Abstract
Coupling reactions of amines and alcohols are of central importance for applications in chemistry and biology. These transformations typically involve the use of a reagent, activated as an electrophile, onto which nucleophile coupling results in the formation of a carbon-nitrogen or a carbon–oxygen bond. Several promising reagents and procedures have been developed to achieve these bond forming processes in high yields with excellent stereocontrol, but few offer direct coupling without the intervention of a catalyst. Herein, we report the synthesis of chiral donor–acceptor azetines by highly enantioselective [3 + 1]-cycloaddition of enoldiazoacetates with aza-ylides and their selective coupling with nitrogen and oxygen nucleophiles via 3-azetidinones to form amino acid derivatives, including those of peptides and natural products. The overall process is general for a broad spectrum of nucleophiles, has a high degree of electronic and steric selectivity, and retains the enantiopurity of the original azetine.
Subject terms: Homogeneous catalysis, Asymmetric synthesis, Synthetic chemistry methodology
Chiral 3-azetidinones are structural analogues of medicinally relevant β-lactams, however their synthesis and reactivity are underexplored. Here, the authors show a highly enantioselective copper-catalyzed [3 + 1]-cycloaddition generating 2-azetidines, which react with nucleophiles yielding amino acids via 3-azetidinones.
Introduction
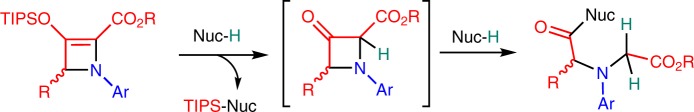
Irreversible ring opening of the strained 2-azetidinone four-membered ring, which is one of the key biomolecular events during both the antibiotic action of β-lactams and their inhibition by β-lactamases1, is a model for nucleophile coupling. Because of their chemically controlled ring opening, 2-azetidinones are widely used for the synthesis of heterocycles, β-amino acids, and their derivatives2–4. 3-Azetidinones, by contrast, are less well established5 even though they have the potential for analogous nucleophilic carbonyl-carbon cleavage to form amine derivatives (Fig. 1a) if an activating electron-withdrawing group (EWG) is located at the 2-position; but the key to realizing this potential lies in the design of a 3-azetidinone capable of nucleophile coupling.
Fig. 1.

Strain-induced ring opening of azetidinones and [3 + 1]-cycloaddition. a Amide ring opening of 2-azetidinones compared with retro-Claisen ring opening of EWG-activated 3-azetidinones. b Generation of 3-azetidinones via catalytic [3 + 1]-cycloaddition of silyl-protected enoldiazoacetates with imido sulfur ylides.
A classic approach to nucleophile coupling is the retro-Claisen reaction of β-ketoesters6 that would require the construction of 2-carboxylate substituted 3-azetidinones, but the basic methods available for their ring-closing formation are the same as those desired for their ring-opening which is favored by ring strain7–10. Although one example of N-Cbz protected 2-carboxylate substituted 3-azetidinones is reported, its synthetic utility has not been pursued due to its inherent instability to nucleophilic ring opening11. Alternative methodologies proceeding to 2-azetine-2-carboxylate structures applied to the formation of the 3-azedidinone analogues, either through [2 + 2]-cycloaddition12, from 3-substituted 2-azetines (lithiation)13–17, or with N-Boc-3-azetidinone (coupling reactions)18, have not been suitable for 2-carboxylate derivatives. In addition, attempted copper(I)-catalyzed [3 + 1] cycloaddition of alkenyldiazoacetates and iminoiodinanes to form the requisite 3-azetidinones has also been unsuccessful19. To circumvent these approaches, we present the formation of an enolate precursor to 2-carboxylate substituted 3-azetidinones that appears to be available by [3 + 1]-cycloaddition (Fig. 1b).
Previous research from our laboratory has established that [3 + 1]-cycloaddition of silyl group protected enoldiazoacetates with α-acyl sulfur ylides is effective in forming donor–acceptor cyclobutene derivatives in good yields and high stereocontrol21. With a corresponding 2-azetine derivative obtained in good yield and high enantioselectivity, desilylation, forming the desired 3-keto-2-carboxylate, could provide the chemical framework for nucleophile coupling that would attach the amino acid framework of the 3-azetidinone to the nucleophile.
In order to achieve strain-induced ring opening of 3-azetidinones, we present here the synthesis of highly optically enriched donor–acceptor azetines and their facile ring opening in high yield with a broad spectrum of nucleophiles under mild conditions. Highly enantioselective [3 + 1]-cycloaddition of silyl-protected enoldiazoacetates occurs with aza-ylides using chiral copper(I) catalysis. The 2-azetidine cycloaddition products generate 3-azetidinones by reactions with nucleophiles that produce a broad spectrum of amino acid derivatives with high efficacy and complete retention of enantiopurity. This retro-Claisen reaction aided by strain release uncovers a methodology for the attachment of chiral amide, peptide and ester units to a variety of amines and alcohols, and tolerates a broad scope of nucleophiles, including naturally occurring amines, alcohols, amino acids, and other nitrogen-based nucleophiles. Mechanistic studies confirm initial desilylation followed by nucleophilic retro-Claisen ring opening. The mild reaction conditions, high enantiocontrol, broad scope of nucleophiles for the ring opening of donor–acceptor azetines, and ability to perform the reaction in aqueous media demonstrated in this work portray a process that will have wide applications.
Results
Development of the [3 + 1]-cycloaddition reaction
Application of N-acylimido sulfur ylides21–23 and enoldiazoacetates to the same catalysts and conditions that were successful with their carbon analogues was unsuccessful even at elevated temperatures due to a lack of reactivity of the N-acylimido ylide. Use of N-arylimido sulfur ylides (S,S-disubstituted N-arylsulfilimines)24–26, however, allowed cycloaddition to proceed smoothly at room temperature. As previously described for the corresponding [3 + 1]-cycloaddition that formed donor–acceptor cyclobutene derivatives21, only copper(I) catalysis was effective for this transformation; and Cu(MeCN)4PF6 was the catalyst of choice in the formation of 2-azetines. Product yields were the highest in dichloromethane, and diphenylsulfur ylides gave higher product yields than their dimethyl or methylphenyl analogues (see Supplementary Table 1). Reactions were performed at room temperature to avoid electrocyclic ring opening of the azetine27–29. [3 + 1]-Cycloaddition occurred with the triisopropylsilyl(TIPS)-protected enoldiazoacetate, but not with the tert-butyldimethylsilyl(TBS)-protected enoldiazoacetate. With these optimizations methyl N-(p-chlorophenyl)-3-OTIPS-2-azetine-2-carboxylate 3 was formed in 80% isolated yield (Fig. 2a).
Fig. 2.
Azetine ring construction. a [3 + 1]-Cycloaddition of methyl 3-triisopropylsil(TIPS)oxy-2-diazo-3-butenoate (1a) with S,S-diphenyl N-(p-chlorophenyl)sulfinylimine (2a) catalyzed by copper(I) tetrakis(acetonitrile). b Asymmetric synthesis of donor–acceptor azetine using copper(I) catalysis with chiral sabox ligand L1.
To introduce chirality into the 2-azetine-2-carboxylate, a substituent at the terminal vinyl position of enoldiazoacetate 1 is required. Previous reports on enoldiazoacetates described the synthesis and uses of only two TBS- and TIPS-protected enoldiazoacetates having terminal vinyl substituents (4-Me and 4-Ar)20,30–36, and both of their geometrical isomers were formed in the case of TIPS-derivatives. We have provided a synthetic solution to this challenge that allows dominant formation of the Z-isomer (Z:E = > 20:1) for these substituted enoldiazoacetates37 and only the Z-isomer undergoes [3 + 1]-cycloaddition39. To effect asymmetric induction for 2-azetine ring formation, we initially selected methyl (Z)-3-OTIPS-2-diazo-3-pentenoate 1b with N-(p-chlorophenyl)imido diphenylsulfur ylide 2a and performed the cycloaddition reaction under the optimized conditions with catalysis by Cu(MeCN)4PF6 coordinated to chiral side-arm bisoxazoline (sabox) ligand L1 (Fig. 2b). The use of ligand L1 resulted in the highest yield and enantioselectivity (71% yield, 75% ee).
Although the yield and enantioselectivity for 3b obtained with L1 were only moderate, we proceeded to vary substituents at the 4-position of enoldiazoacetate 1 in order to determine if these substituents influence product formation and selectivity. A general procedure was established for the introduction of substituents to the 4-position of enoldiazoacetate 138; and, using 2a as the optimum sulfilimine, [3 + 1]-cycloaddition was performed under optimum conditions. The initial reaction of 1b (Z:E = 3:1) with a 50% molar excess of 2a showed complete loss of Z-1b, but retention of E-1b and a 75% ee for 3b (Fig. 2b). This observation prompted us to use an excess of the 4-substituted enoldiazoacetate over sulfilimine 2a to reflect the actual stoichiometric amount of the Z-isomer in the Z-1/E-1 mixture. When the reaction of 1b (Z:E = > 20:1) with 2a was repeated using a (1.2):1 ratio 1b/2a [vs. 1:(1.5) reported in Fig. 2b], this modification resulted in an increased yield of 3b to 82% (entry 1, Table 1) with the same ee value of 75%. To our good fortune, changing the methyl substituent at the 4-position of 1 to ethyl not only improved the enantioselectivity for the [3 + 1]-cycloaddition to 90% ee but also resulted in an increase of the isolated yield (92%) of 3c (entry 2, Table 1). Further elaboration of the substituent at the 4-position with benzyl (3d), isopropyl (3e), and n-octyl (3f) under the same conditions led to a modest decrease in reactivity, apparently due to steric effects, and lowered product yields, but % ee values were comparable with or higher than that of 3c (90−97% ee).
Table 1.
Scope of enoldiazoacetates: effect of the R-substituent.
 | |||
|---|---|---|---|
| Entrya | R | Yield 3 (%)b | ee (%)c |
| 1 | Me | 3b 82 | 75 |
| 2 | Et | 3c 92 | 90 |
| 3d | Bn | 3d 73 | 90 |
| 4d | iPr | 3e 70 | 92 |
| 5d | n-C8H17 | 3f 63 | 97 |
aAll reactions were carried out on a 0.20 -mmol scale in 4.0 mL of DCM: 2a (0.20 mmol), 1a (0.24 mmol)
bIsolated yield after flash-chromatography
cDetermined by chiral HPLC analysis
dReaction time was 72 h
To identify a possible further improvement in enantiocontrol, we investigated the influence of the carboxylate ether group (size and electronic effects) of enoldiazoacetates 1. With an Et (R1) substituent at the 4-position (Table 2) introduction of an isopropyl group as R2 (1g) resulted in a decrease of azetine yield without a change in enantioselectivity (entry 1; Table 2). Notably, the corresponding tert-butyl enoldiazoacetate (R2 = tBu) resulted in only trace amounts of the [3 + 1]-cycloaddition product. Neither benzyl (1h) nor 4-bromobenzyl (1i) substituted enoldiazoacetates provided any noticeable improvement in enantiocontrol (90−92% ee) and yields (87−90%) (entries 2,3; Table 2). Surprisingly, the p-methoxybenzyl (PMB) ester provided a remarkable level of enantiocontrol (99% ee) and also produced 3j in 95% yield (entry 4; Table 2). A very similar ee value (98% ee) was obtained for the 3,4,5-trimethoxybenzyl derivative 3k, however, the reaction time for this reaction was extended to 48 h in order to achieve full conversion (entry 5; Table 2). As expected, the presence of the electron-withdrawing CF3 group at the 4-position of phenyl ring (1l) resulted in a decrease of both the yield (73%) and enantioselectivity (87% ee) of azetine 3l. To determine that the effect of the PMB group as R2 might be general, we prepared p-methoxybenzyl 3-OTIPS-2-diazo-3-pentenoate 1m and performed the [3 + 1]-cycloaddition reaction (entry 7; Table 2): enantioselectivity was improved from 75% (3b, R2 = Me) to 88% ee (3m, R2 = PMB).
Table 2.
Scope of enoldiazoacetates: effect of the carboxylate group.
 | ||||
|---|---|---|---|---|
| Entrya | R1 | R2 | Yield 3 (%)b | ee (%)c |
| 1 | Et | iPr | 3g 82 | 89 |
| 2 | Et | Bn | 3h 87 | 92 |
| 3 | Et | 4-BrBn | 3i 90 | 90 |
| 4 | Et | 4-OMeBn | 3j 95 | 99 |
| 5d | Et | 3,4,5-triOMeBn | 3k 70 | 98 |
| 6 | Et | 4-CF3Bn | 3l 73 | 87 |
| 7 | Me | 4-OMeBn | 3m 77 | 88 |
aAll reactions were carried out on a 0.20 mmol scale in 4.0 mL DCM: 2a (0.20 mmol), 1a (0.24 mmol)
bIsolated yield after flash chromatography
cDetermined by chiral HPLC analysis
dReaction time was 48 h
Nucleophilic ring opening of donor–acceptor azetines
That ring opening would be a facile process of these donor–acceptor azetines was not initially obvious. Five- and six-membered ring silyl-protected β-enolcarboxylates are well known to form β-ketoesters after desilylation39–42. However, when azetine 3b was treated with the classic TBAF to effect desilylation, a mixture of ring-opened products was obtained under typically mild conditions. This observation suggested that initial enolate formation had occurred and that subsequent nucleophilic reaction on the β-keto ester or its equivalent effected strain-induced ring opening. To determine the extent of nucleophilic ring opening with strain release of donor–acceptor azetines, we have treated them with a variety of nitrogen and oxygen nucleophiles. We assumed that TIPS group removal from 2-azetine-2-carboxylates 3 occurs under mild conditions to generate the 3-azetidinone carboxylate structure, which then undergoes ring opening with the excess of a nucleophile (Fig. 3). This concept of strain release through carbon–carbon σ-bond cleavage from 3-azetidinone carboxylates bond is uncovered in this work, and this nucleophile coupling opens doors to enormous opportunities in the synthesis of chiral amino acid derivatives and relevant substances of biological interest with high optical purity.
Fig. 3.
Strain-induced nucleophilic ring opening of donor–acceptor azetines. Desilylation of the azetine forms the activated 3-azetidinone that undergoes nucleophilic retro-Claisen ring opening.
2-Azetine-2-carboxylates 3c and 3j were the substrates of choice in most cases because of their availability and optical purity (90 and 99% ee, respectively). Initial assessment of reactivity was carried out by reactions of 3c and 3j with 2.5 equiv. of benzylamine in DCM at room temperature (Table 3). Ring-opened products that contain one amide bond were formed within 48 h in excellent isolated yields (4a–d, 5; 90–96%). Moreover, we observed the complete retention of optical purity in the products of the nucleophile coupling reactions (see Supplementary Figs. 159–170). The scope of reactions between 2-azetine-2-carboxylate 3 and amines tolerates substituents other than chloride in the aromatic ring of 3. We did not see a substantial difference in either product yields or enantioselectivities with electron-withdrawing substituents on the benzene ring (4a–c). However, with the electron-donating methyl group at the meta position of the N-aryl group, the corresponding 2-azetine-2-carboxylate was obtained with diminished enantiopurity (74% ee) that was carried over to the ring-opened product 4d. The ester functionality remained intact under these reaction conditions even when 4 equiv. of benzylamine was used. However, consistent with nucleophilic reactions that form ionic intermediates43,44, changing the polarity of the solvent played a significant role in increasing the reactivity of 3c toward benzylamine, so that with 4 equiv. of benzylamine or 4-bromobenzylamine in THF/water 1:1 (v/v) chiral diamide derivatives 6a,b were formed in high yields (93 and 85%, respectively) within 12 h. This was an unexpected observation because the classic reaction of an ester with amines is very sluggish at room temperature. A control reaction of monoamide 4 with an excess of benzylamine (2 equiv.) resulted in a quantitative yield of diamide 6 in 12 h. Apparently, formation of the first amide unit activates the carbonyl group of the ester via intramolecular hydrogen bonding in water (polar protic solvent), and therefore favors the nucleophilic substitution by benzylamine on the ester group.
Table 3.
Ring-opening reactions of 2-azetine-2-carboxylates 3 with amines.
We investigated the regioselectivity of the ring-opening reaction of 3c with N-cyclohexyl-1,3-propanediamine (primary-secondary diamine). Formation of the amide bond occurred exclusively at the primary amine position of the diamine, and the monoamination product 7 was isolated in 92% yield. Inspired by the results with benzylamine, the remarkable levels of regioselectivity with N-cyclohexyl-1,3-propanediamine, and the solvent effect on product formation, we examined reactions of 2-azetines 3c and 3f with biologically relevant amines and natural amino acids. Monoamide derivatives of tryptamine (8 and 9), benzyl-protected L-proline (10), and glutamic acid diethyl ester (14), for example, were obtained in 84–88% isolated yields in reactions carried out in dichloromethane. Ring opening of 3c with a natural polyamine, spermine, in DCM occurred selectively at the terminal primary amine position of spermine but, unlike with other amines, formed chiral diamide 15 as the major product (63% yield). The reaction of 3c with tert-butylamine, a sterically hindered primary amine, occurred with similar efficacy as that with benzylamine (92% yield). Treatment of azetine 3c with aromatic amines, which are weaker nucleophiles, showed negligible conversion in DCM at room temperature, but heating 3c with aniline (3 equiv.) at 65 °C in 1,2-dichloroethane (DCE) for 24 h resulted in ring-opening nucleophilic coupling; however, 13a was formed in only 65% yield together with the product from the known thermal electrocyclic ring opening of 3c27–29. Use of electron-rich 4-(dimethylamino)aniline with 3c in nitromethane at room temperature increased the yield of the ring-opened product to 93% (13b). The reaction of 3c with (R)-phenylglycinol (2.5 equiv.) in DCM at room temperature was sluggish but highly selective toward the amino group, affording monoamidation product 11 (d.r. >20:1) in 90% yield after 4 days. We were also interested if L-lysine methyl ester (basic form) was able to provide high regioselectivity in the ring-opening reaction with 3c carried out in DCM. Indeed, remarkable regiocontrol (at the terminal amino group) was achieved in the formation of monoamide derivative 16 (90% yield of a single diastereomer). The same regiocontrol was obtained in the reaction of 2-azetine carboxylate 3c with L-lysine (4 equiv.) in THF/water 1:1 (v/v), but this reaction also resulted in hydrolysis of the ester to the carboxylic acid (17, 75% yield) under the reaction conditions. Reactions with tert-butylamine and pyrrolidine in THF/water 1:1 (v/v), unlike that with benzylamine, resulted in monoamidation and hydrolysis of the ester to form amidocarboxylic acids 18 (80%) and 19 (72%), rather than in the formation of a diamide.
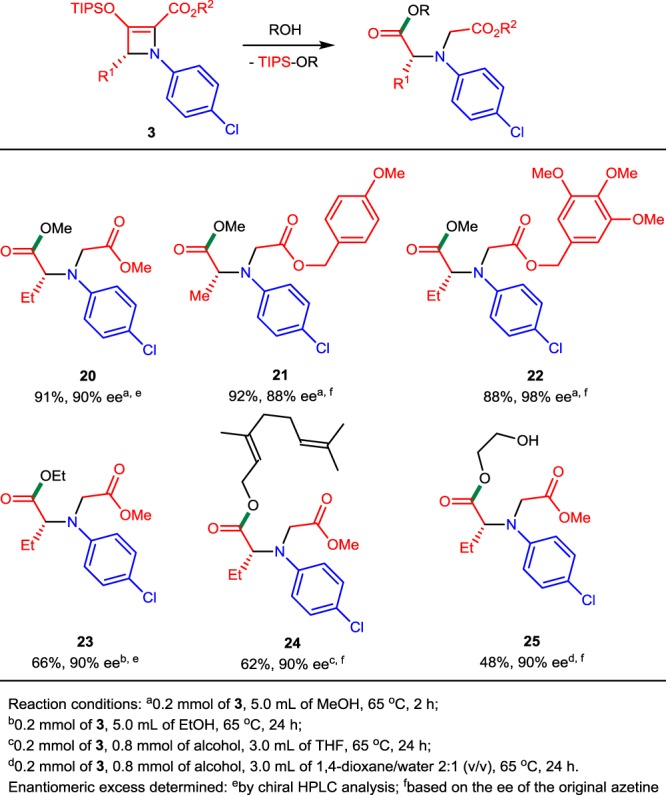
As expected, the ring-opening reactions of 2-azetine-2-carboxylates with the weaker alcohol nucleophiles occurred at slower rates (Table 4). However, very high yields (up to 92%) of chiral diesters 20–22 were obtained with complete retention of enantiopurity from the reactions of 3c, 3j, and 3k with methanol used as the solvent at 65 °C. Use of higher-molecular-weight primary alcohols resulted in a decrease of their reactivity with 2-azetine-2-carboxylates. The yield of diester 23 obtained with ethanol (66%) at 65 °C after 24 h was similar to that obtained from the reaction with aniline (65%), but a much larger excess of the nucleophile was used in this case (ethanol was used as the solvent). Geraniol (a naturally occurring primary alcohol)45,46 and ethylene glycol also formed the corresponding diesters 24 and 25 in moderate yields at 65 °C after 24 h using only 4 equiv. of alcohol. In all reactions performed at elevated temperatures, the main competing reaction was electrocyclic ring opening of 2-azetine carboxylate 3c27–29.
Table 4.
Ring-opening reactions of 2-azetine-2-carboxylates 3 with alcohols.
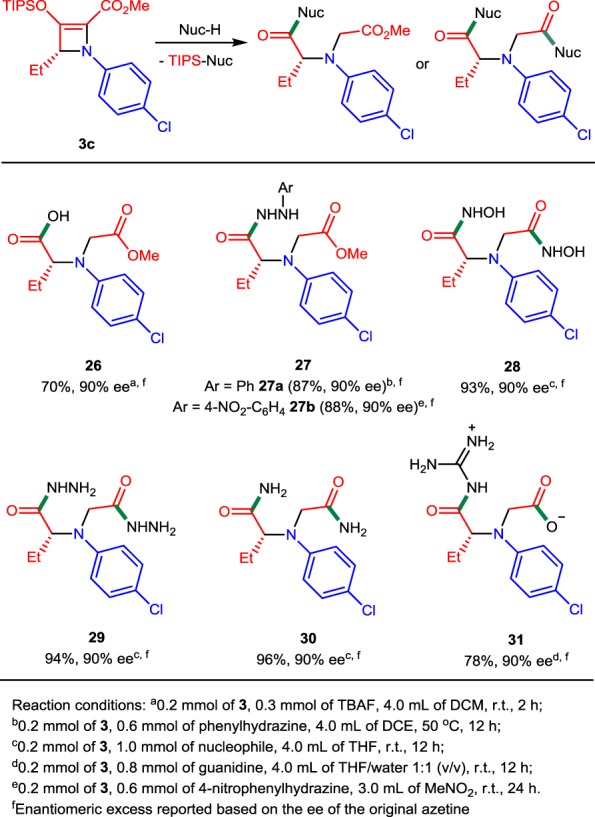
Besides amines, amino acids, and alcohols, we have tested other relatively strong nitrogen-based nucleophiles and tetrabutylammonium fluoride (TBAF) (Table 5). The monomethyl ester of chiral dicarboxylic acid 26 was obtained in 70% yield by a simple treatment of 2-azetine-2-carboxylate 3c with a THF solution of TBAF. Alternatively, compound 26 was obtained in near quantitative yield by treatment of azetine 3c with 5 equiv. of water in nitromethane at room temperature in 12 h. The reactivity of 3c with phenylhydrazine was higher than that with aniline, and chiral monohydrazide 27a was obtained in 87% yield at 50 °C in DCE after 12 h together with minor amounts (<10%) of the electrocyclic ring-opening product from 3c. The reaction of electron-deficient 4-nitrophenylhydrazine with 3c in nitromethane at room temperature resulted in high yield (88%) of the ring-opened product 27b. The use of aqueous solutions of hydroxylamine, hydrazine, and ammonia for the reaction with 3c in THF led to the formation of two C–N bonds and afforded chiral dihydroxamic acid 28, dihydrazide 29, and diamide 30 in excellent yields (up to 96%) in 12 h. Efforts to perform selective reactions leaving the ester group intact were not successful. An interesting example of guanidine-based chiral amino acid 31 was obtained in the reaction of 3c with guanidine in THF/water 1:1 (v/v). Use of 4 equiv. of guanidine produced the zwitterionic compound 31 in 78% isolated yield. The product of the attachment of two molecules of guanidine was detected in the reaction mixture by LC/MS but not isolated.
Table 5.
Ring-opening reactions of 2-azetine-2-carboxylate 3c with other nucleophiles.
Mechanistic studies
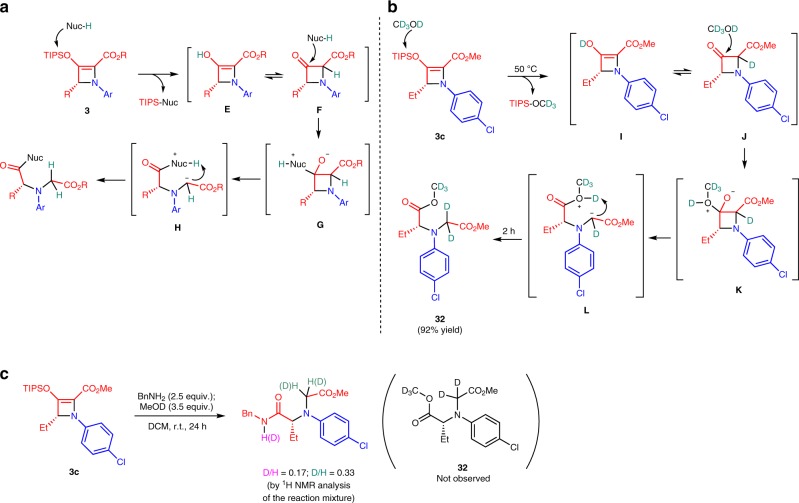
That the nucleophilic ring-opening reaction carried out in DCM requires two molecules of the nucleophile is based on: (1) TIPS-Nuc was isolated as the by-product, and (2) only half of the azetine was converted to product when one equivalent of the nucleophile was used. The same outcome was observed when the reaction of 3c was performed in THF/H2O (1:1, v/v) with one equivalent of benzylamine as the nucleophile. However, complete conversion of 3c was observed in THF within 12 h when only one equivalent each of benzylamine and water was used, which afforded ring-opened product 4a in 92% isolated yield. We propose a sequential pathway of steps in the reaction mechanism to show all relevant reaction intermediates, including the 3-azetidiniones (Fig. 4a). The initial abstraction of the silyl (TIPS) group by a nucleophile forms 2-azetine enol E, the tautomer of which is 3-azetidinone carboxylate F. The carbonyl group of F then undergoes attack by the second molecule of the nucleophile to form zwitterionic four-membered ring intermediate G followed by ring opening to the acyclic zwitterion H. Rapid intramolecular proton abstraction by the carbanion forms the final amino acid derivative. We attempted to trap intermediate H using benzyl bromide (5 equiv.), methyl iodide (5−10 equiv.), and even dimethyl disulphide47–51 (5 equiv.) as the electrophile, but the absence of product from substitution suggested a much higher rate for intramolecular proton transfer. To support this mechanism, we carried out deuterium incorporation experiments on the reaction of 3c with methanol-d4 used as the solvent (Fig. 4b).
Fig. 4.
Mechanistic studies. a Proposed mechanism for nucleophilic ring opening of 2-azetine-2-carboxylates 3. b Deuterium incorporation experiment. c Proton–deuterium exchange with MeOD.
The reaction mechanism includes a set of intermediates I−L that are the same as those shown in Fig. 4a. A preparative 0.2 -mmol scale ring opening reaction of 3c with methanol-d4 afforded deuterated compound 32 in 92% isolated yield in 2 h without detection of intermediates I−L by the NMR method (see Supplementary Fig. 1). To investigate a possibility of intermolecular proton transfer from intermediate H to the final product, we have performed a competing reaction of 3c with benzylamine and methanol-d4 (Fig. 4c). The formation of 4 as the major product confirmed the intramolecular proton transfer as the major reaction pathway. However, minor amounts of deuterated products were observed in the 1H NMR spectrum of the reaction mixture (see Supplementary Fig. 2) as the result of deuterium exchange on the benzylic nitrogen (~17% of D) or incorporation from methanol-d4, suggesting minor competition from intermolecular proton transfer from methanol-d4 during the ring opening (~33% of D). Moreover, diester 32 as the product of reaction of 3c with benzylamine and MeOD was not observed in the reaction mixture.
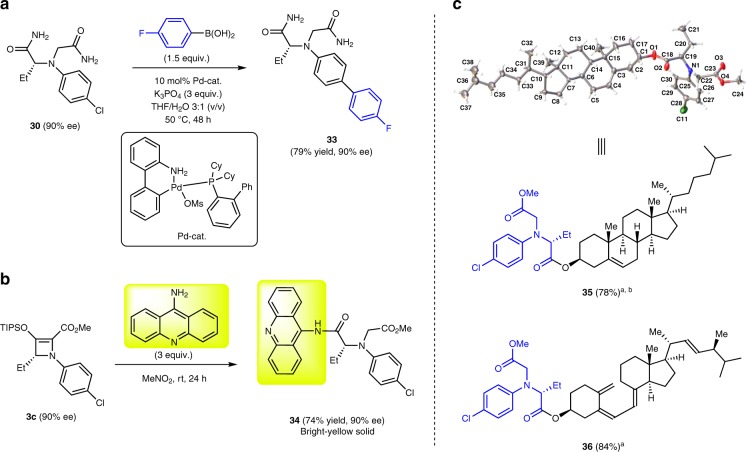
To expand the scope of the ring-opened products and the synthetic applicability of the chlorine atom attached to the benzene ring, we have performed the Suzuki−Miyaura sp2−sp2 cross-coupling with diamide 30 (Fig. 5a). A compound containing a fluorine atom (diamide 33) was obtained in 79% yield by treatment of diamide 30 with 4-fluorophenylboronic acid using the air and moisture stable Buchwald’s third generation precatalyst [a powerful source of Pd(0)]52 that was synthesized from commercially available precursors. Notably, amide functional groups remained intact under these reaction conditions; however, significant amounts of hydrolysis products were formed at temperatures over 100 °C (conversion of the amide to carboxylates).
Fig. 5.
Functionalization of ring-opened products. a Representative example of Suzuki−Miyaura cross-coupling of a ring-opened product. b Attachment of a fluorescent unit to chiral amino acid. c Functionalization of cholesterol and ergocalciferol (vitamin D2) using chiral amino acid attachment.
The use of fluorophores as sensors is common in chemical biology53,54 and plays an important role in rapid detection of peptides55–58. Herein, we report a robust protocol for the attachment of a fluorescent unit using the ring-opening reaction of azetine 3c with 4-aminoacridine as a fluorophore-carrying nucleophile. Bright yellow chiral amino acid derivative 34 was obtained in high yield (74%) in nitromethane as the most suitable solvent (Fig. 5b). The UV spectrum of 34 showed maximum absorptions at λ = 380, 399, and 421 nm; and the fluorescence spectrum showed maximum emissions at λ = 428, 453, and 480 nm (see Supplementary Figs. 3, 4).
As shown in Table 4, methanol and primary alcohols, but not secondary alcohols, were suitable for the ring opening of azetines. To solve the problem with secondary alcohols, we have developed a two-step protocol: synthesis of chiral monoester 26 and its reaction with naturally occurring secondary alcohols, cholesterol and ergocalciferol (vitamin D2), using a classic base-catalyzed N,N’-dicyclohexylcarbodiimide (DCC) coupling reaction (Fig. 5c). Chiral diester derivatives 35 and 36 were obtained under mild conditions in 78 and 84% yields, respectively, and the structure of 35 was confirmed by X-ray crystallography establishing the absolute configuration of 26 as (R). This experimental evidence allowed us to assign absolute configurations of all chiral materials—azetines and ring-opened products.
Discussion
We have developed a methodology for the strain-induced ring opening of chiral 3-azetidinone carboxylates that form glycine derivatives having high enantiopurity in high yield. The key to the success of this process is the highly enantioselective catalytic [3 + 1]-cycloaddition of silyl-protected enoldiazoacetates with aza-ylides that form donor–acceptor 3-triisopropylsiloxyazetine-2-carboxylates that are precursors to 3-azetidinone carboxylates. Catalysis by cationic copper(I) with a sabox chiral ligand ensures high enantiocontrol for azetine formation. Silyl group removal from the azetine by nucleophilic displacement produces the unstable 3-azetidinone-carboxylate that undergoes nucleophilic retro-Claisen ring opening. Reactions with aliphatic amines occur at room temperature, but those with the less nucleophilic arylamines and alcohols require higher temperatures. Mechanistic details, which include deuterium labeling experiments, confirm that proton transfers from the nucleophile to the azetine in the desilylation step and to the azetidinone in the retro-Claisen step are predominantly intramolecular (Fig. 4). Under specific reaction conditions, water may be the nucleophile that removes the silyl group so that the nucleophile used for the retro-Claisen ring opening can be employed in stoichiometric amounts. The rate for ring opening is dependent on the strength of the nucleophile and on the steric encumbrance around the nucleophilic center. When the reaction is performed at elevated temperatures the major competing reaction is electrocyclic ring opening of the 2-azetine carboxylate. The reaction process that is introduced provides a straightforward methodology for the functionalization of a broad selection of nucleophiles with peptide-like amine derivatives. The retro-Claisen products can be further functionalized by aryl group coupling and by esterification, suggesting broad future applications. Future efforts will be directed toward expanding the applicable nucleophiles and methods for further functionalization, as well as applying the retro-Claisen methodology to other products of catalytic asymmetric [3 + 1]-cycloaddition.
Methods
General procedure for [3 + 1]-cycloaddition
To an 8-mL oven-dried screw-capped vial equipped with a magnetic stirring bar were sequentially added Cu(MeCN)4PF6 (3.72 mg, 0.0100 mmol, 5 mol%), sabox ligand L1 (8.81 mg, 0.012 mmol, 6 mol%), and 2.00 mL of dry DCM under a nitrogen atmosphere. The resulting solution was stirred at room temperature for 1 h. N-Arylsulfilimine 2a (62.2 mg, 0.200 mmol) was then introduced to the reaction solution under a flow of nitrogen, followed by dropwise addition (over 1 min) of enoldiazoacetate 1 (0.24 mmol) in dry DCM (2.00 mL). The vial was capped, and stirring was continued at room temperature for 24–72 h. Subsequently, the reaction mixture was concentrated under reduced pressure, and the residue was purified by flash chromatography on silica gel using a gradient of hexane/ethyl acetate 49:1 to 4:1 (v/v) as eluent to afford donor–acceptor azetine 3 as a pale yellow oil.
General procedure for retro-Claisen ring opening
To a 8-mL screw-capped vial equipped with a magnetic stirring bar an azetine 3 (0.20 mmol), an amine (0.50 mmol) and DCM (4.0 mL) were sequentially introduced. The vial was capped, and stirring was continued at room temperature for 48 h [for 4, 5, 7, 8, 9, 12, 15, 16] or 96 h [for 10, 11, 14]. After completion of the reaction (monitored by TLC), DCM was evaporated, and the residue was purified by flash chromatography on silica gel using a gradient hexane/ethyl acetate 2:1 (v/v) to pure ethyl acetate [for 4, 5, 8−12, 14] or a mixture DCM/methanol 9:1 (v/v) [for 7, 15, 16] as eluents to afford the ring opening product.
Supplementary Information
Acknowledgements
We acknowledge the U.S. National Science Foundation (CHE-1763168) and the Max and Minni Voelcker Fund for their support of this research. K.D. acknowledges the support from China Scholarship Council (CSC). We thank N. Greco for the synthesis of substrates and racemic samples, K. Schanze’s group for the fluorescence analyses, and W.G. Griffith for mass spectral analyses. The U.S. National Science Foundation supported the acquisition of a NMR spectrometer used in this study (CHE-1625963).
Author contributions
K.O.M. performed experiments on the synthesis of chiral azetines, their ring opening and fuctionalization, and did mechanistic studies. K.D., L.D.A., L.A.M., and K.W. performed synthesis of starting materials, racemic azetines and their ring-opened products. Y.D. performed catalyst, ligand, and sulfilimine optimizations. H.A. analyzed a sample of 35 by X-ray diffraction. K.O.M. and M.P.D. conceived and directed the project and wrote the paper. All authors discussed the results and commented on the paper.
Data availability
The authors declare that the data supporting the findings of this study are available within the paper and its Supplementary Information. Crystallographic data for compound 35 is available free of charge from the Cambridge Crystallographic Date Centre (www.ccdc.cam.ac.uk) under reference number CCDC 1910314.
Competing interests
The authors declare no competing interests.
Footnotes
Peer review information Nature Communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information is available for this paper at 10.1038/s41467-019-13326-8.
References
- 1.Fisher JF, Meroueh SO, Mobashery S. Bacterial resistance to β-lactam antibiotics: compelling opportunism, compelling opportunity. Chem. Rev. 2005;105:395–424. doi: 10.1021/cr030102i. [DOI] [PubMed] [Google Scholar]
- 2.Palomo, C. & Oiarbide, M. in Heterocyclic Scaffolds I. Topics in Heterocyclic Chemistry Vol. 22 (ed. Banik, B.) 211–259 (Springer, Berlin, Heidelberg, 2010).
- 3.Crowder MW, Spencer J, Vila AJ. Metallo-β-lactamases: novel weaponry for antibiotic resistance in bacteria. Acc. Chem. Res. 2006;39:721–728. doi: 10.1021/ar0400241. [DOI] [PubMed] [Google Scholar]
- 4.Kamath A, Ojima I. Advances in the chemistry of β-lactam and its medicinal applications. Tetrahedron. 2012;68:10640–10664. doi: 10.1016/j.tet.2012.07.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dejaegher Y, Kuz’menok NM, Zvonok AM, De Kimpe N. The chemistry of azetidin-3-ones, oxetan-3-ones, and thietan-3-ones. Chem. Rev. 2002;102:29–60. doi: 10.1021/cr990134z. [DOI] [PubMed] [Google Scholar]
- 6.Jukic M, Sterk D, Casar Z. Recent advances in the retro-Claisen reaction and its synthetic applications. Curr. Org. Synth. 2012;9:488–512. doi: 10.2174/157017912802651438. [DOI] [Google Scholar]
- 7.Gianatassio R, et al. Strain-release amination. Science. 2016;351:241–246. doi: 10.1126/science.aad6252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lopchuk JM, et al. Strain-release heteroatom functionalization: development, scope and stereospecifcity. J. Am. Chem. Soc. 2017;139:3209–3226. doi: 10.1021/jacs.6b13229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fawcett A, Biberger T, Aggarwal VK. Carbopalladation of C–C σ-bonds enabled by strained boronate complexes. Nat. Chem. 2019;11:117–122. doi: 10.1038/s41557-018-0181-x. [DOI] [PubMed] [Google Scholar]
- 10.Fawcett A, Murtaza A, Gregson CHU, Aggarwal VK. Strain-release-driven homologation of boronic esters: application to the modular synthesis of azetidines. J. Am. Chem. Soc. 2019;141:4573–4578. doi: 10.1021/jacs.9b01513. [DOI] [PubMed] [Google Scholar]
- 11.Moyer MP, Feldman PL, Rappoport H. Intramolecular N-H, O-H, and S-H insertion reactions. Synthesis of heterocycles from α-diazo ß-keto esters. J. Org. Chem. 1985;50:5223–5230. doi: 10.1021/jo00225a047. [DOI] [Google Scholar]
- 12.Pang S, et al. Intermolecular [2 + 2] cycloaddition/isomerization of allenyl imides and unactivated imines for the synthesis of 1-azadienes catalyzed by a Ni(ClO4)2·6H2O Lewis acid. ACS Catal. 2018;8:5193–5199. doi: 10.1021/acscatal.8b01454. [DOI] [Google Scholar]
- 13.Hodgson DM, Kloesges J. Lithiation–electrophilic substitution of N‐thiopivaloylazetidine. Angew. Chem. Int. Ed. 2010;49:2900–2903. doi: 10.1002/anie.201000058. [DOI] [PubMed] [Google Scholar]
- 14.Hodgson DM, Pearson CI, Kazmi M. Generation and electrophile trapping of N-Boc-2-lithio-2-azetine: synthesis of 2-substituted 2-azetines. Org. Lett. 2014;16:856–859. doi: 10.1021/ol403626k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burkhard JA, Carreira EM. 2,6-Diazaspiro[3.3]heptanes: synthesis and application in Pd-catalyzed aryl amination reactions. Org. Lett. 2008;10:3525–3526. doi: 10.1021/ol801293f. [DOI] [PubMed] [Google Scholar]
- 16.Burkhard JA, et al. Synthesis of azaspirocycles and their evaluation in drug discovery. Angew. Chem. Int. Ed. 2010;49:3524–3527. doi: 10.1002/anie.200907108. [DOI] [PubMed] [Google Scholar]
- 17.Burkhard JA, et al. Synthesis and structural analysis of a new class of azaspiro[3.3]heptanes as building blocks for medicinal chemistry. Org. Lett. 2010;12:1944–1947. doi: 10.1021/ol1003302. [DOI] [PubMed] [Google Scholar]
- 18.Baumann AN, et al. Methods for the synthesis of substituted azetines. Org. Lett. 2017;19:5681–5684. doi: 10.1021/acs.orglett.7b02847. [DOI] [PubMed] [Google Scholar]
- 19.Barluenga J, et al. Copper(I)‐catalyzed [3 + 1] cycloaddition of alkenyldiazoacetates and iminoiodinanes: easy access to substituted 2‐azetines. Chem. − Eur. J. 2012;18:9221–9224. doi: 10.1002/chem.201200998. [DOI] [PubMed] [Google Scholar]
- 20.Deng Y, Massey LA, Zavalij P, Doyle MP. Catalytic asymmetric [3 + 1]-cycloaddition reaction of ylides with electrophilic metallo-enolcarbene intermediates. Angew. Chem. Int. Ed. 2017;56:7479–7483. doi: 10.1002/anie.201704069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yoshimura T, Omata T. Preparation and physical and chemical properties of “free” sulfilimines. J. Org. Chem. 1976;41:1728–1733. doi: 10.1021/jo00872a013. [DOI] [Google Scholar]
- 22.Bizet V, Buglioni L, Bolm C. Light-induced ruthenium-catalyzed nitrene transfer reactions: a photochemical approach towards N-acyl sulfimides and sulfoximines. Angew. Chem. Int. Ed. 2014;53:5639–5642. doi: 10.1002/anie.201310790. [DOI] [PubMed] [Google Scholar]
- 23.Hayashi R, et al. Metal-free benzylic C−H amination via electrochemically generated benzylaminosulfonium ions. Chem. − Eur. J. 2017;23:61–64. doi: 10.1002/chem.201604484. [DOI] [PubMed] [Google Scholar]
- 24.Gilchrist TL, Moody CJ. The chemistry of sulfilimines. Chem. Rev. 1977;77:409–435. doi: 10.1021/cr60307a005. [DOI] [Google Scholar]
- 25.García Ruano JL, et al. Product class 4: acyclic dialkyl sulfoxides and derivatives. Sci. Synth. 2007;39:245–390. [Google Scholar]
- 26.Tian X, et al. Sulfilimines as versatile gold nitrene transfer reagents: facile access to aza-heterocyclic diversity. Angew. Chem. Int. Ed. 2019;58:3589–3593. doi: 10.1002/anie.201812002. [DOI] [PubMed] [Google Scholar]
- 27.Lopez SA, Houk KN. Substituent effects on rates and torquoselectivities of electrocyclic ring-openings of N-substituted 2-azetines. J. Org. Chem. 2014;79:6189–6195. doi: 10.1021/jo500919s. [DOI] [PubMed] [Google Scholar]
- 28.Shindoh N, Kitaura K, Takemoto Y, Takasu K. Catalyst-controlled torquoselectivity switch in the 4π ring-opening reaction of 2-amino-2-azetines giving β-substituted α,β-unsaturated amidines. J. Am. Chem. Soc. 2011;133:8470–8473. doi: 10.1021/ja202576e. [DOI] [PubMed] [Google Scholar]
- 29.Mangelinckx S, et al. Experimental and computational study of the conrotatory ring opening of various 3-chloro-2-azetines. J. Org. Chem. 2008;73:5481–5488. doi: 10.1021/jo800522b. [DOI] [PubMed] [Google Scholar]
- 30.Wang X, et al. Enantioselective carbene cascade: an effective approach to cyclopentadienes and applications in Diels-Alder reactions. Adv. Synth. Catal. 2016;358:1571–1576. doi: 10.1002/adsc.201501106. [DOI] [Google Scholar]
- 31.Deng Y, Jing C, Doyle MP. Dinitrogen extrusion from enoldiazo compounds under thermal conditions: synthesis of donor–acceptor cyclopropenes. Chem. Commun. 2015;51:12924–12927. doi: 10.1039/C5CC05006E. [DOI] [PubMed] [Google Scholar]
- 32.Xu X, Wang X, Zavalij PY, Doyle MP. Straightforward access to the [3.2.2]nonatriene structural framework via intramolecular cyclopropenation/Buchner reaction/Cope rearrangement cascade. Org. Lett. 2015;17:790–793. doi: 10.1021/ol503498n. [DOI] [PubMed] [Google Scholar]
- 33.Xu X, Zavalij PJ, Doyle MP. A donor-acceptor cyclopropene as a dipole source for a silver(I) catalyzed asymmetric catalytic [3 + 3]-cycloaddition with nitrones. Chem. Commun. 2013;49:10287–10289. doi: 10.1039/c3cc46415f. [DOI] [PubMed] [Google Scholar]
- 34.Qian Y, et al. Rhodium(II)- and copper(II)-catalyzed reactions of enol diazoacetates with nitrones: metal carbene versus Lewis acid directed pathways. Angew. Chem. Int. Ed. 2012;51:5900–5903. doi: 10.1002/anie.201202525. [DOI] [PubMed] [Google Scholar]
- 35.Zhu C, Xu G, Sun J. Gold-catalyzed formal [4+1]/[4+3] cycloadditions of diazo esters with triazines. Angew. Chem. Int. Ed. 2016;55:11867–11871. doi: 10.1002/anie.201606139. [DOI] [PubMed] [Google Scholar]
- 36.Lian Y, Hardcastle KI, Davies HML. Computationally guided stereocontrol of the combined C-H functionalization/Cope rearrangement. Angew. Chem. Int. Ed. 2011;50:9370–9373. doi: 10.1002/anie.201103568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dong K, Marichev KO, Xu X, Doyle MP. High stereocontrol in the preparation of silyl-protected γ-substituted enoldiazoacetates. Synlett. 2019;30:1457–1461. doi: 10.1055/s-0037-1610315. [DOI] [Google Scholar]
- 38.Dong K, Marichev KO, Doyle MP. Role of donor-acceptor cyclopropenes in metal carbene reactions. Conversion of E-substituted enoldiazoacetates to Z-substituted metallo-enolcarbenes. Organometallics. 2019;38:4043–4050. doi: 10.1021/acs.organomet.9b00427. [DOI] [Google Scholar]
- 39.Smith AG, Davies HML. Rhodium-catalyzed enantioselective vinylogous addition of enol ethers to vinyldiazoacetates. J. Am. Chem. Soc. 2012;134:18241–18244. doi: 10.1021/ja3092399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Deng Y, Yglesias MV, Arman H, Doyle MP. Catalytic asymmetric synthesis of cyclopentyl β-amino esters by [3+2] cycloaddition of enecarbamates with electrophilic metalloenolcarbene intermediates. Angew. Chem. Int. Ed. 2016;55:10108–10112. doi: 10.1002/anie.201605438. [DOI] [PubMed] [Google Scholar]
- 41.Xu X, Zavalij PJ, Doyle MP. A donor–acceptor cyclopropene as a dipole source for a silver(I) catalyzed asymmetric catalytic [3+3]-cycloaddition with nitrones. Chem. Commun. 2013;49:10287–10289. doi: 10.1039/c3cc46415f. [DOI] [PubMed] [Google Scholar]
- 42.Xu X, Zavalij PY, Doyle MP. Synthesis of tetrahydropyridazines by a metal–carbene-directed enantioselective vinylogous N−H insertion/Lewis acid-catalyzed diastereoselective Mannich addition. Angew. Chem. Int. Ed. 2012;51:9829–9833. doi: 10.1002/anie.201203962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shawali ASAS, Biechler SS. Aminolysis of esters. I. Kinetics and mechanism in anhydrous dioxane. J. Am. Chem. Soc. 1967;89:3020–3026. doi: 10.1021/ja00988a039. [DOI] [PubMed] [Google Scholar]
- 44.Adalsteinsson H, Bruice TC. What is the mechanism of catalysis of ester aminolysis by weak amine bases? Comparison of experimental studies and theoretical investigation of the aminolysis of substituted phenyl esters of quinoline-6- and -8-carboxylic acids. J. Am. Chem. Soc. 1998;120:3440–3447. doi: 10.1021/ja972162+. [DOI] [Google Scholar]
- 45.Lei Y, Fu P, Jun X, Cheng P. Pharmacological properties of geraniol—a review. Planta. Med. 2019;85:48–55. doi: 10.1055/a-0750-6907. [DOI] [PubMed] [Google Scholar]
- 46.Elsharif SA, Buettner A. Structure–odor relationship study on geraniol, nerol, and their synthesized oxygenated derivatives. J. Agric. Food Chem. 2018;66:2324–2333. doi: 10.1021/acs.jafc.6b04534. [DOI] [PubMed] [Google Scholar]
- 47.Degl’Innocenti A, Mordini A, Pagliai L, Ricci A. Allylsilanes by the regio- and stereocontrolled substitution of metalated homoallylsilanes. Synlett. 1991;3:155–156. doi: 10.1055/s-1991-20660. [DOI] [Google Scholar]
- 48.Klusener PAA, Tip L, Brandsma L. On the direct metalation of isoprene. Tetrahedron. 1991;47:2041–2064. doi: 10.1016/S0040-4020(01)96114-9. [DOI] [Google Scholar]
- 49.Gamage SA, Smith RAJ. Aromatic annulation with naphtho[1,8-de]-1,3-dithiin carbocations. Tetrahedron. 1990;46:2111–2128. doi: 10.1016/S0040-4020(01)89777-5. [DOI] [Google Scholar]
- 50.Reich HJ, Medina MA, Bowe MD. Stereochemistry of a cyclohexyllithium reagent. A case of higher configurational stability in strongly coordinating media. J. Am. Chem. Soc. 1992;114:11003–11004. doi: 10.1021/ja00053a071. [DOI] [Google Scholar]
- 51.Stanetty P, Koller H, Mihovilovic M. Directed ortho lithiation of phenylcarbamic acid 1,1-dimethylethyl ester (N-BOC-aniline). Revision and improvements. J. Org. Chem. 1992;57:6833–6837. doi: 10.1021/jo00051a030. [DOI] [Google Scholar]
- 52.Bruno, N. C. & Buchwald, S. L. Phosphine-Ligated Palladium Sulfonate Palladacycles. (WO2013184198 A1, 2013).
- 53.Lavis LD, Raines RT. Bright ideas for chemical biology. Acs. Chem. Biol. 2008;3:142–155. doi: 10.1021/cb700248m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lavis LD, Raines RT. Bright building blocks for chemical biology. Acs. Chem. Biol. 2014;9:855–866. doi: 10.1021/cb500078u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pazos E, Vázquez O, Mascareňas JL, Vázquez ME. Peptide-based fluorescent biosensors. Chem. Soc. Rev. 2009;38:3348–3359. doi: 10.1039/b908546g. [DOI] [PubMed] [Google Scholar]
- 56.Kobayashi H, Ogawa M, Alford R, Choyke PL, Urano Y. New strategies for fluorescent probe design in medical diagnostic imaging. Chem. Rev. 2010;110:2620–2640. doi: 10.1021/cr900263j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee S, Xie J, Chen X. Peptide-based probes for targeted molecular imaging. Biochemistry. 2010;49:1364–1376. doi: 10.1021/bi901135x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Staderinia M, Megia-Fernandez A, Dhaliwal K, Bradley M. Peptides for optical medical imaging and steps towards therapy. Bioorg. Med. Chem. 2018;26:2816–2826. doi: 10.1016/j.bmc.2017.09.039. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors declare that the data supporting the findings of this study are available within the paper and its Supplementary Information. Crystallographic data for compound 35 is available free of charge from the Cambridge Crystallographic Date Centre (www.ccdc.cam.ac.uk) under reference number CCDC 1910314.