Abstract
Post-mastectomy radiotherapy (PMRT) has been shown to improve the overall survival for invasive breast cancer patients, and many advanced radiotherapy technologies were adopted for PMRT. The purpose of our study is to compare various advanced PMRT techniques including fixed-beam intensity-modulated radiotherapy (IMRT), non-coplanar volumetric modulated arc therapy (NC-VMAT), multiple arc VMAT (MA-VMAT), and Tomotherapy (TOMO). Results of standard VMAT and mixed beam therapy that were published by our group previously were also included in the plan comparisons. Treatment plans were produced for nine PMRT patients previously treated in our clinic. The plans were evaluated based on planning target volume (PTV) coverage, dose homogeneity index (DHI), conformity index (CI), dose to organs at risk (OARs), normal tissue complication probability (NTCP) of pneumonitis, lifetime attributable risk (LAR) of second cancers, and risk of coronary events (RCE). All techniques produced clinically acceptable PMRT plans. Overall, fixed-beam IMRT delivered the lowest mean dose to contralateral breast (1.56 ± 0.4 Gy) and exhibited lowest LAR (0.6 ± 0.2%) of secondary contralateral breast cancer; NC-VMAT delivered the lowest mean dose to lungs (7.5 ± 0.8 Gy), exhibited lowest LAR (5.4 ± 2.8%) of secondary lung cancer and lowest NTCP (2.1 ± 0.4%) of pneumonitis; mixed beam therapy delivered the lowest mean dose to heart (7.1 ± 1.3 Gy) and shown lowest RCE (8.6 ± 7.1%); TOMO plans provided the most optimal target coverage while delivering higher dose to OARs than other techniques. Both NC-VMAT and MA-VMAT exhibited lower values of all OARs evaluation metrics compare to standard VMAT. Fixed-beam IMRT, NC-VMAT, and mixed beam therapy could be the optimal radiation technique for certain breast cancer patients after mastectomy.
Keywords: Post-mastectomy radiotherapy, intensity-modulated radiotherapy, volumetric modulated arc therapy, Tomotherapy
Introduction
About 1 in 8 women will develop invasive breast cancer over the course of her lifetime in the US (www.cancer.org). Due to the prevalence of microscopic diseases after the mastectomy, post-mastectomy radiotherapy (PMRT) is commonly performed on these patients to sterilize the residual tumor cells, and has been shown to improve the overall survival for invasive breast cancer patients by reducing the risk of tumor recurrence and cancer mortality.1
The role of PMRT in breast cancer care is evolving rapidly with the adoption of new radiotherapy technologies: fixed beam intensity-modulated radiotherapy (IMRT) has been shown to be a preferred technique for PMRT patients and has a good balance of target coverage and normal tissue sparing;2 the standard of care for PMRT at our institution has been volumetric modulated arc therapy (VMAT) or Helical Tomotherapy (TOMO).3, 4 Both modalities provide good target coverage and dose homogeneity, but the stray radiation dose to organs at risk (OARs) is a concern;5 bolus electron conformal therapy (BECT) mixed with IMRT and VMAT (mixed beam therapy) for PMRT has been recently evaluated for real patients’ treatment planning by our group and can potentially reduce risks of normal tissue complications.6 Apart from these previously reported PMRT techniques, multiple arc VMAT (MA-VMAT) which consists of 6 small partial arcs showed good feasibility and OAR sparing7 for whole breast radiotherapy but has not been evaluated for PMRT; non-coplanar VMAT (NC-VMAT) has been shown to improve OAR dosimetry for intracranial tumors,8, 9 early stage Hodgkin’s lymphoma,10 liver cancer11 and prostate cancer,12 but has not been investigated for PMRT either.
There have been some treatment planning studies of PMRT,2–4, 6, 13–18 but most of them did not include any or only one advanced PMRT technique. The literature is largely incomplete regarding the systematic comparison of advanced technologies for post-mastectomy patients and these techniques are therefore implemented with very little evidence for safety or efficacy.
The purpose of this study was to compare predicted treatment outcomes (target coverage and risks of developing of radiogenic side effects) for a sample of PMRT patients using various advanced PMRT modalities, including fixed-beam IMRT, NC-VMAT, MA-VMAT, and TOMO. Standard VMAT and mixed beam therapy for PMRT have been reported by our group previously6 for the same sample of patients, and the outcome results using these modalities will be compared with the PMRT techniques investigated in this study.
Methods and Materials
Patient selection
Nine consecutively sampled left-sided post-mastectomy patients were retrospectively selected. All patients received a modified radical mastectomy and were treated at our institution. Computed tomography (CT) scans had been acquired and all patients were scanned in the supine position with the free breathing CT data sets including all anatomy from the top of the head down to the lower abdomen. All CT data sets were anonymized19 and assigned a unique research identifier, CW1 to CW9. The planning target volume (PTV) and organs at risk (OARs) for each patient were previously contoured by the same radiation oncologist. PTV included the left chest wall, left supraclavicular and axillary area, and internal mammary chain area. The patients had a 1-cm thick Superflab bolus (Radiation Products Design, Inc., Albertville, MN, USA) placed on the surface of their ipsilateral chest wall for the purpose of dose buildup.4 OARs included lungs, whole heart, contralateral breast, esophagus, trachea, and spinal cord.
Treatment planning
All plans used a prescribed dose of 50 Gy in 25 fractions. The following criteria were met for each treatment plan to be considered clinically acceptable: the volume of the PTV receiving at least 95% of the prescribed dose is greater than or equal to 95%; the volume of total lungs receiving at least 20 Gy is less than 20%;20 the volume of heart receiving at least 22.5 Gy is less than 20%21.
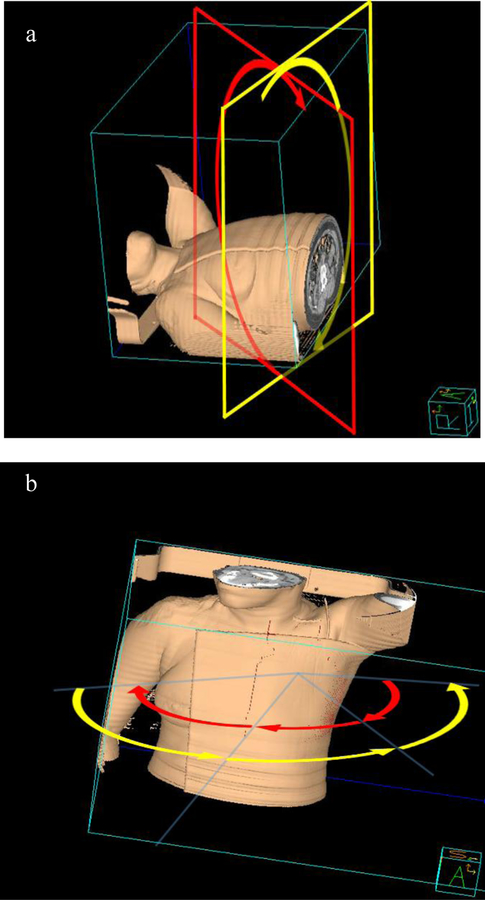
Fixed-beam IMRT plan was generated in a commercial treatment planning system (TPS) (Pinnacle3 v9.8, Philips Medical Systems, Fitchburg, WI, USA) using the direct machine parameter optimization (DMPO) optimization algorithm. Four or five co-planar 6 MV IMRT beams ranging from 150° to 315° were used to give enough PTV coverage. Each beam angle was individualized arranged for every patient in order to limit dose to the surrounding organs. Both NC-VMAT and MA-VMAT plans used 6 MV photon beams and were generated in Pinnacle using the SmartArc optimization algorithm. NC-VMAT plans utilized two partial arcs (Fig. 1 (a)): the first arc was planned to be delivered counterclockwise (CCW) with starting gantry angles between 170° to 180° and stopping gantry angles between 305° to 320° (same as standard VMAT plans that were previously reported by our group6) and with 15° couch angle, the second arc was planned to be delivered clockwise (CW) to over the same range of gantry angle and with 345° couch angle. The collimator was rotated to align with the long axis of PTV in both arcs. MA-VMAT plans consisted of six partial arcs (ARC01 to ARC06), each with 60° or 70° gantry rotations (Fig. 1 (b)). ARC01 to ARC03 were delivered CW and ARC04 to ARC06 were delivered CCW. The ARC01 started between 170° to 180° and ARC03 stopped between 305° to 320°. The ARC04 started between 305° to 320° and ARC06 stopped between 170° to 180°. The starting angle of ARC01 and stopping angle of ARC03 were the same as the standard VMAT plans. The collimator was always rotated to align with the long axis of PTV in each arc. For TOMO planning, the CT images and contours in Pinnacle were imported into TomoTherapy® Hi∙Art TPS (Accuray, Madison, WI) for plan optimization. Parameters for TOMO plan optimization included a pitch of 0.287, a modulation factor of 2.8 and a field width of 5.02 cm. The final TOMO dose distributions were transferred back to Pinnacle for comparison with other plans. The details of standard VMAT and mixed beam therapy treatment planning can be found in our previous publication.6
Fig. 1.
Three-dimensional display of (a) two non-coplanar partial arcs for NC-VMAT. Red plane represents gantry plane at 15° couch angle and the yellow plane represents gantry plane at 345° couch angle; (b) six partial arcs for MA-VMAT. The CW arcs display in yellow curvature and CCW arcs in red curvature.
Plan comparison metrics
Dose-volume histograms (DVHs) and dose-volume metrics were calculated for target volume, total lungs, heart and contralateral breast. Dose homogeneity index (DHI)22 and conformity index (CI)23 were calculated for the target coverage. Risks of developing of radiogenic side effects were calculated including lifetime attributable risk (LAR) of secondary lung and contralateral breast cancer, normal tissue complication probability (NTCP) for pneumonitis, and radiation-induced risk of coronary events (RCE).
LAR was calculated as the integration of excess absolute risk (EAR) using BEIR VII model 24:
where e is age at exposure, a is attained age, L is a risk-free latent period, is the probability of surviving to age conditional on survival to age e.25 EAR is calculated using following equations:
where OED is organ equivalent dose, β is dose response initial slope (βLung=7.5, βBreast= 9.2)26, µ is age correction factor, VT is the total organ volume, and vi is the volume receiving dose Di. µ was calculated for each patient according to Schneider et al.26 as follows:
where the age modifying factor and (γe,Lung = 0.002, γa,Lung = 4.23, γe,Breast = −0 037, γa,Breast =1.7) were taken from by Schneider et al.26
The Lyman-Kutcher-Burman (LKB) model27–29 was used to calculate NTCP for pneumonitis using the following equations:
where TD50 is the uniform dose given to the entire lung that results in 50% complication risk (TD50 = 30.8 Gy), m is a measure of the slope of the dose-response curve (m = 0.37), n is the volume effect parameter (n = 0.99), and v is the fractional volume irradiation to the uniform dose D.29
RCE was estimated using the dose-response model reported by Darby et al. 30:
where D (Gy) is the mean heart dose, Rbaseline is the baseline risk of coronary events and was calculated using Reynolds risk model31 assuming medium risk type.
Statistical analysis
The post hoc Tukey test was used to determine the statistical significance of the differences between two PMRT techniques. All statistical analyses were conducted with R software (version 3.2.3) and the differences were considered significant when p < 0.05.
Results
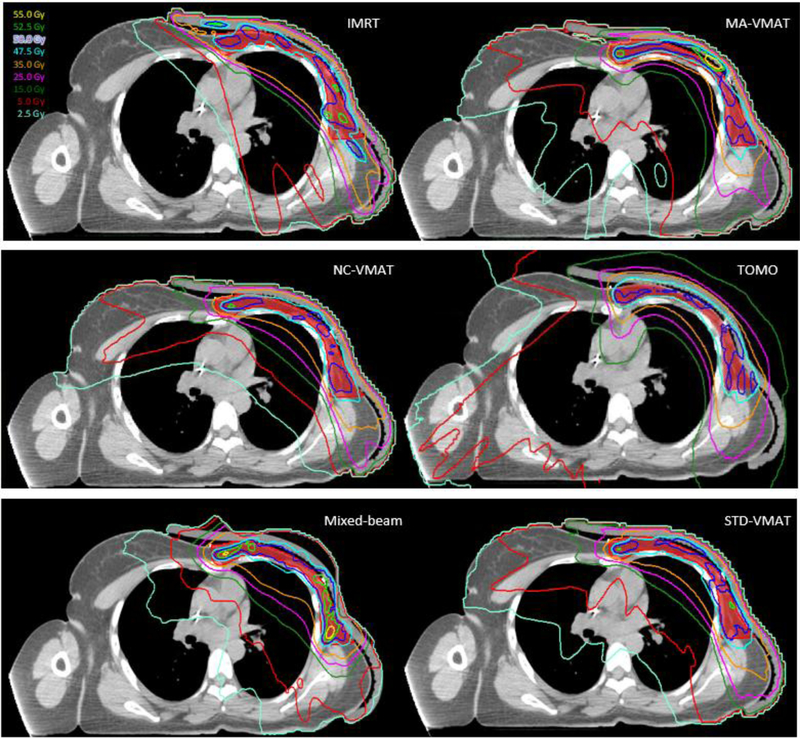
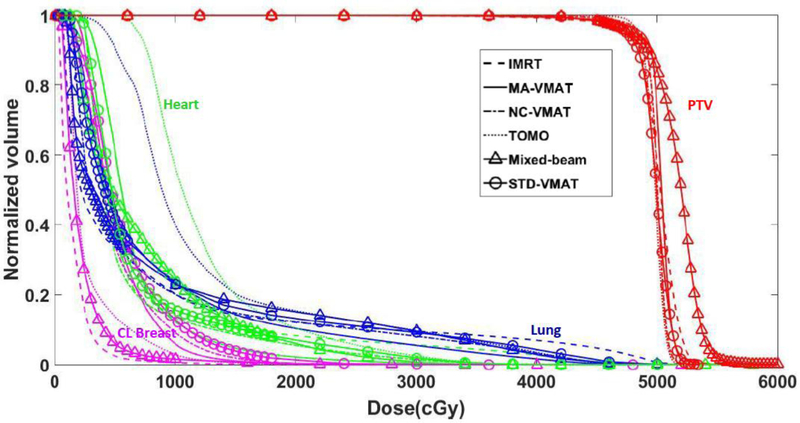
The dose distributions and DVHs for a representative patient are shown in Figs. 2 and 3, respectively. Table 1 lists the total average number of monitor units (MU), PTV and OARs evaluation metrics for various advanced PMRT techniques. The results of post hoc Tukey tests and p values are shown in Table 2.
Fig. 2.
Axial view of isodose distribution for fixed-beam IMRT, MA-VMAT, NC-VMAT, TOMO, mixed-beam therapy and standard VMAT plans for a typical PMRT patient. The red color wash represents the PTV.
Fig. 3.
DVHs for fixed-beam IMRT, MA-VMAT, NC-VMAT, TOMO, mixed-beam therapy and standard VMAT plans for a typical PMRT patient.
Table 1.
MU, PTV and OAR evaluation metrics (mean ± standard deviation) for nine PMRT patients. NC-VMAT: non-coplanar VMAT; MA-VMAT: multiple-arc VMAT; TOMO: Tomotherapy; Mixed: mixed beam therapy; MU: monitor unit; PTV: planning target volume; CL breast: contralateral breast. LAR: lifetime attributable risk; RCE: risk of coronary events.
| NC-VMAT | MA-VMAT | Fixed-beam IMRT | TOMO | Standard VMATa | Mixeda | |
|---|---|---|---|---|---|---|
| Average total MU | 13000.0 ±1725 | 14108.3 ±1839 | 18130.6 ±2155 | 97347.2 ±8824 | 11833.3 ±792 | 16916.7 ±3949 |
| PTV | ||||||
| Dmean (Gy) | 49.7±0.2 | 49.8±0.3 | 50.0±0.3 | 49.8±0.4 | 49.7 ± 0.3 | 51.6 ± 0.4 |
| Dmax (Gy) | 54.1±1.1 | 54.9±2.3 | 55.9±2.7 | 52.4±0.6 | 53.5 ± 0.7 | 59.9 ± 3.6 |
| V107% (%) | 0.0±0.0 | 0.0±0.0 | 0.0±0.0 | 0.0±0.0 | 0.0 ± 0.1 | 15.0 ± 8.6 |
| CI | 0.7±0.0 | 0.6±0.1 | 0.5±0.1 | 0.6±0.1 | 0.7 ± 0.0 | 0.5 ± 0.1 |
| DHI | 0.1±0.0 | 0.1±0.0 | 0.1±0.0 | 0.1±0.0 | 0.1 ± 0.0 | 0.2 ± 0.0 |
| Lungs | ||||||
| Dmean (Gy) | 7.5±0.8 | 7.7±0.9 | 7.9±0.6 | 10.6±1.5 | 8.7 ± 0.6 | 8.4 ± 0.9 |
| Dmax (Gy) | 47.7±1.9 | 48.5±2.6 | 52.8±1.7 | 47.0±1.7 | 51.1 ± 1.6 | 52.0 ± 2.2 |
| V5 | 33.3±4.9 | 35.7±4.9 | 34.4±3.1 | 69.5±20.3 | 43.5 ± 5.8 | 33.5 ± 2.6 |
| V10 | 20.0±2.2 | 21.3±3.6 | 24.9±4.0 | 31.2±7.9 | 24.3 ± 2.4 | 23.4 ± 2.9 |
| V20 | 12.3±1.0 | 12.3±1.8 | 15.2±5.1 | 12.9±2.1 | 13.0 ± 1.0 | 15.5 ± 2.6 |
| NTCP (%) | 2.1±0.4 | 2.2±0.5 | 2.3±0.3 | 3.8±1.3 | 2.7 ± 0.3 | 2.7 ± 0.5 |
| LAR (%) | 5.4±2.8 | 5.5±2.8 | 5.7±2.8 | 7.2±3.8 | 6.3±3.1 | 6.3±3.3 |
| Heart | ||||||
| Dmean (Gy) | 7.4±1.2 | 7.7±1.1 | 8.53±1.33 | 10.3±2.2 | 9.3 ± 1.1 | 7.1 ± 1.3 |
| Dmax (Gy) | 41.4±4.7 | 40.1±2.7 | 48.8±5.4 | 38.7±4.3 | 42.8 ± 3.6 | 38.9 ± 4.6 |
| V5 | 44.6±13.6 | 48.6±12.3 | 54.4±14.4 | 84.3±18.9 | 66.9 ± 13.0 | 44.3 ± 7.6 |
| V10 | 20.7±5.0 | 21.2±6.1 | 23.3±5.0 | 39.1±18.3 | 25.3 ± 4.1 | 21.0 ± 5.7 |
| V22.5 | 6.5±2.3 | 7.2±3.7 | 8.8±2.0 | 6.3±3.0 | 9.8 ± 1.9 | 4.5 ± 3.4 |
| V30 | 2.6±2.2 | 2.9±2.7 | 5.3±2.5 | 2.0±1.7 | 5.0 ± 2.6 | 1.3 ± 1.8 |
| RCE (%) | 8.9±7.3 | 8.9±7.3 | 9.5±8.0 | 9.8±7.7 | 9.7±8.0 | 8.6±7.1 |
| CL breast | ||||||
| Dmean (Gy) | 3.3±1.0 | 3.4±0.9 | 1.56±0.4 | 3.9±1.7 | 4.0 ± 1.1 | 1.8 ± 0.6 |
| Dmax (Gy) | 19.6±9.1 | 22.1±5.9 | 28.3±7.6 | 18.5±10.5 | 27.1 ± 8.4 | 26.6 ± 7.7 |
| V5 | 16.8±12.5 | 21.8±12.9 | 5.1±2.4 | 24.4±21.8 | 24.2 ± 12.1 | 4.6 ± 3.2 |
| LAR (%) | 1.2±0.8 | 1.4±0.7 | 0.6±0.2 | 1.6±0.8 | 1.7±0.8 | 1.1±0.6 |
Data taken from our previous work.6
Table 2.
p values for statistic comparison of six advanced PMRT techniques using post hoc Tukey test. NC-VMAT: non-coplanar VMAT; MA-VMAT: multiple-arc VMAT; TOMO: Tomotherapy; Mixed: mixed beam therapy; STD VMAT: standard VMAT; PTV: planning target volume; CL breast: contralateral breast.
| Variable | NC-VMAT vs MA-VMAT |
NC-VMAT vs Fixed beam IMRT |
NC-VMAT vs TOMO |
NC-VMAT vs STD VMAT |
NC-VMAT vs Mixed |
MA-VMAT vs Fixed beam IMRT |
MA-VMAT vs TOMO |
MA-VMAT vs STD VMAT |
MA-VMAT s Mixed |
Fixed-beam MRT vs TOMO |
Fixed-beam MRT vs STD VMAT |
Fixed-beam IMRT vs Mixed |
TOMO vs STD VMAT |
TOMO vs Mixed |
STD VMAT vs Mixed |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PTV | |||||||||||||||
| Dmean | 0.999 | 0.526 | 1.000 | 0.997 | <0.001* | 0.776 | 1.000 | 0.953 | <0.001* | 0.595 | 0.243 | <0.001* | 0.992 | <0.001* | <0.001* |
| Dmax | 0.971 | 0.057 | 0.487 | 0.982 | <0.001* | 0.328 | 0.111 | 0.676 | <0.001* | <0.001* | 0.006* | 0.028* | 0.895 | <0.001* | <0.001* |
| V107 % (%) | 1.000 | 1.000 | 1.000 | 1.000 | <0.001* | 1.000 | 1.000 | 1.000 | <0.001* | 1.000 | 1.000 | <0.001* | 1.000 | <0.001* | <0.001* |
| CI | 0.0943 | <0.001* | <0.001* | 1.000 | <0.001* | <0.001* | <0.001* | 0.124 | <0.001* | 0.516 | <0.001* | 1.000 | <0.001* | 0.734 | <0.001* |
| DHI | 1.000 | 0.158 | 0.253 | 0.525 | <0.001* | 0.202 | 0.202 | 0.601 | <0.001* | <0.001* | 0.984 | <0.001* | 0.001* | <0.001* | <0.001* |
| Lung | |||||||||||||||
| Dmean | 0.990 | 0.845 | <0.001* | 0.004* | 0.0712 | 0.993 | <0.001* | 0.034* | 0.286 | <0.001* | 0.152 | 0.643 | <0.001* | <0.001* | 0.952 |
| Dmax | 0.955 | <0.001* | 0.956 | 0.636 | <0.001* | <0.001* | 0.515 | 0.984 | <0.001* | <0.001* | <0.001* | 0.938 | 0.155 | <0.001* | 0.004* |
| V5 | 0.988 | 0.999 | <0.001* | 0.063 | 1.000 | 0.999 | <0.001* | 0.268 | 0.993 | <0.001* | 0.127 | 0.999 | <0.001* | <0.001* | 0.073 |
| V10 | 0.981 | 0.058 | <0.001* | 0.136 | 0.369 | 0.291 | <0.001* | 0.495 | 0.814 | 0.004* | 0.999 | 0.959 | <0.001* | <0.001* | 0.996 |
| V20 | 1.000 | 0.159 | 0.996 | 0.989 | 0.088 | 0.164 | 0.996 | 0.990 | 0.091 | 0.411 | 0.487 | 0.999 | 1.000 | 0.271 | 0.333 |
| NTCP | 0.999 | 0.942 | <0.001* | 0.078 | 0.187 | 0.995 | <0.001* | 0.193 | 0.382 | <0.001* | 0.486 | 0.727 | <0.001* | <0.001* | 0.999 |
| LAR (%) | 0.999 | 0.966 | <0.001* | 0.096 | 0.143 | 0.999 | <0.001* | 0.235 | 0.321 | <0.001* | 0.469 | 0.580 | 0.116 | 0.076 | 1.000 |
| Heart | |||||||||||||||
| Dmean | 0.994 | 0.360 | <0.001* | 0.008* | 0.993 | 0.711 | <0.001* | 0.044* | 0.876 | 0.019* | 0.686 | 0.113 | 0.533 | <0.001* | <0.001* |
| Dmax | 1.000 | <0.001* | 0.591 | 0.958 | 0.682 | <0.001* | 0.756 | 0.878 | 0.831 | <0.001* | 0.006* | <0.001* | 0.137 | 1.000 | 0.186 |
| V5 | 0.989 | 0.643 | <0.001* | 0.006* | 1.000 | 0.947 | <0.001* | 0.047* | 0.984 | <0.001* | 0.361 | 0.613 | 0.068 | <0.001* | 0.005* |
| V10 | 0.999 | 0.986 | <0.001* | 0.864 | 1.000 | 0.995 | <0.001* | 0.914 | 1.000 | 0.001* | 0.997 | 0.992 | 0.007* | <0.001* | 0.896 |
| V22.5 | 0.986 | 0.262 | 0.999 | 0.032* | 0.425 | 0.670 | 0.941 | 0.177 | 0.118 | 0.152 | 0.957 | <0.001* | 0.014* | 0.598 | <0.001* |
| V30 | 0.999 | 0.021* | 0.979 | 0.056 | 0.639 | 0.049* | 0.917 | 0.116 | 0.454 | 0.002* | 0.999 | <0.001* | 0.006* | 0.965 | <0.001* |
| RCE (%) | 1.000 | 0.252 | 0.009* | 0.025* | 0.931 | 0.337 | 0.015* | 0.040* | 0.874 | 0.823 | 0.943 | 0.022* | 1.000 | <0.001* | <0.001* |
| CL breast | |||||||||||||||
| Dmean | 0.998 | 0.001* | 0.644 | 0.596 | 0.007* | <0.001* | 0.878 | 0.846 | 0.001* | <0.001* | <0.001* | 0.997 | 1.000 | <0.001* | <0.001* |
| Dmax | 0.905 | 0.006* | 0.005* | 0.029* | <0.001* | 0.135 | 0.113 | 0.342 | 0.027* | 1.000 | 0.997 | 0.992 | 0.995 | 0.995 | 0.898 |
| V5 | 0.930 | 0.215 | 0.688 | 0.712 | 0.181 | 0.017* | 0.996 | 0.997 | 0.127 | 0.003* | 0.003* | 1.000 | 1.000 | 0.002* | 0.002* |
| LAR (%) | 0.965 | 0.259 | 0.760 | 0.528 | 0.997 | 0.035* | 0.995 | 0.949 | 0.788 | 0.006* | 0.002* | 0.548 | 0.999 | 0.450 | 0.245 |
indicates statistical significant.
The four PMRT techniques evaluated in this study as well as two techniques studied previously all meet clinical requirement of PTV coverage. Overall, TOMO plans exhibit the most optimal PTV coverage by showing the lowest Dmax in PTV, but deliver relatively higher dose to OARs than other plans: significantly higher Dmean, V5, V10 and NTCP for lung, significantly higher V10 for heart, the highest LAR for lung, the highest Dmean, V5 for heart. Fixed-beam IMRT plans induce the lowest Dmean, V5 and LAR for contralateral breast, but induce the highest V30 for heart and the highest Dmax for lung, and yield the significantly higher Dmax for heart and contralateral breast than other techniques. Compared with standard VMAT, both NC-VMAT and MA-VMAT significantly reduce Dmean for lungs, heart and contralateral breast, and also significantly reduce V5 and RCE for heart. NC-VMAT plans exhibit the minimum Dmean, V5, V10, V20, NTCP and LAR values for lungs and the minimum V10 for the heart compared with other plans. Mixed-beam therapy plans show significantly higher Dmax, DHI and V107% for PTV than other techniques, and the highest V20 for lungs, but provide the lowest Dmean, V5, V22.5, V30 and RCE for heart, the lowest V5 and the second lowest Dmean and LAR for contralateral breast compared with other techniques.
Discussion
This study evaluated four advanced radiotherapy techniques for treating post-mastectomy breast cancer patients, and these techniques were compared with another two PMRT techniques in the literature. Dosimetric and radiobiological endpoints were used to assess the dose to the target and normal tissues. All six techniques provide acceptable dose coverage to target region. Fixed-beam IMRT exhibits the best sparing of contralateral breast, but increases dose to lungs and heart. NC-VMAT provides the best sparing of lungs. Mixed beam therapy provides the best sparing of heart and good sparing of contralateral breast at the cost of inducing less homogenous dose to PTV.
Wang et al.2 drew a conclusion that four fields IMRT has the best balance of target coverage and normal tissue sparing compared with conventional tangential beams, tangential IMRT and single arc VMAT. In our study, we used four to five IMRT beams in fixed-beam IMRT plans because the separation between PTV and OARs is small in some patients and four beams will introduce significantly high dose to OARs. Fixed-beam IMRT plans provide the lowest doses to contrlateral breast, which is mainly because this technique is characterized by the limited gantry angles and low dose spread to the organs. On the other hand, also due to the limited gantry angles that are used to cover the entire PTV, the edge of some IMRT beams need to transmit through lungs, heart and contralateral breast in some plans. As a result, the fixed beam IMRT plans yield the highest maximum dose to OARs, which is contradictory to what has been reported in Wang et al.2 These results show that fixed-beam IMRT is not the optimal technique for all PMRT patients and its application should be judged based on the complexity of the target and patient geometry.
Both NC-VMAT and MA-VMAT provide superior OAR sparing than standard VMAT and NC-VMAT offers the best sparing of lungs, which indicates OARs can be spared more for PMRT patients by adjusting the couch angle or splitting a single arc into multiple partial ones since these will provide more degrees of freedom for plan optimizations. Instead of using 50° gantry rotation for each arc in MA-VMAT as reported by Tsai et al.7 for whole breast, we chose larger rotation angle (60° or 70°) in this study in order to achieve enough PTV coverage and optimal OAR sparing. For NC-VMAT, non-coplanar geometries are fixed (couch angle and collimator angle are fixed) in this study, while studies have shown that dynamic couch/gantry rotation and dynamic collimator rotation during VMAT delivery can further improve target coverage or normal tissue sparing9, 32, 33 and should be investigated for PMRT in the future.
The mean lung and heart doses from TOMO are the highest among all the PMRT techniques and can be explained by the fact that radiation to these organs is not limited enough due to the characteristic of TOMO (the beam is delivered from 360 degrees around the body). The other group in our institution independently evaluated TOMO and VMAT for PMRT previously4. Our study shows lower lung and heart doses than theirs, which can be explained by the fact different planning goals (they were trying to achieve 90% volume of the PTV receiving the prescribed dose) were used in these two studies and only one optimization objective could be specified for a given OAR in their TOMO TPS, i.e., it was not feasible for them to include more dose objectives to further optimize their plans then. In contrast, the latest TOMO planning system used in our study is capable of including multiple objectives for one OAR.
The CT data representation, contouring and dose calculation algorithm may introduce uncertainties to the dosimetric values. Previous studies34, 35 reported that using thick CT slice thickness may underdose target volume. Slice thickness of 2.5mm is an optimal and standard choice and was used in our study, and effect of CT data uncertainty on dose values should be minimal. Inter-observer variability in contouring is a major contributor to uncertainty in radiation treatment planning36, 37. Kirli et al.38 reported variability in intra-observer contouring was similar to inter-observer variability and can be reduced by following certain contouring atlas. In our study, all the contours were generated by the same physician following the RTOG atlas and were used for the comparison between different techniques. Thus the uncertainty of inter-observer contouring does not exist and the uncertainty of intra-observer contouring should be minimal. For dose calculation algorithm, we did a test and calculated dose distributions for several patients using adaptive algorithm and fast convolution algorithm besides the standard collapsed-cone convolution superposition algorithm in Pinnacle treatment planning system. The dose differences among three different algorithms for PTV and OARs were very small (within 4%). The we do not expect dramatic changes of relative values, i.e. the rank of alternative RT techniques.
Deep inspiration breath hold has been shown to significantly reduce cardiac exposure in patients receiving PMRT,39 which translates to the reduction of risk of heart disease. However, free breathing is the standard of care for PMRT patients in our clinic and breath hold was not adopted for the patients used in this study, neither in most of the previous PMRT studies. Acquiring patients’ CT images with breath hold and comparing various advanced radiotherapy techniques for breath hold patients will be further investigated in the near future.
We only compared various photon and electron radiotherapy techniques while did not include proton therapy. Actually proton PMRT was also evaluated by our group previously,18 and the superior dose distribution makes proton PMRT dominant among all PMRT techniques. However, due to the limited availability and much higher cost, proton PMRT is not as popular as photon or electron PMRT. Robust proton treatment planning is more challenging compared with photon treatment due to uncertainties related to imaging, setup, proton range, dose calculation algorithm, biological effectiveness etc.40, although Hernandez et al 18 reported that relative plan comparisons between standard VMAT and proton plans were robust to patient setup errors (up to 1 cm), proton range uncertainty (up to 10%) and uncertainty in dose-risk models. Evidence on the effectiveness and safety of proton therapy from clinical trials is lacking and will not be available until years or decades later, and it is controversial if the additional cost of proton therapy is justified by the potential advantages.
Conclusions
Four advanced PMRT techniques were evaluated in this study and were compared with another two PMRT techniques in the literature. Our analysis shows it is feasible to use NC-VMAT and MA-VMAT for PMRT patients. Among all techniques, fixed-beam IMRT might reduce contralateral breast dose, NC-VMAT could reduce lungs dose, and mixed beam therapy might lower the heart and contralateral breast doses. Based on evaluated target coverage and estimated risks for OARs, fixed-beam IMRT, NC-VMAT, mixed-beam therapy might be the appropriate PMRT techniques for certain patients who are prone to develop radiogenic side effects.
Acknowledgement
This work was partially supported by National Institutes of Health (NIH) through a National Cancer Institute (NCI) grant K22CA204464 and Louisiana State University (LSU) Economic Development Assistantship Award.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
The authors have no conflicts of interest.
References
- 1.McGale P, Taylor C, Correa C, Cutter D, Duane F, Ewertz M, Gray R, Mannu G, Peto R, Whelan T, Wang Y, Wang Z and Darby S, “Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials,” Lancet 383, 2127–2135 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang J, Li X, Deng Q, Xia B, Wu S, Liu J and Ma S, “Postoperative radiotherapy following mastectomy for patients with left-sided breast cancer: A comparative dosimetric study,” Med Dosim 40, 190–194 (2015). [DOI] [PubMed] [Google Scholar]
- 3.Ashenafi M, Boyd RA, Lee TK, Lo KK, Gibbons JP, Rosen II, Fontenot JD and Hogstrom KR, “Feasibility of postmastectomy treatment with helical TomoTherapy,” Int J Radiat Oncol Biol Phys 77, 836–842 (2010). [DOI] [PubMed] [Google Scholar]
- 4.Nichols GP, Fontenot JD, Gibbons JP and Sanders ME, “Evaluation of volumetric modulated arc therapy for postmastectomy treatment,” Radiat Oncol 9, 66 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yoon J, Heins D, Zhao X, Sanders M and Zhang R, “Measurement and modeling of out-of-field doses from various advanced post-mastectomy radiotherapy techniques,” Phys Med Biol 62, 9039–9053 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang R, Heins D, Sanders M, Guo B and Hogstrom K, “Evaluation of a mixed beam therapy for postmastectomy breast cancer patients: Bolus electron conformal therapy combined with intensity modulated photon radiotherapy and volumetric modulated photon arc therapy,” Med Phys 45, 2912–2924 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsai PF, Lin SM, Lee SH, Yeh CY, Huang YT, Lee CC and Hong JH, “The feasibility study of using multiple partial volumetric-modulated arcs therapy in early stage left-sided breast cancer patients,” J Appl Clin Med Phys 13, 3806 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Uto M, Mizowaki T, Ogura K and Hiraoka M, “Non-coplanar volumetric-modulated arc therapy (VMAT) for craniopharyngiomas reduces radiation doses to the bilateral hippocampus: a planning study comparing dynamic conformal arc therapy, coplanar VMAT, and non-coplanar VMAT,” Radiat Oncol 11, 86 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smyth G, Evans PM, Bamber JC, Mandeville HC, Welsh LC, Saran FH and Bedford JL, “Non-coplanar trajectories to improve organ at risk sparing in volumetric modulated arc therapy for primary brain tumors,” Radiother Oncol 121, 124–131 (2016). [DOI] [PubMed] [Google Scholar]
- 10.Fiandra C, Filippi AR, Catuzzo P, Botticella A, Ciammella P, Franco P, Borca VC, Ragona R, Tofani S and Ricardi U, “Different IMRT solutions vs. 3D-conformal radiotherapy in early stage Hodgkin’s Lymphoma: dosimetric comparison and clinical considerations,” Radiat Oncol 7, 186 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Woods K, Nguyen D, Tran A, Yu VY, Cao M, Niu T, Lee P and Sheng K, “Viability of Non-Coplanar VMAT for Liver SBRT as Compared to Coplanar VMAT and Beam Orientation Optimized 4pi IMRT,” Adv Radiat Oncol 1, 67–75 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tran A, Zhang J, Woods K, Yu V, Nguyen D, Gustafson G, Rosen L and Sheng K, “Treatment planning comparison of IMPT, VMAT and 4pi radiotherapy for prostate cases,” Radiat Oncol 12, 10 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pierce LJ, Butler JB, Martel MK, Normolle DP, Koelling T, Marsh RB, Lichter AS and Fraass BA, “Postmastectomy radiotherapy of the chest wall: dosimetric comparison of common techniques,” Int J Radiat Oncol Biol Phys 52, 1220–1230 (2002). [DOI] [PubMed] [Google Scholar]
- 14.MacDonald SM, Jimenez R, Paetzold P, Adams J, Beatty J, DeLaney TF, Kooy H, Taghian AG and Lu HM, “Proton radiotherapy for chest wall and regional lymphatic radiation; dose comparisons and treatment delivery,” Radiat Oncol 8, 71 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Opp D, Forster K, Li W, Zhang G and Harris EE, “Evaluation of bolus electron conformal therapy compared with conventional techniques for the treatment of left chest wall postmastectomy in patients with breast cancer,” Med Dosim 38, 448–453 (2013). [DOI] [PubMed] [Google Scholar]
- 16.Gauer T, Engel K, Kiesel A, Albers D and Rades D, “Comparison of electron IMRT to helical photon IMRT and conventional photon irradiation for treatment of breast and chest wall tumours,” Radiother Oncol 94, 313–318 (2010). [DOI] [PubMed] [Google Scholar]
- 17.Rudat V, Alaradi AA, Mohamed A, Ai-Yahya K and Altuwaijri S, “Tangential beam IMRT versus tangential beam 3D-CRT of the chest wall in postmastectomy breast cancer patients: a dosimetric comparison,” Radiat Oncol 6, 26 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hernandez M, Zhang R, Sanders ME and Newhauser W, “A treatment planning comparison of volumetric modulated arc therapy and proton therapy for a sample of breast cancer patients treated with post-mastectomy radiotherapy,” Jour Proton Ther 1, 119 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Newhauser WD, Jones T, Swerdloff S, Newhauser W, Cilia M, Carver R, Halloran A and Zhang R, “Anonymization of DICOM electronic medical records for radiation therapy,” Computers in Biology and Medicine 53, 134–140 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marks LB, Bentzen SM, Deasy JO, Kong FM, Bradley JD, Vogelius IS, El Naqa I, Hubbs JL, Lebesque JV, Timmerman RD, Martel MK and Jackson A, “Radiation dose-volume effects in the lung,” Int J Radiat Oncol Biol Phys 76, S70–76 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hardenbergh PH, Munley MT, Bentel GC, Kedem R, Borges-Neto S, Hollis D, Prosnitz LR and Marks LB, “Cardiac perfusion changes in patients treated for breast cancer with radiation therapy and doxorubicin: preliminary results,” Int J Radiat Oncol Biol Phys 49, 1023–1028 (2001). [DOI] [PubMed] [Google Scholar]
- 22.Wu Q, Mohan R, Morris M, Lauve A and Schmidt-Ullrich R, “Simultaneous integrated boost intensity-modulated radiotherapy for locally advanced head-and-neck squamous cell carcinomas. I: dosimetric results,” Int J Radiat Oncol Biol Phys 56, 573–585 (2003). [DOI] [PubMed] [Google Scholar]
- 23.van’t Riet A, Mak AC, Moerland MA, Elders LH and van der Zee W, “A conformation number to quantify the degree of conformality in brachytherapy and external beam irradiation: application to the prostate,” Int J Radiat Oncol Biol Phys 37, 731–736 (1997). [DOI] [PubMed] [Google Scholar]
- 24.NRC, Health Risks from Exposure to Low Levels of Ionizing Radation: BEIR VII - Phase 2 Nation Research Council of the National Academies, 2006. [PubMed] [Google Scholar]
- 25.Arias E and Xu J, “United States Life Tables, 2015,” Natl Vital Stat Rep 67, 1–64 (2018). [PubMed] [Google Scholar]
- 26.Schneider U, Sumila M and Robotka J, “Site-specific dose-response relationships for cancer induction from the combined Japanese A-bomb and Hodgkin cohorts for doses relevant to radiotherapy,” Theor Biol Med Model 8, 27 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lyman JT, “Complication probability as assessed from dose-volume histograms,” Radiat Res Suppl 8, S13–19 (1985). [PubMed] [Google Scholar]
- 28.Kutcher GJ and Burman C, “Calculation of complication probability factors for non-uniform normal tissue irradiation: the effective volume method,” Int J Radiat Oncol Biol Phys 16, 1623–1630 (1989). [DOI] [PubMed] [Google Scholar]
- 29.Seppenwoolde Y, Lebesque JV, de Jaeger K, Belderbos JS, Boersma LJ, Schilstra C, Henning GT, Hayman JA, Martel MK and Ten Haken RK, “Comparing different NTCP models that predict the incidence of radiation pneumonitis. Normal tissue complication probability,” Int J Radiat Oncol Biol Phys 55, 724–735 (2003). [DOI] [PubMed] [Google Scholar]
- 30.Darby SC, Ewertz M, McGale P, Bennet AM, Blom-Goldman U, Bronnum D, Correa C, Cutter D, Gagliardi G, Gigante B, Jensen MB, Nisbet A, Peto R, Rahimi K, Taylor C and Hall P, “Risk of ischemic heart disease in women after radiotherapy for breast cancer,” N Engl J Med 368, 987–998 (2013). [DOI] [PubMed] [Google Scholar]
- 31.Ridker PM, Buring JE, Rifai N and Cook NR, “Development and validation of improved algorithms for the assessment of global cardiovascular risk in women: the Reynolds Risk Score,” JAMA 297, 611–619 (2007). [DOI] [PubMed] [Google Scholar]
- 32.Popescu CC, Beckham WA, Patenaude VV, Olivotto IA and Vlachaki MT, “Simultaneous couch and gantry dynamic arc rotation (CG-Darc) in the treatment of breast cancer with accelerated partial breast irradiation (APBI): a feasibility study,” J Appl Clin Med Phys 14, 4035 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Webb S, “Does the option to rotate the Elekta Beam Modulator MLC during VMAT IMRT delivery confer advantage?--a study of ‘parked gaps’,” Phys Med Biol 55, N303–319 (2010). [DOI] [PubMed] [Google Scholar]
- 34.Prabhakar R, Ganesh T, Rath GK, Julka PK, Sridhar PS, Joshi RC and Thulkar S, “Impact of different CT slice thickness on clinical target volume for 3D conformal radiation therapy,” Med Dosim 34, 36–41 (2009). [DOI] [PubMed] [Google Scholar]
- 35.Somigliana A, Zonca G, Loi G and Sichirollo AE, “How thick should CT/MR slices be to plan conformal radiotherapy? A study on the accuracy of three-dimensional volume reconstruction,” Tumori 82, 470–472 (1996). [DOI] [PubMed] [Google Scholar]
- 36.Jameson MG, Holloway LC, Vial PJ, Vinod SK and Metcalfe PE, “A review of methods of analysis in contouring studies for radiation oncology,” J Med Imaging Radiat Oncol 54, 401–410 (2010). [DOI] [PubMed] [Google Scholar]
- 37.Vinod SK, Jameson MG, Min M and Holloway LC, “Uncertainties in volume delineation in radiation oncology: A systematic review and recommendations for future studies,” Radiother Oncol 121, 169–179 (2016). [DOI] [PubMed] [Google Scholar]
- 38.Kirli M, Akcay D, Baris MM and Gorken IB, “A heart atlas for breast radiation therapy and the influence of delination education on both intra and interobserver variability,” Jpn J Radiol (2019). [DOI] [PubMed]
- 39.Lin A, Sharieff W, Juhasz J, Whelan T and Kim DH, “The benefit of deep inspiration breath hold: evaluating cardiac radiation exposure in patients after mastectomy and after breast-conserving surgery,” Breast Cancer 24, 86–91 (2017). [DOI] [PubMed] [Google Scholar]
- 40.Unkelbach J and Paganetti H, “Robust Proton Treatment Planning: Physical and Biological Optimization,” Semin Radiat Oncol 28, 88–96 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]