechanisms and accelerate the translation of new phenotyping methods to critically ill patients. Overall, MPSs have tremendous potential to reveal patient-specific biological endotypes, which would improve personalized outcomes of importance to patients.
ACKNOWLEDGMENTS
We are thankful for financial support from the NIH (No. HL136141 to S.T. and J.B.G.; No. AG061687 to S.T. and L.H.; No. HL126603 to R.T.; No. 5T32EB006343-08 to H.V.). L.H. was supported by the Office of the Assistance Secretary of Defense for Health Affairs, through the Peer Reviewed Medical Research Program under Award No. W81XWH-17-1-0443; the Veterans Administration Health System Grant No. 1 I01 BX003919-01A1; and NIH Grant No. 1R41HL140741. J.R.G. was supported by the Atlanta Pediatric Scholars Program (No. NICHD K12 HD072245). J.C. was supported by the NSF Graduate Research Fellowship Program (No. 1650114). Opinions, interpretations, conclusions, and recommendations are those of the authors and are not necessarily endorsed by the Department of Defense, Department of Veterans Affairs, Atlanta Pediatric Scholars Program, National Science Foundation, or NIH. Figures 1, 2, 3, and 5 were created with BioRender.
Note: This paper is part of the special topic on Microphysiological Systems.
Contributor Information
Hannah Viola, Email: .
Shuichi Takayama, Email: .
References
- 1. Albelda S. M., “ Endothelial and epithelial cell adhesion molecules,” Am. J. Respir. Cell Mol. Biol. 4(3), 195–203 (1991). 10.1165/ajrcmb/4.3.195 [DOI] [PubMed] [Google Scholar]
- 2. Albert R. K., “ The role of ventilation-induced surfactant dysfunction and atelectasis in causing acute respiratory distress syndrome,” Am. J. Respir. Crit. Care Med. 185(7), 702–708 (2012). 10.1164/rccm.201109-1667PP [DOI] [PubMed] [Google Scholar]
- 3. Aogáin M. M., Tiew P. Y., Lim A. Y. H. et al. , “ Distinct “immunoallertypes” of disease and high frequencies of sensitization in non-cystic fibrosis bronchiectasis,” Am. J. Respir. Crit. Care Med. 199(7), 842–853 (2019). 10.1164/rccm.201807-1355OC [DOI] [PubMed] [Google Scholar]
- 4. Ariel A. and Timor O., “ Hanging in the balance: Endogenous anti-inflammatory mechanisms in tissue repair and fibrosis,” J. Pathol. 229(2), 250–263 (2013). 10.1002/path.4108 [DOI] [PubMed] [Google Scholar]
- 5. Ballotta V., Driessen-Mol A., Bouten C. V. C., and Baaijens F., “ Strain-dependent modulation of macrophage polarization within scaffolds,” Biomaterials 35(18), 4919–4928 (2014). 10.1016/j.biomaterials.2014.03.002 [DOI] [PubMed] [Google Scholar]
- 6. Barkal L. J., Procknow C. L., Álvarez-García Y. R. et al. , “ Microbial volatile communication in human organotypic lung models,” Nat. Commun. 8(1), 1770 (2017). 10.1038/s41467-017-01985-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Beers M. F. and Morrisey E. E., “ The three R's of lung health and disease: Repair, remodeling, and regeneration,” J. Clin. Invest. 121(6), 2065–2073 (2011). 10.1172/JCI45961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bellingan G. J., “ The pulmonary physician in critical care c 6: The pathogenesis of ALI/ARDS,” Throax 57, 540–546 (2002). 10.1136/thorax.57.6.540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Benam K. H., Novak R., Nawroth J. et al. , “ Matched-comparative modeling of normal and diseased human airway responses using a microengineered breathing lung chip,” Cell Syst. 3(5), 456–466.e4 (2016). 10.1016/j.cels.2016.10.003 [DOI] [PubMed] [Google Scholar]
- 10. Bilek A. M., Dee K. C., and Gaver D. P., “ Mechanisms of surface-tension-induced epithelial cell damage in a model of pulmonary airway reopening,” J. Appl. Physiol. 94(2), 770–783 (2003). 10.1152/japplphysiol.00764.2002 [DOI] [PubMed] [Google Scholar]
- 11. Blank R. and Napolitano L. M., “ Epidemiology of ARDS and ALI,” Crit. Care Clin. 27(3), 439–458 (2011). 10.1016/j.ccc.2011.05.005 [DOI] [PubMed] [Google Scholar]
- 12. Blázquez-Prieto J., López-Alonso I., Huidobro C., and Albaiceta G. M., “ The emerging role of neutrophils in repair after acute lung injury,” Am. J. Respir. Cell Mol. Biol. 59(3), 289–294 (2018). 10.1165/rcmb.2018-0101PS [DOI] [PubMed] [Google Scholar]
- 13. Bos L. D., Schouten L. R., van Vught L. A. et al. , “ Identification and validation of distinct biological phenotypes in patients with acute respiratory distress syndrome by cluster analysis,” Thorax 72(10), 876–883 (2017). 10.1136/thoraxjnl-2016-209719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Breen E. C., “ Mechanical strain increases type I collagen expression in pulmonary fibroblasts in vitro,” J. Appl. Physiol. 88(1), 203–209 (2000). 10.1152/jappl.2000.88.1.203 [DOI] [PubMed] [Google Scholar]
- 15. Burnham E. L., Janssen W. J., Riches D. W. H., Moss M., and Downey G. P., “ The fibroproliferative response in acute respiratory distress syndrome: Mechanisms and clinical significance,” Eur. Respir. J. 43(1), 276–285 (2014). 10.1183/09031936.00196412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Button B., Okada S. F., Frederick C. B., Thelin W. R., and Boucher R. C., “ Mechanosensitive ATP release maintains proper mucus hydration of airways,” Sci. Signaling 6(279), ra46 (2013). 10.1126/scisignal.2003755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Calfee C. S., Delucchi K., Parsons P. E. et al. , “ Subphenotypes in acute respiratory distress syndrome: Latent class analysis of data from two randomised controlled trials,” Lancet Respir. Med. 2(8), 611–620 (2014). 10.1016/S2213-2600(14)70097-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Calfee C. S., Delucchi K. L., Sinha P. et al. , “ Acute respiratory distress syndrome subphenotypes and differential response to simvastatin: Secondary analysis of a randomised controlled trial,” Lancet Respir. Med. 6(9), 691–698 (2018). 10.1016/S2213-2600(18)30177-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Castro C. Y., “ ARDS and diffuse alveolar damage: A pathologist's perspective,” Semin. Thorac. Cardiovasc. Surg. 18(1), 13–19 (2006). 10.1053/j.semtcvs.2006.02.001 [DOI] [PubMed] [Google Scholar]
- 20. Chapman K. E., Sinclair S. E., Zhuang D., Hassid A., Desai L. P., and Waters C. M., “ Cyclic mechanical strain increases reactive oxygen species production in pulmonary epithelial cells,” Am. J. Physiol. 289(5), L834–L841 (2005). 10.1152/ajplung.00069.2005 [DOI] [PubMed] [Google Scholar]
- 21. Chesnutt A. N., Matthay M. A., Tibayan F. A., and Clark J. G., “ Early detection of type III procollagen peptide in acute lung injury,” Am. J. Respir. Crit. Care Med. 156(3), 840–845 (1997). 10.1164/ajrccm.156.3.9701124 [DOI] [PubMed] [Google Scholar]
- 22. Cheung A. M., Tansey C. M., Tomlinson G. et al. , “ Two-year outcomes, health care use, and costs of survivors of acute respiratory distress syndrome,” Am. J. Respir. Crit. Care Med. 174(5), 538–544 (2006). 10.1164/rccm.200505-693OC [DOI] [PubMed] [Google Scholar]
- 23. Choe M. M., Sporn P. H. S., and Swartz M. A., “ Extracellular matrix remodeling by dynamic strain in a three-dimensional tissue-engineered human airway wall model,” Am. J. Respir. Cell Mol. Biol. 35(3), 306–313 (2006). 10.1165/rcmb.2005-0443OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chu E. K., Foley J. S., Cheng J., Patel A. S. et al. , “ Bronchial epithelial compression regulates epidermal growth factor receptor family ligand expression in an autocrine manner,” Am. J. Respir. Cell Mol. Biol. 32(5), 373–380 (2005). 10.1165/rcmb.2004-0266OC [DOI] [PubMed] [Google Scholar]
- 25. Cochrane C. G., Spragg R. G., Revak S. D., Cohen A. B., and McGuire W. W., “ The presence of neutrophil elastase and evidence of oxidation activity in bronchoalveolar lavage fluid of patients with adult respiratory distress syndrome,” Am. Rev. Respir. Dis. 127(2), 2 (1983). [DOI] [PubMed] [Google Scholar]
- 26. Coiffard B. and Papazian L., “ Time to evaluate biomarkers for use in directing treatment strategies in ARDS patients,” Intensive Care Med. 44(9), 1553–1555 (2018). 10.1007/s00134-018-5324-4 [DOI] [PubMed] [Google Scholar]
- 27. Crosby L. M. and Waters C. M., “ Epithelial repair mechanisms in the lung,” Am. J. Physiol. 298(6), L715–L731 (2010). 10.1152/ajplung.00361.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. D'Angelo E., Pecchiari M., and Gentile G., “ Dependence of lung injury on surface tension during low-volume ventilation in normal open-chest rabbits,” J. Appl. Physiol. 102(1), 174–182 (2007). 10.1152/japplphysiol.00405.2006 [DOI] [PubMed] [Google Scholar]
- 29. Derosa S., Borges J. B., Segelsjö M. et al. , “ Reabsorption atelectasis in a porcine model of ARDS: Regional and temporal effects of airway closure, oxygen, and distending pressure,” J. Appl. Physiol. 115(10), 1464–1473 (2013). 10.1152/japplphysiol.00763.2013 [DOI] [PubMed] [Google Scholar]
- 30. Desai L. P., Chapman K. E., and Waters C. M., “ Mechanical stretch decreases migration of alveolar epithelial cells through mechanisms involving Rac1 and Tiam1,” Am. J. Physiol. 295(5), L958–L965 (2008). 10.1152/ajplung.90218.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dolinay T., Himes B. E., Shumyatcher M., Lawrence G. G., and Margulies S. S., “ Integrated stress response mediates epithelial injury in mechanical ventilation,” Am. J. Respir. Cell Mol. Biol. 57(2), 193–203 (2017). 10.1165/rcmb.2016-0404OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Douville N. J., Zamankhan P., Tung Y.-C. et al. , “ Combination of fluid and solid mechanical stresses contribute to cell death and detachment in a microfluidic alveolar model,” Lab Chip 11(4), 609–619 (2011). 10.1039/C0LC00251H [DOI] [PubMed] [Google Scholar]
- 33. Dunn I. and Pugin J., “ Mechanical ventilation of various human lung cells in vitro: Identification of the macrophage as the main producer of inflammatory mediators,” Chest 116, 95S–97S (1999). 10.1378/chest.116.suppl_1.95S [DOI] [PubMed] [Google Scholar]
- 34. Ekpenyong A. E., Toepfner N., Chilvers E. R., and Guck J., “ Mechanotransduction in neutrophil activation and deactivation,” Biochim. Biophys. Acta 1853(11, Part B), 3105–3116 (2015). 10.1016/j.bbamcr.2015.07.015 [DOI] [PubMed] [Google Scholar]
- 35. Eworuke E., Major J. M., and Gilbert McClain L. I., “ National incidence rates for Acute Respiratory Distress Syndrome (ARDS) and ARDS cause-specific factors in the United States (2006–2014),” J. Crit. Care 47, 192–197 (2018). 10.1016/j.jcrc.2018.07.002 [DOI] [PubMed] [Google Scholar]
- 36. Felder M., Stucki A. O., Stucki J. D., Geiser T., and Guenat O. T., “ The potential of microfluidic lung epithelial wounding: Towards in vivo-like alveolar microinjuries,” Integr. Biol. 6(12), 1132–1140 (2014). 10.1039/C4IB00149D [DOI] [PubMed] [Google Scholar]
- 37. Felder M., Trueeb B., Stucki A. O. et al. , “ Impaired wound healing of alveolar lung epithelial cells in a breathing lung-on-a-chip,” Front. Bioeng. Biotechnol. 7, 3 (2019). 10.3389/fbioe.2019.00003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fielding-Singh V., Matthay M. A., and Calfee C. S., “ Beyond low tidal volume ventilation: Treatment adjuncts for severe respiratory failure in acute respiratory distress syndrome,” Crit. Care Med. 46(11), 1820–1831 (2018). 10.1097/CCM.0000000000003406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Filoche M., Tai C.-F., and Grotberg J. B., “ Three-dimensional model of surfactant replacement therapy,” Proc. Natl. Acad. Sci. 112(30), 9287–9292 (2015). 10.1073/pnas.1504025112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fine N., Dimitriou I. D., Rullo J. et al. , “ GEF-H1 is necessary for neutrophil shear stress–induced migration during inflammation,” J. Cell Biol. 215(1), 107–119 (2016). 10.1083/jcb.201603109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Fronius M., Bogdan R., Althaus M., Morty R. E., and Clauss W. G., “ Epithelial Na+ channels derived from human lung are activated by shear force,” Respir. Physiol. Neurobiol. 170(1), 113–119 (2010). 10.1016/j.resp.2009.11.004 [DOI] [PubMed] [Google Scholar]
- 42. Fujioka H., Takayama S., and Grotberg J. B., “ Unsteady propagation of a liquid plug in a liquid-lined straight tube,” Phys. Fluids 20(6), 062104 (2008). 10.1063/1.2938381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Fulcher L. M., Gabriel S., Burns K. A., Yankaskas J. R., and Randell S. H., “ Well-differentiated human airway epithelial cell cultures,” Human Cell Culture Protocols ( Humana Press, New Jersey, 2004), Vol. 107, pp. 183–206. [DOI] [PubMed] [Google Scholar]
- 44. Genschmer K. R., Russell D. W., Lal C. et al. , “ Activated PMN exosomes: Pathogenic entities causing matrix destruction and disease in the lung,” Cell 176(1-2), 113–126.e15 (2019). 10.1016/j.cell.2018.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ghadiali S. N. and Gaver D. P., “ Biomechanics of liquid-epithelium interactions in pulmonary airways,” Respir. Physiol. Neurobiol. 163(1-3), 232–243 (2008). 10.1016/j.resp.2008.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gilbert J. A., “ Advancing towards precision medicine in ARDS,” Lancet Respir. Med. 6(7), 494–495 (2018). 10.1016/S2213-2600(18)30156-5 [DOI] [PubMed] [Google Scholar]
- 47. Glindmeyer H. W., Smith B. J., and Gaver D. P., “ In situ enhancement of pulmonary surfactant function using temporary flow reversal,” J. Appl. Physiol. 112(1), 149–158 (2011). 10.1152/japplphysiol.00643.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Grommes J. and Soehnlein O., “ Contribution of neutrophils to acute lung injury,” Mol. Med. 17(3), 293–307 (2011). 10.2119/molmed.2010.00138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Grotberg J. B., “ Crackles and wheezes: Agents of injury?,” Ann. Am. Thorac. Soc. 16(8), 967–969 (2019). 10.1513/AnnalsATS.201901-022IP [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Grotberg J. B., Filoche M., Willson D. F., Raghavendran K., and Notter R. H., “ Did reduced alveolar delivery of surfactant contribute to negative results in adults with acute respiratory distress syndrome?,” Am. J. Respir. Crit. Care Med. 195(4), 538–540 (2017). 10.1164/rccm.201607-1401LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Grunwell J. R., Giacalone V. D., Stephenson S. et al. , “ Neutrophil dysfunction in the airways of children with acute respiratory failure due to lower respiratory tract viral and bacterial coinfections,” Sci. Rep. 9(1), 2874 (2019). 10.1038/s41598-019-39726-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Halbertsma F. J., Vaneker M., Scheffer G. J., and Van J der H., “ Cytokines and biotrauma in ventilator-induced lung injury: A critical review of the literature,” Neth. J Med. 63(10), 382–392 (2005). [PubMed] [Google Scholar]
- 53. Hamlington K. L., Bates J. H. T., Roy G. S. et al. , “ Alveolar leak develops by a rich-get-richer process in ventilator-induced lung injury,” PLoS One 13(3), e0193934 (2018). 10.1371/journal.pone.0193934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hassan E. A., Uzgoren E., Fujioka H., Grotberg J. B., and Shyy W., “ Adaptive Lagrangian-Eulerian computation of propagation and rupture of a liquid plug in a tube,” Int. J. Numer. Methods Fluids 67(11), 1373–1392 (2011). 10.1002/fld.2422 [DOI] [Google Scholar]
- 55. Henry O. F. H., Villenave R., Cronce M., Leineweber W., Benz M., and Ingber D., “ Organs-on-chips with integrated electrodes for trans-epithelial electrical resistance (TEER) measurements of human epithelial barrier function,” Lab Chip 17(13), 2264–2271 (2017). 10.1039/C7LC00155J [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hiemstra P. S., McCray P. B., and Bals R., “ The innate immune function of airway epithelial cells in inflammatory lung disease,” Eur. Respir. J. 45(4), 1150–1162 (2015). 10.1183/09031936.00141514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Higuita-Castro N., Mihai C., Hansford D. J., and Ghadiali S. N., “ Influence of airway wall compliance on epithelial cell injury and adhesion during interfacial flows,” J. Appl. Physiol. 117(11), 1231–1242 (2014). 10.1152/japplphysiol.00752.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Higuita-Castro N., Nelson M. T., Shukla V. et al. , “ Using a novel microfabricated model of the alveolar-capillary barrier to investigate the effect of matrix structure on atelectrauma,” Sci. Rep. 7(1), 1–13 (2017). 10.1038/s41598-017-12044-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Holm Å., Sundqvist T., Öberg Å., and Magnusson K.-E., “ Mechanical manipulation of polymorphonuclear leukocyte plasma membranes with optical tweezers causes influx of extracellular calcium through membrane channels,” Med. Biol. Eng. Comput. 37(3), 410–412 (1999). 10.1007/BF02513321 [DOI] [PubMed] [Google Scholar]
- 60. Huang Y., Crawford M., Higuita-Castro N., Nana-Sinkam P., and Ghadiali S. N., “ miR-146a regulates mechanotransduction and pressure-induced inflammation in small airway epithelium,” FASEB J. 26(8), 3351–3364 (2012). 10.1096/fj.11-199240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Huh D., Fujioka H., Tung Y.-C. et al. , “ Acoustically detectable cellular-level lung injury induced by fluid mechanical stresses in microfluidic airway systems,” Proc. Natl. Acad. Sci. U. S. A. 104(48), 18886–18891 (2007). 10.1073/pnas.0610868104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Huh D., Leslie D. C., Matthews B. D. et al. , “ A human disease model of drug toxicity–induced pulmonary edema in a lung-on-a-chip microdevice,” Sci. Transl. Med. 4(159), 159ra147 (2012). 10.1126/scitranslmed.3004249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Huh D., Matthews B. D., Mammoto A., Montoya-Zavala M., Hsin H. Y., and Ingber D. E., “ Reconstituting organ-level lung functions on a chip,” Science 328(5986), 1662–1668 (2010). 10.1126/science.1188302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Hussain M., Xu C., Ahmad M. et al. , “ Acute respiratory distress syndrome: Bench-to-bedside approaches to improve drug development,” Clin. Pharmacol. Ther. 104(3), 484–494 (2018). 10.1002/cpt.1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Ichikado K., Muranaka H., Gushima Y. et al. , “ Fibroproliferative changes on high-resolution CT in the acute respiratory distress syndrome predict mortality and ventilator dependency: A prospective observational cohort study,” BMJ Open 2(2), e000545 (2012). 10.1136/bmjopen-2011-000545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Imai Y., Parodo J., Kajikawa O. et al. , “ Injurious mechanical ventilation and end-organ epithelial cell apoptosis and organ dysfunction in an experimental model of acute respiratory distress syndrome,” JAMA 289(16), 2104–2112 (2003). 10.1001/jama.289.16.2104 [DOI] [PubMed] [Google Scholar]
- 67. Ingbar D. H., “ Mechanisms of repair and remodeling following acute lung injury,” Clin. Chest Med. 21(3), 589–616 (2000). 10.1016/S0272-5231(05)70168-4 [DOI] [PubMed] [Google Scholar]
- 68. Ingber D. E., “ Developmentally inspired human “organs on chips”,” Development 145(16), dev156125 (2018). 10.1242/dev.156125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Jacob A.-M. and Gaver D. P., “ Atelectrauma disrupts pulmonary epithelial barrier integrity and alters the distribution of tight junction proteins ZO-1 and claudin 4,” J. Appl. Physiol. 113(9), 1377–1387 (2012). 10.1152/japplphysiol.01432.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Kay S. S., Bilek A. M., Dee K. C., and Gaver D. P., “ Pressure gradient, not exposure duration, determines the extent of epithelial cell damage in a model of pulmonary airway reopening,” J. Appl. Physiol. 97(1), 269–276 (2004). 10.1152/japplphysiol.01288.2003 [DOI] [PubMed] [Google Scholar]
- 71. King G. G., James A., Harkness L., and Wark P., “ Pathophysiology of severe asthma: We've only just started,” Respirology 23(3), 262–271 (2018). 10.1111/resp.13251 [DOI] [PubMed] [Google Scholar]
- 72. Laffey J. G. and Kavanagh B. P., “ Fifty years of research in ARDS. Insight into acute respiratory distress syndrome. From models to patients,” Am. J. Respir. Crit. Care Med. 196(1), 18–28 (2017). 10.1164/rccm.201612-2415CI [DOI] [PubMed] [Google Scholar]
- 73. Leckband D. E., le Duc Q., Wang N., and de Rooij J., “ Mechanotransduction at cadherin-mediated adhesions,” Curr. Opin. Cell Biol. 23(5), 523–530 (2011). 10.1016/j.ceb.2011.08.003 [DOI] [PubMed] [Google Scholar]
- 74. Lewis J. F. and Brackenbury A., “ Role of exogenous surfactant in acute lung injury,” Crit. Care Med. 31(4), S324 (2003). 10.1097/01.CCM.0000057911.19145.9F [DOI] [PubMed] [Google Scholar]
- 75. Li Q., Ge Y.-L., Li M. et al. , “ miR-127 contributes to ventilator-induced lung injury,” Mol. Med. Rep. 16(4), 4119–4126 (2017). 10.3892/mmr.2017.7109 [DOI] [PubMed] [Google Scholar]
- 76. Liu M., Tanswell A. K., and Post M., “ Mechanical force-induced signal transduction in lung cells,” Am. J. Physiol. 277(4), L667–L683 (1999). 10.1152/ajplung.1999.277.4.L667 [DOI] [PubMed] [Google Scholar]
- 77. Liu Z., Yago T., Zhang N. et al. , “ L-selectin mechanochemistry restricts neutrophil priming in vivo,” Nat. Commun. 8, 15196 (2017). 10.1038/ncomms15196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Ma X., Schickel M. E., Stevenson M. D. et al. , “ Fibers in the extracellular matrix enable long-range stress transmission between cells,” Biophys. J. 104(7), 1410–1418 (2013). 10.1016/j.bpj.2013.02.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Manuyakorn W., Smart D. E., Noto A. et al. , “ Mechanical strain causes adaptive change in bronchial fibroblasts enhancing profibrotic and inflammatory responses,” PLoS One 11(4), e0153926 (2016). 10.1371/journal.pone.0153926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Marshall R., Bellingan G., and Laurent G., “ The acute respiratory distress syndrome: Fibrosis in the fast lane,” Thorax 53(10), 815–817 (1998). 10.1136/thx.53.10.815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Martin C., Papazian L., Payan M.-J., Saux P., and Gouin F., “ Pulmonary fibrosis correlates with outcome in adult respiratory distress syndrome,” Chest 107(1), 196–200 (1995). 10.1378/chest.107.1.196 [DOI] [PubMed] [Google Scholar]
- 82. Martin T. R., Hagimoto N., Nakamura M., and Matute-Bello G., “ Apoptosis and epithelial injury in the lungs,” Proc. Am. Thorac. Soc. 2(3), 214–220 (2005). 10.1513/pats.200504-031AC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Martin T. R., Pistorese B. P., Hudson L. D., and Maunder R. J., “ The function of lung and blood neutrophils in patients with the adult respiratory distress syndrome: Implications for the pathogenesis of lung infections,” Am. Rev. Respir. Dis. 144(2), 254–262 (1991). 10.1164/ajrccm/144.2.254 [DOI] [PubMed] [Google Scholar]
- 84. Matthay M. A., Zemans R. L., Zimmerman G. A. et al. , “ Acute respiratory distress syndrome,” Nat. Rev. Dis. Primer 5(1), 1–22 (2019). 10.1038/s41572-019-0069-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. McWhorter F. Y., Davis C. T., and Liu W. F., “ Physical and mechanical regulation of macrophage phenotype and function,” Cell Mol. Life Sci. 72(7), 1303–1316 (2015). 10.1007/s00018-014-1796-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Meduri G. U., “ Late adult respiratory distress syndrome,” New Horiz. 1(4), 563 (1993). [PubMed] [Google Scholar]
- 87. Meduri G. U., “ Pulmonary fibroproliferation and death in patients with late ARDS,” Chest 107(1), 5–6 (1995). 10.1378/chest.107.1.5 [DOI] [PubMed] [Google Scholar]
- 88. Meduri G. U., Annane D., Chrousos G. P., Marik P. E., and Sinclair S. E., “ Activation and regulation of systemic inflammation in ARDS,” Chest 136(6), 1631–1643 (2009). 10.1378/chest.08-2408 [DOI] [PubMed] [Google Scholar]
- 89. Meduri G. U. and Eltorky M. A., “ Understanding ARDS-associated fibroproliferation,” Intensive Care Med. 41(3), 517–520 (2015). 10.1007/s00134-014-3613-0 [DOI] [PubMed] [Google Scholar]
- 90. Meduri G. U., Kohler G., Headley S., Tolley E., Stentz F., and Postlethwaite A., “ Inflammatory cytokines in the BAL of patients with ARDS. Persistent elevation over time predicts poor outcome,” Chest 108(5), 1303–1314 (1995). 10.1378/chest.108.5.1303 [DOI] [PubMed] [Google Scholar]
- 91. Mertz D. R., Ahmed T., and Takayama S., “ Engineering cell heterogeneity into organs-on-a-chip,” Lab Chip 18(16), 2378–2395 (2018). 10.1039/C8LC00413G [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Moazzam F., DeLano F. A., Zweifach B. W., and Schmid-Schönbein G. W., “ The leukocyte response to fluid stress,” Proc. Natl. Acad. Sci. 94(10), 5338–5343 (1997). 10.1073/pnas.94.10.5338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Mokres L. M., Parai K., Hilgendorff A. et al. , “ Prolonged mechanical ventilation with air induces apoptosis and causes failure of alveolar septation and angiogenesis in lungs of newborn mice,” Am. J. Physiol. 298(1), L23–L35 (2009). 10.1152/ajplung.00251.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Mourgeon E., Isowa N., Keshavjee S., Zhang X., Slutsky A. S., and Liu M., “ Mechanical stretch stimulates macrophage inflammatory protein-2 secretion from fetal rat lung cells,” Am. J. Physiol. 279(4), L699–L706 (2000). 10.1152/ajplung.2000.279.4.L699 [DOI] [PubMed] [Google Scholar]
- 95. Muradoglu M., Romanò F., Fujioka H., and Grotberg J. B., “ Effects of surfactant on propagation and rupture of a liquid plug in a tube,” J. Fluid Mech. 872, 407–437 (2019). 10.1017/jfm.2019.333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Nemzek J. A., Call D. R., Ebong S. J., Newcomb D. E., Bolgos G. L., and Remick D. G., “ Immunopathology of a two-hit murine model of acid aspiration lung injury,” Am. J. Physiol. 278(3), L512–L520 (2000). 10.1152/ajplung.2000.278.3.L512 [DOI] [PubMed] [Google Scholar]
- 97. Nieman G. F., Andrews P., Satalin J. et al. , “ Acute lung injury: How to stabilize a broken lung,” Crit. Care 22(1), 136 (2018). 10.1186/s13054-018-2051-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Nieman G. F., Gatto L. A., and Habashi N. M., “ Impact of mechanical ventilation on the pathophysiology of progressive acute lung injury,” J. Appl. Physiol. 119(11), 1245–1261 (2015). 10.1152/japplphysiol.00659.2015 [DOI] [PubMed] [Google Scholar]
- 99. Palumbo S., Shin Y.-J., Ahmad K. et al. , “ Dysregulated Nox4 ubiquitination contributes to redox imbalance and age-related severity of acute lung injury,” Am. J. Physiol. 312(3), L297–L308 (2017). 10.1152/ajplung.00305.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Park J.-A., Sharif A. S., Tschumperlin D. J. et al. , “ Tissue factor–bearing exosome secretion from human mechanically stimulated bronchial epithelial cells in vitro and in vivo,” J. Allergy Clin. Immunol. 130(6), 1375–1383 (2012). 10.1016/j.jaci.2012.05.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Parker J. C., Hernandez L. A., and Peevy K. J., “ Mechanisms of ventilator-induced lung injury,” Crit. Care Med. 21(1), 131–143 (1993). 10.1097/00003246-199301000-00024 [DOI] [PubMed] [Google Scholar]
- 102. Pinheiro de Oliveira R., Hetzel M. P., dos Anjos Silva M., Dallegrave D., and Friedman G., “ Mechanical ventilation with high tidal volume induces inflammation in patients without lung disease,” Crit. Care 14(2), R39 (2010). 10.1186/cc8919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Plötz F. B., Vreugdenhil H. A., Slutsky A. S., Zijlstra J., Heijnen C. J., and van Vught H., “ Mechanical ventilation alters the immune response in children without lung pathology,” Intensive Care Med. 28(4), 486–492 (2002). 10.1007/s00134-002-1216-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Reiss L. K., Schuppert A., and Uhlig S., “ Inflammatory processes during acute respiratory distress syndrome: A complex system,” Curr. Opin. Crit. Care 24(1), 1–9 (2018). 10.1097/MCC.0000000000000472 [DOI] [PubMed] [Google Scholar]
- 105. Ressler B., Lee R. T., Randell S. H., Drazen J. M., and Kamm R. D., “ Molecular responses of rat tracheal epithelial cells to transmembrane pressure,” Am. J. Physiol. 278(6), L1264–L1272 (2000). 10.1152/ajplung.2000.278.6.L1264 [DOI] [PubMed] [Google Scholar]
- 106. Ruan S.-Y., Lin H.-H., Huang C.-T., Kuo P.-H., Wu H.-D., and Yu C.-J., “ Exploring the heterogeneity of effects of corticosteroids on acute respiratory distress syndrome: A systematic review and meta-analysis,” Crit. Care 18(2), R63 (2014). 10.1186/cc13819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. dos Santos C. C. and Slutsky A. S., “ The contribution of biophysical lung injury to the development of biotrauma,” Annu. Rev. Physiol. 68(1), 585–618 (2006). 10.1146/annurev.physiol.68.072304.113443 [DOI] [PubMed] [Google Scholar]
- 108. Sapru A., Flori H., Quasney M. W., and Dahmer M. K., “ Pathobiology of acute respiratory distress syndrome,” Pediatr. Crit. Care Med. 16, S6–S22 (2015). 10.1097/PCC.0000000000000431 [DOI] [PubMed] [Google Scholar]
- 109. Savla U. and Waters C. M., “ Mechanical strain inhibits repair of airway epithelium in vitro,” Am. J. Physiol. 274(6), L883–L892 (1998). 10.1152/ajplung.1998.274.6.L883 [DOI] [PubMed] [Google Scholar]
- 110. Schenck E. J., Oromendia C., Torres L. K., Berlin D. A., Choi A. M. K., and Siempos I. I., “ Rapidly improving ARDS in therapeutic randomized controlled trials,” Chest 155(3), 474–482 (2019). 10.1016/j.chest.2018.09.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Sellgren K. L., Butala E. J., Gilmour B. P., Randell S. H., and Grego S., “ A biomimetic multicellular model of the airways using primary human cells,” Lab Chip 14(17), 3349–3358 (2014). 10.1039/C4LC00552J [DOI] [PubMed] [Google Scholar]
- 112. Seok J., Warren H. S., Cuenca A. G. et al. , “ Genomic responses in mouse models poorly mimic human inflammatory diseases,” Proc. Natl. Acad. Sci. 110(9), 3507–3512 (2013). 10.1073/pnas.1222878110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Shin H. Y., Simon S. I., and Schmid‐Schönbein G. W., “ Fluid shear-induced activation and cleavage of CD18 during pseudopod retraction by human neutrophils,” J. Cell. Physiol. 214(2), 528–536 (2008). 10.1002/jcp.21235 [DOI] [PubMed] [Google Scholar]
- 114. Sidhaye V. K., Schweitzer K. S., Caterina M. J., Shimoda L., and King L. S., “ Shear stress regulates aquaporin-5 and airway epithelial barrier function,” Proc. Natl. Acad. Sci. U. S. A. 105(9), 3345–3350 (2008). 10.1073/pnas.0712287105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Singer W., Frick M., Haller T., Bernet S., Ritsch-Marte M., and Dietl P., “ Mechanical forces impeding exocytotic surfactant release revealed by optical tweezers,” Biophys. J. 84(2), 1344–1351 (2003). 10.1016/S0006-3495(03)74950-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Sinha P. and Calfee C. S., “ Phenotypes in acute respiratory distress syndrome: Moving towards precision medicine,” Curr. Opin. Crit. Care 25(1), 12–20 (2019). 10.1097/MCC.0000000000000571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Song J. W., Paek J., Park K.-T., Seo J., and Huh D., “ A bioinspired microfluidic model of liquid plug-induced mechanical airway injury,” Biomicrofluidics 12(4), 042211 (2018). 10.1063/1.5027385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Stapleton R. D., Wang B. M., Hudson L. D., Rubenfeld G. D., Caldwell E. S., and Steinberg K. P., “ Causes and timing of death in patients with ARDS,” Chest 128(2), 525–532 (2005). 10.1378/chest.128.2.525 [DOI] [PubMed] [Google Scholar]
- 119. Stucki A. O., Stucki J. D., Hall S. R. R. et al. , “ A lung-on-a-chip array with an integrated bio-inspired respiration mechanism,” Lab Chip 15(5), 1302–1310 (2015). 10.1039/C4LC01252F [DOI] [PubMed] [Google Scholar]
- 120. Summers C., “ Chasing the “Holy Grail”: Modulating neutrophils in inflammatory lung disease,” Am. J. Respir. Crit. Care Med. 200, 2 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Swartz M. A., Tschumperlin D. J., Kamm R. D., and Drazen J. M., “ Mechanical stress is communicated between different cell types to elicit matrix remodeling,” Proc. Natl. Acad. Sci. U. S. A. 98(11), 6180–6185 (2001). 10.1073/pnas.111133298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Sweeney T. E., Lofgren S., Khatri P., and Rogers A. J., “ Gene expression analysis to assess the relevance of rodent models to human lung injury,” Am. J. Respir. Cell Mol. Biol. 57(2), 184–192 (2017). 10.1165/rcmb.2016-0395OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Szabari M. V., Takahashi K., Feng Y. et al. , “ Relation between respiratory mechanics, inflammation, and survival in experimental mechanical ventilation,” Am. J. Respir. Cell Mol. Biol. 60(2), 179–188 (2019). 10.1165/rcmb.2018-0100OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Tan S.-Y. and Weninger W., “ Neutrophil migration in inflammation: Intercellular signal relay and crosstalk,” Curr. Opin. Immunol. 44, 34–42 (2017). 10.1016/j.coi.2016.11.002 [DOI] [PubMed] [Google Scholar]
- 125. Tarran R., Button B., Picher M. et al. , “ Normal and cystic fibrosis airway surface liquid homeostasis. The effects of phasic shear stress and viral infections,” J. Biol. Chem. 280(42), 35751–35759 (2005). 10.1074/jbc.M505832200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Tavana H., Zamankhan P., Christensen P. J., Grotberg J. B., and Takayama S., “ Epithelium damage and protection during reopening of occluded airways in a physiologic microfluidic pulmonary airway model,” Biomed. Microdevices 13(4), 731–742 (2011). 10.1007/s10544-011-9543-5 [DOI] [PubMed] [Google Scholar]
- 127. Thampatty B. P. and Wang J.-C., “ Mechanobiology of fibroblasts,” in Mechanosensitive Ion Channels, Mechanosensitivity in Cells and Tissues, edited by Kamkin A. and Kiseleva I. ( Springer, Dordrecht, Netherlands, 2008), pp. 351–378. [Google Scholar]
- 128. Treggiari-Venzi M., Ricou B., Romand J.-A., and Suter P. M., “ The response to repeated nitric oxide inhalation is inconsistent in patients with acute respiratory distress syndrome,” Anesthesiology 88(3), 634–641 (1998). 10.1097/00000542-199803000-00013 [DOI] [PubMed] [Google Scholar]
- 129. Tremblay L. N., Miatto D., Hamid Q., Govindarajan A., and Slutsky A. S., “ Injurious ventilation induces widespread pulmonary epithelial expression of tumor necrosis factor-α and interleukin-6 messenger RNA*,” Crit. Care Med. 30(8), 1693 (2002). 10.1097/00003246-200208000-00003 [DOI] [PubMed] [Google Scholar]
- 130. Tremblay L. N. and Slutsky A. S., “ Ventilator-induced injury: From barotrauma to biotrauma,” Proc. Assoc. Am. Physicians 110(6), 482–488 (1998). [PubMed] [Google Scholar]
- 131. Tschumperlin D. J. and Drazen J. M., “ Mechanical stimuli to airway remodeling,” Am. J. Respir. Crit. Care Med. 164(supplement_2), S90–S94 (2001). 10.1164/ajrccm.164.supplement_2.2106060 [DOI] [PubMed] [Google Scholar]
- 132. Tschumperlin D. J., Shively J. D., Swartz M. A. et al. , “ Bronchial epithelial compression regulates MAP kinase signaling and HB-EGF-like growth factor expression,” Am. J. Physiol. 282(5), L904–L911 (2002). 10.1152/ajplung.00270.2001 [DOI] [PubMed] [Google Scholar]
- 133. Vaporidi K., Vergadi E., Kaniaris E. et al. , “ Pulmonary microRNA profiling in a mouse model of ventilator-induced lung injury,” Am. J. Physiol. 303(3), L199–L207 (2012). 10.1152/ajplung.00370.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Ventura B. D., Lemerle C., Michalodimitrakis K., and Serrano L., “ From in vivo to in silico biology and back,” Nature 443(7111), 527–533 (2006). 10.1038/nature05127 [DOI] [PubMed] [Google Scholar]
- 135. Virk H., Arthur G., and Bradding P., “ Mast cells and their activation in lung disease,” Transl. Res. 174, 60–76 (2016). 10.1016/j.trsl.2016.01.005 [DOI] [PubMed] [Google Scholar]
- 136. Vlahakis N. E., Schroeder M. A., Limper A. H., and Hubmayr R. D., “ Stretch induces cytokine release by alveolar epithelial cells in vitro,” Am. J. Physiol. 277(1), L167–L173 (1999). 10.1152/ajplung.1999.277.1.L167 [DOI] [PubMed] [Google Scholar]
- 137. Waters C. M., Roan E., and Navajas D., “ Mechanobiology in lung epithelial cells: Measurements, perturbations, and responses,” in Comprehensive Physiology, edited by Terjung R. ( John Wiley & Sons, Inc, Hoboken, NJ, USA, 2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Wiener-Kronish J. P., Albertine K. H., and Matthay M. A., “ Differential responses of the endothelial and epithelial barriers of the lung in sheep to Escherichia coli endotoxin,” J. Clin. Invest. 88(3), 864–875 (1991). 10.1172/JCI115388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Wirtz H. R. and Dobbs L. G., “ Calcium mobilization and exocytosis after one mechanical stretch of lung epithelial cells,” Science 250(4985), 1266–1269 (1990). 10.1126/science.2173861 [DOI] [PubMed] [Google Scholar]
- 140. Wirtz H. R. and Dobbs L. G., “ The effects of mechanical forces on lung functions,” Respir. Physiol. 119(1), 1–17 (2000). 10.1016/S0034-5687(99)00092-4 [DOI] [PubMed] [Google Scholar]
- 141. Wolthuis E. K., Vlaar A. P., Choi G., Roelofs J. J., Juffermans N. P., and Schultz M. J., “ Mechanical ventilation using non-injurious ventilation settings causes lung injury in the absence of pre-existing lung injury in healthy mice,” Crit. Care 13(1), R1 (2009). 10.1186/cc7688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Yalcin H. C., Perry S. F., and Ghadiali S. N., “ Influence of airway diameter and cell confluence on epithelial cell injury in an in vitro model of airway reopening,” J. Appl. Physiol. 103(5), 1796–1807 (2007). 10.1152/japplphysiol.00164.2007 [DOI] [PubMed] [Google Scholar]
- 143. Yang J.-H., Sakamoto H., Xu E. C., and Lee R. T., “ Biomechanical Regulation of Human Monocyte/Macrophage Molecular Function,” Am. J. Pathol. 156(5), 1797–1804 (2000). 10.1016/S0002-9440(10)65051-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Yap B. and Kamm R. D., “ Cytoskeletal remodeling and cellular activation during deformation of neutrophils into narrow channels,” J. Appl. Physiol. 99(6), 2323–2330 (2005). 10.1152/japplphysiol.00503.2005 [DOI] [PubMed] [Google Scholar]
- 145. Yu X., Tan J., and Diamond S. L., “ Hemodynamic force triggers rapid NETosis within sterile thrombotic occlusions,” J. Thromb. Haemostasis 16(2), 316–329 (2018). 10.1111/jth.13907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Zemans R. L., Colgan S. P., and Downey G. P., “ Transepithelial migration of neutrophils: Mechanisms and implications for acute lung injury,” Am. J. Respir. Cell Mol. Biol. 40(5), 519–535 (2009). 10.1165/rcmb.2008-0348TR [DOI] [PMC free article] [PubMed] [Google Scholar]