Abstract
In this research, the binding of cellulolytic enzymes in Cellic® CTec2 on six lignin isolates obtained from alkali (0.5, 1.0, and 1.5% NaOH at 121 °C for 30 min) and acid (1, 2, and 3% H2SO4 at 121 °C for 60 min) pretreated switchgrass was investigated. Briefly, the hydrolysis of cellobiose and Avicel with and without (control) lignin isolates was performed via CTec2 (5 and 10 FPU g−1 carbohydrate) to determine whether the presence of lignin and binding of cellulolytic enzymes to the isolated lignin can affect the sugar production using three carbohydrate-lignin loadings, namely, 0.5:0.25, 0.5:0.5, and 0.5:1.0% (wv−1). Based on SDS-PAGE results, β-glucosidase (BG) was significantly bound to all lignin isolates. Some enzymes in CTec2 presumed to be cellobiohydrolases, endo-1,4-β-glucanases, and xylanase, were also observed to partially bind to the lignin isolates. Up to 0.97 g glucose g−1 cellobiose was produced via hydrolysis (72 h and pH 4.8) with CTec2 (5 and 10 FPU g−1 carbohydrate). Similarly, up to 0.23 and 0.46 g glucose g−1 Avicel were produced via hydrolysis (72 h and pH 4.8) with 5 and 10 FPU g−1 carbohydrate, respectively. Results indicated that the addition of lignin isolates during cellobiose and Avicel hydrolysis did not significantly (p > 0.05) reduce glucose production regardless of type and amount of lignin isolate. Hence, even though BG was significantly bound to lignin isolates, it could maintain its functionality as a biological catalyst in this study.
Keywords: Switchgrass, Lignin, Pretreatment, Enzyme binding, Cellulases, β-Glucosidase
Introduction
Lignin is an organic, non-carbohydrate, aromatic polymer whose main function is to provide physical and structural protection to cell wall polysaccharides (Ponnusamy et al. 2019). Lignin hinders the production of fermentable sugars during enzymatic hydrolysis of the polysaccharides in lignocelluloses (Kim et al. 2016; Zabed et al. 2016). Its inhibitory action is mainly attributed to the impediment of the enzyme (cellulase) access to plant polysaccharides and non-productive enzyme adsorption on lignin instead of cellulose and hemicellulose (Vermaas et al. 2015). The extent of enzyme adsorption on lignin varies depending on the type of lignin and the method of extraction, hydrolysis time and the enzyme loading, and the pH during the hydrolysis processes (Kumar et al. 2012; Li et al. 2014, 2018; Li and Zheng 2017; Lou et al. 2013).
Non-productive interaction of cellulase and lignin is facilitated via electrostatic, hydrophobic, hydrogen bonding effects (Li and Zheng 2017). However, electrostatic binding between cellulose and lignin due to opposing charges may be impacted by various factors. Rahikainen et al. (2011) investigated non-productive cellulase binding on lignin and the results indicated that lower amounts of cellulase were bound to lignin at higher pH. The authors employed cellulases from M. albomyces (Cel45A, endoglucanase) and mutated Cel45A with carbohydrate binding modules (CBMs) whose isoelectric points (pI) ranged from 3.4 to 4.4. At higher pH, negatively charged cellulase and the negatively charged phenolic carboxyl groups of lignin resulted in repulsive interaction and thus prevented enzyme adsorption. Hydrophobic interaction between cellulose and lignin also plays a significant role in non-productive cellulase adsorption on lignin (Heiss-Blanquet et al. 2011; Pareek et al. 2013). In addition, it was also reported that the lignin-induced inhibition is highly dependent on temperature and the specific enzyme that was employed (Kellock et al. 2017; Rahikainen et al. 2013a, b).
A few authors investigated cellulase (Cellic® CTec2, Novozymes) binding on lignin residue from steam-pretreated wheat straw and mixed hardwood chips pretreated by liquid hot water pretreatment and observed that a significant amount of β-glucosidase (BG) was bound to the lignin residue (Haven and Jørgensen 2013; Ko et al. 2015). It was also found that when higher guaicyl units are present in lignin a majority of the enzymatic activity was lost from the supernatant (Ko et al. 2015). Nevertheless, BG can remain active even when it is intensively bound to the lignin residue (Haven and Jørgensen 2013). Even though it has been shown that cellulolytic enzymes have varying tendencies to be bound to lignin during hydrolysis of lignocellulosic biomass, the effect of varying pretreatments on enzyme adsorption and functionality is not clear.
In this study, lignin was isolated from switchgrass pretreated with sodium hydroxide (NaOH) and dilute sulfuric acid (H2SO4) at various concentrations to investigate (1) which cellulolytic enzymes interact with isolated lignin and (2) if lignin isolates from different pretreatment conditions influence enzyme binding. The lignin isolates were incubated with Cellic® CTec2 enzyme cocktail and enzymatic hydrolysis of mixtures of model carbohydrates and lignin isolates was also performed to study if cellulase bound to lignin isolate can affect the production of monomeric sugars.
Materials and methods
Sample preparation
Alamo switchgrass was obtained from Mountain Horticultural Crops Research and Extension Center, Mills River, North Carolina. The switchgrass was ground and passed through a 2-mm sieve by a Thomas Wiley Laboratory Mill (Model No. 4, Philadelphia, PA, USA). Extractives removal was achieved by Soxhlet extraction in cellulose thimbles with acetone reflux for 24 h to prevent potential interference with lignin analysis and during processes for recovering lignin isolates. Extractive-free switchgrass was used as raw material for alkali and acid pretreatment.
Alkali and acid pretreatment
Switchgrass was pretreated using two pretreatment agents: NaOH and dilute H2SO4. Three chemical concentrations were used for pretreatment at 121 °C for predetermined treatment times (NaOH: 0.5, 1.0 and 1.5% for 30 min, designated as 0.5 N, 1.0 N, and 1.5 N, respectively, and H2SO4: 1, 2 and 3% for 60 min, designated as 1.0H, 2.0H, and 3.0H, respectively). Briefly, 10 g of extractive-free biomass and 100 mL NaOH or H2SO4 solution of desired concentration were mixed at 10% (wv−1) solid loading in 125 mL glass serum bottles which were crimp sealed prior to pretreating in the autoclave at 121 °C (15 psi) (Model 3021, Amsco, Mentor, OH, USA). Pretreated biomass was washed with 500 mL deionized (DI) water and recovered by vacuum filtration. In all, 50 g of switchgrass was pretreated at each condition and biomass from the 5 serum bottles was combined into one 500 mL polypropylene bottle after pretreatment to obtain a well-mixed bigger batch.
Enzyme hydrolysis and lignin isolation
Cellic® CTec2 and HTec2 enzyme cocktails (Novozymes North America, INC, Franklinton, NC, USA) were used in excess for maximum removal of structural carbohydrates from the pretreated switchgrass. The densities of CTec2 and HTec2 were measured to be 1.23 and 1.16 g mL−1, respectively. Cellulase activity of CTec2 was estimated as 103.5 FPU mL−1 according to National Renewable Energy Laboratory (NREL)’s Laboratory Analytical Procedures (LAP) (Adney and Baker 1996).
Lignin isolation
Alkali and acid pretreated biomass at 8% solid loading (wv−1) was hydrolyzed in 0.05 M sodium citrate buffer (pH 4.8) with excessive Cellic® CTec2 dosage, equivalent to 140 FPU g−1 dry pretreated biomass and supplemented with HTec2 (0.25 g of enzyme g−1 dry pretreated biomass) to maximize enzyme saccharification efficiency. Tetracycline (40 µg mL−1) was added during enzymatic hydrolysis to prevent microbial contamination and hydrolysis was performed for 120 h at 50 °C (150 rpm) in an air bath shaker (Series 25 incubator shaker, New Brunswick Scientific Co., INC, Edison, NJ). After hydrolysis, the suspension was centrifuged (model 5810R, Eppendorf, Hauppauge, NY) and the supernatant removed. The remaining solids were washed thrice with DI water adjusted to a pH of 2.5 by adding hydrochloric acid (HCl) to prevent lignin solubilization during the washing step (Rahikainen et al. 2011). A commercial bacterial protease (Type (XXIV), EC # 232-752-2, Sigma-Aldrich Co., St. Louis, MO) was used to remove residual enzymes bound to the lignin-rich hydrolysate. The protease treatment method was modified from Berlin et al. (2006), Rahikainen et al. (2013a, b) and Tamminen and Hortling (1999). Briefly, washed solids were incubated overnight in 0.05 M phosphate buffer (pH 8.5) containing 0.1 mg protease 50 mg−1 lignin in an air bath shaker at 37 °C. After treatment, the proteases were deactivated by placing the samples at 90 °C in a convection oven for 2 h. Protease treated solids were further washed with DI water adjusted to pH 2.5 with HCl three times. Finally, the solids were dried in a 40 °C vacuum oven and used as lignin isolates (LI).
The composition analysis including glucan, xylan, and lignin of the lignin isolates as well as raw switchgrass was performed according to NREL’s LAP prior to conducting the lignin-enzyme binding study (Sluiter et al. 2012). To investigate the visual structural changes, micrographs of raw switchgrass and lignin isolates from alkali and acid pretreated switchgrass were collected using a Hitachi S-3200 N variable pressure scanning electron microscope (VPSEM) available at North Carolina State University’s Analytical Instrumental Facility (AIF). Typically, dried samples were placed on a sticky stub with carbon tape, followed by sputter coating with Gold–Palladium (Au–Pd) in high vacuum mode. Subsequently, the Au–Pd-coated samples were placed in a chamber and analyzed via VPSEM.
Lignin-enzyme binding study
Six types of lignin isolates (LI) were prepared from switchgrass pretreated with three NaOH and three H2SO4 pretreatments, to study lignin-enzyme binding. Enzyme binding was investigated in microcentrifuge tubes by mixing 1% (wv−1) lignin isolate in 1.5 mL 0.05 M sodium citrate buffer (pH 4.8) with CTec2 equivalent to 5 FPU g−1 LI. As a control, 1% (wv−1) LI was suspended in 1.5 mL of 0.05 M sodium citrate buffer only (pH 4.8). The experimental and control tubes were incubated for conventional enzymatic hydrolysis (at 50 °C, 150 rpm for 72 h). Subsequently, the supernatant and LI were separated by centrifugation at 14,000 rpm for 10 min.
Sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) was used to visualize enzyme-lignin isolate binding. For supernatant analysis (liquid fraction (LF)), 0.3 mL of 5X SDS sample buffer was mixed with the supernatant (approximately 1.5 mL) and the mixture was incubated at 90 °C in a heat block for 10 min. Enzyme binding with the lignin isolates (solid fraction (SF)) was studied by washing the isolates with 1.5 mL of 0.05 M sodium citrate buffer (pH 4.8) followed by centrifugation to remove the wash liquid. The LI was re-suspended in 1.5 mL of 0.05 M sodium citrate buffer (pH 4.8) and mixed with 0.3 mL of 5X SDS sample buffer. The mixture was incubated at 90 °C in the heat block for 10 min and centrifuged to obtain the liquid for SDS-PAGE analysis using Mini-Protean® TGX™ Precast Gels (Bio-Rad, Hercules, CA, USA) with 10 wells and Tris/glycine/SDS running buffer. Eight microliter protein standard ladder (Precision Plus Protein Kaleidoscope Standards, Bio-Rad, Hercules, CA, USA) and 20 µl samples (from liquid and solid fractions) were loaded into the wells on the gel. The gels were run at 120 V (constant) for approximately 60 min. Coomassie blue staining solution was applied to the gel for protein staining, followed by the application of a de-staining solution to remove the stain from the non-protein portions of the gel. Gel Documentation EQ System (Bio-RAD, Hercules, CA, USA) was used to take gel images and analyze enzyme binding with LI.
Carbohydrate hydrolysis with or without lignin isolate
Cellobiose (Sigma-Aldrich) and crystalline Avicel (Sigma-Aldrich) were individually hydrolyzed via CTec2 in the presence and absence of LI to investigate how binding of cellulolytic enzymes with the LI affects the production of glucose from these two carbohydrates. Generally, BG plays a significant role in converting cellobiose into glucose while BG, cellobiohydrolases (CBH), and endo-1,4-β-glucanases (EG) are needed to convert Avicel into glucose. Therefore, BG’s conversion efficiency with cellobiose may be explained when hydrolysis is performed with and without lignin isolates. Similarly, changes in CBH and EG conversion efficiencies can be indirectly estimated by analyzing the amount of sugar generated from Avicel in the presence and absence of lignin isolates.
In order to better understand the functionality and binding characteristics of cellulolytic enzymes, the following experimental variations were investigated during hydrolysis of cellobiose and Avicel with and without lignin isolates: (a) CTec2 at 5 and 10 FPU g−1 carbohydrates was used to study how changes in enzyme loading affect glucose production, and (b) the amounts of carbohydrate and lignin in the hydrolysate were varied to simulate various carbohydrate : lignin ratios to determine if higher lignin content affected sugar production from the two carbohydrate types (cellobiose and Avicel). The LI: carbohydrate ratios tested were 0.25% (wv−1, equivalent to 2.5 g L−1) LI:0.5% (wv−1, equivalent to 5 g L−1) carbohydrate, 0.5:0.5%, and 1.0:0.5%. Hydrolysis was performed by mixing the desired amounts of cellobiose or Avicel and LI with 1.5 mL of 0.05 M sodium citrate buffer (pH 4.8) in a microcentrifuge with a CTec2 loading of 5 and 10 FPU g−1 carbohydrate. A number of control samples were also prepared to establish baseline values for (i) blank control (just 0.05 M sodium citrate buffer), (ii) substrate control (cellulose and Avicel only), (iii) CTec2 control (5 and 10 FPU g−1 carbohydrate) and (iv) lignin isolate only. Glucose contents of the hydrolysate were determined by centrifuging and analyzing the supernatant with a YSI 2950 Biochemistry Analyzer (Xylem Inc., Yellow Springs, USA).
All experiments were performed in duplicates. The collected data were analyzed via a generalized linear model (GLM) procedure with Tukey adjustment at 95% confidence level in SAS 9.3 (Cary, NC, USA) to study the effects of pretreatment method (0.5 N, 1.0 N, 1.5 N, 1.0H, 2.0H, and 3.0H), lignin to substrate ratio (1:2, 1:1, and 2:1), and CTec2 loading (5 FPU and 10 FPU g−1) on glucose production from Cellobiose and Avicel.
Results and discussion
Chemical compositions of raw switchgrass and lignin isolates
Composition analysis for raw switchgrass and lignin isolates obtained from switchgrass pretreated by NaOH and H2SO4 at the desired conditions was performed to determine residual carbohydrates (e.g. glucan and xylan) and lignin contents (Table 1). Raw switchgrass contained 35.1% glucan, 23.4% xylan and 24.4% total lignin (AIL + ASL). Lignin in the isolates obtained from NaOH pretreated switchgrass ranged from 36.3 to 58.8%. Higher NaOH concentration resulted in higher lignin content in the isolates. Even though excessive CTec2 and HTec2 loadings were used to maximize the removal of structural carbohydrates, significant amounts of glucan and xylan remained in lignin isolates from NaOH pretreatment, especially those pretreated by 0.5% NaOH. Therefore, the lignin isolate from 0.5% NaOH pretreated switchgrass was subjected to repeat enzymatic hydrolysis at the same conditions as the first enzymatic hydrolysis to remove the remaining glucan and xylan and improve lignin content. However, no significant change (p > 0.05) in lignin content occurred (data not shown). It is inferred that the remaining glucan and xylan were resistant to any further cellulolytic enzyme action for conversion to soluble polysaccharides or monomeric sugars. Thus, these isolates were considered suitable for use in lignin-binding studies. In comparison to NaOH, lignin isolates from H2SO4 pretreatment had higher lignin contents ranging from 63.1 to 70.4%. Xylan was not detected in the isolates potentially because H2SO4 pretreatment in known to efficiently solubilize hemicellulose like xylan (Dien et al. 2006; Yang et al. 2009).
Table 1.
Composition of raw switchgrass and lignin isolate
| Material | Type of LI | Glucan (%) | Xylan (%) | Lignin (%) |
|---|---|---|---|---|
| Raw | 35.1 ± 0.51 | 23.4 ± 0.46 | 24.4 ± 0.26 | |
| Lignin isolates | 0.5N | 35.4 ± 0.11 | 9.2 ± 0.56 | 36.3 ± 2.07 |
| 1.0N | 25.5 ± 0.09 | 6.9 ± 0.75 | 50.8 ± 0.49 | |
| 1.5N | 20.4 ± 0.50 | 4.1 ± 0.95 | 58.8 ± 1.36 | |
| 1.0H | 29.2 ± 0.68 | NDa | 63.1 ± 0.32 | |
| 2.0H | 19.4 ± 1.92 | ND | 69.6 ± 2.15 | |
| 3.0H | 16.9 ± 1.04 | ND | 70.4 ± 0.73 |
aNot detected
Visualizing the impact of pretreatment conditions on the structure of switchgrass
Scanning electron microscopy (SEM) was performed to observe the visual structural changes in lignin isolates generated from switchgrass pretreated by alkali and acid at various conditions. According to SEM analysis, no significant difference was apparent in the structure of lignin isolates due to a change in the concentration of NaOH or H2SO4. Representative SEM images of raw switchgrass and lignin residues pretreated by 1% NaOH and 3% H2SO4 are presented in Fig. 1. While raw switchgrass showed a clear and intact outer core, its surface was relatively rough (Fig. 1a). Lignin isolated from 1% NaOH pretreated switchgrass showed significant disruption compared to raw switchgrass (Fig. 1b) and resulted in an elongated structure that is consistent with cellulose microfibril bundles (Boudet et al. 2003; Kumar et al. 2009). Based on the SEM image (Fig. 1b), the elongated structures may be related to glucan and the clumped substance may be related to lignin and xylan complex. Composition analysis of this lignin isolate showed 25.5% glucan, 6.9% xylan, and 50.8% lignin (Table 1). The lignin isolate from 3% H2SO4 pretreatment (Fig. 1c) predominately resembled a clumped shapeless structure which consisted 70.4% lignin and 16.9% glucan (with no xylan detected), Overall, both LI obtained from NaOH and H2SO4-pretreated switchgrass had significant structural changes with relatively higher lignin content than raw switchgrass.
Fig. 1.
SEM Images of a raw switchgrass b lignin-rich switchgrass pretreated by 1% NaOH at 121 °C for 30 min, and c lignin-rich switchgrass pretreated by 3% H2SO4 at 121 °C for 60 min (at 500 X magnification)
SDS-PAGE visualization of cellulolytic enzyme binding on lignin isolates
Lignin isolates were incubated with CTec2 cellulolytic enzyme cocktail to investigate how (if any) enzyme binding on lignin occurred. To interpret protein bands on the SDS-PAGE gel, information about the molecular weight of proteins is needed. Cellic® CTec2 (Novozymes North America, INC, USA) used in this study is a commercial enzyme blend including (1) cellulases like CBH and EG, (2) BG, and (3) hemicellulase like xylanase (XYN) (Novozymes). Based on the results presented by Zanchetta et al. (2018), Ctec2 was separated into 11 fractions based on the molecular weights (25-150 kDa). Similarly, Ko et al. (2015) noted that Ctec2 from Trichoderma reesei consists of at least eight proteins including (1) BG (EC 3.2.1.21) with additional BG, (2) at least the two main CBH (EC 3.2.1.91: Cel6A as CBHI and Cel7A as CBHII) and five kinds of EG (EC 3.2.1.4: EGI, EGII, EGIII, EGIV, and EGV), which are Cel7B, Cel5A, Cel12A, Cel61A, and Cel45A, respectively. Further, the proteins in Ctec 2 were grouped into three main fractions based on their molecular weights, namely, large, medium, and small molecular weight fragments. Therefore, for the ease of interpretation, in our research, the protein bands were divided into 4 zones on the basis of CTec2 control lane in the gel. Zone 1, covering molecular weights between 75 and 120 kDa, was identified as being related to BG which has relatively high molecular weight compared to other cellulases (Haven and Jørgensen 2013; Ko et al. 2015). Zone 2 (approximately 50 to 75 kDa) is expected to be related to CBHI (60 ~ 70 kDa), CBHII (60 ~ 70 kDa), and EGI (50 ~ 60 kDa) (Pribowo et al. 2013; Rahikainen et al. 2011). Zone 3 (approximately 30 to 50 kDa) is likely related to EGII, EGIV, XYNIII (xyn10a) and Zone 4 (approximately 20 to 30 kDa) to EGIII, EGV, XYNI (xyn11a), and XYNII (xyn11b) (Ko et al. 2015).
Electrophoresis of samples drawn from lignin isolates incubated in buffer without CTec2 showed that there were no proteins/enzymes in the liquid (LF, supernatant) and solid fractions (SF, lignin residue) regardless of lignin isolate type (Fig. 2). The absence of enzymes in the solid fraction indicated that no residual cellulolytic enzymes (from hydrolysis performed to prepare the isolates) were present on the lignin isolates. On the other hand, SDS-PAGE images of samples drawn from lignin isolates incubated with CTec2 showed several protein bands (Fig. 3).
Fig. 2.
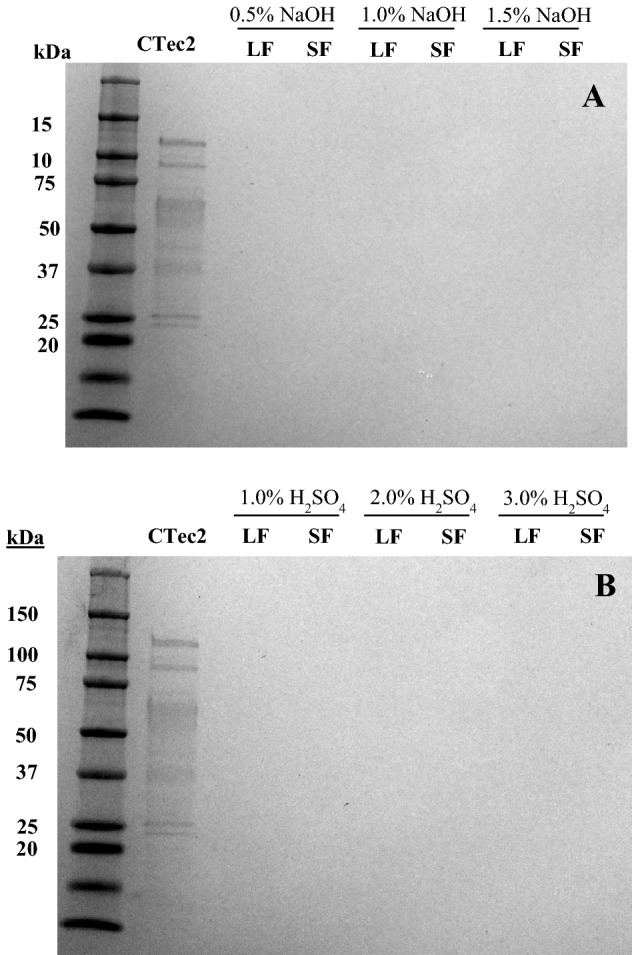
SDS-PAGE for liquid (LF) and solid fraction (SF) obtained after control hydrolysis of lignin isolated from a NaOH pretreated switchgrass and b H2SO4 pretreated switchgrass without CTec2
Fig. 3.
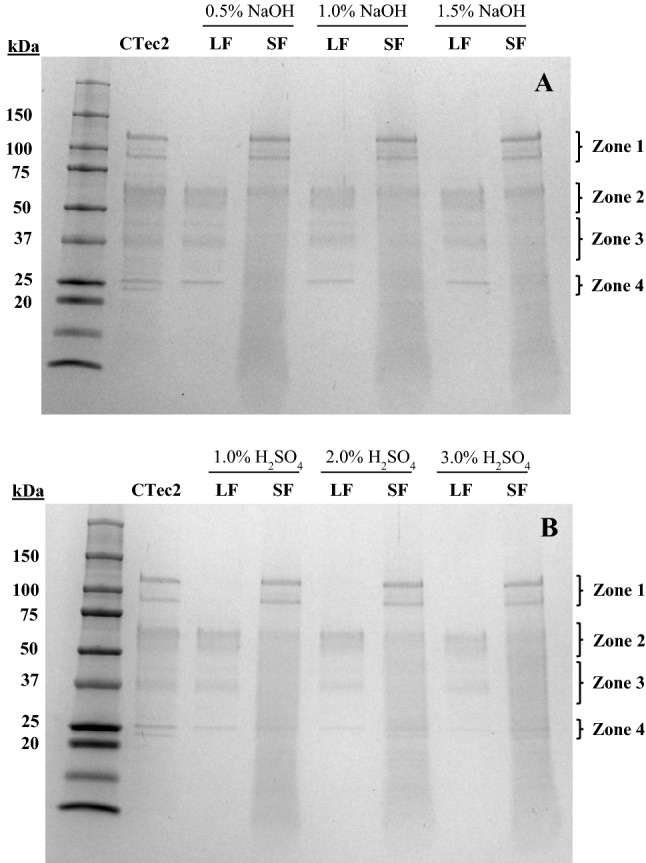
SDS-PAGE for liquid (LF) and solid fraction (SF) after hydrolysis of lignin isolated from a NaOH pretreated switchgrass and b H2SO4 pretreated switchgrass with CTec2 (5 FPU/g lignin isolate)
In Zone 1, related to BG, intense bands were seen for solid fractions of lignin isolates from both NaOH and H2SO4 pretreatments (Fig. 3a, b). The liquid fraction lanes did not seem to show BG bands regardless of chemical type and concentration. This demonstrates that BG was significantly bound to all lignin isolate solids. These results are consistent with those of Yarbrough et al. (2015) who investigated the binding of Ctec2 on lignin extracted from corn stover. Based on their results from the activities of para-nitrophenol substrates coupled with lignin adsorption studies, it was suggested that BG (> 80 kDa) and xylanses (< 30 kDa) tend to bind to lignin. Similarly, adsorption of Ctec2 and Novozyme 188 on lignin derived from sugar cane bagasse via acid and enzymatic hydrolysis as a function of temperature was studied recently (Zanchetta et al. 2018). It was reported that BG adsorbed strongly on lignin; however, the extent of adsorption depended on how lignin was extracted. Interestingly, BG appeared to prefer to bind acid-derived lignin relative to enzymatically-derived lignin. Further, the strong binding of BG on LI may also be due to the pH 4.8 in our system. It was shown that at elevated pH (5.5) lignin binding is less pronounced (Lou et al. 2013). It was suggested that pH 4.8 could have promoted an electrostatic interaction between negatively charged lignin and a positively charged BG and at higher pH BG would have acquired a negative charge resulting in a decreased interaction with lignin (Zanchetta et al. 2018). Besides, in our research, some cellulases related to CBHI, CBHII, and EGI in Zone 2 were detected on SF, with bands appearing to be more intense for NaOH derived lignin isolates than H2SO4 derived isolates. Since no clear protein bands could be seen below zone 2, enzyme binding on SF of lignin isolates in zone 3 could not be determined. The presence of bands related to zone 3 in LF lanes shows that there may have been limited enzyme-lignin binding in this zone. In zone 4, two distinguishable protein bands potentially related to some EG and XYN were observed on CTec2 control lane. However, only one upper band was detected in LF lanes (Fig. 3a) indicating that enzymes related to the lower band may be adsorbed on lignin isolates from NaOH pretreatment. In addition, higher H2SO4 concentration pretreatments seemed to draw more enzymes to the solid fraction.
Guo et al. (2014) also investigated the cellulase (from Penicillium oxalicum) adsorption on lignin from six types of lignocellulosic biomass (aspen, pine, corn, kenaf, and two Arabidopsis lines). These six lignins were extracted from aqueous dioxane (96%) solution. They found that lignin with low syringyl/guaiacyl (S/G) ratio had a high enzyme adsorption capacity, and also suggested that the amount of p-hydroxyphenyl (H) units from lignin may not affect the enzyme capacity. Based on our previous research, NaOH concentration during pretreatment affected H/G ratio (p < 0.05) and not S/G ratio (p > 0.05) (Jung et al. 2018). Nonetheless, the difference in the enzyme binding was not detected based on SDS-PAGE (Fig. 3b). As inferred by Guo et al. (2014) changes in the amount of H unit may not significantly affect CTec2 binding capacity on the lignin isolates.
Glucose production during hydrolysis of carbohydrate model compounds
Since lignin isolates were not homogenous materials containing only lignin, the carbohydrates present in the isolates had the potential to produce glucose through enzymatic hydrolysis during lignin-enzyme binding studies. Hence, a preliminary experiment focused on the hydrolysis of lignin isolates was performed with 5 and 10 FPU g−1 CTec2 to establish baseline levels of glucose, which were then subtracted from glucose generated during the model (carbohydrate) compound-lignin isolate hydrolysis (Table 2).
Table 2.
Glucose yield from glucan remaining in lignin isolates during hydrolysis
| Type of LI | (FPU g−1 LI) | Glucose yield (g g−1) | ||
|---|---|---|---|---|
| CTec2 loading | 0.25% LI | 0.5% LI | 1.0% LI | |
| 0.5 N | 5 | 0.02 ± 0.009 | 0.02 ± 0.022 | 0.01 ± 0.000 |
| 10 | 0.03 ± 0.010 | 0.02 ± 0.004 | 0.02 ± -0.003 | |
| 1.0 N | 5 | 0.07 ± 0.005 | 0.07 ± 0.001 | 0.06 ± 0.004 |
| 10 | 0.08 ± 0.004 | 0.08 ± 0.011 | 0.09 ± 0.002 | |
| 1.5 N | 5 | 0.06 ± 0.005 | 0.08 ± 0.007 | 0.08 ± 0.009 |
| 10 | 0.07 ± 0.021 | 0.08 ± 0.006 | 0.09 ± 0.011 | |
| 1.0H | 5 | 0.02 ± 0.006 | 0.02 ± 0.002 | 0.01 ± 0.003 |
| 10 | 0.03 ± 0.009 | 0.03 ± 0.017 | 0.02 ± 0.001 | |
| 2.0H | 5 | 0.02 ± 0.006 | 0.02 ± 0.002 | 0.02 ± 0.003 |
| 10 | 0.05 ± 0.003 | 0.03 ± 0.003 | 0.02 ± 0.000 | |
| 3.0H | 5 | 0.03 ± 0.003 | 0.03 ± 0.011 | 0.02 ± 0.002 |
| 10 | 0.04 ± 0.003 | 0.03 ± 0.007 | 0.04 ± 0.003 | |
Glucose yields from glucan remaining in lignin isolates from NaOH and H2SO4 pretreatments ranged from 0.01 to 0.09 g g−1 and 0.01 to 0.05 g g−1, respectively. Cellobiose and Avicel hydrolysis with and without lignin isolates was performed to determine if binding of enzymes on lignin isolates affected their conversion to glucose. Glucose yields from cellobiose (0.5% wv−1) at CTec2 loadings of 5 and 10 FPU g−1 cellobiose were 0.97 g g−1 with no significant difference between the values (Table 3). Since the role of BG is to convert cellobiose into glucose, high conversion efficiency showed that BG in 5 FPU g−1 of CTec2 was enough to produce glucose from cellobiose. On the other hand, glucose yield from Avicel was 0.30 g g−1 with a CTec2 dose of 5 FPU g−1 Avicel and increased significantly to 0.46 g g−1 when 10 FPU g−1 was employed (Table 3). Avicel is crystalline cellulose with a relatively more resistant structure than amorphous cellulose in cellobiose and needs BG, CBH, and EG for efficient conversion to glucose. Thus, it may be inferred that higher CBH and EG loading might be needed to improve glucose production from Avicel.
Table 3.
Glucose production through hydrolysis of Cellobiose and Avicel
| Sample type | Glucose (g L−1) | Glucose Yield (g g−1 carbohydrate) |
|---|---|---|
| Buffer only | 0 | – |
| Carbohydrates only | ||
| 0.5% (w/v) Cellobiose | 0.02 ± 0.007 | 0.003 ± 0.0013 |
| 0.5% (w/v) Avicel | 0 | 0 |
| CTec2 only | ||
| 5 FPU/g carbohydrates | 0.01 ± 0.005 | – |
| 10 FPU/g carbohydrates | 0.02 ± 0.007 | – |
| Cellobiose + CTec2 | ||
| 5 FPU/g carbohydrates | 5.06 ± 0.210 | 0.97 ± 0.04 |
| 10 FPU/g carbohydrates | 5.07 ± 0.170 | 0.97 ± 0.03 |
| Avicel + CTec2 | ||
| 5 FPU/g carbohydrates | 1.62 ± 0.264 | 0.30 ± 0.05 |
| 10 FPU/g carbohydrates | 2.53 ± 0.156 | 0.46 ± 0.03 |
| Lignin isolate only | ||
| 0.5, 1.0, 1.5% NaOH | 0 | – |
| 1, 2, 3% H2SO4 | 0 | – |
Includes data from controls
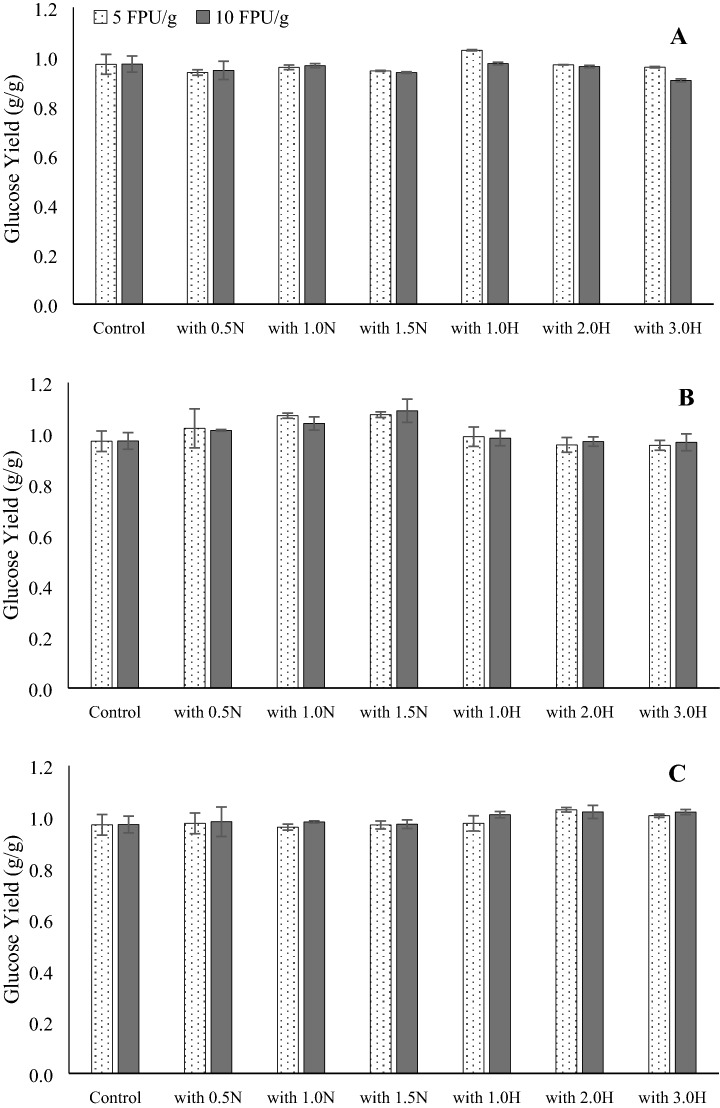
Interestingly, the glucose yields from the hydrolysis of cellobiose in the presence of various lignin isolates were not significantly different (p > 0.05) from those obtained from cellobiose only hydrolysis (Fig. 4). In addition, the type of pretreatment agent, its concentrations, and the relative proportion of lignin within the cellobiose-lignin mixture did not substantially affect the glucose yields. Somewhat similar results were also reported by Zhang et al. (2017), who investigated the effects of five types of lignins (derived from sugarcane bagasse, softwood, and hardwood) on enzyme hydrolysis efficiencies of cellulose. Out of five types of lignin tested, lignin isolated via organosolv and sulfite pulping did not exhibit any inhibition while lignin derived from CO2 pretreatment and soda pulping were found to inhibit hydrolysis of cellulose under similar conditions. Based on the reports in the literature, lignin can theoretically impede the hydrolysis processes either by serving as a non-productive active site for the enzyme or via steric hindrance due to physical blockade of cellulose by lignin or chemical inhibition of enzyme via lignin-derived products (dos Santos et al. 2018; Vermaas et al. 2015). However, experimental results indicate that the actual lignin-induced inhibition depends on lignin type, its origin, chemistry, and the method of isolation. In their report, Li et al. (2018) found that the inhibition of lignin was inversely correlated with the molecular weight and concentration of carboxylic moieties while the presence of hydroxyl groups promoted inhibition, similar to the observations reported by Rahikainen et al. (2013a, b) and Sun et al. (2016).
Fig. 4.
Glucose production through hydrolysis of cellobiose and lignin isolates from various pretreatments mixed as a 0.25% LI:0.5% cellobiose, b 0.5% LI:0.5% cellobiose, and c 1.0% LI:0.5% cellobiose
In addition, the presence of lignin may not always result in the inhibition of hydrolysis (Saini et al. 2016). This is especially true during longer hydrolysis periods (Li et al. 2014). The authors observed a reduction in hydrolysis (29.3–39.7% for low and high enzyme loadings) during the early stages of enzymatic hydrolysis of Avicel-impregnated lignin. However, after 72 h, the inhibition was limited to only 6% (low enzyme loading) or 0%-no inhibition (high enzyme loading) suggesting that non-specific binding of enzymes with lignin does not always inhibit enzymatic hydrolysis. In our research, despite the binding of BG to lignin, no inhibition of hydrolysis was observed. These results are consistent with those of Haven and Jorgensen (2013) who also noted that BG was active despite bonded to lignin. Further, as reported by Kumar et al. (2012), the inhibitory effect of lignin binding was relevant only at lower enzyme concentrations. Thus, the low lignin loading (0.25 and 1%) coupled with longer (72 h) hydrolysis time may have overcome the inhibitory effects of lignin. Similar observations were reported by Kumar et al. (2012), who investigated hydrolysis of steam pretreated softwoods. Their results indicated that when higher hydrolysis time (48 h) was coupled with relatively higher enzyme loading (10 FPU g−1 cellulose), the effects of lignin inhibition was not significant.
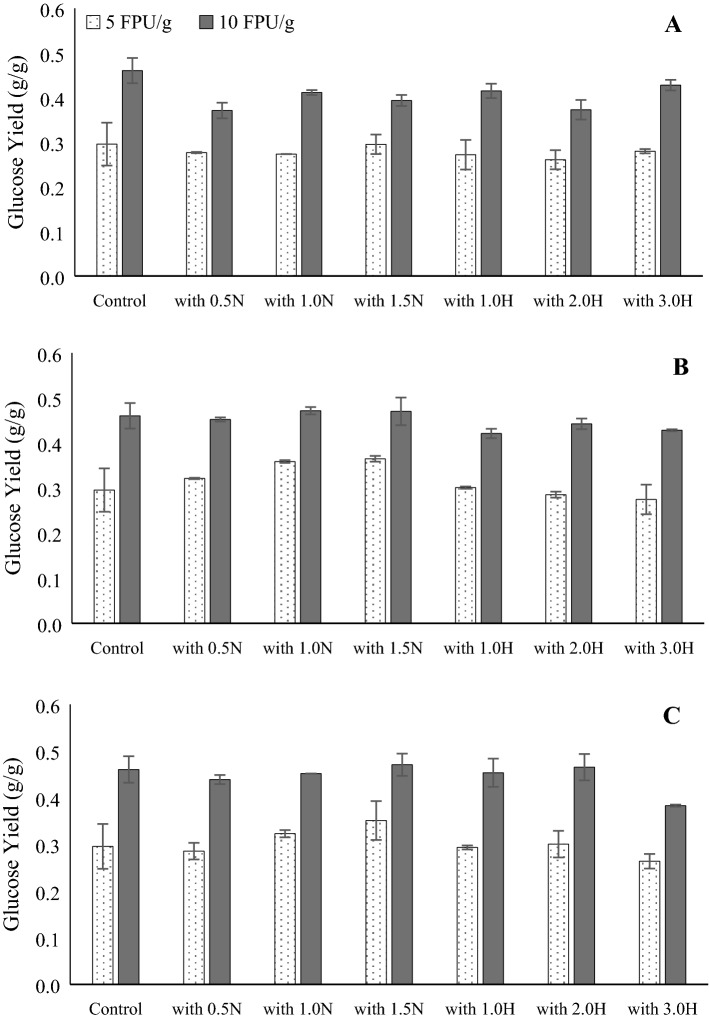
On the other hand, hydrolysis of Avicel in the presence of various lignin isolates at conditions similar to those for cellobiose hydrolysis showed that a CTec2 loading of 10 FPU g−1 generated significantly (p < 0.05) more glucose than 5 FPU g−1 (Fig. 5).
Fig. 5.
Glucose production through hydrolysis of Avicel and lignin isolates from various pretreatments mixed as a 0.25% LI:0.5% Avicel, b 0.5% LI:0.5% Avicel, and c 1.0% LI:0.5% Avicel (All treatments significantly different except for 1.5 N in (c))
As suggested by Kumar et al. (2012) and Zhang et al. (2017), increased enzyme loading may have provided an increased number of active sites for hydrolysis resulting in higher hydrolysis yields. Nonetheless, the presence of lignin isolate as well as its type and amount did not have a significant effect on glucose yield (p > 0.05). As with cellobiose hydrolysis, glucose production during Avicel hydrolysis did not change even though cellulase related to CBH and EG seemed to be partially bound to the lignin isolates (Fig. 3). This may be attributed to longer hydrolysis time, which may have overcome the inhibition due to lignin caused by binding (Li et al. 2014). In addition, the effects of lignin binding are perhaps insignificant when the substrate offers high accessibility for enzyme interaction (Kumar et al. 2012). Overall, our results suggest that the binding of lignin to the enzyme did not play any significant role in the inhibition of hydrolysis of cellobiose and Avicel after 72 h of hydrolysis, even at low enzyme loadings (5 and 10 FPU g−1).
Conclusions
The amount of lignin in isolates derived from NaOH and H2SO4 pretreated switchgrass increased with an increase in chemical concentration. Though BG was observed to be significantly bound to lignin isolates from NaOH and H2SO4 pretreated switchgrass, it exhibited sufficient activity during carbohydrate hydrolysis. Some cellulases related to CBH and EG also showed a tendency to bind to lignin isolates. The addition of lignin isolates at various levels during hydrolysis of carbohydrates model compounds (cellobiose and Avicel) did not impact glucose production by CTec2 though more glucose was produced from Avicel hydrolyzed at higher enzyme loading. Overall, the binding of enzymes in the cellulolytic enzyme cocktail on lignin isolates did not negatively impact glucose production. It might be possible that other cellulases bound to the lignin isolates can remain active like BG. Also, cellulases in the liquid fraction (not bound to the lignin isolate) may have catalytic activity which is sufficient for Avicel hydrolysis.
Acknowledgements
The authors thank Drs. Rongda Qu (NC State University Crop Science) and Dhanalekshmi Savithri (NC State University Department of Forest Biomaterials) for help with sample analyses.
Compliance with ethical standards
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Contributor Information
Ratna Sharma-Shivappa, Phone: +1-919-513-9797, Email: ratna_sharma@ncsu.edu.
Praveen Kolar, Email: pkolar@ncsu.edu.
References
- Adney B, Baker J (1996) Measurement of cellulase activities. NREL Lab Anal Proced NREL/TP-510-42628
- Berlin A, Balakshin M, Gilkes N, Kadla J, Maximenko V, Kubo S, Saddler J. Inhibition of cellulase, xylanase and β-glucosidase activities by softwood lignin preparations. J Biotechnol. 2006;125:198–209. doi: 10.1016/j.jbiotec.2006.02.021. [DOI] [PubMed] [Google Scholar]
- Boudet AM, Kajita S, Grima-Pettenati J, Goffner D. Lignins and lignocellulosics: a better control of synthesis for new and improved uses. Trends Plant Sci. 2003;8:576–581. doi: 10.1016/j.tplants.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Dien BS, Jung HG, Vogel KP, Casler MD, Lamb JF, Iten L, Mitchell RB, Sarath G. Chemical composition and response to dilute-acid pretreatment and enzymatic saccharification of alfalfa, reed canarygrass, and switchgrass. Biomass Bioenergy. 2006;30:880–891. doi: 10.1016/j.biombioe.2006.02.004. [DOI] [Google Scholar]
- dos Santos AC, Ximenes E, Kim Y, Ladisch MR. Lignin–enzyme interactions in the hydrolysis of lignocellulosic biomass. Trends Biotechnol. 2018;37:518–531. doi: 10.1016/j.tibtech.2018.10.010. [DOI] [PubMed] [Google Scholar]
- Guo F, Shi W, Sun W, Li X, Wang F, Zhao J, Qu Y. Differences in the adsorption of enzymes onto lignins from diverse types of lignocellulosic biomass and the underlying mechanism. Biotechnol Biofuels. 2014;7:38. doi: 10.1186/1754-6834-7-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haven MØ, Jørgensen H. Adsorption of β-glucosidases in two commercial preparations onto pretreated biomass and lignin. Biotechnol Biofuels. 2013;6:165. doi: 10.1186/1754-6834-6-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiss-Blanquet S, Zheng D, Ferreira NL, Lapierre C, Baumberger S. Effect of pretreatment and enzymatic hydrolysis of wheat straw on cell wall composition, hydrophobicity and cellulase adsorption. Bioresour Technol. 2011;102:5938–5946. doi: 10.1016/j.biortech.2011.03.011. [DOI] [PubMed] [Google Scholar]
- Jung W, Savithri D, Sharma-Shivappa R, Kolar P. Changes in lignin chemistry of Switchgrass due to delignification by sodium hydroxide pretreatment. Energies. 2018;11:376. doi: 10.3390/en11020376. [DOI] [Google Scholar]
- Kellock M, Rahikainen J, Marjamaa K, Kruus K. Lignin-derived inhibition of monocomponent cellulases and a xylanase in the hydrolysis of lignocellulosics. Bioresour Technol. 2017;232:183–191. doi: 10.1016/j.biortech.2017.01.072. [DOI] [PubMed] [Google Scholar]
- Kim JS, Lee YY, Kim TH. A review on alkaline pretreatment technology for bioconversion of lignocellulosic biomass. Bioresour Technol. 2016;199:42–48. doi: 10.1016/j.biortech.2015.08.085. [DOI] [PubMed] [Google Scholar]
- Ko JK, Ximenes E, Kim Y, Ladisch MR. Adsorption of enzyme onto lignins of liquid hot water pretreated hardwoods. Biotechnol Bioeng. 2015;112:447–456. doi: 10.1002/bit.25359. [DOI] [PubMed] [Google Scholar]
- Kumar P, Barrett DM, Delwiche MJ, Stroeve P. Methods for pretreatment of lignocellulosic biomass for efficient hydrolysis and biofuel production. Ind Eng Chem Res. 2009;48:3713–3729. doi: 10.1021/ie801542g. [DOI] [Google Scholar]
- Kumar L, Arantes V, Chandra R, Saddler J. The lignin present in steam pretreated softwood binds enzymes and limits cellulose accessibility. Bioresour Technol. 2012;103:201–208. doi: 10.1016/j.biortech.2011.09.091. [DOI] [PubMed] [Google Scholar]
- Li X, Zheng Y. Lignin-enzyme interaction: mechanism, mitigation approach, modeling, and research prospects. Biotechnol Adv. 2017;35:466–489. doi: 10.1016/j.biotechadv.2017.03.010. [DOI] [PubMed] [Google Scholar]
- Li H, Pu Y, Kumar R, Ragauskas AJ, Wyman CE. Investigation of lignin deposition on cellulose during hydrothermal pretreatment, its effect on cellulose hydrolysis, and underlying mechanisms. Biotechnol Bioeng. 2014;111:485–492. doi: 10.1002/bit.25108. [DOI] [PubMed] [Google Scholar]
- Li X, Li M, Pu Y, Ragauskas AJ, Klett AS, Thies M, Zheng Y. Inhibitory effects of lignin on enzymatic hydrolysis: the role of lignin chemistry and molecular weight. Renew Energy. 2018;123:664–674. doi: 10.1016/j.renene.2018.02.079. [DOI] [Google Scholar]
- Lou H, Zhu JY, Lan TQ, Lai H, Qiu X. pH-Induced lignin surface modification to reduce nonspecific cellulase binding and enhance enzymatic saccharification of lignocelluloses. Chemsuschem. 2013;6:919–927. doi: 10.1002/cssc.201200859. [DOI] [PubMed] [Google Scholar]
- Pareek N, Gillgren T, Jönsson LJ. Adsorption of proteins involved in hydrolysis of lignocellulose on lignins and hemicelluloses. Bioresour Technol. 2013;148:70–77. doi: 10.1016/j.biortech.2013.08.121. [DOI] [PubMed] [Google Scholar]
- Ponnusamy VK, Nguyen DD, Dharmaraja J, Shobana S, Banu JR, Saratale RG, Chang SW, Kumar G. A review on lignin structure, pretreatments, fermentation reactions and biorefinery potential. Bioresour Technol. 2019;271:462–472. doi: 10.1016/j.biortech.2018.09.070. [DOI] [PubMed] [Google Scholar]
- Pribowo AY, Hu J, Arantes V, Saddler JN. The development and use of an ELISA-based method to follow the distribution of cellulase monocomponents during the hydrolysis of pretreated corn stover. Biotechnol Biofuels. 2013;6:80. doi: 10.1186/1754-6834-6-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahikainen J, Mikander S, Marjamaa K, Tamminen T, Lappas A, Viikari L, Kruus K. Inhibition of enzymatic hydrolysis by residual lignins from softwood—study of enzyme binding and inactivation on lignin-rich surface. Biotechnol Bioeng. 2011;108:2823–2834. doi: 10.1002/bit.23242. [DOI] [PubMed] [Google Scholar]
- Rahikainen JL, Evans JD, Mikander S, Kalliola A, Puranen T, Tamminen T, Marjamaa K, Kruus K. Cellulase–lignin interactions—the role of carbohydrate-binding module and pH in non-productive binding. Enzyme Microb Technol. 2013;53:315–321. doi: 10.1016/j.enzmictec.2013.07.003. [DOI] [PubMed] [Google Scholar]
- Rahikainen JL, Moilanen U, Nurmi-Rantala S, Lappas A, Koivula A, Viikari L, Kruus K. Effect of temperature on lignin-derived inhibition studied with three structurally different cellobiohydrolases. Bioresour Technol. 2013;146:118–125. doi: 10.1016/j.biortech.2013.07.069. [DOI] [PubMed] [Google Scholar]
- Saini JK, Patel AK, Adsul M, Singhania RR. Cellulase adsorption on lignin: a roadblock for economic hydrolysis of biomass. Renew Energy. 2016;98:29–42. doi: 10.1016/j.renene.2016.03.089. [DOI] [Google Scholar]
- Sluiter A, Hames B, Ruiz R, Scarlata C, Sluiter J, Templeton D, Crocker D (2012) Determination of structural carbohydrates and lignin in biomass. NREL Lab Anal Proced (NREL/TP-510-42618)
- Sun S, Huang Y, Sun R, Tu M. The strong association of condensed phenolic moieties in isolated lignins with their inhibition of enzymatic hydrolysis. Green Chem. 2016;18:4276–4286. doi: 10.1039/C6GC00685J. [DOI] [Google Scholar]
- Tamminen TL, Hortling BR (1999) Isolation and characterization of residual lignin. In: Argyropoulos DS (ed) Advances in lignocellulosics characterization. Atlanta, pp 1–41
- Vermaas JV, Petridis L, Qi X, Schulz R, Lindner B, Smith JC. Mechanism of lignin inhibition of enzymatic biomass deconstruction. Biotechnol Biofuels. 2015;8:217. doi: 10.1186/s13068-015-0379-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novozymes CELLIC Ctec and Htec2—Enzymes for Hydrolysis of Lignocellulosic Materials:01
- Yang Y, Sharma-Shivappa R, Burns JC, Cheng JJ. Dilute acid pretreatment of oven-dried switchgrass germplasms for bioethanol production. Energy Fuels. 2009;23:3759–3766. doi: 10.1021/ef900043z. [DOI] [Google Scholar]
- Yarbrough JM, Mittal A, Mansfield E, Taylor LE, Hobdey SE, Sammond DW, Bomble YJ, Crowley MF, Decker SR, Himmel ME. New perspective on glycoside hydrolase binding to lignin from pretreated corn stover. Biotechnol Biofuels. 2015;8:214. doi: 10.1186/s13068-015-0397-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabed H, Sahu JN, Boyce AN, Faruq G. Fuel ethanol production from lignocellulosic biomass: an overview on feedstocks and technological approaches. Renew Sustain Energy Rev. 2016;66:751–774. doi: 10.1016/j.rser.2016.08.038. [DOI] [Google Scholar]
- Zanchetta A, dos Santos ACF, Ximenes E, Nunes CDCC, Boscolo M, Gomes E, Ladisch MR. Temperature dependent cellulase adsorption on lignin from sugarcane bagasse. Bioresour Technol. 2018;252:143–149. doi: 10.1016/j.biortech.2017.12.061. [DOI] [PubMed] [Google Scholar]
- Zhang H, Wu S, Xie J. Evaluation of the effects of isolated lignin on enzymatic hydrolysis of cellulose. Enzyme Microb Technol. 2017;101:44–50. doi: 10.1016/j.enzmictec.2017.03.001. [DOI] [PubMed] [Google Scholar]