Abstract
Research using electroencephalography (EEG) as a measure of brain function and maturation has demonstrated links between cortical activity and cognitive processes during infancy and early childhood. The current study examines whether neonatal EEG is correlated with later atypical socioemotional behaviors or neurocognitive delays. Parental-report developmental assessments were administered to families with children ages 24 to 36 months who had previously participated in a neonatal EEG study (N = 129). Significant associations were found between neonatal EEG (higher frequencies in the frontal-polar, temporal, and parietal brain regions) and BITSEA ASD risk scores. Infants with lower EEG power in these brain areas were more likely to have higher risk of socio-emotional problems. When examining sex differences, significant links were found for males but not for females. These results demonstrate some promising associations between early neural biomarkers and later risk for atypical behaviors, which may shape early neurobehavioral development and could lead to earlier identification and intervention.
Keywords: EEG, BITSEA, brain activity, cognition, autism risk, socioemotional development
Introduction
The course of children’s development is mapped by milestones in domains including motor, social, language, and cognition. Patterns of growth may vary widely, as factors such as culture or environment can greatly impact developmental trajectories. Neurodevelopmental disorders are heterogeneous conditions characterized by a delay or disruption in the acquisition of skills within these developmental domains (Levy, 2018). Common diagnoses include intellectual disability (ID), attention deficit hyperactivity disorder (ADHD), and autism spectrum disorder (ASD). Although there is wide variation in trajectories of typical development, particularly during the first three years of life, early identification of atypical behaviors or developmental delay is important, as this could lead to prompt intervention efforts.
As early identification is key, many researchers have investigated possible brain-based biomarkers for neurodevelopmental disorders (e.g., Dudley, Häßler, & Thome, 2014; Goldani, Downs, Widjaja, Lawton, & Hendren, 2014; Hsiao et al., 2013; Jeste, Frohlich, & Loo, 2015; Reeb-Sutherland & Fox, 2013; Varcin & Nelson, 2016), but a consensus has not yet been reached on the best methodologies. Electroencephalography (EEG) may be a possible candidate. There is heterogeneity in the underlying genetic and environmental pathways, but hypothesized mechanisms of neurodevelopmental disorders include atypical neural connectivity due to disruption of cortical excitation/inhibition balance and abnormalities of cortical interneurons and/or gamma-aminobutyric acid (GABA) receptors (Levitt, Eagleson, & Powell, 2004; Dani et al., 2005; Rubenstein, 2010; Wang et al., 2013). As EEG is a sensitive measure of cortical function, reflecting locally synchronous transmembrane currents in pyramidal neurons, this direct measure of the brain can index underlying neural activity with great temporal resolution; allowing for possible delineation between typical and atypical neurodevelopment. Additionally, EEG recordings are cost-effective and can be collected with difficult populations (e.g., infants, children with special needs) in a variety of settings.
Previous prospective studies have investigated the association between EEG power and later cognitive development. Gou and colleagues (2011) reported significant associations between baseline EEG gamma power (31–50 Hz) during toddlerhood and individual differences in language and cognition during preschool (Gou, Choudhury, & Benasich, 2011). Examining links between neonatal EEG and cognitive outcomes at 15-months, Brito et al. (2016) found that, independent of socioeconomic status, higher frontal gamma power (24–48 Hz) was significantly correlated with better recognition memory and that higher parietal gamma power (24–35 Hz) was significantly related to increased scores in language comprehension. Finally, in a higher-risk sample of infants born with congenital heart disease, Williams and colleagues (2012) observed significant correlations between neonatal EEG and Bayley cognitive scores (BSID-III) at 18-months, with higher frontal power in beta (12–24 Hz), low-gamma (24–35 Hz), and gamma (36–48 Hz) frequencies associated with higher cognitive scores.
In typically developing children, there is a developmental decrease in EEG power of low-frequency rhythms (e.g., delta and theta) and an increase in high-frequency oscillations (e.g., beta and gamma) across different ages (Matousek & Petersen, 1973). Relative to typically developing children, children with learning or attention disorders often demonstrate higher levels of low-frequency power and lower levels of high-frequency power (Barry, Clarke, & Johnstone, 2003). For example, Tierney and colleagues (2012) reported that 6-month-olds who were at high risk for ASD (younger siblings of children with ASD) demonstrated lower EEG power in the frontal lobe across all frequency bands, compared to their low-risk peers. Although group differences in delta (2–4 Hz), theta (4–6 Hz), and beta (13–30 Hz) bands disappeared by age 2, EEG power in alpha (6–13 Hz) and gamma (30–50 Hz) bands remained lower for high-risk infants compared to their lower-risk peers. Levin et al (2017) reported significant differences in frontal EEG power at 3 months of age for infants at high versus low-risk for ASD, with infants in the high-risk group demonstrating reduced power in both high-alpha (9–13 Hz) and beta (13–30 Hz) frequency bands. Links between 3-month frontal high-alpha power and expressive language scores at 12-months of age were also found, with reduced high-alpha power associated with poorer expressive language scores. No significant correlations were detected between 3-month baseline EEG and cognitive/language scores at 18, 24, or 36 months of age.
These studies demonstrate that early EEG activity may be a useful tool in identifying neurodevelopmental risk, specifically ASD, before overt behavioral symptoms are observable. ASD is a neurodevelopmental disorder characterized by impairments in social interaction, communication, and the presence of stereotypic behaviors or restricted interests (DSM-IV, 2013). Past studies have reported links between ASD and abnormalities in learning, attention, and sensory processing (Johnson & Myers, 2007); ASD is reported to occur in all racial, ethnic, and socioeconomic groups (Durkin et al., 2010), but is much more common among boys (1 in 42) than among girls (1 in 189) (Baio, 2014). Although sex differences are present in many developmental disorders, sexual dimorphism also exists in typical development. Prior research has demonstrated sexual dimorphism of human brain maturation beginning in the fetal period and extending throughout early postnatal development (Giedd, Castellanos, Rajapakse, Vaituzis, & Rapoport, 1997; De Lacoste, Horvath, & Woodward, 1991). Sex differences have been demonstrated in EEG coherence where females demonstrate higher intrahemispheric coherence than males across all frequency bands apart from alpha (Marosi et al., 1993; Clarke, Barry, McCarthy, & Selikowitz, 2001). Principal component analysis of EEG coherence across development has revealed synchronized EEG coherence in females is concurrently associated with cognition and social skills whereas for males this link was found for cognitive skills only (Hanlon, Thatcher, & Cline, 1999). In infants at-risk for autism, males have been shown to demonstrate higher resting state EEG power than females in higher frequencies (13 – 50 Hz) (Tierney, Gabard-Durnam, Vogel-Farley, Tager-Flusberg, & Nelson, 2012).
Several studies have investigated links between ASD risk/diagnosis and EEG activity, but there have not been any studies using prospective samples from birth. The current study examined associations between neonatal EEG and later cognitive and socioemotional outcomes, including ASD risk, in a community sample of toddlers aged 24–36 months. The median age of ASD diagnosis is often not until age 4, with lower socioeconomic status associated with later diagnosis. Yet, CDC statistics suggest that 80% of parents are expressing concerns about their children by age 2 (Centers for Disease Control and Prevention, 2009). Here we focus on ASD as (1) infants at risk for ASD are often also at risk for other neurodevelopmental disorders (Jones, Gliga, Bedford, Charman, & Johnson, 2014), (2) behavioral symptoms characteristic of ASD typically appear between 12 and 24 months (American Psychiatric Association, 2000), and (3) parent-report measures like the Modified Checklist for Autism in Toddlers (MCHAT-R/F) and Brief Infant Toddler Social Emotional Assessment (BITSEA) have been utilized as Level I screeners (identify children who are at-risk in the general population) for ASD (Gardner et al., 2013; Robins et al., 2014). We hypothesized that neurodevelopmental risk would be associated with lower EEG power for higher frequencies (13–48 Hz), primarily in the frontal and parietal lobes at birth. We also hypothesized that the association between EEG power at birth and neurodevelopmental disorder risk would be sexually dimorphic.
Methods
Participants
Participants were selected from a subset of infants participating in a large, longitudinal study investigating the relation between prenatal exposures and birth outcomes (Dukes et al., 2014). The present study took place at participating clinic sites in an urban Midwest community. The final sample included 129 infants (55 males; gestational age at birth M: 39.4 weeks, SD: 1.1) who had both neonatal EEG and later developmental assessments. Data was collected 12 to 96 hours after birth, then again between 24 and 36 months of age (M: 30.29 months, SD: 4.28). Participants were excluded from participating in the present study on the basis of birth before 37 weeks’ gestation, multiple births, or NICU admission. All caregivers provided informed consent for their family’s participation in this study. Research procedures were approved by the Columbia University Medical Center IRB and the Sanford Health IRB.
Measures
Respiration and IBR.
Respiration waveforms were collected by means of a respiratory inductance belt (Ambulatory Monitoring Inc., Ardsley, NY) and were digitized at 20 samples per second. The respiration waveform was then smoothed with a three-sample moving average and custom software (Ledano Solutions, Inc.) was used to mark the peaks. Marked peaks were verified by visual inspection and corrected if needed. Breath-to-breath intervals derived from subtracting successive peak times were then inverted to produce the instantaneous breathing rate (IBR). IBR values greater than five times the interquartile range from the median were considered outliers and removed.
Sleep States.
For each minute, the variance of IBR was used to determine sleep state using the quantitative method described in Isler, Thai, Myers, & Fifer (2016). Briefly, IBR variance is much higher during active sleep (AS) than in quiet sleep (QS). Isler et al. (2016) used data from 3 separate laboratories to find an optimal threshold of IBR variance that dichotomized AS from QS with the highest concordance to sleep state coded more traditionally.
Neonatal EEG.
While the infant was asleep, electroencephalogram (EEG) data were collected using a hybrid system of a 28-lead high-impedance electrode net (Electrical Geodesics, Eugene OR) and a miniature amplifier and recording device (ATES, Colognola ai Colli, Italy). The EEG net was soaked for 5 to 10 minutes in a saline solution before being placed on the infant’s head, ensuring all EEG leads were properly placed with good scalp contact and secured with a chinstrap. When checking for impedance, at least 26 electrodes needed to be less than 50 kOhms. If the other 1–2 electrodes were less then 75 kOhms then data acquisition proceeded. EEG was recorded for 10 minutes with the baby in the supine position followed by three 45-degree head-up tilts of approximately 2.75 minutes duration with approximately 2.75 minutes between successive tilts. During recording, the EEG voltage from each lead referenced to the vertex electrode was recorded through a hardware filter (96 Hz low-pass) and digitized with 16 bits per sample at a rate of 250 samples per second. After recording, data were bandpass filtered in software with a 16,000-order finite impulse response filter with passbands of 0.1Hz to 58 Hz and 62 Hz to 118 Hz to avoid AC line noise frequencies.
EEG power spectra were computed for 60-second epochs using the Welch method, averaging over fast Fourier transforms (FFTs) taken each second (Bendat and Piersol, 2000). Data were demeaned and a Hanning window was applied prior to computing the FFT for each second. To determine the leads and times contaminated by movement-related or other sources of electrical artifact, we applied multiple criteria on a second by second basis to data from each lead. Criteria were as follows: standard deviation of voltage less than 50 µV and greater than 0.001 µV; sample-to-sample change less than 50 µV; absolute value of voltage less than 300 µV; log-log spectral slope of raw data between 20 and 120 Hz less than −0.1 (to screen for muscle artifact); log-log spectral slope of raw data between 10 and 30 Hz less than −1 (to screen for ECG artifact). If more than 5 leads had artifact during any one second, that second was excluded. Remaining data were re-referenced to the average over all leads at each sample. Finally, minute by minute power was the average of the squared FFT’s over the accepted seconds, requiring at least 30 acceptable seconds per minute for each lead.
Average power was calculated for 12 scalp regions (left frontal-polar, right frontal-polar, left frontal, right frontal, left central, right central, left parietal, right parietal, left temporal, right temporal, left occipital, and right occipital). Minute by minute EEG power was aligned with simultaneous sleep state codes and averaged over AS and QS minutes within each study. The natural log was taken of EEG power for all analyses. Higher frequencies (13–36 Hz) in six bands (13–15 Hz, 16–18 Hz, 19–21 Hz, 22–24 Hz, 25–36 Hz, and 37–48 Hz) were analyzed as higher frequencies have been correlated to have irregular power spectra, showing reduced power spectra in children at risk for developmental delays when compared to typically developing children (Brito et al., 2016; Gou et al., 2011; Tierney et al., 2012; Tomalski et al., 2013).
Developmental Assessments at 24–36 Months.
Three developmental assessments were given over the phone to the parents of the subjects who participated in the neonatal EEG study once they aged into the 24 to 36-month range. This follow-up study was designed after the completion of the neonatal data collection. To increase the number of participants at the second-time point, parental self-report measures were selected rather than observer-rated measures as many families had moved out of the immediate area and were unable to return for a lab visit. The phone assessments, administered by trained research assistants, took 45 to 60 minutes to complete. Any outliers were winsorized.
The Modified Checklist for Autism in Toddlers.
The Modified Checklist for Autism in Toddlers, Revised with Follow-Up (M-CHAT-R/F), is a 20-item (yes or no) guardian/parental report checklist (Robins et al., 2001; 2014). Questions pertain to a child’s social, communicative, and play behaviors (e.g. “Does your child try to attract your attention to his/her own activity?” “Does your child enjoy playing peek-a-boo/hide-and-seek?”), as well as other behaviors that are associated with ASD (e.g. “Does your child ever seem oversensitive to noise?”). The M-CHAT is validated to screen children between 16 and 30 months for early signs of ASD or developmental delay. Risk classifications include: low risk (total score: 0–2; requires no further evaluation unless other risk factors are present), medium risk (total score: 3–7; requires administration of the M-CHAT-Follow-Up to determine whether referrals are warranted), and high risk (total score: 8–20; warrants immediate referral for evaluation and intervention). The M-CHAT is designed to maximize sensitivity for ASD, meaning that there will be a high false positive rate; most (80–90%) of children who fail the M-CHAT will not be diagnosed with ASD, but importantly, these children are at risk for other developmental delays (Chlebowski, Robins, Barton, & Fein, 2013).
The Brief Infant Toddler Social Emotional Assessment (BITSEA).
The BITSEA is a quick and efficient tool to evaluate potential risk for social and emotional developmental problems or delays in competence amongst children 12 to 36 months of age (Briggs-Gowan, Carter, Irwin, Wachtel, & Cicchetti, 2002). The assessment contains 42 questions, each requiring one of three responses: true/rarely, somewhat true/sometimes, or very true/often. The assessment gives two scores: total problem score and total competence score. This parental-report measure of socioemotional ability has been found to significantly correlate with evaluator ratings of infant competence and internalizing problems, as well as experimenter conducted assessments measures like the Infant Mullen Scales of Early Learning and Vineland Adaptive Behavior Scales for Children (Briggs-Gowan & Carter, 2007). Although the BITSEA was developed to identify a broad range of behavior problems, it also incorporates autism-specific items. The BITSEA ASD risk score was calculated using 17 of the questions (each question scoring 0–2) on the assessment that relate directly to behaviors typical of ASD.
The Parent Report of Children’s Abilities-Revised (PARCA-R).
The PARCA-R is an assessment for examining potential risk for linguistic and cognitive development delays in toddlers (Blaggan et al., 2014). The caregiver report consists of multiple sections to assess language and cognition. Sections of the report include nonverbal cognition (34 questions), vocabulary (list of 100 common words), and sentence complexity (12 questions). The vocabulary and sentence complexity scores were summed to create the linguistic score.
Results
EEG Power Not Associated with MCHAT-R/F or PARCA-R Scores.
All subsequent analyses controlled for sex, gestational age at birth, maternal education, age at the time of toddler assessments, and prenatal exposures to alcohol, cigarettes, recreational drugs, or psychiatric drugs. Descriptive statistics for variables of interest are provided in Table 1. EEG power was analyzed separately for data during active and quiet sleep. EEG Power in quiet sleep was not significantly associated with any of the outcomes of interest. This may possibly be due to less EEG data during quiet sleep, as neonates spend much more of their time in active vs. quiet sleep (Barnard, 1999). Therefore, in the subsequent results only EEG power in active sleep will be reported. After controlling for covariates, neonatal EEG was not significantly associated with MCHAT-R/F, PARCA-R cognitive, or PARCA-R linguistic scores.
Table 1.
Descriptive Statistics
| Mean (SD; Range) or N (%) | |
|---|---|
| Gestational Age at Birth | 39.42 weeks (1.1; 37.14 – 41.86) |
| Age at Assessment | 30.29 months (4.3; 24 – 36) |
| Sex | |
| Male | 55 (42.6%) |
| Female | 74 (57.4%) |
| Parental education | 15.06 years (2.1; 10 – 18) |
| MCHAT | .37 (0.8; 0 – 4) |
| BITSEA Problem | 8.69 (5.0; 0 – 32) |
| BITSEA Competence | 18.67 (2.2; 12 – 22) |
| BITSEA ASD Risk | 3.43 (2.7; 0 – 18) |
| PARCA-R Cognitive | 28.21 (4.0; 14 – 34) |
| PARCA-R Linguistic | 82.42 (29.1; 1 – 124) |
EEG Power Associated with BITSEA ASD Risk Scores.
Controlling for covariates, significant associations were found between EEG activity in active sleep in all higher frequencies of interest (primarily in the frontal and parietal regions of the brain) and both BITSEA problem and BITSEA competence scores; however, after running false discovery rate (FDR) to correct for multiple comparisons, significance did not hold. Even after multiple comparison correction, BITSEA ASD Risk scores were significantly linked to higher frequency EEG power in all higher frequencies of interest except within 37–48 Hz. Specifically, lower BITSEA risk scores were significantly associated with increased EEG power in right frontal-polar (13–15, 16–18, and 22–24 Hz), left parietal (13–36 Hz), and right temporal (13–15, 16–18, 22–24, and 25–36 Hz) brain regions (Table 2, Figure 1).
Table 2.
BITSEA ASD Risk Results by Frequencies
| Brain Region | EEG Frequency Range | Coefficient (p-value; adjusted R2) |
|---|---|---|
| Right Frontal-Polar | 13−15 Hz | −.247 (0.012; 0.13) |
| 16−18 Hz | −.292 (0.003; 0.16) | |
| 22−24 Hz | −.288 (0.003; .15) | |
| Left Parietal | 13−15 Hz | −.245 (0.010; 0.11) |
| 16−18 Hz | −.263 (0.006; 0.11) | |
| 19−21 Hz | −.252 (0.008; 0.11) | |
| 22−24 Hz | −.255 (0.007; 0.11) | |
| 25−36 Hz | −.230 (0.015; 0.10) | |
| Right Temporal | 13−15 Hz | −.222 (0.12; .13) |
| 16−18 Hz | −.215 (0.015; 0.12) | |
| 22−24 Hz | −.229 (0.009; 0.133) | |
| 25−36 Hz | −.222 (0.011; 0.130) | |
Figure 1. EEG Power and ASD Risk.
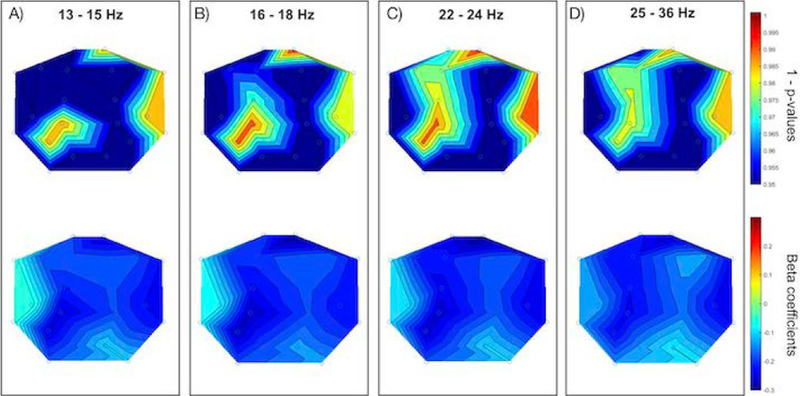
Each panel displays the scalp topography of 1 - p-values (upper) and beta coefficients (lower) for EEG power in active sleep and BITSEA autism risk scores. Panel A displays 13 – 15 Hz, Panel B displays 16 – 18 Hz, Panel C displays 22 – 24 Hz, and Panel D displays 25 – 36 Hz.
Sex Specific Analyses.
As sex specific effects have been reported relating to ASD and other neurodevelopmental disorders (Clark, Barry, McCarthy, & Selikowitz, 2001; Werling & Geschwind, 2013), analyses were then re-run for males (N= 55) and females (N = 74) separately. For males, when controlling for gestational age at birth, maternal education, and age at the time of 2nd assessment, significant FDR-corrected associations were between neonatal EEG power and both BITSEA competence and ASD risk scores. Specifically, higher BITSEA competence scores were associated with: increased higher frequency EEG power in the right frontal-polar (13–36 Hz), left frontal-polar (16–18 Hz, 22–36 Hz), right frontal (13–18 Hz, 22–24 Hz), left frontal (13–18 Hz, 22–36 Hz),left parietal (13–24 Hz), right temporal (13–36 Hz), and left occipital (13–36 Hz) brain regions (Table 3, Figure 2). Lower BITSEA ASD Risk scores were significantly associated with: increased EEG power in right temporal (13–36 Hz), and right frontal (22–24 Hz) brain regions. No FDR corrected associations were found between any of the BITSEA scores and the 37–48 Hz frequency range. Additionally, for males, there were no significant associations between neonatal EEG and the other developmental assessments.
Table 3.
BITSEA Results by Frequencies for Males Only
| Brain Region | EEG Frequency Range | Coefficient (p-value; adjusted R2) | |
|---|---|---|---|
| BITSEA Competence | Right Frontal-Polar | 13–15 Hz | .455 (0.004; 0.17) |
| 16–18 Hz | .548 (0.001; 0.25) | ||
| 19–21 Hz | .450 (0.007; 0.15) | ||
| 22–24 Hz | .520 (0.001; 0.21) | ||
| 25–36 Hz | .493 (0.003; 0.18) | ||
| Left Frontal-Polar | 16–18 Hz | .441 (0.008; 0.13) | |
| 22–24 Hz | .410 (0.016; 0.10) | ||
| 25–36 Hz | .463 (0.007; 0.14) | ||
| Right Frontal | 13–15 Hz | .347 (0.016; 0.10) | |
| 16–18 Hz | .370 (0.01; 0.12) | ||
| 22–24 Hz | .370 (0.009; 0.12) | ||
| Left Frontal | 13–15 Hz | .401 (0.007; 0.11) | |
| 16–18 Hz | .474 (0.001; 0.17) | ||
| 22–24 Hz | .406 (0.008; 0.11) | ||
| 25–36 Hz | .390 (0.012; 0.09) | ||
| Left Parietal | 13–15 Hz | .473 (0.002; 0.20) | |
| 16–18 Hz | .536 (0.0004; 0.25) | ||
| 19–21 Hz | .483 (0.002; 0.20) | ||
| 22–24 Hz | .463 (0.002; 0.20) | ||
| Right Temporal | 13–15 Hz | .424 (0.004; 0.16) | |
| 16–18 Hz | .451 (0.002; 0.18) | ||
| 19–21 Hz | .455 (0.002; 0.18) | ||
| 22–24 Hz | .507 (0.0004; 0.24) | ||
| 25–36 Hz | .462 (0.001; 0.21) | ||
| Left Occipital | 13–15 Hz | .456 (0.002; 0.19) | |
| 16–18 Hz | .519 (0.0003; 0.26) | ||
| 19–21 Hz | .506 (0.001; 0.24) | ||
| 22–24 Hz | .505 (0.001; 0.24) | ||
| 25–36 Hz | .445 (0.003; 0.19) | ||
| BITSEA ASD Risk | Right Frontal | 22–24 Hz | −.398 (0.004; 0.17) |
| Right Temporal | 13–15 Hz | −.452 (0.002; 0.17) | |
| 16–18 Hz | −.456 (0.002; 0.17) | ||
| 19–21 Hz | −.443 (0.004; 0.16) | ||
| 22–24 Hz | −.502 (0.0005; 0.22) | ||
| 25–36 Hz | −.458 (0.001; 0.20) | ||
Figure 2. EEG Power at 16 – 18 Hz , ASD Risk, and Social-Emotional Competence.
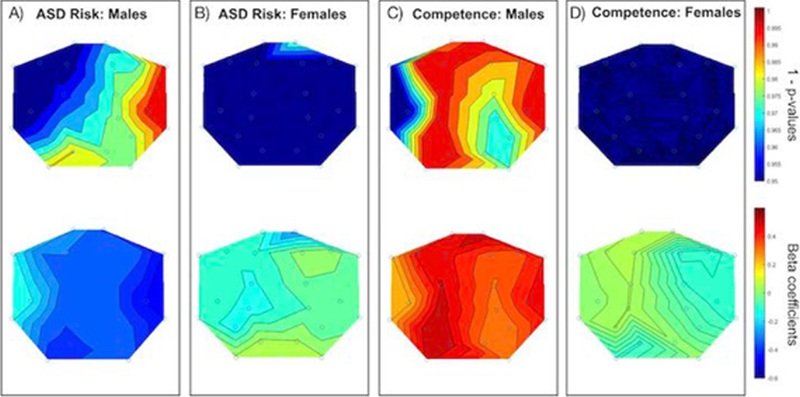
Panels A and B display the scalp topography of 1 - p-values (upper) and beta coefficients (lower) for EEG power at 16 – 18 Hz in active sleep and BITSEA autism risk scores broken down by sex (Panel A: Males; Panel B: Females). Panels C and D are the same as A and B but for BITSEA competence scores.
For females, no FDR corrected associations were found for neonatal EEG and BITSEA scores (Figure 1) or any of the other developmental assessments.
Post Hoc Analyses: EEG Asymmetry.
Past studies have reported that individuals with ASD often show abnormal hemispheric asymmetry. To probe if the association between neonatal EEG and BITSEA scores were driven by differences in lateralization, asymmetry values were calculated (difference between right and left power values in each brain region of interest). No significant correlations were found among any of the asymmetry values and BITSEA scores (Problem, Competence, or ASD Risk).
Exploratory Analyses in Lower Frequencies.
Although hypotheses were proposed specifically for higher frequencies, to gain a more complete picture of how oscillations of varying rates may impact development, exploratory analyses were completed to examine correlations in within the lower frequencies (1–12 Hz). Significant correlations were found for right frontal power (1–3 Hz) to PARCAR-R Linguistic scores and EEG power (left frontal-polar, left frontal, and left parietal) to BITSEA competency scores, but these did not survive FDR correction. EEG power (left frontal-polar, left parietal, and right temporal) within 4–12 Hz were significantly linked to BITSEA ASD Risk scores, but only 10–12 Hz in the left parietal and right temporal brain regions passed multiple comparisons correction.
Discussion
The aim of the current study was to examine whether neonatal EEG was associated with later neurodevelopmental outcomes, particularly those related to ASD specific behaviors during toddlerhood. We found no significant associations between neonatal EEG power and later outcome scores for the MCHAT-R/F or PARCA-R cognitive assessments. We did find significant associations among neonatal EEG power for higher frequencies in frontal-polar, temporal, and parietal brain regions and the ASD risk score from the BITSEA assessment. Specifically, we found that increased EEG power was associated with lower autism risk, consistent with our hypothesis that children at risk for neurodevelopmental disorders would have reduced neonatal EEG power. Re-running analyses separately for each sex, no significant links were found between neonatal EEG and later developmental outcomes for females, but analyses yielded significant correlations for males. For males, higher EEG power within higher frequencies was related to both higher BITSEA socio-emotional competence scores and lower BITSEA ASD risk scores.
While several significant results were found when examining the BITSEA socio-emotional scores, no significant associations were discovered between neonatal EEG and later outcomes in the PARCA-R or MCHAT-R/F scores. Despite previous research showing positive correlations between EEG power at birth and 12- (Levin et al., 2017) and 15-month language outcomes (Brito et al., 2016), we found no associations between neonatal EEG and PARCA-R linguistic or cognitive scores. Prior studies reporting these links had used experimenter elicited/observed language measures (Levin et al., 2017: Mullen Scales of Early Learning; Brito et al., 2016: Preschool Language Scale); parent-report assessments of vocabulary and linguistic complexity could potentially be biased and have led to decreased variability within the scores. Similarly, no significant associations were found between neonatal EEG and MCHAT-R/F scores. The MCHAT is a short assessment with only 24 questions but has demonstrated high sensitivity and specificity for a level 1 ASD screening tool (Robins et al., 2014). Since only 1 in 68 children will most likely be diagnosed with autism (Christensen et al., 2016), we could not expect to have a high number of children displaying overt behavioral symptoms typical of ASD out of our small participant pool. Only 3 of the 129 participants failed the follow up MCHAT-R/F assessment, thus there was not enough data to make any statement regarding EEG values at birth and later MCHAT ASD outcomes.
In addition to spectral power differences, past studies have reported that individuals with ASD often show abnormal hemispheric asymmetry, with more power in the left compared with the right hemisphere across various frequency bands (Gabard-Durnam, Tierney, Vogel-Farley, Tager-Flusberg, & Nelson, 2015; Stroganova et al., 2007; Sutton et al., 2005). Gabard-Durnam and colleagues (2015) reported left frontal asymmetry for 6-month-old infants at higher risk for ASD (i.e., infants who had older siblings with ASD) relative to low-risk infants who demonstrated right frontal asymmetry. The researchers noted that low-risk children followed a developmental pattern of initial relative right frontal asymmetry towards relative left frontal asymmetry, whereas the high-risk children showed the opposite pattern. Interestingly, there were no group differences in asymmetry values at 18-months of age (Gabard-Durnam et al., 2015). Within the current study, post hoc analyses were conducted examining links between asymmetry and developmental outcomes at 24 to 36 months, but no significant associations were found. As past studies have reported reduced coherence among individuals with ASD between frontal regions and all other scalp regions (Murias, Webb, Greenson, & Dawson, 2007) and poor interhemispheric connectivity (Isler, Martien, Grieve, Stark, & Herbert, 2010), future studies should also examine EEG coherence and relative power in addition to absolute power values. Within our current analyses, as the most significant findings we found were in the right frontal polar and left parietal brain regions, it would be interesting to systematically examine inter-region connectivity in future analyses in children at higher risk for autism compared to those at lower risk.
The current findings demonstrate some potential associations between early neural biomarkers and later risk for ASD. Parental report BITSEA scores have been shown to predict emotional/behavioral problems in early elementary school (Briggs-Gowan & Carter, 2008) and a recent study found that the BITSEA had good discriminative power to differentiate children with and without ASD and may therefore be helpful in the detection of early neurodevelopmental risk in relation to ASD (Kruizinga, et al., 2014). Interestingly, significant associations within the current study were found for males and not females, mimicking the sex differences often reported in ASD studies. The BITSEA Autism risk score has previously been shown to differentiate children with and without ASD with high accuracy (Karabekiroglua, et al., 2014). Here we show links between higher-frequency oscillations, particularly in the frontal-polar, parietal and temporal regions, and potential risk for ASD, with reduced EEG power associated with increased risk. Examining higher frequencies within the EEG literature, gamma oscillations have been implicated in in cognitive and sensory processing (Lee & Jones, 2013; Tiesinga & Sejnowski, 2009), and while the beta frequency range has most prominently been related to sensorimotor activity (Har & Salmelin, 1997), it has increasingly been associated with a wider range of cognitive functions including visual perception (Kloosterman et al., 2014), language processing (Weiss & Mueller, 2012), and memory (Hanslmayr et al., 2016). The results from this study support previous findings with both typical children (Brito et al., 2016; Gou et al., 2011; Williams et al., 2012) and children at higher-risk for ASD (Tierney et al., 2012; Levin et al., 2017) and suggest that links between EEG activity within higher frequencies (i.e., over 13 Hz) may be associated with individual differences in developmental outcomes reflective of higher order cognitive processing. Although parental report may not always accurately reflect a child’s true status, the BITSEA does have a strong predictive validity, with early BITSEA ratings strongly correlated with later ratings (Briggs-Gowan & Carter, 2008; Briggs-Gowan et al., 2004)
This is the first study, to our knowledge, to capitalize on neonatal EEG measures to examine early electrophysiology and subsequent parental report of neurodevelopmental disorder risk, but it is important to note limitations with the current study. First, we do not have a measure of whether these children do go on to be diagnosed with ASD or any other neurodevelopmental disorder. These are correlational results and cannot be treated as deterministic on any level. Rather, these results suggest that reduced EEG power in the higher frequencies may indicate an increase in an individual’s susceptibility for later atypical symptoms or delays in behaviors. As such, neonatal EEG activity may be a suitable biomarker to identify children who may be at a higher risk of neurodevelopmental disorders, but this does not mean that these children will go on to develop these conditions. Second, although there was variation in maternal educational attainment within the sample, as infants were primarily from middle to upper-middle-class Caucasian families, this study needs to be replicated within a larger cohort of infants from a wider range of socioeconomic backgrounds and experiences. As we excluded infants who were born before 37 weeks gestational age or admitted to the NICU at birth, this resulted in a sample of infants with potentially fewer adverse prenatal exposures, e.g., alcohol, smoking, recreational drugs, and/or psychiatric medications. In other studies, infants with high prenatal exposure to alcohol have been shown to demonstrate hypersynchrony, resulting in significantly higher power in a wide range of frequencies (Havlicek, Childiaeva, & Chernick, 1977; Chernick, Childiaeva, & Ioffe, 1983; Stephen et al., 2018), therefore the directionality of EEG power and developmental outcomes may vary based on the characteristics of the sample.
ASD is typically diagnosed around four years of age, at which point the opportunity for very early intervention has been missed. Early intervention alters brain development and results in developmental gains in communication, social interaction, and cognitive ability (Woods & Wetherby, 2003). Implementing routine non-invasive EEG data collection at birth and/or at well-baby visits could provide an easily attainable rich source of data. Baseline EEG could be used as major component of a screening profile for infants at high risk of developing neurodevelopmental disorders and could facilitate intervention for the highest risk infants before behavioral symptoms emerge. Diagnosing children after infancy deprives them of the opportunity to benefit from effective early interventions. Determining a profile of perinatal, neonatal, and social risk factors linked to the development of neurodevelopmental disorders will help researchers and clinicians better target prevention, early diagnosis, and early intervention efforts.
Acknowledgements:
Authors would like to thank Luke Mack, Erin Holahan, and Daianna Rodriguez for their invaluable help in collecting and coding data. This publication was supported by the Rita G. Rudel Prize and Sackler Parent-Infant Project Fellowship to NHB, T32MH016434-40 fellowship funding to LCS and NIH grants: R00HD086255-03, UL1TR000040, U01 HD55155, U01 HD045935 & R37 HD032774.
References
- American Psychiatric Association. (2016). Diagnostic and Statistical Manual of Mental Disorders Text revision Washington, DC: American Psychiatric Association; 2000; 771–774. DSM-IV TR, 679. [Google Scholar]
- Baio J (2014). Developmental Disabilities Monitoring Network Surveillance Year 2010 Principal Investigators; Centers for Disease Control and Prevention (CDC). Prevalence of autism spectrum disorder among children aged 8 years—Autism and developmental disabilities monitoring network, 11 sites, United States, 2010. MMWR Surveill. Summ, 63, 1–21. [PubMed] [Google Scholar]
- Barnard KE (1999). Beginning rhythms: The emerging process of sleep wake behaviors and self-regulation Seattle: NCAST, University of Washington [Google Scholar]
- Barry RJ, Clarke AR, & Johnstone SJ (2003). A review of electrophysiology in attention-deficit/hyperactivity disorder: I. Qualitative and quantitative electroencephalography. Clinical neurophysiology, 114(2), 171–183. [DOI] [PubMed] [Google Scholar]
- Blaggan S, Guy A, Boyle EM, Spata E, Manktelow BN, Wolke D, & Johnson S (2014). A parent questionnaire for developmental screening in infants born late and moderately preterm. Pediatrics, 134(1), e55–e62. [DOI] [PubMed] [Google Scholar]
- Briggs-Gowan MJ, & Carter AS (2007). Applying the infant-toddler social & emotional assessment (ITSEA) and brief-ITSEA in early intervention. Infant Mental Health Journal, 28(6), 564–583. [DOI] [PubMed] [Google Scholar]
- Briggs-Gowan MJ, & Carter AS (2008). Social-emotional screening status in early childhood predicts elementary school outcomes. Pediatrics, 121(5), 957–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs-Gowan MJ, Carter AS, Irwin JR, Wachtel K, & Cicchetti DV (2002). Brief Infant-Toddler Social and Emotional Assessment (BITSEA) manual, version 2.0 [DOI] [PubMed]
- Brito NH, Fifer WP, Myers MM, Elliott AJ, & Noble KG (2016). Associations among family socioeconomic status, EEG power at birth, and cognitive skills during infancy. Developmental Cognitive Neuroscience, 19, 144–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (2009). Prevalence of autism spectrum disorders--Autism and Developmental Disabilities Monitoring Network, 14 sites, United States (2008). Autism and Developmental Disabilities Monitoring Network Surveillance Year 2008 Principal Investigators; National Library of Medicine. Morbidity and mortality weekly report. Surveillance summaries (Washington, D.C. :2002) Vol. 61, Iss. 3, (March 30, 2012): 1–19. [PubMed] [Google Scholar]
- Chernick V, Childiaeva R, & Ioffe S (1983). Effects of maternal alcohol intake and smoking on neonatal electroencephalogram and anthropometric measurements. American Journal of Obstetrics & Gynecology, 146(1), 41–47. [DOI] [PubMed] [Google Scholar]
- Christensen DL, Bilder DA, Zahorodny W, Pettygrove S, Durkin MS, Fitzgerald RT, … & Yeargin-Allsopp M (2016). Prevalence and characteristics of autism spectrum disorder among 4-year-old children in the autism and developmental disabilities monitoring network. Journal of Developmental & Behavioral Pediatrics, 37(1), 1–8. [DOI] [PubMed] [Google Scholar]
- Clarke AR, Barry RJ, McCarthy R, & Selikowitz M (2001). Age and sex effects in the EEG: development of the normal child. Clin Neurophysiol, 112(5), 806–814. [DOI] [PubMed] [Google Scholar]
- Clarke AR, Barry RJ, McCarthy R, & Selikowitz M (2001). Excess beta activity in children with attention-deficit/hyperactivity disorder: an atypical electrophysiological group. Psychiatry Research, 103(2), 205–218. [DOI] [PubMed] [Google Scholar]
- Dani VS, Chang Q, Maffei A, Turrigiano GG, Jaenisch R, & Nelson SB (2005). Reduced cortical activity due to a shift in the balance between excitation and inhibition in a mouse model of Rett syndrome. Proceedings of the National Academy of Sciences of the United States of America, 102(35), 12560–12565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lacoste MC, Horvath DS, & Woodward DJ (1991). Possible sex differences in the developing human fetal brain. J Clin Exp Neuropsychol, 13(6), 831–846. doi: 10.1080/01688639108405101 [DOI] [PubMed] [Google Scholar]
- Dudley E, Häßler F, & Thome J (2011). Profiling for novel proteomics biomarkers in neurodevelopmental disorders. Expert review of proteomics, 8(1), 127–136. [DOI] [PubMed] [Google Scholar]
- Dukes KA, Burd L, Elliott AJ, Fifer WP, Folkerth RD, Hankins GD, … & Signore C (2014). The S afe P assage S tudy: Design, Methods, Recruitment, and Follow-Up Approach. Pediatric and Perinatal Epidemiology, 28(5), 455–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durkin MS, Maenner MJ, Meaney FJ, Levy SE, DiGuiseppi C, Nicholas JS, … & Schieve LA (2010). Socioeconomic inequality in the prevalence of autism spectrum disorder: evidence from a US cross-sectional study. PLoS One, 5(7), e11551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabard-Durnam L, Tierney AL, Vogel-Farley V, Tager-Flusberg H, & Nelson CA (2015). Alpha asymmetry in infants at risk for autism spectrum disorders. Journal of Autism and Developmental Disorders, 45(2), 473–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner LM, Murphy L, Campbell JM, Tylavsky F, Palmer FB, & Graff JC (2013). Screening accuracy for risk of autism spectrum disorder using the Brief Infant-Toddler Social and Emotional Assessment (BITSEA). Research in Autism Spectrum Disorders, 7(5), 591–600. [Google Scholar]
- Giedd JN, Castellanos FX, Rajapakse JC, Vaituzis AC, & Rapoport JL (1997). Sexual dimorphism of the developing human brain. Prog Neuropsychopharmacol Biol Psychiatry, 21(8), 1185–1201. [DOI] [PubMed] [Google Scholar]
- Goldani AA, Downs SR, Widjaja F, Lawton B, & Hendren RL (2014). Biomarkers in autism. Frontiers in Psychiatry, 5, 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gou Z, Choudhury N, & Benasich AA (2011). Resting frontal gamma power at 16, 24 and 36 months predicts individual differences in language and cognition at 4 and 5 years. Behavioural Brain Research, 220(2), 263–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanslmayr S, Staresina BP, & Bowman H (2016). Oscillations and episodic memory: addressing the synchronization/desynchronization conundrum. Trends in neurosciences, 39(1), 16–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havlicek V, Childiaeva R, & Chernick V (1977). EEG frequency spectrum characteristics of sleep states in infants of alcoholic mothers. Neuropaediatrie, 8(04), 360–373. [DOI] [PubMed] [Google Scholar]
- Hsiao EY, McBride SW, Hsien S, Sharon G, Hyde ER, McCue T, … & Patterson PH (2013). Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell, 155(7), 1451–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isler JR, Martien KM, Grieve PG, Stark RI, & Herbert MR (2010). Reduced functional connectivity in visual evoked potentials in children with autism spectrum disorder. Clinical Neurophysiology, 121(12), 2035–2043. [DOI] [PubMed] [Google Scholar]
- Isler JR, Thai T, Myers MM, Fifer WP (2016). An automated method for coding sleep states in human infants based on respiratory rate variability. Developmental Psychobiology 58(8):1108–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeste SS, Frohlich J, & Loo SK (2015). Electrophysiological biomarkers of diagnosis and outcome in neurodevelopmental disorders. Current opinion in neurology, 28(2), 110–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson CP, & Myers SM (2007). Identification and evaluation of children with autism spectrum disorders. Pediatrics, 120(5), 1183–1215. [DOI] [PubMed] [Google Scholar]
- Jones EJ, Gliga T, Bedford R, Charman T, & Johnson MH (2014). Developmental pathways to autism: a review of prospective studies of infants at risk. Neuroscience & Biobehavioral Reviews, 39, 1–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloosterman NA, Meindertsma T, Hillebrand A, van Dijk BW, Lamme VA, & Donner TH (2014). Top-down modulation in human visual cortex predicts the stability of a perceptual illusion. Journal of neurophysiology, 113(4), 1063–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruizinga I, Visser JC, van Batenburg-Eddes T, Carter AS, Jansen W, & Raat H (2014). Screening for autism spectrum disorders with the brief infant-toddler social and emotional assessment. PloS One, 9(5), e97630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, & Jones SR (2013). Distinguishing mechanisms of gamma frequency oscillations in human current source signals using a computational model of a laminar neocortical network. Frontiers in human neuroscience, 7, 869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin AR, Varcin KJ, O’Leary HM, Tager-Flusberg H, & Nelson CA (2017). EEG power at 3 months in infants at high familial risk for autism. Journal of Neurodevelopmental Disorders, 9(1), 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt P, Eagleson KL, & Powell EM (2004). Regulation of neocortical interneuron development and the implications for neurodevelopmental disorders. Trends in Neurosciences, 27(7), 400–406. [DOI] [PubMed] [Google Scholar]
- Levy Y (2018). ‘Developmental Delay’reconsidered: The critical role of age-dependent, co-variant development. Frontiers in psychology, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marosi E, Harmony T, Becker J, Bernal J, Reyes A, Rodriguez M, & Fernandez T (1993). Sex differences in EEG coherence in normal children. Int J Neurosci, 72(1–2), 115–121. [DOI] [PubMed] [Google Scholar]
- Matoušek M, & Petersén I (1973). Automatic evaluation of EEG background activity by means of age-dependent EEG quotients. Electroencephalography and Clinical Neurophysiology, 35(6), 603–612. [DOI] [PubMed] [Google Scholar]
- Murias M, Webb SJ, Greenson J, & Dawson G (2007). Resting state cortical connectivity reflected in EEG coherence in individuals with autism. Biological Psychiatry, 62(3), 270–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orekhova EV, Stroganova TA, Nygren G, Tsetlin MM, Posikera IN, Gillberg C, & Elam M (2007). Excess of high frequency electroencephalogram oscillations in boys with autism. Biological Psychiatry, 62(9), 1022–1029. [DOI] [PubMed] [Google Scholar]
- Reeb-Sutherland BC, & Fox NA (2015). Eyeblink conditioning: a non-invasive biomarker for neurodevelopmental disorders. Journal of Autism and Developmental Disorders, 45(2), 376–394. [DOI] [PubMed] [Google Scholar]
- Robins DL, Casagrande K, Barton M, Chen CMA, Dumont-Mathieu T, & Fein D (2014). Validation of the modified checklist for autism in toddlers, revised with follow-up (M-CHAT-R/F). Pediatrics, 133(1), 37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins DL, Fein D, Barton ML, & Green JA (2001). The Modified Checklist for Autism in Toddlers: an initial study investigating the early detection of autism and pervasive developmental disorders. Journal of Autism and Developmental Disorders, 31(2), 131–144. [DOI] [PubMed] [Google Scholar]
- Rubenstein JL (2010). Three hypotheses for developmental defects that may underlie some forms of autism spectrum disorder. Current Opinion in Neurology, 23(2), 118–123. [DOI] [PubMed] [Google Scholar]
- Stephen JM, Flynn L, Kabella D, Schendel M, Cano S, Savage DD, … & Bakhireva LN (2018). Hypersynchrony in MEG spectral amplitude in prospectively-identified 6-month-old infants prenatally exposed to alcohol. NeuroImage: Clinical, 17, 826–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton SK, Burnette CP, Mundy PC, Meyer J, Vaughan A, Sanders C, & Yale M (2005). Resting cortical brain activity and social behavior in higher functioning children with autism. Journal of Child Psychology and Psychiatry, 46(2), 211–222. [DOI] [PubMed] [Google Scholar]
- Tierney AL, Gabard-Durnam L, Vogel-Farley V, Tager-Flusberg H, & Nelson CA (2012). Developmental trajectories of resting EEG power: an endophenotype of autism spectrum disorder. PloS One, 7(6), e39127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiesinga P, & Sejnowski TJ (2009). Cortical enlightenment: are attentional gamma oscillations driven by ING or PING?. Neuron, 63(6), 727–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomalski P, Moore DG, Ribeiro H, Axelsson EL, Murphy E, Karmiloff-Smith A, … & Kushnerenko E (2013). Socioeconomic status and functional brain development–associations in early infancy. Developmental Science, 16(5), 676–687. [DOI] [PubMed] [Google Scholar]
- Varcin KJ, & Nelson CA III (2016). A developmental neuroscience approach to the search for biomarkers in autism spectrum disorder. Current Opinion in Neurology, 29(2), 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Barstein J, Ethridge LE, Mosconi MW, Takarae Y, & Sweeney JA (2013). Resting state EEG abnormalities in autism spectrum disorders. Journal of Neurodevelopmental Disorders, 5(1), 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S, & Mueller HM (2012). “Too many betas do not spoil the broth”: the role of beta brain oscillations in language processing. Frontiers in Psychology, 3, 201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werling DM, & Geschwind DH (2013). Sex differences in autism spectrum disorders. Current Opinion in Neurology, 26(2), 146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams IA, Tarullo AR, Grieve PG, Wilpers A, Vignola EF, Myers MM, & Fifer WP (2012). Fetal Cerebrovascular Resistance and Neonatal EEG Predict 18-month Neurodevelopmental Outcome in Infants with Congenital Heart Disease. Ultrasound in Obstetrics & Gynecology 40(3), 304–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods JJ, & Wetherby AM (2003). Early identification of and intervention for infants and toddlers who are at risk for autism spectrum disorder. Language, Speech, and Hearing Services in Schools, 34(3), 180–193. [DOI] [PubMed] [Google Scholar]


