Abstract
Seleno-short-chain chitosan (SSCC) is a derivative of chitosan. In the present study, we sought to investigate the underlying antitumor mechanism of SSCC on human gastric cancer BGC-823 cells in vitro. MTT assay suggested that SSCC exhibited a dose-dependent inhibitory effect on the proliferation of BGC-823 cells. We found the SSCC-treated cells showed typical morphological characteristics of apoptosis in a dose dependent manner by observing on microscope. Annexin V-FITC/PI double staining and cell cycle assay identified that SSCC could induce BGC-823 cells apoptosis by triggering G2/M phase arrest. Our research provided the first evidence that SSCC could effectively induce the apoptosis of BGC-823 cells via an intrinsic mitochondrial pathway, as indicated by inducing the disruption of mitochondrial membrane potential (MMP), the excessive accumulation of reactive oxidative species (ROS), the increase of Bax/Bcl-2 ratio and the activation of caspase 3, caspase 9 and cytochrome C (Cyt-C) in BGC-823 cells. These combined results clearly indicated that SSCC could induce BGC-823 cells apoptosis by the involvement of mitochondrial signaling pathway, which provided precise experimental evidence for SSCC as a potential agent in the prevention and treatment of human gastric cancer.
Keywords: SSCC, Human gastric cancer, Cell apoptosis, Mitochondrial pathway
Introduction
Cancer is a leading cause of death in the world and its incidence rates are high in many Asian countries including China (Liu et al. 2015; Nagappan et al. 2012). Unfortunately, because of the malignant properties of cancer, especially metastasis, it is difficult to find effective treatments. Nowadays, the most common way to treat cancer is the combination of chemotherapy, radiation therapy and surgical intervention (Fan et al. 2017). However, the clinical treatment benefits remain modest with many problems such as hepatotoxicity and drug resistance in current therapeutics. So it urges researchers to develop new treatment strategy without side effects and find alternative treatments (Xie et al. 2016).
As an ingredient in many multivitamins and dietary supplements, selenium (Se) plays an important role in maintaining the health of living organisms. In the meanwhile, as the active center of diverse enzymes in the human body, Se has crucial effects in the disease resistance (Jia et al. 2015). In recent studies, Se compounds had shown higher selectivity and sensitivity in malignant cells and possessed antitumor and immunomodulating activities (Gandin et al. 2018). For example, Endo et al. (2017) indicated that selenite exhibited significant antitumor effects on HSC-3 cells. Cheng et al. (2018a) demonstrated that Se-containing tea polysaccharides could effectively inhibit the tumor growth in a dose-dependent manner. These researches showed Se compounds might have the potency of chemotherapeutic agents (Sinha and Ei-Bayoumy 2004). Recently, we paid much attention to Selenium-polysaccharides, especially their chemically synthesized derivatives, because of the various bioactivities such as cancer-therapeutic benefit, antibacterial, antioxidant and hypolipidemic activities (Wang et al. 2013; Lü et al. 2014; Liu et al. 2013). Chitosan, a natural cationic polymer and important derivative of chitin, is easily extracted from the exoskeletons of shrimps or crabs. For its compatibility, degradability and relative non-toxicity (Laskar et al. 2017; Bano et al. 2017), chitosan is widely accepted as a potential antitumor agent. Therefore, chemicals composed of selenium and chitosan would be a potential substance for the prevention and treatment of cancer. Based on the above theoretical research, our laboratory synthesized a new selenium compound called seleno-short-chain chitosan (SSCC).
Apoptosis is a well-characterised process of cell death presenting unique morphological and biochemical features (Ma et al. 2018). Apoptotic cells are distinguished by blebbing, cell shrinking, nuclear fragmentation, chromatin condensation and nuclear fragmentation (Redza-Dutordoir and Averill-Bates 2016; Ma et al. 2018). Most of the chemotherapeutic drugs kill tumor cells by inducing apoptotic signaling pathways (Lee and Hong 2010). There are two classic signaling pathways related to apoptosis: a pathway mediated by death receptor (the extrinsic pathway) and a pathway mediated by mitochondrion (the intrinsic pathway) (Li et al. 2018). The mitochondrial pathway plays a central role in the induction of apoptosis, accompanied by many biological activities such as the loss of mitochondrial membrane potential, the generation of ROS and the changed expression of apoptosis-related proteins (Liu et al. 2018; Tao et al. 2018). Our previous studies indicated that SSCC was a synthesized chitosan by binding more stable selenic acid groups (–SeO3–) to –OH or –NH2 groups with the molecular weight distribution of 41 kDa. The related research has demonstrated that SSCC could induce apoptosis of human erythrocyte leukemia K562 cells and lung cancer A549 cells in vitro (Liu et al. 2008; Zhao et al. 2017). However, it is still undiscovered whether SSCC has an antitumor activity on human gastric cancer BGC-823 cells in vitro. Therefore, to further confirm the anti-proliferative activity of SSCC on BGC-823 cells, the present study investigated the effects of SSCC in some ways, such as the growth inhibition, cell cycle arrest and apoptosis induction of human gastric cancer BGC-823 cells and revealed a potential apoptosis signal pathway to elaborate on SSCC-induced antitumor molecular mechanisms.
Materials and methods
Reagents
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) and dimethyl sulfoxide (DMSO) were purchased from Sigma Chemical Co. (St. Louis, MO, USA). RPMI-1640 Medium and fetal bovine serum (FBS) were provided by GIBCO (Carlsbad, CA, USA). AnnexinV-FITC/PI apoptosis detection kit was obtained from Keygen Biotech (Nanjing, China). RT-PCR Kit and SYBR Premix Ex Taq TM II Kit were purchased from Takara Bio Co. (Dalian, China). Propidium Iodide(PI), Reactive oxygen species (ROS), Rhodamin 123 assay kit, Bradford Protein Assay Kit, cytosolic protein lysis buffer, Enhanced chemiluminescence (ECL) kit were obtained from Solarbio Science &Technology Co, Ltd (Beijing, China). The antibodies specific to β-actin, Bax, Bcl-2, Cytochrome C (Cyt-C), Caspase-3, and Caspase-9 were provided by Tianjin Sungene Biotech Co. (Tianjin, China). The other chemical regents were of analytical grade.
Cell culture
The human gastric carcinoma cell lines were purchased from Tianjin Medical University and cultured in RPMI-1640 Medium with 10% heat inactivated FBS, 100 U/mL penicillin and 100 μg/mL streptomycin. The human gastric mucosal cells GES-1 were provided by American type culture collection (ATCC). The cells were maintained at 37 °C in a humidified incubator containing 5% CO2 with 95% humidity. One day after growing, the cells were treated with SSCC in various concentrations.
Preparation of SSCC
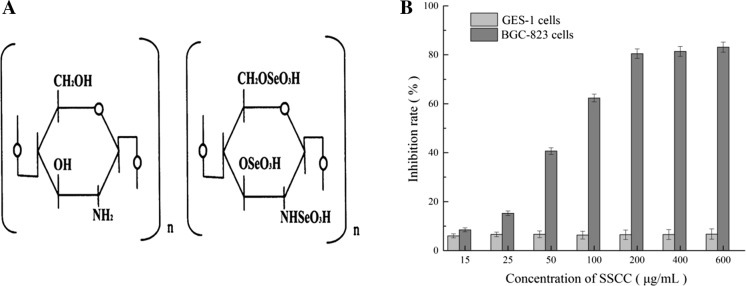
Seleno-short-chain chitosan (SSCC) was synthesized as described previously (Liu et al. 2008). Briefly, the chitosan solution (1%, in 0.5% acetic acid solution) was degraded with 3% hydrogen peroxide (H2O2) at room temperature. Then the supernatants of chitosan were harvested under stirring (200 rpm/4 g) for 12 h. Next, SeO2 (1:10 w/v) was added to the chitosan solution, and the reaction was completed in 3 h. After the synthesis, the mixture was dialyzed (using 5 kDa cut-off dialysis membrane, Sigma Chemical) for 24 h at room temperature and precipitated by ethanol to get SSCC (Patent Number CN1600793A). The chemical structure of SSCC was showed in Fig. 1a. When co-cultured with cells, SSCC would be dissolved in the RPMI-1640 medium and diluted to different concentrations.
Fig. 1.
Growth inhibitory effect of SSCC on GES-1 cells and BGC-823 cells. a Chemical structure of chitosan and seleno-shortchain chitosan (SSCC). b Cells were cultured in 96-well plate and treated with different doses of SSCC for 24 h. The cells inhibition rate was analyzed by MTT assay
MTT assay
The BGC-823 and GES-1 cells were placed in 96-well flat-bottomed plates at a final density of 2.5 × 104 cells/well overnight and treated with different concentrations of SSCC (10–600 μg/mL) for 24 h. At the end of the incubation period, the cells were exposed to 20 μL MTT (5 mg/mL) and the resulting formazan crystals were dissolved in 150 μL DMSO. Finally, the absorbance was measured at 570 nm using an ELISA reader (Model 680; BioRad, Hercules, CA, USA). Inhibition rate (%) = (a − b)/a × 100, where a and b stand for the absorbance of the untreated and experimental groups, respectively (Zhang et al. 2017b).
Cell morphological observation
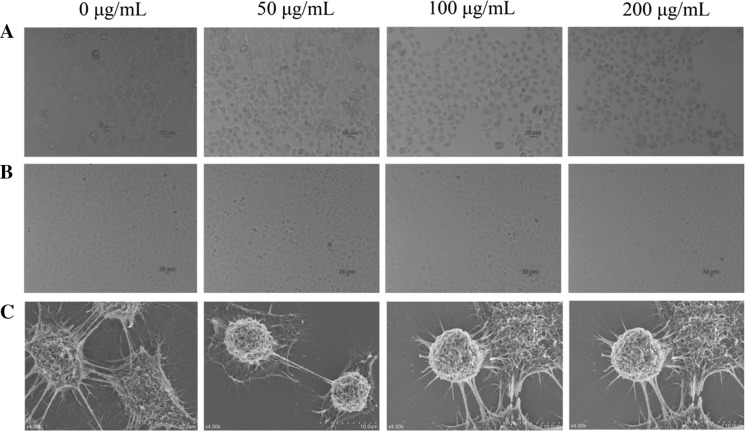
After incubation for 24 h, the BGC-823 cells were cultivated with different concentrations of SSCC (0, 50, 100 and 200 μg/mL) for another 24 h. Then the medium was removed and the cells were fixed with 4% paraformaldehyde for 10 min after washed three times with PBS. Finally, the morphological changes of BGC-823 cells were observed and photographed under an inverted light microscope (Nikon, Tokyo, Japan).
Cell apoptosis observation by scanning electron microscope
After incubation in 6-well plates for 24 h, the BGC-823 cells were cultivated with different concentrations of SSCC (0, 50, 100 and 200 μg/mL) for another 24 h. The cells were centrifuged for 5 min at 1300 rpm and removed the supernatant. This process was repeated for three times. After the step, the cells were maintained with 3% (v/v) glutaraldehyde solution for 3 h in order to fix the morphology and then centrifuged for 5 min by 1300 rpm. The specimens were dehydrated with gradient ethanol alcohol of 30%, 50%, 70%, 80%, 90%, 95% (v/v), respectively. Finally, the samples dried by carbon dioxide critical point and coated with gold using a metal ions spattering instrument. The surface morphology was examined by a scanning electron microscope (Hitachi, Japan).
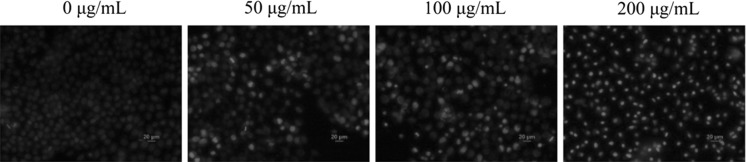
Hoechst 33258 staining
In order to observe the apoptosis in BGC-823 cells, 3 mL cells (1 × 105 cells/mL) were seeded in 6-well plates and treated with different concentrations of SSCC (0, 50, 100 and 200 μg/mL). After incubated for 24 h, the cells were harvested, washed with 500 μL PBS and fixed with freshly prepared 4% paraformaldehyde for about 15 min. Later cells were washed with 500 μL of PBS three times, stained with freshly prepared 500 μL Hoechst 33258 solution (10 μg/mL) and incubated at room temperature for 10 min. Finally, the cells were observed under an inverted fluorescence microscope.
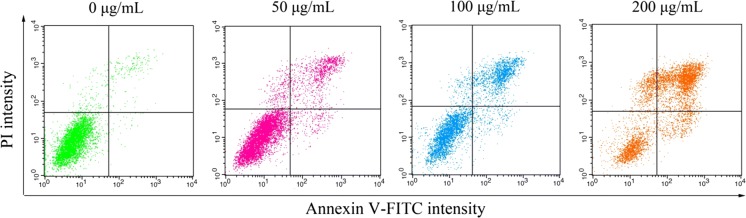
AnnexinV-FITC/PI staining assay
This assay was performed as described previously (Perumalsamy et al. 2017) and the Annexin V-FITC apoptosis detection kit (Keygen Biotech, Nanjing, China) was used according to the manufacturer’s manual. Briefly, after treated with SSCC (0, 50, 100 and 200 μg/mL) for 24 h, the BGC-823 cells were harvested, washed with PBS and incubated with the solution containing 500 μL binding buffer, 5 μL AnnexinV-FITC and 5 μL PI at room temperature in darkness for 10 min. Stained cells were immediately detected by a flow cytometry (BD FACSCalibur, San Jose, CA, USA).
Cell cycle analysis
BGC-823 cell cycle phase distribution was investigated by the published method (Xiao et al. 2017) with a little difference. Briefly, BGC-823 cells (1 × 106 cells/well) were seeded in 6-well plates and incubated with different concentrations of SSCC (0, 50, 100 and 200 μg/mL) for 24 h. Subsequently, the cells were harvested via trypsinisation, centrifuged at 1300 rpm for 5 min, washed twice with PBS, and then fixed with 70% cold ethanol (stored at -20 °C overnight. Subsequently, the fixed cells were washed with PBS three times, exposed to 50 μg/mL RNase A at 37 °C for 30 min and 50 μg/mL PI dye at 4 °C for 10 min in dark. The results were obtained by flow cytometer (BD FACSC alibur, San Jose, CA, USA) equipped with a flow cytometry software and analyzed by the ModFit LT software.
Measurement of MMP
A lot of protons out of the inner membrane caused transmembrane potential, which can be estimated by the uptake of the cationic fluorescent dye Rhodamine 123 (Rh-123) (Song et al. 2012). In brief, after incubated with SSCC (0, 50, 100, and 200 μg/mL), the cells were harvested, washed with PBS and re-suspended in ultra-pure water containing 5 mg/mL Rh-123 at 37 °C in a 5% CO2 incubator for 30 min in the dark. After incubation, the cells were gently washed three times with PBS, re-suspended with 500 μL PBS and detected by flow cytometry.
Production of intracellular ROS assay
The production of intracellular ROS was estimated using a ROS assay kit (Solarbio). After incubation in 6-well plates for 24 h, BGC-823 cells were incubated with SSCC (0, 50, 100, and 200 μg/mL) for another 24 h. Then the cells were collected and incubated with PBS containing 10 μM 2′,7′-dichlorodihydrofluorescein-diacetate (DCFH-DA) at 37 °C for 30 min in the dark. Finally, the cells were analyzed by flow cytometry.
Western blotting
Western blotting assay was performed to detect the expression of mitochondrial apoptotic proteins. Briefly, BGC-823 cells were treated with SSCC (0, 50, 100 and 200 μg/mL) for 24 h at 37 °C. Then the cells were collected and washed twice with cold PBS before lysed in lysis buffer with fresh-added protease inhibitor. The lysate was placed on ice for 30 min, followed by centrifugation (12,000 rpm, 5 min) at 4 °C. The supernatant fraction was collected and the concentration of proteins from the supernatant was detected using BCA protein assay kit (Solarbio). Then, the supernatants were separated on 12% sodium dodecyl sulfate polyacrylamide (SDS-PAGE) gel and electrically transferred onto polyvinylidene difluoride (PVDF) membrane (Millipore, USA) in transfer buffer. After incubation with 5% bovine serum albumin for 2 h at room temperature, the membranes were subsequently immunoblotted with the primary antibodies at 4 °C overnight. Subsequently, the membranes were washed three times with 0.1% Tween-20 in TBS and incubated with the corresponding horseradish peroxidase (HRP)-conjugated secondary antibodies for 1.5 h at room temperature on the shaker. Finally, the protein bands were processed with the chemiluminescence (ECL) reagent according to the instructions and exposed to X-ray films in a darkroom. The relative density of the immunoreactive bands was visualized with Quantity One software and the relative amounts of proteins in each lane were obtained after normalization with the β-actin values in the same lane.
Real-time quantitative PCR (qRT-PCR)
The mRNA levels of Bax and Bcl-2 were detected by real-time PCR in order to explore apoptosis mechanism. BGC-823 cells were treated with SSCC (0, 50, 100, and 200 μg/mL) for 24 h at 37 °C, and total RNA was extracted using Trizol according to the protocol supplied with the reagent. The RNA concentration was obtained using BioPhotometer plus of Eppendorf Company (Hamburg, Germany) and the cDNA was transcribed from 2 μg RNA by using Bio-rad MyCycler Thermal Cycler according to the procedure provided by RT-PCR Kit (TaKaRa, Dalian, China). The qRT-PCR analysis was performed using qTOWER 2.2 of Analytik Jena Company (Jena, Germany) according to SYBR Premix Ex Taq TM II Kit (Takara, Dalian, China). β-actin was used as the internal control. The primer sequences were summarized in Table 1. The quantitative results were evaluated by the 2-DDCt method.
Table 1.
List of primers for real time PCR
| Gene name | Primer sequences (5′-3′) | Orientation |
|---|---|---|
| β-actin | CATGTACGTTGCTATCCAGGC CTCCTTAATGTCACGCACGAT |
Forward Reverse |
| Bax | CCCGAGAGGTCTTTTTCCGAG CCAGCCCATGATGGTTCTGAT |
Forward Reverse |
| Bcl-2 | GGTGGGGTCATGTGTGTGG CGGTTCAGGTACTCAGTCATCC |
Forward Reverse |
Statistical analysis
Statistical analyses were performed using SPSS version 19.0 (IBM SPSS, Armonk, Ny, USA). Statistical significance of the different groups was estimated by Student’s t-test and all the data were presented as the mean ± SD. P < 0.05 was considered to be significant.
Results
Inhibitory effect of SSCC on BGC-823 cells
To access the inhibition effects of SSCC on human gastric cancer cells, different concentrations of SSCC (15–600 μg/mL) were seeded into BGC-823 cells for 24 h, conducted by MTT assay. As shown in Fig. 1b, the cell viability of BGC-823 cells was decreased obviously in a SSCC dose dependent manner. After treatment with 100 μg/mL SSCC for 24 h, the cell inhibition rate of BGC-823 cells were increased by nearly 60%. Notably, when the dose of SSCC was 200 μg/mL, the inhibitory effect of SSCC on BGC-823 cells arrived at the platform stage. Furthermore, the results suggested that SSCC had no influence upon the proliferation of human gastric mucosal cells GES-1 which indicated that SSCC had less cytotoxicity on organisms.
Effect of SSCC on cell morphology
The morphological changes of BGC-823 cells after exposure to SSCC for 24 h were displayed in Fig. 2a, c. The results suggested that the experimental cells showed typical morphological characteristics of apoptosis in a dose-dependent manner, such as a reduction in cell density, gradual disintegration of the nuclear membrane, detachment of cells, diminished cell volume and marked shape changes. However, as shown in Fig. 2b, after treatment with SSCC, no obvious apoptotic characteristics were observed in the GES-1 cells. The results were consistent with MTT data, which further confirmed that SSCC had no obvious toxic and side effects on normal cells.
Fig. 2.
Effects of SSCC on cell morphology. a BGC-823 cells were exposed to different concentrations of SSCC (0, 50, 100 and 200 μg/mL) for 24 h and cell morphological changes were assessed by inverted light microscope. b GES-1 cells were exposed to different concentrations of SSCC (0, 50, 100 and 200 μg/mL) for 24 h and cell morphological changes were assessed by inverted light microscope. c The SSCC treatment cells were dehydrated with gradient ethanol alcohol and dried by carbon dioxide critical point and coated with gold using a metal ions spattering instrument. The surface morphology of BGC-823 cells was examined by a scanning electron microscope (SEM)
The result of Hoechst 33258 staining
Stained BGC-823 cells with Hoechst 33258 dye suggested that apoptosis was the cause of diminishing cell viability. As shown in Fig. 3, the untreated group was stained equally with blue fluorescence, which suggested the steady chromatin distribution in nucleus. Conversely, the SSCC-treated cells demonstrated typical characteristics of apoptosis, such as emitting bright fluorescence and forming nucleus fragments due to chromatin congregation and nucleus shrinkage.
Fig. 3.
Hochest 33,258 staining fluorescence images. After treatment with SSCC (0, 50, 100 and 200 μg/mL) for 24 h, BGC-823 cells were stained with Hochest 33,258 and then observed under a fluorescence microscope
Annexin V-FITC/PI labeling for detection of apoptosis
To further confirm the effects of SSCC on BGC-823 cells, we operated flow cytometry based on the method of Annexin V-FITC/PI staining. As shown in Fig. 4 and Table 2, compared with the untreated cells, the administration of SSCC obviously increased the apoptosis of BGC-823 cells in a dose-dependent manner. The percentage of late apoptotic cells increased from 3.34 to 53.43%, which further verified that the growth inhibition of SSCC on BGC-823 cells was caused by apoptosis induction.
Fig. 4.
Apoptosis rate of BGC-823 cells induced by SSCC. BGC-823 cells treatment with SSCC at concentrations of 0, 50, 100 and 200 μg/mL for 24 h. The percentage of apoptotic and necrotic BGC-823 cells were determined by Annexin V-FITC/PI staining method
Table 2.
The results of the Annexin V-FITC/PI staining BGC-823 cells
| Concentration (μg/mL) | Early apoptotic (%) | Late apoptotic (%) |
|---|---|---|
| 0 | 0.57 ± 0.29 | 3.34 ± 0.18 |
| 50 | 3.62 ± 0.57* | 13.61 ± 0.34* |
| 100 | 5.45 ± 0.48* | 31.12 ± 0.28* |
| 200 | 8.47 ± 0.61* | 53.43 ± 0.49* |
*P < 0.05, compared to untreated group
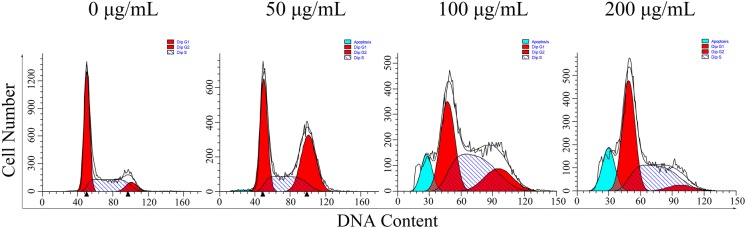
Effect of SSCC on cell cycle
Flow cytometry was applied to evaluate the distribution of cell cycle phase in order to gain further insights into the mechanisms involved in the antiproliferative activity of SSCC on BGC-823 cells. As observed in Fig. 5 and Table 3, compared with the untreated group, the SSCC treatment significantly changed the percentages of BGC-823 cells in G0/G1, S, and G2/M phase. The percentages of cells in G2/M phase increased from 8.66% (0 μg/mL) to 37.91% (50 μg/mL), and eventually dropped to 5.72% (200 μg/mL). The results indicated that the growth suppression effect of SSCC on BGC-823 cells was associated with the cell cycle arrest at G2/M phase.
Fig. 5.
The effect of SSCC on BGC-823 cells cell cycle distribution. BGC-823 cells were exposed to different concentrations of SSCC (0, 50, 100, 200 μg/mL) for 24 h and then stained with PI. The number of cells was analyzed by flow cytometry
Table 3.
The statistical results of BGC-823 cell cycle
| Concentration (μg/Ml) | G0/G1 (%) | S (%) | G2/M (%) | Apoptosis (%) |
|---|---|---|---|---|
| 0 | 55.83 ± 2.62 | 35.51 ± 3.21 | 8.66 ± 4.38 | 0 |
| 50 | 37.99 ± 2.33* | 24.10 ± 3.12* | 37.91 ± 3.01* | 1.09 ± 2.04* |
| 100 | 36.97 ± 1.85* | 44.31 ± 2.9* | 18.72 ± 3.24* | 10.62 ± 4.52* |
| 200 | 52.84 ± 3.48* | 41.44 ± 2.36* | 5.72 ± 3.15* | 17.47 ± 3.49* |
*P < 0.05, compared to untreated group
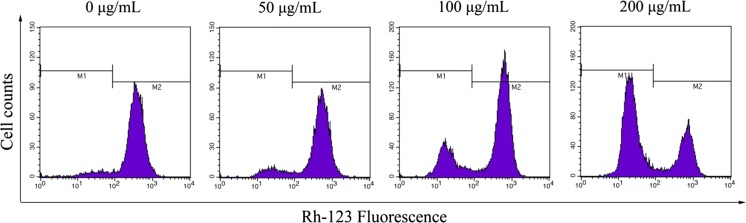
Effect of SSCC on MMP
To explore whether the apoptotic effects of SSCC was associated with the mitochondrial pathway, we investigated the change of MMP on the BGC-823 cells using flow cytometry. As shown in Fig. 6, the MMP of SSCC-treated BGC-823 cells obviously decreased in a dose-dependent manner. With the increase of SSCC concentration, the proportions of Rh-123 positive cells rapidly decreased from 94.33 to 89.45%, 75.38%, 32.17% (P < 0.05), respectively, which suggested that SSCC could affect the collapse of MMP in BGC-823 cells (Table 4).
Fig. 6.
The change of MMP on BGC-823 cells. BGC-823 cells were treated with SSCC (0, 50, 100, 200 μg/mL) for 24 h. After incubation, cells were stained with Rh-123 and analyzed by flow cytometry. The reduced fluorescence of Rh-123 was determined as the reduced MMP
Table 4.
Effect of SSCC on MMP in BGC-823 cells
| Concentration (μg/mL) | MMP (%) |
|---|---|
| 0 | 94.33 ± 2.19 |
| 50 | 89.45 ± 2.97* |
| 100 | 75.38 ± 1.36* |
| 200 | 32.17 ± 3.23* |
*P < 0.05, compared to untreated group
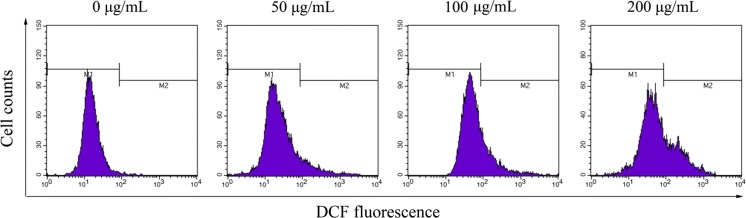
Effect of SSCC on production of intracellular ROS
The changes of the mitochondrial situation were considered involving the intracellular ROS levels. Therefore, we examined the ROS production on BGC-823 cells treated with SSCC by flow cytometry. Compared with the untreated group (Fig. 7), SSCC-treatment induced a rapidly rise in the intracellular ROS levels in a dose-dependent manner. After treatment with SSCC (0, 50, 100 and 200 μg/mL), the intracellular ROS levels increased from 0.12 to 8.87%, 20.16% and 42.17% (P < 0.05), respectively. The results suggested that SSCC-induced apoptosis in BGC-823 cells was triggered by improving the levels of intracellular ROS (Table 5).
Fig. 7.
SSCC caused the apoptosis on BGC-823 cells through the generation of ROS. BGC-823 cells were treated with SSCC (0, 50, 100 and 200 μg/mL) for 24 h and ROS generation were estimated by flow cytometry
Table 5.
Effect of SSCC on ROS generation of BGC-823 cells
| Concentration (μg/mL) | Intracelluar ROS (%) |
|---|---|
| 0 | 0.12 ± 0.08 |
| 50 | 8.87 ± 2.76* |
| 100 | 20.16 ± 1.39* |
| 200 | 42.17 ± 3.41* |
*P < 0.05, compared to untreated group
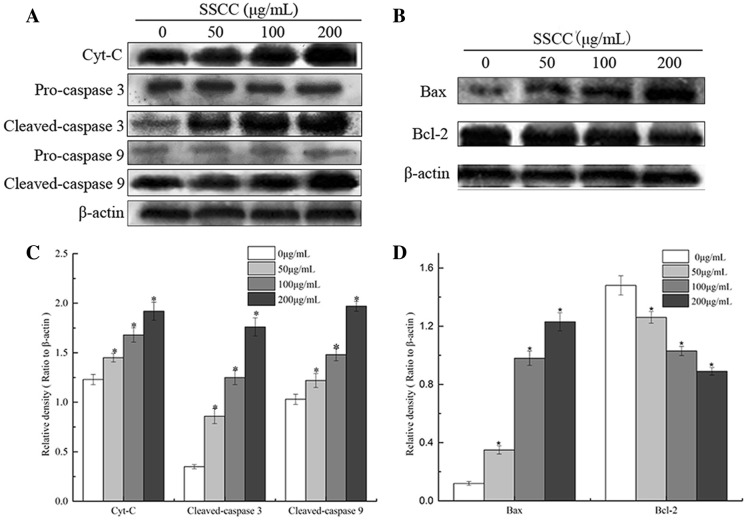
Western blot analysis
The release of Cyt-C from the mitochondria to the cytosol would subsequently give rise to apoptosis by activating caspases, including caspase 3 and caspase 9. The Bcl-2 family numbers were also important regulators in the mitochondrial apoptosis pathway. To further confirm cell apoptosis induced by SSCC was through mitochondrial apoptosis pathway, we analyzed the expression of Cyt-C, Cleaved-caspase 3, Cleaved-caspase 9, Bax and Bcl-2 by western blotting. Compared with the untreated group (Fig. 8), the expression of Cyt-C, Cleaved-caspase 3, Cleaved-caspase 9 and Bax was significantly increased (P < 0.05) and the levels of Bcl-2 remarkably reduced (P < 0.05) in BGC-823 cells in a dose-dependent manner.
Fig. 8.
The expression level of apoptosis-related proteins in BGC-823 cells exposed to SSCC (0-200 μg/mL) for 24 h as measured by Western blotting. a Western blot analysis of Cyt-C, Pro-caspase 3, Cleaved-caspase 3, Pro-caspase 9 and Cleaved-caspase 9 expressions on BGC-823 cells. b Western blot analysis of Bax,Bcl-2 expressions in BGC-823 cells. c Quantitative analysis for Cleaved-caspase 3, Cleaved-caspase 9 and Cyt-C levels normalised to β-actin. d Quantitative analysis for Bax and Bcl-2 levels normalised to β-actin. Equal amounts of total protein (40 μg) were loaded and β-actin serves as an internal control. Data are expressed as mean ± S.D. of three independent experiments. *P < 0.05, compared to untreated group
The changes of Bcl-2 and Bax expression
BGC-823 cells were exposed to different concentrations of SSCC (0, 50, 100 and 200 μg/mL) for 24 h and the mRNA expressions of both Bcl-2 and Bax genes were quantified. As shown in Fig. 9, the Bcl-2 transcripts showed steady decrease in expressions after treatment with SSCC (P < 0.05), meanwhile Bax’s mRNA was obviously increased in a dose-dependent manner (P < 0.05).
Fig. 9.
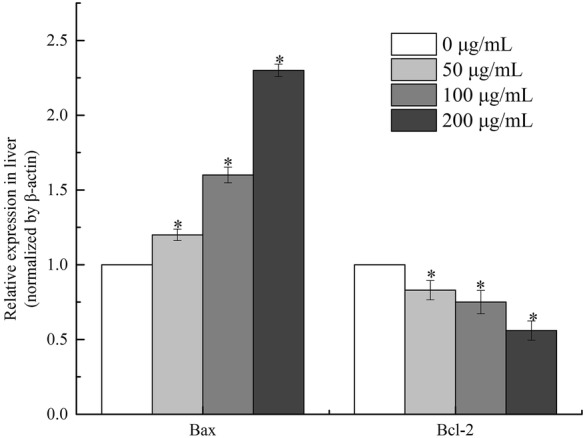
Cells were treated with SSCC at the concentration of 0, 50, 100, 200 μg/mL SSCC respectively for 24 h. The effects of SSCC on relative mRNA levels of Bax and Bcl-2 were detected by real-time PCR. *P < 0.05, compared to untreated group
Discussion
Gastric cancer (GC) is one of the most common cancers in the world, with approximately one million new cases diagnosed each year. Because of its complex molecular mechanisms and clinical heterogeneity, GC is the fifth most common malignancy and the third leading cause of cancer-related deaths worldwide (Qiao et al. 2018; Zhang et al. 2017a). Fortunately, some researches have demonstrated that selenium (Se) could exhibit potential anti-tumor activity and reduce cancer mortality and morbidity (Cui et al. 2018). Compared with general inorganic selenium, organic selenium compounds retained better bioavailability and higher biological activity with fewer side effects and lower toxicity (Gandin et al. 2018; He et al. 2017). Therefore, the studies of organic selenium compounds have attracted a lot of attention in recent years (He et al. 2017). Cheng et al. (2018b) demonstrated that Selenium (Se)-containing polysaccharide generally exhibited higher antitumor activity than regular polysaccharides. In order to improve the selectivity, specificity, efficacy and to reduce the toxicity, we had done a lot of work on selenium modification and synthesized an organic Se polysaccharide (SSCC).
In our study, the antitumor effects of SSCC on BGC-823 cells were investigated in many ways. Firstly, we evaluated the cytotoxicity of SSCC on BGC-823 cells by the MTT method. The MTT assay is a common tool in estimating the proliferation activity of living cells. The test is based on enzymatic reduction of the yellow MTT to its formazan of intense purple-blue color in the mitochondria of living cells, which could be quantified by an ELISA reader (Grela et al. 2018; Perez et al. 2017). The Fig. 1 indicated that SSCC could obviously produce cytotoxicity on BGC-823 cells and prevent the proliferation of BGC-823 cells effectively in a dose-dependent manner. Meanwhile, to explore the process of programmed cell death or apoptosis, the morphological alterations of SSCC-treated BGC-823 cells were observed.
The changes of BGC-823 cells morphology were appraised by invert microscope and SEM (Fig. 2), including cell shrinkage, nuclear pyknosis and deformities, reduction of cell number and increase of cell gap, which suggested that SSCC could induce BGC-823 cells apoptosis. Through Hoechst 33258 fluorescence dye, we found that the untreated cells exhibited normal nuclei without any nuclear condensation or DNA degradation and appeared in blue. Whereas, the SSCC-treated cells were observed bright blue fluorescence pointing chromatin condensation of DNA and formed apoptotic bodies. Hoechst 33258 staining provided direct evidence in this research that SSCC could induce apoptosis on BGC-823 cells. To further confirm the results of morphological analysis, we operated flow cytometry based on Annexin V-FITC/PI staining. Phosphatidylserine (PS) was existed in the inner side of the plasma membrane, which would be translated to the outer layers after inducing cell apoptosis. So the changes in position of PS was a crucial marker for detection of early apoptosis (Perumalsamy et al. 2017). Annexin V was a Ca2+-dependent phospholipid-binding protein that specifically binds to PS residues with high affinity. It could be combined with propidium iodide (PI) to discriminate normal, apoptotic and necrotic cells by flow cytometry. In the test, Annexin-V−/PI− cells, Annexin-V+/PI− cells, Annexin-V+/PI+ cells, Annexin-V+/PI− cells were regarded as viable cells, early apoptotic cells, late apoptotic cells and necrotic cells, respectively (Sawai and Domae 2011). The flow cytometry results (Fig. 4) revealed that SSCC could effectively increase the percentage of early apoptotic cells, late apoptotic cells and necrotic cells in a dose-dependent manner, validating that induction of apoptosis lead to the growth inhibition of BGC-823 cells.
Cell cycle deregulation is a common change that happened at tumor development. For this reason, the cell cycle arrest is regarded as an effective target for cancer therapy (Liu et al. 2015). Numerous researches indicated that many antitumor drugs aimed to block cell cycle at a specific checkpoint and thereby increased the ratio of apoptotic cells (Chang et al. 2013; Zhang et al. 2015). Our investigation suggested that SSCC induced apoptosis by leaking the nuclear DNA out in G2/M phase.
The mitochondrial apoptosis pathway is one of the most crucial mechanism for cell apoptosis (Jia et al. 2018; Chen et al. 2013). In the early stage of cell apoptosis, the mitochondrial pathway could be activated by different signals and the mitochondrial structure and function would be changed. Previous researches indicated that the collapse of MMP was always associated with the mitochondrial dysfunction and also regarded as an important sign in the early apoptosis via mitochondrial pathway (Ya 2017). Meanwhile, mitochondria were recognized as the crucial source of producing ROS. Excessive ROS generation could trigger mitochondrial oxidative damage and increase the mitochondrial membrane permeability (Cao et al. 2017). Therefore, the generation of ROS played important roles in regulation of cell apoptosis. In our present study (Figs. 6 and 7), we found that SSCC could induce the disruption of MMP and improve the levels of intracellular ROS thereby triggering cell apoptosis.
As the most classical apoptosis factors, the apoptosis protein inhibitor Bcl-2 and pro-apoptotic protein Bax play important roles in the mitochondrial apoptosis pathway (Birkinshaw and Czabotar 2017). Bcl-2, the first anti-death gene discovered, possesses the abilities of suppressing apoptosis. Bax can be transferred to the mitochondrial outer membrane and form a transmembrane channel which can allow Cyt-C to be released, therefore inducing the mitochondria apoptosis (Liu et al. 2017). The lower ratio of Bcl-2/Bax was the indicator for the higher permeability of mitochondrial outer membrane, leading to the release of various apoptogenic proteins and other cell apoptosis mediators. In our study, a significant dose-dependent downregulation of Bcl-2 and an up-regulation of Bax were observed in SSCC-treated BGC-823 cells by western blot and real-time PCR, which further confirmed that SSCC could induce cell apoptosis. Furthermore, intracellular death signals could be induced by the release of Cyt-C in mitochondrial. Cyt-C is a small heme-containing metalloprotein which played an important role in cell apoptosis as an important intermediate (Pur et al. 2018). Caspase 3 is a crucial executioner of apoptosis, which is activated by an initiator caspase such as Caspase 9 during apoptosis (Cao et al. 2017). After Cyt-C, Caspase 9 and Caspase 3 were released and activated, the apoptosis process and substrate degradation would be triggered. In this study, we found that SSCC induced the activation of Cyt-C, Caspase 3 and Caspase 9 in a dose-dependent manner, which could stimulate the molecular cascades for apoptosis (Zhou et al. 2018).
Conclusion
In conclusion, our research provided direct evidence that SSCC could be regarded as a safe and potent antitumor agent against gastric cancer cell BGC-823 in vitro. However, further study was needed to perform in vivo for the therapeutic use of SSCC against gastric cancer in humans.
Compliance with ethical standards
Conflicts of interest
All authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Bano I, Arshad M, Yasin T, Ghauri MA, Younus M. Chitosan: a potential biopolymer for wound management. Int J Biol Macromol. 2017;102:380–383. doi: 10.1016/j.ijbiomac.2017.04.047. [DOI] [PubMed] [Google Scholar]
- Birkinshaw RW, Czabotar PE. The BCL-2 family of proteins and mitochondrial outer membrane permeabilisation. Semin Cell Dev Biol. 2017;72:152–162. doi: 10.1016/j.semcdb.2017.04.001. [DOI] [PubMed] [Google Scholar]
- Cao H, Xu H, Zhu G, Liu S. Isoquercetin ameliorated hypoxia/reoxygenation-induced H9C2 cardiomyocyte apoptosis via a mitochondrial-dependent pathway. Biomed Pharmacother. 2017;95:938–943. doi: 10.1016/j.biopha.2017.08.128. [DOI] [PubMed] [Google Scholar]
- Chang CC, Hung CM, Yang YR, Lee MJ, Hsu YC. Sulforaphane induced cell cycle arrest in the G2/M phase via the blockade of cyclin B1/CDC2 in human ovarian cancer cells (Article) J Ovarian Res. 2013;6:7. doi: 10.1186/1757-2215-6-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen GC, Zhang PY, Huang TT, Yu WQ, Lin J, Li P, et al. Polysaccharides from Rhizopus nigricans mycelia induced apoptosis and G2/M arrest in BGC-823 cells (Article) Carbohyd Polym. 2013;97:800–808. doi: 10.1016/j.carbpol.2013.05.068. [DOI] [PubMed] [Google Scholar]
- Cheng L, Chen L, Yang Q, Wang Y, Wei X. Antitumor activity of Se-containing tea polysaccharides against sarcoma 180 and comparison with regular tea polysaccharides and Se-yeast. Int J Biol Macromol. 2018;120:853–858. doi: 10.1016/j.ijbiomac.2018.08.154. [DOI] [PubMed] [Google Scholar]
- Cheng L, Chen L, Yang Q, Wang Y, Wei X. Antitumor activity of Se-containing tea polysaccharides against sarcoma 180 and comparison with regular tea polysaccharides and Se-yeast. Int J Biol Macromol. 2018 doi: 10.1016/j.ijbiomac.2018.08.154. [DOI] [PubMed] [Google Scholar]
- Cui D, Yan C, Miao J, Zhang X, Chen J, Sun L, et al. Synthesis, characterization and antitumor properties of selenium nanoparticles coupling with ferulic acid. Mater Sci Eng C. 2018;90:104–112. doi: 10.1016/j.msec.2018.04.048. [DOI] [PubMed] [Google Scholar]
- Endo M, Hasegawa H, Kaneko T, Kanno C, Monma T, Kano M, et al. Antitumor activity of selenium compounds and its underlying mechanism in human oral squamous cell carcinoma cells: a preliminary study. J Oral Maxillofac Surg Med Pathol. 2017;29:17–23. doi: 10.1016/j.ajoms.2016.08.006. [DOI] [Google Scholar]
- Fan S, Zhang J, Nie W, Zhou W, Jin L, Chen X, et al. Antitumor effects of polysaccharide from Sargassum fusiforme against human hepatocellular carcinoma HepG2 cells. Food Chem Toxicol. 2017;102:53–62. doi: 10.1016/j.fct.2017.01.020. [DOI] [PubMed] [Google Scholar]
- Gandin V, Khalkar P, Braude J, Fernandes AP. Organic selenium compounds as potential chemotherapeutic agents for improved cancer treatment. Free Radical Biol Med. 2018 doi: 10.1016/j.freeradbiomed.2018.05.001. [DOI] [PubMed] [Google Scholar]
- Grela E, Kozłowska J, Grabowiecka A. Current methodology of MTT assay in bacteria—a review. Acta Histochem. 2018;120:303–311. doi: 10.1016/j.acthis.2018.03.007. [DOI] [PubMed] [Google Scholar]
- He J, Wu Z, Pan D, Guo Y, Zeng X. Effect of selenylation modification on antitumor activity of peptidoglycan from Lactobacillus acidophilus. Carbohyd Polym. 2017;165:344–350. doi: 10.1016/j.carbpol.2017.02.031. [DOI] [PubMed] [Google Scholar]
- Jia X, Liu Q, Zou S, Xu X, Zhang L. Construction of selenium nanoparticles/β-glucan composites for enhancement of the antitumor activity. Carbohyd Polym. 2015;117:434–442. doi: 10.1016/j.carbpol.2014.09.088. [DOI] [PubMed] [Google Scholar]
- Jia Z-Q, Li S-Q, Qiao W-Q, Xu W-Z, Xing J-W, Liu J-T, et al. Ebselen protects mitochondrial function and oxidative stress while inhibiting the mitochondrial apoptosis pathway after acute spinal cord injury. Neurosci Lett. 2018;678:110–117. doi: 10.1016/j.neulet.2018.05.007. [DOI] [PubMed] [Google Scholar]
- Laskar K, Faisal SM, Rauf A, Ahmed A, Owais M. Undec-10-enoic acid functionalized chitosan based novel nano-conjugate: an enhanced anti-bacterial/biofilm and anti-cancer potential. Carbohyd Polym. 2017;166:14–23. doi: 10.1016/j.carbpol.2017.02.082. [DOI] [PubMed] [Google Scholar]
- Lee JS, Hong EK. Hericium erinaceus enhances doxorubicin-induced apoptosis in human hepatocellular carcinoma cells. Cancer Lett. 2010;297:144–154. doi: 10.1016/j.canlet.2010.05.006. [DOI] [PubMed] [Google Scholar]
- Li FN, Dong XW, Lin P, Jiang JL. Regulation of Akt/FoxO3a/Skp2 axis is critically involved in berberine-induced cell cycle arrest in hepatocellular carcinoma cells (Article) Int J Mol Sci. 2018;19:13. doi: 10.3390/ijms19020327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu A, et al. Growth inhibition and apoptosis of human leukemia K562 cells induced by seleno-short-chain chitosan. Methods Find Exp Clin Pharmacol. 2008;3:181–186. doi: 10.1358/mf.2008.30.3.1213209. [DOI] [PubMed] [Google Scholar]
- Liu Y, Sun J, Rao S, Su Y, Li J, Li C, et al. Antidiabetic activity of mycelia selenium-polysaccharide from Catathelasma ventricosum in STZ-induced diabetic mice. Food Chem Toxicol. 2013;62:285–291. doi: 10.1016/j.fct.2013.08.082. [DOI] [PubMed] [Google Scholar]
- Liu L, Wang D, Wang J, Ji H, Zhang Y. NOAD, a novel nitric oxide donor, induces G2/M phase arrest and apoptosis in human hepatocellular carcinoma Bel-7402 cells. Toxicol In Vitro. 2015;29:1289–1297. doi: 10.1016/j.tiv.2015.05.003. [DOI] [PubMed] [Google Scholar]
- Liu WB, Xie F, Sun HQ, Meng M, Zhu ZY. Anti-tumor effect of polysaccharide from Hirsutella sinensis on human non-small cell lung cancer and nude mice through intrinsic mitochondrial pathway. Int J Biol Macromol. 2017;99:258–264. doi: 10.1016/j.ijbiomac.2017.02.071. [DOI] [PubMed] [Google Scholar]
- Liu L, Chang X, Zhang Y, Wu C, Li R, Tang L, et al. Fluorochloridone induces primary cultured Sertoli cells apoptosis: involvement of ROS and intracellular calcium ions-mediated ERK1/2 activation. Toxicol In Vitro. 2018;47:228–237. doi: 10.1016/j.tiv.2017.12.006. [DOI] [PubMed] [Google Scholar]
- Lü H, Gao Y, Shan H, Lin Y. Preparation and antibacterial activity studies of degraded polysaccharide selenide from Enteromorpha prolifera. Carbohyd Polym. 2014;107:98–102. doi: 10.1016/j.carbpol.2014.02.045. [DOI] [PubMed] [Google Scholar]
- Ma Z-J, Lu L, Yang J-J, Wang X-X, Su G, Wang Z-L, et al. Lariciresinol induces apoptosis in HepG2 cells via mitochondrial-mediated apoptosis pathway. Eur J Pharmacol. 2018;821:1–10. doi: 10.1016/j.ejphar.2017.12.027. [DOI] [PubMed] [Google Scholar]
- Nagappan A, Park KI, Park HS, Kim JA, Hong GE, Kang SR, et al. Vitamin C induces apoptosis in AGS cells by down-regulation of 14-3-3sigma via a mitochondrial dependent pathway. Food Chem. 2012;135:1920–1928. doi: 10.1016/j.foodchem.2012.06.050. [DOI] [PubMed] [Google Scholar]
- Perez MG, Fourcade L, Mateescu MA, Paquin J. Neutral Red versus MTT assay of cell viability in the presence of copper compounds. Anal Biochem. 2017;535:43–46. doi: 10.1016/j.ab.2017.07.027. [DOI] [PubMed] [Google Scholar]
- Perumalsamy H, Sankarapandian K, Kandaswamy N, Balusamy SR, Periyathambi D, Raveendiran N. Cellular effect of styrene substituted biscoumarin caused cellular apoptosis and cell cycle arrest in human breast cancer cells. Int J Biochem Cell Biol. 2017;92:104–114. doi: 10.1016/j.biocel.2017.09.019. [DOI] [PubMed] [Google Scholar]
- Pur MRK, Hosseini M, Faridbod F, Ganjali MR, Hosseinkhani S. Early detection of cell apoptosis by a cytochrome C label-Free electrochemiluminescence aptasensor. Sens Actuators B Chem. 2018;257:87–95. doi: 10.1016/j.snb.2017.10.138. [DOI] [Google Scholar]
- Qiao Y, Li T, Zheng S, Wang H. The Hippo pathway as a drug target in gastric cancer. Cancer Lett. 2018;420:14–25. doi: 10.1016/j.canlet.2018.01.062. [DOI] [PubMed] [Google Scholar]
- Redza-Dutordoir M, Averill-Bates DA. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim Biophys Acta. 2016;1863:2977–2992. doi: 10.1016/j.bbamcr.2016.09.012. [DOI] [PubMed] [Google Scholar]
- Sawai H, Domae N. Discrimination between primary necrosis and apoptosis by necrostatin-1 in Annexin V-positive/propidium iodide-negative cells. Biochem Biophys Res Commun. 2011;411:569–573. doi: 10.1016/j.bbrc.2011.06.186. [DOI] [PubMed] [Google Scholar]
- Sinha R, Ei-Bayoumy K. Apoptosis is a critical cellular event in cancer chemoprevention and chemotherapy by selenium compounds (Review) Curr Cancer Drug Targets. 2004;4:13–28. doi: 10.2174/1568009043481614. [DOI] [PubMed] [Google Scholar]
- Song F, Zhang L, Yu HX, Lu RR, Bao JD, Tan C, et al. The mechanism underlying proliferation-inhibitory and apoptosis-inducing effects of curcumin on papillary thyroid cancer cells. Food Chem. 2012;132:43–50. doi: 10.1016/j.foodchem.2011.10.024. [DOI] [PubMed] [Google Scholar]
- Tao J, Xu J, Chen F, Xu B, Gao J, Hu Y. Folate acid-Cyclodextrin/Docetaxel induces apoptosis in KB cells via the intrinsic mitochondrial pathway and displays antitumor activity in vivo. Eur J Pharm Sci. 2018;111:540–548. doi: 10.1016/j.ejps.2017.10.039. [DOI] [PubMed] [Google Scholar]
- Wang Y, Chen J, Zhang D, Zhang Y, Wen Y, Li L, et al. Tumoricidal effects of a selenium (Se)-polysaccharide from Ziyang green tea on human osteosarcoma U-2 OS cells. Carbohyd Polym. 2013;98:1186–1190. doi: 10.1016/j.carbpol.2013.07.022. [DOI] [PubMed] [Google Scholar]
- Xiao X, Liu H, Li X. Orlistat treatment induces apoptosis and arrests cell cycle in HSC-3 oral cancer cells. Microb Pathog. 2017;112:15–19. doi: 10.1016/j.micpath.2017.09.001. [DOI] [PubMed] [Google Scholar]
- Xie P, Fujii I, Zhao J, Shinohara M, Matsukura M. A novel polysaccharide derived from algae extract induces apoptosis and cell cycle arrest in human gastric carcinoma MKN45 cells via ROS/JNK signaling pathway. Int J Oncol. 2016;49:1561–1568. doi: 10.3892/ijo.2016.3658. [DOI] [PubMed] [Google Scholar]
- Ya G. A Lentinus edodes polysaccharide induces mitochondrial-mediated apoptosis in human cervical carcinoma HeLa cells. Int J Biol Macromol. 2017;103:676–682. doi: 10.1016/j.ijbiomac.2017.05.085. [DOI] [PubMed] [Google Scholar]
- Zhang CG, Huang JC, Liu T, Li XY. Anticancer effects of bishydroxycoumarin are mediated through apoptosis induction, cell migration inhibition and cell cycle arrest in human glioma cells. J Buon. 2015;20:1592–1600. [PubMed] [Google Scholar]
- Zhang C, Powell SE, Betel D, Shah MA. The gastric microbiome and its influence on gastric carcinogenesis: current knowledge and ongoing research. Hematol Oncol Clin North Am. 2017;31:389–408. doi: 10.1016/j.hoc.2017.01.002. [DOI] [PubMed] [Google Scholar]
- Zhang RJ, Shi Y, Zheng J, Mao XM, Liu ZY, Chen QX, et al. Effects of polysaccharides from abalone viscera (Haliotis discus hannai Ino) on MGC 803 cells proliferation. Int J Biol Macromol. 2017 doi: 10.1016/j.ijbiomac.2017.08.055. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Zhang S, Wang P, Fu S, Wu D, Liu A. Seleno-short-chain chitosan induces apoptosis in human non-small-cell lung cancer A549 cells through ROS-mediated mitochondrial pathway. Cytotechnology. 2017;69:851–863. doi: 10.1007/s10616-017-0098-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Zhong X, Lin J, Hong Z. Qianliening capsule promotes mitochondrial pathway mediated the apoptosis of benign prostatic hyperplasia epithelial-1 cells by regulating the miRNA-181a. Int J Gerontol. 2018 doi: 10.1016/j.ijge.2018.04.002. [DOI] [Google Scholar]