Abstract
Stem cells provide a new strategy for the treatment of cardiac diseases; however, their effectiveness in dilated cardiomyopathy (DCM) has not been investigated. In this study, cardiosphere-derived cells (CDCs) were isolated from infants (≤ 24 months) and identified by the cell surface markers CD105, CD90, CD117 and CD45, which is consistent with a previous report, although increased CD34 expression was observed. The molecular expression profile of CDCs from infants was determined by RNA sequencing and compared with adult CDCs, showing that infant CDCs have almost completely altered gene expression patterns compared with adult CDCs. The upregulated genes in infant CDCs are mainly related to the biological processes of cell morphogenesis and differentiation. The molecular profile of infant CDCs was characterized by lower expression of inflammatory cytokines and higher expression of stem cell markers and growth factors compared to adult CDCs. After intramyocardial administration of infant CDCs in the heart of DCM rats, we found that infant CDCs remained in the heart of DCM rats for at least 7 days, improved DCM-induced cardiac function impairment and protected the myocardium by elevating the left ventricular ejection fraction and fraction shortening. However, the effectiveness of transplanted CDCs was reversed later, as increased fibrosis formation instead of angiogenesis was observed. We concluded that infant CDCs, with higher expression of stem cell markers and growth factors, exhibit non-durable heart protection due to limited residence time in the heart of DCM animals, suggesting that multiple administrations of the CDCs or post-regulation after transplantation may be the key for cell therapy in the future.
Electronic supplementary material
The online version of this article (10.1007/s10616-019-00328-z) contains supplementary material, which is available to authorized users.
Keywords: Cardiosphere-derived cells (CDCs), Dilated cardiomyopathy (DCM), RNA sequence, Doxorubicin
Introduction
Dilated cardiomyopathy (DCM) is a serious life-threatening disorder that is characterized by enlargement of the left ventricular chamber associated with systolic dysfunction (Kopecky and Gersh 1987). The incidence of DCM is approximately 80/100,000 (Tavazzi 1997), and nearly 50% of paediatric cardiomyopathies are DCM (Towbin et al. 2006). Because a refractory paediatric condition can eventually lead to heart failure, heart transplantation remains the ultimate treatment for DCM (Silva and Canter 2010). Due to the invasive and costly nature of heart transplantation, alternative therapies with higher accessibility are imperative for paediatric DCM.
Cellular therapies hold significant promise for patients with heart disease. Among the stem cells, cardiosphere-derived cells (CDCs) have emerged as a candidate cell type for regenerative therapy post-myocardial infarction (Smith et al. 2007), as these cells have been reported to improve cardiac function in DCM (Aminzadeh et al. 2015). However, the effectiveness of CDCs on heart repair has been challenged by some studies (Kasai-Brunswick et al. 2017). Therefore, the efficacy of CDCs in DCM is still unclear.
Some researchers showed that the total number of CDCs is the most abundant in newborns and infants (Mishra et al. 2011), and the CDCs from newborns have much stronger repair ability in preventing adverse cardiac remodelling, maintaining myocardial function and promoting angiogenesis (Simpson et al. 2012). However, contradictory results emerged recently and showed a stronger repair ability of CDCs from adults instead of CDCs from young patients (Walravens et al. 2018). Elucidation of the molecular characteristics of infant CDCs may provide us insight into the mechanism underlying these conflicts.
In the present study, we determined the key molecular characteristics of infant CDCs and investigated the effectiveness of infant CDCs in a doxorubicin-induced DCM rat model, which can provide useful information to optimize the strategy for stem cell therapy in DCM.
Materials and methods
Isolation and identification of infant CDCs
Different infant myocardial tissue was collected from paediatric congenital heart surgery from the Heart Center of the Guangzhou Women and Children’s Medical Center (detail in supplement Table 1). The procedures involving infant myocardial tissue were approved by the Ethics Committee of Guangzhou Women and Children’s Medical Center (number 2018102201). Infant CDCs were isolated following the protocol previously described (Smith et al. 2007). In brief, the tissue samples were cut into smaller biopsy-sized pieces and placed onto a fibronectin-coated flask and covered with 1 ml of complete explant medium (IMDM, 20% foetal bovine serum (FBS), 1% penicillin–streptomycin, 1% l-glutamine, 0.1 mM 2-mercaptoethanol). Once these outgrowth cells were approximately 70–80% confluent, small, round and bright cells derived from explants were harvested and seeded into a poly-d-lysine coated flask and cultured in cardiosphere-growth medium (35% IMDM and 65% DMEM/F-12 mix, 3.5% FBS, 1% penicillin–streptomycin, 1% l-glutamine, 0.1 mM 2-mercaptoethanol, 1 unit/mL thrombin, 2% B-27 supplement, 80 ng/mL bFGF, 25 ng/mL EGF and 4 ng/mL cardiotrophin-1). After the formation of the cardiospheres, cardiospheres (CSps) were then collected from poly-d-lysine-coated flasks and replated onto fibronectin-coated surfaces to produce adherent CDCs. The CDCs were identified with antibodies against CD105, CD90, CD117 (c-kit), CD34 and CD45 (BioLegend, USA) by flow cytometry.
RNA sequencing
Total RNA from three infant CDCs samples was isolated using QiAamp RNA Blood mini kits according to the manufacturer’s instructions and then sent to Novogene Co., Ltd. (Beijing, China) for sequencing on an Illumina HiSeq 4000 platform.
Establishment of the DCM model
SPF-grade male Sprague-Dawley rats (average body weight 150 ± 20 g) were purchased from the Guangdong Animal Experiment Center (Foshan, Guangdong, China). The rats were intraperitoneally administered doxorubicin (3.5 mg per kg body weight) once a week for 4 consecutive weeks. The development of DCM was examined and verified by echocardiography. All the procedures involving the rats were approved by the Ethics Committee in Animal Research of Guangzhou Medical University according to the university regulations for animal research.
Infant CDCs transplantation
The DCM rats were randomly divided into the PBS and CDCs groups. A total of 2 × 106 infant CDCs cells in 50 µl PBS were administered with a microsyringe into the left ventricular anterior free wall at five locations. PBS served as a control of CDCs administration. Immunosuppression with cyclosporine A was started 2 days before cell administration at a dose of 10 mg/kg/day and maintained for 10 days for both groups.
Bioluminescence
Bioluminescence of infant CDCs after transplantation in vivo followed the protocol previously described (Kasai-Brunswick et al. 2017). In brief, infant CDCs were transfected with luciferase 2 and mCheery lentivirus, and then, the luciferase 2- and mCheery-transfected CDCs were intramyocardially administered to animals. In vivo bioluminescence was performed using In-Vivo FX PRO (Bruker Corporation, US) after intraperitoneal injection of 150 mg/kg of D-luciferin.
Echocardiographic analysis
Animals were anaesthetized by isoflurane, and their cardiac function was examined by using a Vevo2100 Echocardiography System (America). The left ventricular long-axis view and the apical four-chamber view were examined by two-dimensional and M-mode echocardiography. All animal experiments were conducted following the Ethics Committee in Animal Research of Guangzhou Medical University.
Immunohistochemistry
The rat heart was fixed for 4 h with 2% paraldehyde and then replaced with 10% sucrose and 30% sucrose before embedding with O.C.T. The primary antibodies used in these assays included rabbit anti-rat CD31 and rabbit anti-rat Tunel, all purchased from Abcam (Cambridge). The images were taken by using a Leica DMi8 microscope (Leica, Germany) and analysed by using Image-Pro Plus 6.0 software (USA).
Statistical analysis
Values are expressed as the mean ± SEM. Data analyses were performed using the independent-samples T test for comparisons between two groups. Statistical analysis was performed with SPSS 19.0. A value of P < 0.05 was considered significant.
Results
CDCs isolated from infants were characterized by lower expression of inflammatory cytokines and higher expression of stem cells and growth factors
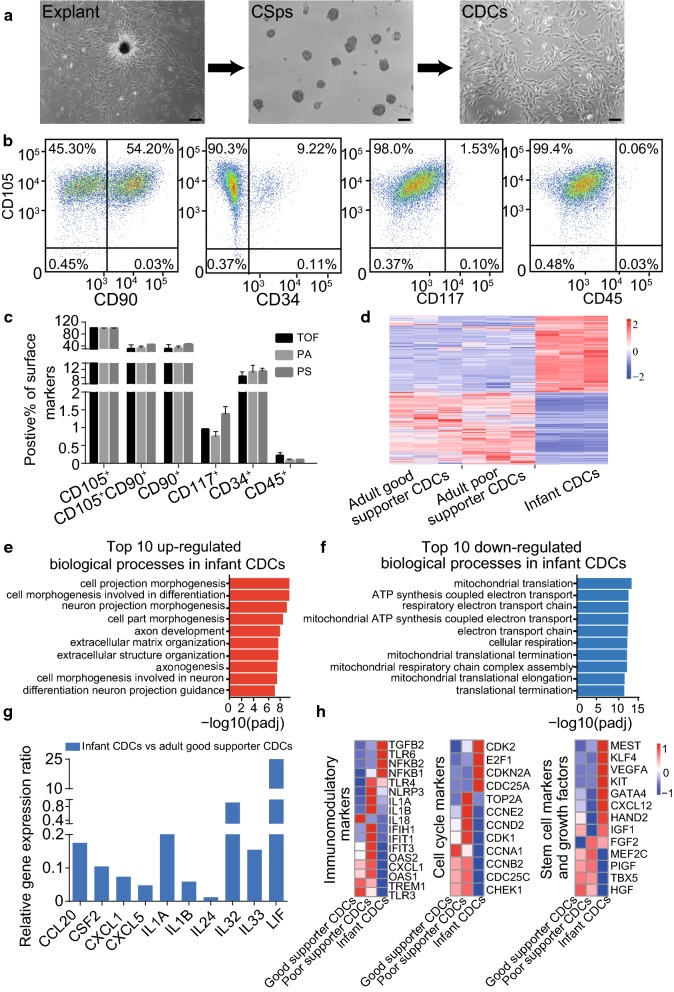
All myocardial tissue was collected from paediatric congenital heart surgery of individuals aged ≤ 24 months (Supplement Table 1), and CDCs were isolated by tissue explants. The small, round and bright cells derived from explants were collected for culture on a poly-d-lysine-coated flask, where they formed cardiospheres (CSps) and were then transferred to a fibronectin-coated flask, giving rise to CDCs (Fig. 1a). Flow cytometry showed a similar result as previous research that showed that the infant CDCs expressed all the key cell surface markers: CD105 (99.07% ± 0.40%), CD90 (61.22% ± 7.59%), CD45 (0.33% ± 0.05%) and CD117 (1.36% ± 0.43%), except that CD34 (14.96% ± 4.61%) was higher than previously reported (Fig. 1b, supplement Table 2). There was no significant difference observed in the expression of cell-surface markers among the infant CDCs isolated from different congenital heart diseases (Fig. 1c). The expression of cell surface markers remained consistent in infant CDCs even after multiple generations of culture and cryopreservation (supplemental Fig. 3a-b). These results indicated that the characteristics of infant CDCs exhibited less variability among infant patients and were very stable during isolation, amplification and storage, which is important for clinical applications.
Fig. 1.
Characteristic identification and RNA-sequencing analysis of infant CDCs. a Isolation and culture of CDCs from infant patients shown as explant culture (left panel), cardiosphere culture (middle panel) and cardiosphere-driven cell culture (right panel). Scale bars = 100 μm. b Cell surface markers of infant CDCs were analysed by flow cytometry. (n = 3). c Expression of cell surface markers of infant CDCs isolated from different congenital heart diseases (all graphs show the mean ± S.E.M. *p < 0.05, n = 3, TOF, tetralogy of Fallot; PA, pulmonary atresia; PS, pulmonary stenosis). (d) Heatmap of Spearman correlations with the differences observed among adult good supporter CDCs, adult poor supporter CDCs and infant CDCs. (The colour bar is included. Red = upregulated; Blue = downregulated) e The top 10 GO terms of biological processes enriched in upregulated gene of infant CDCs shown as d. f The top 10 GO terms of biological processes enriched in downregulated genes of infant CDCs are shown in d. g Differential expression of inflammatory cytokines related to the angiogenesis support in infant CDCs versus adult good supporter CDCs based on the RNA-sequencing assay. h Heatmaps of immunomodulatory genes, cell cycle markers and stem cell markers and growth factors among adult good supporter CDCs, poor supporter CDCs and infant CDCs. (Color figure online)
To identify more details about the biological differences between infant CDCs and adult CDCs, we performed RNA sequencing of infant CDCs and compared them with the RNA sequence data of adult CDCs available in the GEO database (access number GSE81827). The RNA sequence data of adult CDCs were provided by Emma Harvey who divided adult CDCs into two subgroups based on angiogenesis characteristics: good supporter CDCs and poor supporter CDCs (Harvey et al. 2017). The cluster heatmap (Fig. 1d) showed almost completely altered gene expression patterns between infant CDCs and adult CDCs (including good supporter CDCs and poor supporter CDCs). The upregulated genes in infant CDCs were downregulated in adult CDCs; in contrast, the downregulated genes in infant CDCs were highly expressed in adult CDCs. We analysed the GO terms of biological processes enriched in upregulated and downregulated genes in infant CDCs and found that the enriched upregulated genes in infant CDCs were mostly related to cell morphogenesis and differentiation (Fig. 1e). However, the enriched downregulated genes in infant CDCs were mostly related to cell metabolism, such as mitochondrial translation and ATP synthesis coupled electron transport. (Figure 1f). The difference in the biological processes GO terms between infant CDCs and adult CDCs suggests that infant CDCs have more stem cell characteristics and may possess better transdifferentiating ability than adult CDCs.
The vascular supportive function of CDCs is important for therapeutic potential, so it is critical to find CDCs with robust vascular function. Emma Harvey reported that poor supporter CDCs have an enhanced inflammatory profile that leads to a reduced capability to support the tubule formation of endothelial cells, whereas good supporter CDCs have a robust vascular supportive ability and high proliferative potential with lower expression of inflammatory cytokines such as CCL20, CSF2, IL8, IL1B, etc. (Harvey et al. 2017). Compared with adult good supporter CDCs, infant CDCs expressed relatively lower expression of inflammatory cytokines (Fig. 1g), which indicates stronger angiogenesis support.
Although researchers showed a better regenerative capacity of neonatal CDCs in vivo, Ann-Sophie Walravens recently reported that adult CDCs possess a more notable regeneration ability than young CDCs for higher expression of immunomodulatory markers, cell cycle markers and stem markers and growth factors (Walravens et al. 2018). We singled out these genes from our RNA sequence result for comparison of adult and infant CDCs (Fig. 1h). We observed an altered expression of these markers between infant CDCs and adult CDCs, which indicated that infant CDCs overall displayed a lower activation of immunomodulatory and cell cycle pathways but had a higher potential capacity for proliferation and transdifferentiation. Compared with the results of Ann-Sophie Walravens, the main discrepancy is the expression of stem cell markers and growth factors. She suggested that most of these genes showed much higher expression in adult CDCs than in young CDCs, which may be the reason that adult CDCs exhibited a better transdifferentiating ability in their hands. However, our results showed that most of these genes had higher expression in infant CDCs than in adult CDCs, which is consistent with a previous report that neonatal CDCs have a stronger regeneration ability. Thus, the expression level of stem cell markers and growth factors is an important index to evaluate the therapeutic potential of CDCs.
Infant CDCs exhibit non-durable heart protection in dilated cardiomyopathy rats
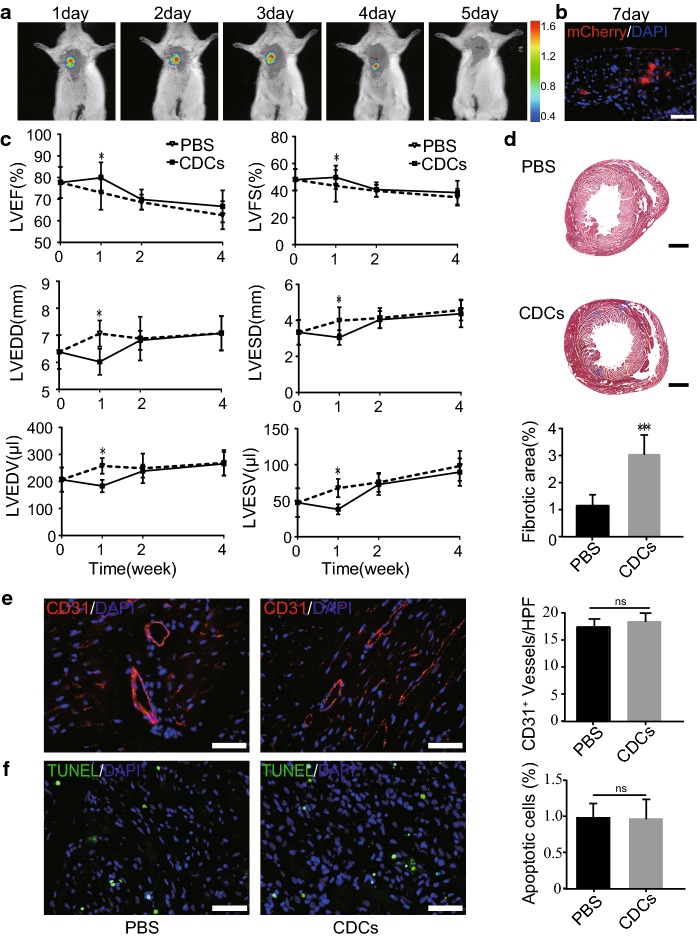
To track the bioluminescent signal in vivo, infant CDCs were transfected with luciferase 2 and mCheery lentivirus. Figure 2a shows representative bioluminescent images after intramyocardial administration of infant CDCs. The rats were pre-administered cyclosporine A for immunosuppression. The bioluminescent signal was detectable in the thoracic region at day 5 post-administration. Seven days after administration of infant CDCs, the heart was taken for frozen sections and examined by fluorescence microscopy. The fluorescent images showed that infant CDCs could still be observed in the heart (Fig. 2b), indicating that infant CDCs could survive in rats for at least 7 days after intramyocardial administration.
Fig. 2.
Cardiac function of infant CDCs in DCM rats after transplantation. a Live imaging technology was used to track the residence time of infant CDCs in rat heart by bioluminescence. b Immunofluorescence image of heart sections 7 days post-intramyocardial administration of infant CDCs carrying mCherry (scale bar = 50 μm). c Cardiac function after treatment of CDCs or PBS in the heart of DCM rats (all graphs show the mean ± S.E.M. *p < 0.05, n = 10). LVEF (left ventricular ejection fraction), LVFS (left ventricular fractional shortening), LVEDD (left ventricular end-diastolic diameter), LVESD (left ventricular end-systolic diameter), LVEDV (left ventricular end-diastolic volume), and LVESV (left ventricular end-systolic volume). d–f Heart sections of DCM rats after administration of PBS or infant CDCs for 4 weeks. d Masson trichrome staining and the quantification of fibrotic areas (n = 8). e Immunofluorescence staining of CD31 and the quantitative data of CD31-positive cells in each high-power field (HPF) (n = 15). f TUNEL staining of apoptosis and the quantitative analysis of TUNEL-positive cells per HPF (n = 15). (All graphs show the mean ± S.E.M. *p < 0.05, scale bar = 50 μm)
To examine whether infant CDCs could attenuate the deterioration of cardiac function in DCM, we established a DCM rat model by administration of doxorubicin for 4 consecutive weeks. Infant CDCs or PBS were intramyocardially administered to DCM rats, and cardiac function in DCM rats was determined weekly by echocardiography. One week later, there was a significant increase in the left ventricular ejection fraction (LVEF) (79.85 ± 5.6 vs 73.03 ± 5.92%) and left ventricular fractional shortening (LVFS) (49.81 ± 5.83 vs 43.52 ± 5.15%). Moreover, the left ventricular end-diastolic diameter (LVEDD), left ventricular end-systolic diameter (LVESD), left ventricular end-diastolic volume (LVEDV) and left ventricular end-systolic volume (LVESV) were significantly reduced in the CDCs group rats compared with the rats in the PBS group (p < 0.05) (Fig. 2c). However, the cardiac function improvement was non-durable and finally reverted back, as no significant difference was observed between the CDCs treatment and PBS treatment groups, suggesting that the effectiveness of infant CDCs may be related to the short residence time of transplanted infant CDCs.
To understand the potential reason for the temporary effect of CDCs in DCM, we investigated fibrosis, apoptosis and angiogenesis in the heart 4 weeks post-administration of infant CDCs. The staining of the endothelial cell marker CD31 and TUNEL staining showed no significant difference between the CDCs group and the PBS group (Fig. 2e–f). However, there was an (p < 0.05) enhancement in the fibrotic area by Masson trichrome staining (Fig. 2d), suggesting that increased fibrosis formation and decreased neovascularization are the main reasons that compromise the effectiveness of CDCs transplantation in the heart of DCM rats.
Discussion
As one of the well-studied progenitor cells from the heart, CDCs have been reported to improve cardiac function in different cardiovascular diseases (Cambier et al. 2018; Gallet et al. 2016; Hensley et al. 2017; Ishigami et al. 2017; Lapchak et al. 2018; Lo et al. 2016; Makkar et al. 2012; Middleton et al. 2017; Nana-Leventaki et al. 2019), animal models (Aminzadeh et al. 2015; Hensley et al. 2017; Kasai-Brunswick et al. 2017; Lapchak et al. 2018; Suzuki et al. 2014) and clinical trials (Makkar et al. 2012). In this study, we isolated and identified the infant CDCs surface markers CD105, CD90, CD117, CD34 and CD45. We found that the expression of infant CDCs surface antigens was mostly the same as adult CDCs, except that CD34 was higher than previously reported (supplemental Table 2). Ann-Sophie Walravens recently reported that adult CDCs possess more notable regeneration than young CDCs, which indicates that cell surface markers are not enough to predict the therapeutic potential of CDCs. Our results showed that infant CDCs have lower inflammatory cytokine expression profiles and higher expression of stem cell markers and growth factors, which may be used as indexes to evaluate the therapeutic potential of CDCs.
The paracrine effect is the combined effect of different factors released from stem cells and is thought to be an important mechanism for stem cell therapy, including antiapoptotic effects on resident myocytes, upregulation of angiogenesis, modulation of inflammatory processes, promotion of cardiomyocyte cell cycle re-entry, and induction of secondary humoural effects in the host tissue. Compared with adult CDCs, we observed that infant CDCs have lower expression of inflammatory cytokines, such as CCL20 (strongly chemotactic for lymphocytes), CSF2 (controls the production, differentiation, and function of granulocytes and macrophages), CXCL1 (neutrophil chemoattractant activity), IL32 (proinflammatory cytokine), IL1B (delays myofibroblast activation), etc. and higher expression of growth factors and stem cell markers from CDCs such as GATA4 (proper mammalian cardiac development), KLF4 (key factor that is essential for inducing pluripotent stem cells), VEGFA (mediating vascular permeability, inducing angiogenesis, vasculogenesis and endothelial cell growth, promoting cell migration, and inhibiting apoptosis), IGF1 (anabolic effects), FGF2 (involved in a variety of biological processes, including embryonic development, cell growth, morphogenesis, tissue repair), etc. (Figure 1g and h). We speculate that the non-durable heart protection of infant CDCs in DCM rats may be due to the expression of these factors and markers. However, if these paracrine effects are not durable, the functional improvement will not last for a long time when the transplanted cells die.
One study reported that mouse CDCs can persist for 3 months in a DCM mouse model (Aminzadeh et al. 2015). In our hands, we found that infant CDCs can only survive for approximately 1 week in the heart even when cyclosporine A was pre-administered for immunosuppression. Four weeks later, we did not observe a significant difference in angiogenesis and cell apoptosis but did detect increased fibrosis.
Research has shown that inflammation can accelerate the formation of fibrosis (Frangogiannis 2015). The relapse of cardiac function deterioration with more fibrosis formation may be due to the complex inflammatory environment. After cardiac injury, infiltrated macrophages play an important role in fibrosis formation. How CDCs exert their protection in myocardial infarction remains unclear. Geoffrey de Couto reported that infarct macrophages can acquire cardioprotective phenotypes that promote survival of ischaemic cardiomyocytes and that these protective effects are conferred by CDCs (de Couto et al. 2015).
DCM is characterized by a continued severe functional decline and is a more aggressive disease than other heart diseases, such as ischaemia reperfusion. The serious and aggressive disorder of DCM may inhibit angiogenesis and endogenous cell repair, including the formation of cardioprotective macrophages, which may be the reason for fibrosis formation after transplantation of infant CDCs in DCM.
Conclusions
This study demonstrates that infant CDCs express more stem cell markers and growth factors than adult CDCs and that these cells can improve cardiac function in DCM for a transient time, suggesting that multiple administrations of the CDCs or post regulation after transplantation may be the future direction to improve the effectiveness of CDCs in DCM.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Detailed clinical information of the infant donors for CDCs isolation (data from the Heart Center of Guangzhou Women and Children Medical Center). (PDF 575 kb)
Reported expression of cell surface markers on CDCs isolated from patients of different ages and the effect of CDCs in vivo after transplantation. References are listed in the last column. (PDF 196 kb)
(a-b) Expression of cell surface markers of infant CDCs by flow cytometry (a) after multiple generation culture and (b) after cryopreservation. (All graphs show the mean±S.E.M. *p<0.05, n=3). (PDF 164 kb)
Acknowledgements
We sincerely thank the Heart Center, Guangzhou Women and Children’s Medical Center for the surgical assistance. We also thank the Nature Science Foundation of China (No. 81670262), the Guangzhou Lingnan Yingjie Project and start-up funding from Guangzhou Women and Children’s Medical Center.
Abbreviations
- CDCs
Cardiosphere-derived cells
- DCM
Dilated cardiomyopathy
- GEO
Gene expression omnibus
- CSps
Explant-derived cardiospheres
- LVEF
Left ventricular ejection fraction
- LVFS
Left ventricular fractional shortening
- LVEDD
Left ventricular end-diastolic diameter
- LVESD
Left ventricular end-systolic diameter
- LVEDV
Left ventricular end-diastolic volume
- LVESV
Left ventricular end-systolic volume
- TOF
Tetralogy of Fallot
- PA
Pulmonary atresia
- PS
Pulmonary stenosis
Author’s contribution
SW, WC, LM and MZ performed this experiment. XC and JD designed the study and wrote the main manuscript. WD and HY collected the data and analysed the results. LS reviewed the data and prepared the pictures. All authors read and approved the final manuscript.
Funding
This work was funded by the Nature Science Foundation of China (No. 81670262), the Guangzhou Lingnan Yingjie Project and the start-up funding from Guangzhou Women and Children’s Medical Center.
Compliance with ethical standards
Conflict of interest
The authors have no conflicts of interest to declare.
Ethical approval
The study was approved by the Animal Ethics Committee of Guangzhou Medical University (G2017-080).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Siyuan Wang and Weidan Chen have contributed equally.
Contributor Information
Xinxin Chen, Email: zingerchen@163.com.
Jinzhu Duan, Email: duanjinzhu@163.com.
References
- Aminzadeh MA, Tseliou E, Sun B, Cheng K, Malliaras K, Makkar RR, Marban E. Therapeutic efficacy of cardiosphere-derived cells in a transgenic mouse model of non-ischaemic dilated cardiomyopathy. Eur Heart J. 2015;36:751–762. doi: 10.1093/eurheartj/ehu196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cambier L, Giani JF, Liu W, Ijichi T, Echavez AK, Valle J, Marban E. Angiotensin ii-induced end-organ damage in mice is attenuated by human exosomes and by an exosomal Y RNA fragment. Hypertension. 2018;72:370–380. doi: 10.1161/hypertensionaha.118.11239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Couto G, Liu W, Tseliou E, Sun B, Makkar N, Kanazawa H, Arditi M, Marban E. Macrophages mediate cardioprotective cellular postconditioning in acute myocardial infarction. J Clin Invest. 2015;125:3147–3162. doi: 10.1172/jci81321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frangogiannis NG. Emerging roles for macrophages in cardiac injury: cytoprotection, repair, and regeneration. J Clin Invest. 2015;125:2927–2930. doi: 10.1172/jci83191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallet R, de Couto G, Simsolo E, Valle J, Sun B, Liu W, Tseliou E, Zile MR, Marban E. Cardiosphere-derived cells reverse heart failure with preserved ejection fraction (hfpef) in rats by decreasing fibrosis and inflammation. JACC Basic Transl Sci. 2016;1:14–28. doi: 10.1016/j.jacbts.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey E, Zhang H, Sepulveda P, Garcia SP, Sweeney D, Choudry FA, Castellano D, Thomas GN, Kattach H, Petersen R, Blake DJ, Taggart DP, Frontini M, Watt SM, Martin-Rendon E. Potency of human cardiosphere-derived cells from patients with ischemic heart disease is associated with robust vascular supportive ability. Stem Cells Transl Med. 2017;6:1399–1411. doi: 10.1002/sctm.16-0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensley MT, Tang J, Woodruff K, Defrancesco T, Tou S, Williams CM, Breen M, Meurs K, Keene B, Cheng K. Intracoronary allogeneic cardiosphere-derived stem cells are safe for use in dogs with dilated cardiomyopathy. J Cell Mol Med. 2017;21:1503–1512. doi: 10.1111/jcmm.13077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishigami S, Ohtsuki S, Eitoku T, Ousaka D, Kondo M, Kurita Y, Hirai K, Fukushima Y, Baba K, Goto T, Horio N, Kobayashi J, Kuroko Y, Kotani Y, Arai S, Iwasaki T, Sato S, Kasahara S, Sano S, Oh H. Intracoronary cardiac progenitor cells in single ventricle physiology: The perseus (cardiac progenitor cell infusion to treat univentricular heart disease) randomized phase 2 trial. Circ Res. 2017;120:1162–1173. doi: 10.1161/circresaha.116.310253. [DOI] [PubMed] [Google Scholar]
- Kasai-Brunswick TH, Costa AR, Barbosa RA, Farjun B, Mesquita FC, Silva Dos Santos D, Ramos IP, Suhett G, Brasil GV, Cunha ST, Brito JO, Passipieri JD, Carvalho AB, Campos de Carvalho AC. Cardiosphere-derived cells do not improve cardiac function in rats with cardiac failure. Stem Cell Res Ther. 2017;8:36. doi: 10.1186/s13287-017-0481-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopecky SL, Gersh BJ. Dilated cardiomyopathy and myocarditis: natural history, etiology, clinical manifestations, and management. Curr Probl Cardiol. 1987;12:569–647. doi: 10.1016/0146-2806(87)90002-8. [DOI] [PubMed] [Google Scholar]
- Lapchak PA, Boitano PD, de Couto G, Marban E. Intravenous xenogeneic human cardiosphere-derived cell extracellular vesicles (exosomes) improves behavioral function in small-clot embolized rabbits. Exp Neurol. 2018;307:109–117. doi: 10.1016/j.expneurol.2018.06.007. [DOI] [PubMed] [Google Scholar]
- Lo CY, Weil BR, Palka BA, Momeni A, Canty JM, Jr, Neelamegham S. Cell surface glycoengineering improves selectin-mediated adhesion of mesenchymal stem cells (mscs) and cardiosphere-derived cells (cdcs): pilot validation in porcine ischemia-reperfusion model. Biomaterials. 2016;74:19–30. doi: 10.1016/j.biomaterials.2015.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makkar RR, Smith RR, Cheng K, Malliaras K, Thomson LE, Berman D, Czer LS, Marban L, Mendizabal A, Johnston PV, Russell SD, Schuleri KH, Lardo AC, Gerstenblith G, Marban E. Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (caduceus): a prospective, randomised phase 1 trial. Lancet. 2012;379:895–904. doi: 10.1016/s0140-6736(12)60195-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton RC, Fournier M, Xu X, Marban E, Lewis MI. Therapeutic benefits of intravenous cardiosphere-derived cell therapy in rats with pulmonary hypertension. PLoS ONE. 2017;12:e0183557. doi: 10.1371/journal.pone.0183557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra R, Vijayan K, Colletti EJ, Harrington DA, Matthiesen TS, Simpson D, Goh SK, Walker BL, Almeida-Porada G, Wang D, Backer CL, Dudley SC, Jr, Wold LE, Kaushal S. Characterization and functionality of cardiac progenitor cells in congenital heart patients. Circulation. 2011;123:364–373. doi: 10.1161/circulationaha.110.971622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nana-Leventaki E, Nana M, Poulianitis N, Sampaziotis D, Perrea D, Sanoudou D, Rontogianni D, Malliaras K. Cardiosphere-derived cells attenuate inflammation, preserve systolic function, and prevent adverse remodeling in rat hearts with experimental autoimmune myocarditis. J Cardiovasc Pharmacol Ther. 2019;24:70–77. doi: 10.1177/1074248418784287. [DOI] [PubMed] [Google Scholar]
- Silva JN, Canter CE. Current management of pediatric dilated cardiomyopathy. Curr Opin Cardiol. 2010;25:80–87. doi: 10.1097/HCO.0b013e328335b220. [DOI] [PubMed] [Google Scholar]
- Simpson DL, Mishra R, Sharma S, Goh SK, Deshmukh S, Kaushal S. A strong regenerative ability of cardiac stem cells derived from neonatal hearts. Circulation. 2012;126:S46–53. doi: 10.1161/circulationaha.111.084699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RR, Barile L, Cho HC, Leppo MK, Hare JM, Messina E, Giacomello A, Abraham MR, Marban E. Regenerative potential of cardiosphere-derived cells expanded from percutaneous endomyocardial biopsy specimens. Circulation. 2007;115:896–908. doi: 10.1161/circulationaha.106.655209. [DOI] [PubMed] [Google Scholar]
- Suzuki G, Weil BR, Leiker MM, Ribbeck AE, Young RF, Cimato TR, Canty JM., Jr Global intracoronary infusion of allogeneic cardiosphere-derived cells improves ventricular function and stimulates endogenous myocyte regeneration throughout the heart in swine with hibernating myocardium. PLoS ONE. 2014;9:e113009. doi: 10.1371/journal.pone.0113009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavazzi L. Epidemiology of dilated cardiomyopathy: a still undetermined entity. Eur Heart J. 1997;18:4–6. doi: 10.1093/oxfordjournals.eurheartj.a015116. [DOI] [PubMed] [Google Scholar]
- Towbin JA, Lowe AM, Colan SD, Sleeper LA, Orav EJ, Clunie S, Messere J, Cox GF, Lurie PR, Hsu D, Canter C, Wilkinson JD, Lipshultz SE. Incidence, causes, and outcomes of dilated cardiomyopathy in children. JAMA. 2006;296:1867–1876. doi: 10.1001/jama.296.15.1867. [DOI] [PubMed] [Google Scholar]
- Walravens AS, Vanhaverbeke M, Ottaviani L, Gillijns H, Trenson S, Driessche NV, Luttun A, Meyns B, Herijgers P, Rega F, Heying R, Sampaolesi M, Janssens S. Molecular signature of progenitor cells isolated from young and adult human hearts. Sci Rep. 2018;8:9266. doi: 10.1038/s41598-018-26969-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Detailed clinical information of the infant donors for CDCs isolation (data from the Heart Center of Guangzhou Women and Children Medical Center). (PDF 575 kb)
Reported expression of cell surface markers on CDCs isolated from patients of different ages and the effect of CDCs in vivo after transplantation. References are listed in the last column. (PDF 196 kb)
(a-b) Expression of cell surface markers of infant CDCs by flow cytometry (a) after multiple generation culture and (b) after cryopreservation. (All graphs show the mean±S.E.M. *p<0.05, n=3). (PDF 164 kb)