Abstract
Background:
Socioeconomic factors have been consistently linked with children’s hippocampal and anterior cingulate cortex (ACC) structure. Chronic stress – as indexed by hair cortisol – may represent an important mechanism underlying these associations. Here, we examined associations between hair cortisol and children’s hippocampal and ACC structure, including across hippocampal subfields, and whether hair cortisol mediated associations between socioeconomic background (family income-to-needs ratio, parental education) and the structure of these brain regions.
Methods:
Participants were 5- to 9-year-old children (N=94; 61% female) from socioeconomically diverse families. Parents and children provided hair samples that were assayed for cortisol. High-resolution, T1-weighted MRI scans were acquired, and FreeSurfer 6.0 was used to compute hippocampal volume and rostral and caudal ACC thickness and surface area (n = 37 with both child hair cortisol and MRI data; n = 41 with both parent hair cortisol and MRI data).
Results:
Higher hair cortisol was significantly associated with smaller CA3 and dentate gyrus hippocampal subfield volumes but not with CA1 or subiculum volume. Higher hair cortisol was also associated with greater caudal ACC thickness. Hair cortisol significantly mediated associations between parental education and CA3 and dentate gyrus volumes; lower parental education was associated with higher hair cortisol which in turn was associated with smaller volume in these subfields.
Conclusions:
These findings point to chronic physiologic stress as a potential mechanism through which lower parental education leads to reduced hippocampal volume. Hair cortisol may be an informative biomarker leading to more effective prevention and intervention strategies aimed at childhood socioeconomic disadvantage.
Keywords: socioeconomic status, stress, cortisol, hippocampus, anterior cingulate cortex, developing brain
Although socioeconomic background has been consistently linked with children’s brain structure (1), the proximal mechanisms underlying these associations are not well understood. Socioeconomic disadvantage is associated with a range of chronic stressors, including family conflict and turmoil, household chaos, noise/crowding, and neighborhood violence (2). Socioeconomic context has also been linked with the physiologic stress response, including hypothalamic-pituitary-adrenal (HPA) axis function, most commonly indexed by salivary cortisol (3). Although salivary cortisol is a robust measure of acute stress, its diurnal variation often requires collection of multiple samples that are less reflective of chronic stress due to susceptibility to situational factors. Recently, socioeconomic factors (family income, parental education) have been linked with hair cortisol concentration (4–6), a relatively novel, reliable and valid measure of chronic stress obtained through a single sample (7, 8).
Socioeconomic disparities have been reported in the structure of brain regions that are particularly sensitive to chronic stress and strongly implicated in risk for multiple psychiatric disorders. Socioeconomic disadvantage has been repeatedly associated with reduced gray matter in the hippocampus (9–18) and anterior cingulate cortex (ACC) (15, 19–22), which are involved in cognitive skills including episodic memory and executive function (EF), respectively (23–26). Structural differences in the hippocampus and ACC have been observed in a number of psychiatric disorders including major depressive disorder and attention-deficit/hyperactivity disorder (24–27).
Animal work has established that chronic stress exerts pronounced effects on morphology of the hippocampus and ACC, which have a high density of glucocorticoid receptors (28–30). These effects represent adaptations to the context in which the brain develops (30). Chronic stress has been associated with dendritic remodeling and reduced dendritic spine density in hippocampal neurons (28) and dendritic shrinkage in ACC regions (28, 29, 31). The hippocampus consists of a number of subfields including the cornu ammonis (CA) 1–4, dentate gyrus (DG), and subiculum (32). The CA3 and DG subfields are particularly susceptible to chronic stress, with stress inducing dendritic remodeling in the CA3 and suppressing neurogenesis in the DG (33, 34). These effects are mediated not only by glucocorticoids, but also by excitatory amino acids (e.g., glutamate) and other cellular mechanisms (35). Thus, socioeconomic disadvantage may increase chronic stress and alter HPA axis function, which in turn influences hippocampal (particularly CA3 and DG) and ACC structure.
In humans, childhood exposure to stressful life events has been associated with hippocampal and ACC structure (12, 14, 36, 37). However, few studies have examined children’s HPA axis function in relation to their brain structure, and results have been inconsistent (37–39). In the only such study to focus on hair cortisol, higher hair cortisol was associated with smaller hippocampal volume in a subsample with Child Behavior Checklist total problems scores above the 91st percentile (39). It is unknown whether hair cortisol may be associated with the same hippocampal subfields in humans as those shown to be altered by chronic stress in animal models. In addition, although stressful life events mediate socioeconomic disparities in children’s hippocampal structure (14) and adults’ prefrontal function (36), no work has examined chronic physiologic stress as a mediator of socioeconomic differences in children’s brain structure.
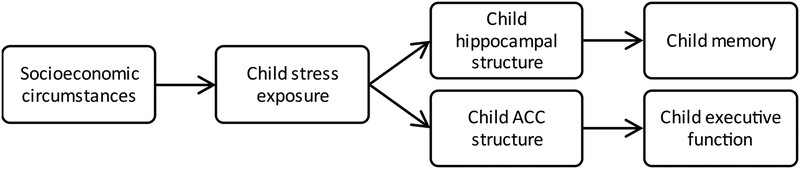
The goals of this study were to examine associations between stress exposure and hippocampal and ACC structure in children and whether stress exposure mediated associations between socioeconomic factors and children’s hippocampal and ACC structure (see Figure 1 for the full model). Family income-to-needs ratio and parental education were examined separately as they represent distinct aspects of children’s environments that relate differentially to their development (40). Indeed, family income-to-needs ratio and parental education have been linked with material resources and the quality of parent-child interactions, respectively (40), which have both been linked with variability in children’s stress exposure (2). Stress exposure was measured at multiple levels (41), with parent-reported perceived stress representing the family’s exposure to stressors (e.g., stressful life events, material hardship) and parent and child hair cortisol representing chronic physiologic stress in the parent and child, respectively.
Figure 1.
Hypothesized mechanistic model.
ACC, anterior cingulate cortex
We hypothesized a priori that lower family income-to-needs ratio and parental education would be associated with greater stress exposure, which in turn would be associated with reduced gray matter in the hippocampus and ACC (16). Further, we hypothesized that stress exposure would be most strongly associated with volume reductions in the DG and CA3 hippocampal subfields (17, 30, 42). In particular, we predicted that stress exposure would be more strongly associated with the CA3 and DG compared to two other components of the hippocampal formation -- the CA1 and subiculum (32). We tentatively predicted that children’s hair cortisol would be the most likely of the three stress measures to relate to their hippocampal and ACC structure since it is the most direct measure of children’s stress. To assess the ramifications of these associations for observable cognitive performance, we also examined the associations of hippocampal and ACC structure with episodic memory and EF, respectively.
Methods
Participants
Participants were recruited in New York, NY by posting flyers and meeting families at local community children’s events. Families were recruited that represented a wide range of parental educational attainment. Interested families were contacted by phone and screened for eligibility. Inclusionary criteria for children were the following: 5–9 years of age; 37+ weeks of gestation at birth; born from a singleton pregnancy; no history of medical or psychiatric problems; spoke primarily English in the home; no contraindications for MRI scanning.
Sample characteristics.
Children ranged from 5.06 to 9.87 years of age (N = 94; 61% female) and were from socioeconomically diverse families (parental education range: 6.50 – 20.00 years; family income-to-needs ratio range: .17 – 15.21). Fifty percent were Hispanic/Latino; 31% were African American; and 14% were European American (see Table 1).
Table 1.
Descriptive statistics for sample characteristics (N = 94)
| M | SD | |
|---|---|---|
| Child age (years) | 7.03 | 1.29 |
| Parental education (years) | 14.14 | 2.64 |
| Family income-to-needs ratio | 2.68 | 2.79 |
| % | n | |
| Child sex (female) | 60.64 | 57 |
| Child race/ethnicity | ||
| African American, non-Hispanic/Latino | 30.85 | 29 |
| Hispanic/Latino | 50.00 | 47 |
| European American, non-Hispanic/Latino | 13.83 | 13 |
| Other | 5.32 | 5 |
| Family income below U.S. poverty thresholda | 29.79 | 28 |
Note. Parental education reflects educational attainment averaged across parents in the household.
Income-to-needs ratio < 1.00
Hair samples and MRI data.
Ninety-four families completed questionnaires and the child testing battery. Hair cortisol data were available for 78/82 parents (95% female) who provided hair samples (see Figure S1 for flow chart). Hair cortisol data were available for 65/67 children (77% female) who provided hair samples (see Figure S1). MRI data were acquired for 66/85 children who enrolled in the MRI portion of the study and participated in a mock scan (see Figure S1). There were 37 children with both MRI and child hair cortisol data and 41 children with both MRI and parent hair cortisol data.
Procedure
Families participated in two campus visits within a month. During the first visit, parents completed questionnaires, hair samples were collected from parents and children, and children completed neurocognitive tasks. Children also participated in a practice MRI session in a mock scanner to familiarize them with scanning. During the second visit, children completed an MRI scanning session. Informed consent/assent was obtained from all families, and all procedures were approved by the Institutional Review Boards at the New York State Psychiatric Institute and Teachers College, Columbia University.
Image Acquisition and Processing
MRI data were acquired on a 3-Tesla General Electric MR750 scanner with a 32-channel head coil. A high-resolution, T1-weighted fast spoiled gradient echo sequence was acquired in the sagittal plane (TR = 7.1 ms; TE = min full; inversion time [TI] = 500 ms; flip angle = 11°; 176 slices; 1.0 mm slice thickness; field of view [FOV] = 25 cm; inplane resolution = 1.0 by 1.0 mm). All images were visually inspected for motion artifacts and ghosting, resulting in the exclusion of 15 participants’ MRI data from analyses. There was no manual editing of imaging data that passed quality control procedures.
Hippocampal volume.
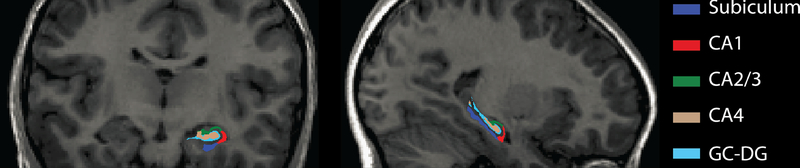
Hippocampal subfield segmentation was conducted using the automated algorithm available in FreeSurfer 6.0 (http://surfer.nmr.mgh.harvard.edu/) (43). A comprehensive description of the pipeline has been published (see also Supplemental Information) (43, 44). For each hemisphere, subfields that are segmented include the CA2/3 (combined in the atlas due to indistinguishable MRI contrast), CA4, granule cell layer of the dentate gyrus (GC-DG), CA1, and subiculum (see Figure 2). The CA4 and GC-DG subfields were combined because they are both components of the DG and because the ability to distinguish the molecular layer in T1-weighted images is limited (17). Measures of whole hippocampal volume were obtained by summing the volumes of the subfields (not including the hippocampal fissure). All hippocampal segmentations passed visual inspection for major errors. Hippocampal subfield segmentation could not be completed for one participant.
Figure 2.
Color-coded depiction of the focal hippocampal subfields in coronal (left) and sagittal (right) views from a representative participant. The subfield volumes are overlaid on the whole-brain T1-weighted processed image.
ACC thickness and surface area.
Images were processed using standard automated procedures in FreeSurfer 6.0. Following cortical surface reconstruction (45, 46), the cortex was parcellated into regions based on gyral and sulcal structure (47, 48). Cortical thickness is computed as the closest distance from the gray/white matter boundary to the gray matter/cerebrospinal fluid boundary at each vertex on the tessellated surface. Surface area is computed as the sum of the areas of each tessellation falling within a given region. FreeSurfer cortical thickness and surface area measurements have been shown to be reliable and are well-validated (49, 50). The boundaries of the rostral and caudal ACC were determined via the Desikan-Killiany atlas (47). Rostral and caudal ACC thickness and surface area data were extracted.
Measures
Socioeconomic factors.
Parents reported their annual household income, the number of adults and children in the household, and their educational attainment in years. The income-to-needs ratio was calculated by dividing household income by the poverty threshold for the size of the family. Family income-to-needs ratio was log-transformed to correct for positive skew. Educational attainment was averaged across all parents in the household.
Parental perceived stress.
Parents completed the Perceived Stress Scale (51), Life Experiences Survey (52), and Material Deprivation Scale (53) (see Supplemental Information). Principal component analysis was used to extract one factor with an eigenvalue > 1.0 (1.69), which explained 56.37% of the total variance. Factor loadings ranged from .63-.85. This factor score (termed ‘perceived stress’) was used in analyses.
Hair cortisol concentration.
A small section of hair (≥15 mg) proximal to the posterior vertex of the participant’s scalp was cut. Each hair sample was approximately 3 cm long, thereby containing cortisol deposited during roughly the past 3 months. Samples were stored at −20°C until being sent for analysis. Samples were processed and analyzed using methods previously validated and described in detail (see also Supplemental Information) (54, 55). Hair cortisol values outside of 3 standard deviations from the mean were excluded (n = 3 parents; n = 5 children), and hair cortisol data were log-transformed to correct for skew (39). Previous studies have been inconsistent in terms of whether certain potential confounding variables are associated with hair cortisol (6). In this study, there were no significant associations between hair cortisol and hair washing frequency, use of steroid medications, use of oral contraceptives, or use of hair dye.
Episodic memory and EF.
Children completed the Picture Sequence Memory, Flanker Inhibitory Control and Attention, List Sorting Working Memory, and Dimensional Change Card Sort Tests from the NIH Toolbox Cognition Battery (56–59). Raw scores were used in analyses. Scores on the latter three tasks, putative measures of core EF components, were significantly correlated, r = .28-.43, p < .001, and were thus standardized and averaged to create an EF composite.
Statistical Analyses
Using SAS software (version 9.4), multiple linear regression analyses were conducted to examine associations between socioeconomic factors and children’s hippocampal and ACC structure (c paths); socioeconomic factors and children’s stress exposure (a paths); and children’s stress exposure and their hippocampal and ACC structure (b paths). Covariates included child (or parent) age, sex, and race/ethnicity. Race/ethnicity was not significant in any of the regression models and did not improve model fit, and was thus dropped from the final models for parsimony. In models predicting hippocampal volume, whole brain volume was included as a covariate. Cortisol has been shown to correlate with cognitive functioning in an inverted U-shaped pattern (60). Thus, a quadratic term (hair cortisol2) was initially included in regression analyses, but it was not significant across models and was dropped from the final analyses. Effect sizes (ηp2) are presented, with values of .01, .06, and .14 indicating small, medium, and large effects, respectively. To control for multiple comparisons, false discovery rate (FDR) correction (61) was applied (via PROC MULTTEST in SAS) to the hippocampal subfield and ACC analyses, separately (α = .05). FDR-corrected p-values are reported in the text in addition to the tables (see Supplementary Materials). For instances in which the a and b paths were both supported in regression analyses, the significance of the indirect or mediated effect (ab path) was assessed using bias-corrected bootstrapping via the PROCESS macro (62, 63). Indirect effects were significant if the 95% confidence intervals did not include zero (62, 64).
Results
Descriptive statistics and zero-order correlations are presented in Tables 2 and S1, respectively.
Table 2.
Descriptive statistics for stress exposure and hippocampal and ACC structure
| N | M | SD | |
|---|---|---|---|
| Perceived Stress Scale score | 94 | 29.38 | 7.54 |
| Life Experiences Survey score | 94 | 10.77 | 10.25 |
| Material Deprivation Scale score | 94 | 2.56 | 2.12 |
| Parent hair cortisol (pg/mg) | 75 | 32.00 | 91.96 |
| Child hair cortisol (pg/mg) | 60 | 25.20 | 25.34 |
| Total hippocampal volume (mm3) | 50 | 3145.65 | 311.86 |
| CA1 volume (mm3) | 50 | 585.15 | 66.70 |
| CA3 volume (mm3) | 50 | 183.55 | 25.71 |
| Dentate gyrus volume (mm3) | 50 | 474.03 | 53.27 |
| Subiculum volume (mm3) | 50 | 392.30 | 37.89 |
| Rostral ACC thickness (mm) | 51 | 3.35 | .24 |
| Caudal ACC thickness (mm) | 51 | 3.05 | .24 |
| Rostral ACC surface area (mm2) | 51 | 1257.78 | 254.50 |
| Caudal ACC surface area (mm2) | 51 | 1301.59 | 228.86 |
Note. Log-transformed hair cortisol values were used in analyses, but raw scores are shown here for ease of interpretation.
ACC, anterior cingulate cortex
Children’s Hippocampal and ACC Structure
Higher parental education was significantly associated with larger total hippocampal volume, β = .18, p = .04, ηp2 = .08, DG volume, β = .22, p = .04, ηp2= .10, and CA1 volume, β = .26, p = .04, ηp2 = .12 (see Table S2). Effect sizes were medium-to-large in the DG, CA1, and CA3 subfields and small-to-medium in the subiculum (see Figure S2). Family income-to-needs ratio was not significantly associated with hippocampal volume.
Higher parental education was significantly associated with reduced rostral, β = −.39, p = .01, ηp2 = .18, and caudal ACC thickness, β = −.30, p = .03, ηp2 = .10, and greater rostral, β = .30, p = .02, ηp2 = .13, and caudal ACC surface area, β = .24, p = .046, ηp2 = .08 (see Table S2). Family income-to-needs ratio was not significantly associated with rostral or caudal ACC thickness or surface area.
Socioeconomic Factors and Children’s Stress Exposure
Higher parental education was significantly associated with lower parental perceived stress, β = −.29, p = .01, ηp2= .08, and lower child hair cortisol, β = −40, p = .01, ηp2= .16 (see Figure S3 and Table S3). The latter association remained significant after additionally controlling for parental hair cortisol, β = −.25, p = .03, ηp2= .10. Parental education was not significantly associated with parent hair cortisol. Family income-to-needs ratio was not significantly associated with the stress exposure measures.
Stress Exposure and Hippocampal Volume in Children
Higher child hair cortisol was significantly associated with smaller volume in the CA3, β = −.26, p = .04, ηp2= .12, and DG, β = −.23, p = .04, ηp2= .13 (see Figure 3), but not in the CA1 or subiculum, after accounting for child age, sex, and whole brain volume. Effect sizes for the CA3 and DG were medium-to-large whereas those for the CA1 and subiculum were negligible-to-small (see Figure S4). When child hair cortisol (p = .07 - .08) and parental education (p = .19 −.20) were entered simultaneously into models predicting CA3 and DG volume, neither remained significant, but child hair cortisol retained its medium-to-large effect size (ηp2= .08 - .09) while parental education did not (ηp2= .04 - .05). Parental perceived stress and hair cortisol were not significantly associated with hippocampal volume (see Table S4).
Figure 3.
Higher hair cortisol (log-transformed) was significantly associated with smaller (A) CA3 volume (mm3) and (B) dentate gyrus volume (mm3) in children (n = 37).
Stress Exposure and ACC Structure in Children
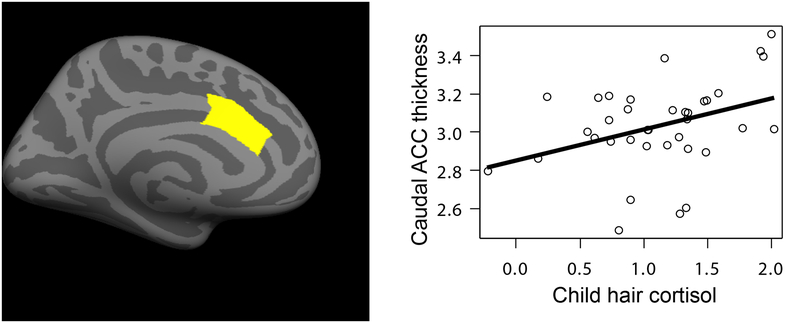
Higher child hair cortisol was significantly associated with greater caudal ACC thickness, after accounting for child age and sex, β = .40, p = .04, ηp2 = .17 (see Figure 4 and Table S4). Parent perceived stress and hair cortisol were not associated with ACC thickness. When child hair cortisol (p = .05) and parental education (p = .10) were entered simultaneously, neither remained significant, but child hair cortisol retained its medium-to-large effect size (ηp2= .10). Parental perceived stress and parent and child hair cortisol were not associated with ACC surface area.
Figure 4.
Higher hair cortisol (log-transformed) was significantly associated with greater thickness (mm) in the caudal anterior cingulate cortex (ACC) in children (n = 37).
Parental Education, Child Hair Cortisol, and Hippocampal and ACC Structure
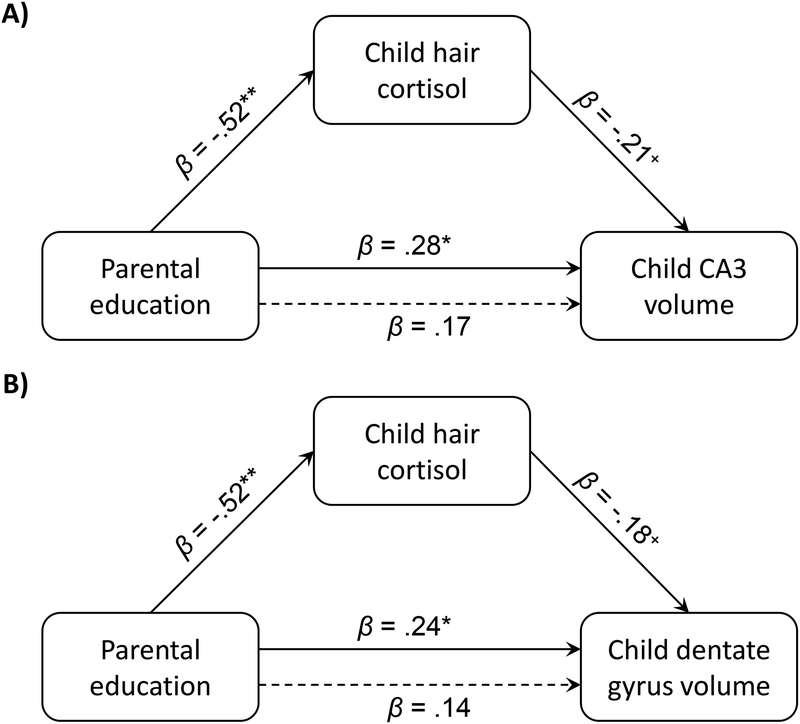
Child hair cortisol significantly mediated the associations between parental education and CA3 volume, ab path = .11, SE = .07, 95% CI: [.0076, .2895], and between parental education and DG volume, ab path = .10, SE = .06, 95% CI: [.0055, .2506]. Lower parental education was associated with higher child hair cortisol, which in turn was associated with smaller CA3 and DG volumes (see Figure 5). Child hair cortisol did not significantly mediate the association between parental education and caudal ACC thickness.
Figure 5.
Hair cortisol significantly mediated the associations between (A) parental education and CA3 volume and (B) parental education and dentate gyrus volume in children (n = 37). The solid and dotted lines from parental education to child CA3/dentate gyrus volume represent the total (c path) and direct associations (c’ path), respectively.
* p < .05, + p < .10
Socioeconomic Factors, Hippocampal and ACC Structure, and Cognitive Functioning
Higher family income-to-needs ratio, β = .21, p = .04, ηp2= .04, and parental education, β = .25, p = .03, ηp2= .06, were significantly associated with higher episodic memory. Higher family income-to-needs ratio was significantly associated with higher EF, β = .26, p = .02, ηp2= .07, but parental education was not (see Table S5). However, hippocampal and ACC structure were not significantly associated with episodic memory or EF task performance, respectively.
Discussion
The goals of this study were to examine associations between children’s stress exposure and their hippocampal and ACC structure, and to investigate stress exposure as a mediator of socioeconomic differences in hippocampal and ACC structure. Stress exposure was measured in terms of parent-reported family stressors and parent and child hair cortisol (41), with parental stress reflecting children’s exposure to stress in the home environment. Animal models have consistently demonstrated that chronic stress impacts hippocampal and ACC structure (29, 30). Yet, no human studies have focused on hair cortisol in both parents and children as biomarkers of chronic stress in relation to children’s brain structure.
Hair Cortisol is Associated with Hippocampal Subfield Volume in Children
This study is the first to report that higher child hair cortisol is significantly associated with reduced volume in the CA3 and DG hippocampal subfields, consistent with animal work showing these subfields to be particularly susceptible to chronic stress (33, 34). While medium-to-large effect sizes were found for the CA3 and DG subfields, negligible-to-small effect sizes were found for the CA1 and subiculum (see Figure S4). These results are consistent with the notion that prolonged exposure to circulating cortisol may alter hippocampal structure (65). At the cellular level, animal research has shown stress-induced reductions in dendritic length and branching in CA3 neurons and impaired neurogenesis in the DG (30). The one previous study of hair cortisol and hippocampal structure in children yielded mixed results (39) that were possibly due in part to variability in associations across hippocampal subfields, which were not assessed in that study (39). In addition to glucocorticoids, excitatory amino acids and other mechanisms are also involved in stress-induced remodeling of hippocampal neurons (30, 35), and future work could help disentangle these processes.
Hair Cortisol Mediates Socioeconomic Differences in Children’s Hippocampal Volume
Parental education was significantly positively associated with DG and CA1 volume, with medium-to-large effect sizes for those subfields plus the CA3 subfield (see Figure S2). Child hair cortisol significantly mediated associations between parental education and CA3 and DG subfield volume. Lower parental education was associated with higher hair cortisol [consistent with previous work on a portion of this sample (4)], which in turn was linked with smaller CA3 and DG volumes. Whereas stressful life events have been found to mediate the association between socioeconomic background and children’s hippocampal volume (14), this is the first study to show that chronic physiologic stress may partially explain these associations. Socioeconomic disadvantage may increase long-term cortisol exposure and in turn alter the structural development of stress-sensitive hippocampal subfields.
Results were specific to parental education rather than family income-to-needs ratio, although the two were strongly correlated (r = .68). Parental education may more directly reflect the quality of parent-child interactions, whereas family income-to-needs ratio may more directly reflect material resources (40). Thus, one possibility is that variation in parent-child interaction quality may be partially responsible for associations between parental education and children’s hair cortisol. In addition, results were specific to child hair cortisol rather than parental hair cortisol or perceived stress. Thus, direct measures of children’s physiologic stress responses may be valuable in understanding stress-related variability in brain structure. More work with larger samples is needed to unpack the ways in which poverty-related stressful environments alter HPA axis function (e.g., circadian disruption due to sleep deprivation) and in turn brain structure.
Hair Cortisol is Associated with ACC Thickness in Children
Higher child hair cortisol was also associated with greater thickness in the caudal (dorsal) ACC, consistent with work identifying the ACC as particularly susceptible to chronic stress (29, 66). This association could be related to the ACC’s role in regulating the HPA axis (41, 67, 68), resolving conflict associated with threat detection, or filtering out threat-related stimuli during cognitive processing (e.g., trying to complete a task in the midst of stressors) (66, 69–71). This result contrasts in direction with animal models of stress showing dendritic shrinkage in ACC (28, 29, 31) and null findings from one previous study of children (39). These discrepancies could be due to differences in the nature of these associations by age or other sample characteristics.
Developmentally, cortical thickness decreases rapidly in childhood and early adolescence, followed by a more gradual thinning, and ultimately plateauing in early- to mid-adulthood (72). One possibility is that lower levels of chronic physiologic stress may foster greater age-related cortical thinning in the caudal ACC. Some evidence has linked positive parenting (often a buffer of children’s stress) with an increased rate of PFC thinning (73) and conversely, maternal aggressive behavior with thickening of superior frontal cortex in adolescents (74). Another possibility is that higher chronic stress may lead to blunting of the HPA axis stress response system (41), leading to paradoxically lower levels hair cortisol, which in turn are associated with reduced ACC thickness. Longitudinal studies that assess chronic stress in relation to rates of cortical thinning over time are needed to address these possibilities.
Children from more advantaged families demonstrated higher episodic memory and EF skills, consistent with a large body of work (1, 3). However, children’s hippocampal and ACC structure were not associated with these cognitive outcomes. In previous research, hippocampal volume has been associated with episodic memory (18, 23), and ACC thickness has been inversely associated with EF (24–26, 75, 76). However, other work has suggested that links between gray matter morphology and cognition vary by age and socioeconomic background (9, 77–79). Thus, it is possible that in this socioeconomically diverse sample of 5- to 9-year-old children, associations between brain structure and cognitive task performance were obscured due to variability in these characteristics across the sample. Furthermore, more work is needed to elucidate the implications of these results for socioeconomic differences in risk for psychiatric disorders (80).
There are several limitations of this study that should be taken into account when interpreting these results. First, causal inferences cannot be made because this was a cross-sectional, correlational study, and all possible confounds (e.g., parent IQ) could not be ruled out. Second, it was more difficult to collect hair samples from boys because their hair was often too short, similar to sampling constraints noted in previous studies (81). And, previous work has identified sex-specific effects of early life stressors on hippocampal volume (82). Third, head motion has a negative effect on estimates of cortical thickness, and younger participants generally move more during acquisition (83). Accordingly, motion-corrupted images were excluded from analyses and all statistical models controlled for age. Fourth, subcortical volume estimates derived from automated segmentation algorithms may differ from those resulting from manual tracing (12, 84, 85). In this study, we used the FreeSurfer 6.0 hippocampal subfield segmentation procedures, which are accurate and reliable (43, 44, 85).
This study showed that higher hair cortisol is significantly associated with smaller CA3 and DG hippocampal subfield volume in children, consistent with animal research underscoring the adaptive plasticity of the hippocampus (30, 35). Hair cortisol significantly mediated associations between socioeconomic background and CA3 and DG volume. Socioeconomic disadvantage may lead to increased long-term cortisol exposure which in turn alters the structural development of the hippocampus. Prevention and intervention strategies that reduce children’s exposure to poverty-related chronic stress may be instrumental in ensuring their healthy neurodevelopment across the lifespan.
Supplementary Material
Acknowledgements
This publication was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through grant numbers UL1TR001873 and UL1RR024156. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Additional funding was provided from the Gertrude H. Sergievsky Center, Columbia University Medical Center; Teachers College, Columbia University; the Russell Sage Foundation; and a National Institute of Mental Health training grant (T32MH13043). We are grateful to the families who participated in this study. We also thank Rachel RouChen Lin, Charles Sisk, Mayra Lemus Rangel, Rebecca Lichtin, Lexi Paul, Samantha Moffett, Julissa Veras, and Victor Issa Garcia for assisting with data collection.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
The authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Farah MJ (2017): The Neuroscience of Socioeconomic Status: Correlates, Causes, and Consequences. Neuron. 96: 56–71. [DOI] [PubMed] [Google Scholar]
- 2.Evans GW, Kim P (2013): Childhood Poverty, Chronic Stress, Self-Regulation, and Coping. Child Dev Perspect. 7: 43–48. [Google Scholar]
- 3.Blair C, Granger DA, Willoughby M, Mills-Koonce R, Cox M, Greenberg MT, et al. (2011): Salivary Cortisol Mediates Effects of Poverty and Parenting on Executive Functions in Early Childhood. Child Dev. 82: 1970–1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ursache A, Merz EC, Melvin S, Meyer J, Noble KG (2017): Socioeconomic status, hair cortisol and internalizing symptoms in parents and children. Psychoneuroendocrinology. 78: 142–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vaghri Z, Guhn M, Weinberg J, Grunau RE, Yu W, Hertzman C (2013): Hair cortisol reflects socio-economic factors and hair zinc in preschoolers. Psychoneuroendocrinology. 38: 331–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gray NA, Dhana A, Van Der Vyver L, Van Wyk J, Khumalo NP, Stein DJ (2018): Determinants of hair cortisol concentration in children: A systematic review. Psychoneuroendocrinology. 87: 204–214. [DOI] [PubMed] [Google Scholar]
- 7.Flom M, St John AM, Meyer JS, Tarullo AR (2017): Infant hair cortisol: associations with salivary cortisol and environmental context. Dev Psychobiol. 59: 26–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Short SJ, Stalder T, Marceau K, Entringer S, Moog NK, Shirtcliff EA, et al. (2016): Correspondence between hair cortisol concentrations and 30-day integrated daily salivary and weekly urinary cortisol measures. Psychoneuroendocrinology. 71: 12–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ellwood-Lowe ME, Humphreys KL, Ordaz SJ, Camacho MC, Sacchet MD, Gotlib IH (2017): Time-varying effects of income on hippocampal volume trajectories in adolescent girls. Dev Cogn Neurosci. 30: 41–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hair NL, Hanson JL, Wolfe BL, Pollak SD (2015): Association of Child Poverty, Brain Development, and Academic Achievement. JAMA Pediatr. 169: 822–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanson JL, Chandra A, Wolfe BL, Pollak SD (2011): Association between Income and the Hippocampus. PLOS ONE. 6: e18712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hanson JL, Nacewicz BM, Sutterer MJ, Cayo AA, Schaefer SM, Rudolph KD, et al. (2015): Behavior Problems After Early Life Stress: Contributions of the Hippocampus and Amygdala. Biol Psychiatry. 77: 314–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jednoróg K, Altarelli I, Monzalvo K, Fluss J, Dubois J, Billard C, et al. (2012): The Influence of Socioeconomic Status on Children’s Brain Structure. PLoS ONE. 7. doi: 10.1371/journal.pone.0042486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luby JL, Belden A, Botteron K, Marrus N, Harms MP, Babb C, et al. (2013): The Effects of Poverty on Childhood Brain Development: The Mediating Effect of Caregiving and Stressful Life Events. JAMA Pediatr. 167: 1135–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Noble KG, Houston SM, Brito NH, Bartsch H, Kan E, Kuperman JM, et al. (2015): Family Income, Parental Education and Brain Structure in Children and Adolescents. Nat Neurosci. 18: 773–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Noble KG, Houston SM, Kan E, Sowell ER (2012): Neural correlates of socioeconomic status in the developing human brain. Dev Sci. 15: 516–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brody GH, Gray JC, Yu T, Barton AW, Beach SRH, Galván A, et al. (2017): Protective Prevention Effects on the Association of Poverty With Brain Development. JAMA Pediatr. 171: 46–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu Q, Daugherty AM, Anderson DM, Nishimura M, Brush D, Hardwick A, et al. (2018): Socioeconomic status and hippocampal volume in children and young adults. Dev Sci. 21: e12561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gianaros PJ, Horenstein JA, Cohen S, Matthews KA, Brown SM, Flory JD, et al. (2007): Perigenual anterior cingulate morphology covaries with perceived social standing. Soc Cogn Affect Neurosci. 2: 161–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lawson GM, Duda JT, Avants BB, Wu J, Farah MJ (2013): Associations between Children’s Socioeconomic Status and Prefrontal Cortical Thickness. Dev Sci. 16: 641–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang J, Liu H, Wei D, Liu W, Meng J, Wang K, et al. (2016): Regional gray matter volume mediates the relationship between family socioeconomic status and depression-related trait in a young healthy sample. Cogn Affect Behav Neurosci. 16: 51–62. [DOI] [PubMed] [Google Scholar]
- 22.McDermott CL, Seidlitz J, Nadig A, Liu S, Clasen LS, Blumenthal JD, et al. (2019): Longitudinally Mapping Childhood Socioeconomic Status Associations with Cortical and Subcortical Morphology. J Neurosci. 39: 1365–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ghetti S, Bunge SA (2012): Neural Changes Underlying the Development of Episodic Memory During Middle Childhood. Dev Cogn Neurosci. 2: 381–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tamnes CK, Østby Y, Walhovd KB, Westlye LT, Due-Tønnessen P, Fjell AM (2010): Neuroanatomical correlates of executive functions in children and adolescents: a magnetic resonance imaging (MRI) study of cortical thickness. Neuropsychologia. 48: 2496–2508. [DOI] [PubMed] [Google Scholar]
- 25.Kharitonova M, Martin RE, Gabrieli JDE, Sheridan MA (2013): Cortical gray-matter thinning is associated with age-related improvements on executive function tasks. Dev Cogn Neurosci. 6. doi: 10.1016/j.dcn.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Squeglia LM, Jacobus J, Sorg SF, Jernigan TL, Tapert SF (2013): Early Adolescent Cortical Thinning Is Related to Better Neuropsychological Performance. J Int Neuropsychol Soc JINS. 19: 962–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burgess N, Maguire EA, O’Keefe J (2002): The human hippocampus and spatial and episodic memory. Neuron. 35: 625–641. [DOI] [PubMed] [Google Scholar]
- 28.Chattarji S, Tomar A, Suvrathan A, Ghosh S, Rahman MM (2015): Neighborhood matters: divergent patterns of stress-induced plasticity across the brain. Nat Neurosci. 18: 1364–1375. [DOI] [PubMed] [Google Scholar]
- 29.McEwen BS, Morrison JH (2013): The brain on stress: vulnerability and plasticity of the prefrontal cortex over the life course. Neuron. 79: 16–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McEwen BS, Nasca C, Gray JD (2016): Stress Effects on Neuronal Structure: Hippocampus, Amygdala, and Prefrontal Cortex. Neuropsychopharmacology. 41: 3–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kolb B, Harker A, Mychasiuk R, de Melo SR, Gibb R (2017): Stress and prefrontal cortical plasticity in the developing brain. Cogn Dev, Current Perspectives on Neuroplasticity in Human Development. 42: 15–26. [Google Scholar]
- 32.Roddy DW, Farrell C, Doolin K, Roman E, Tozzi L, Frodl T, et al. (2019): The Hippocampus in Depression: More Than the Sum of Its Parts? Advanced Hippocampal Substructure Segmentation in Depression. Biol Psychiatry. 85: 487–497. [DOI] [PubMed] [Google Scholar]
- 33.Conrad CD, LeDoux JE, Magariños AM, McEwen BS (1999): Repeated restraint stress facilitates fear conditioning independently of causing hippocampal CA3 dendritic atrophy. Behav Neurosci. 113: 902–913. [DOI] [PubMed] [Google Scholar]
- 34.Pham K, Nacher J, Hof PR, McEwen BS (2003): Repeated restraint stress suppresses neurogenesis and induces biphasic PSA-NCAM expression in the adult rat dentate gyrus. Eur J Neurosci. 17: 879–886. [DOI] [PubMed] [Google Scholar]
- 35.McEwen BS (2016): Stress-induced remodeling of hippocampal CA3 pyramidal neurons. Brain Res. 1645: 50–54. [DOI] [PubMed] [Google Scholar]
- 36.Kim P, Evans GW, Angstadt M, Ho SS, Sripada CS, Swain JE, et al. (2013): Effects of childhood poverty and chronic stress on emotion regulatory brain function in adulthood. Proc Natl Acad Sci U S A. 110: 18442–18447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pagliaccio D, Luby JL, Bogdan R, Agrawal A, Gaffrey MS, Belden AC, et al. (2014): Stress-System Genes and Life Stress Predict Cortisol Levels and Amygdala and Hippocampal Volumes in Children. Neuropsychopharmacology. 39: 1245–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wiedenmayer CP, Bansal R, Anderson GM, Zhu H, Amat J, Whiteman R, Peterson BS (2006): Cortisol Levels and Hippocampus Volumes in Healthy Preadolescent Children. Biol Psychiatry. 60: 856–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen R, Muetzel RL, El Marroun H, Noppe G, van Rossum EFC, Jaddoe VW, et al. (2016): No association between hair cortisol or cortisone and brain morphology in children. Psychoneuroendocrinology. 74: 101–110. [DOI] [PubMed] [Google Scholar]
- 40.Duncan GJ, Magnuson K (2012): Socioeconomic status and cognitive functioning: moving from correlation to causation. Wiley Interdiscip Rev Cogn Sci. 3: 377–386. [DOI] [PubMed] [Google Scholar]
- 41.Hostinar CE, Sullivan RM, Gunnar MR (2014): Psychobiological mechanisms underlying the social buffering of the hypothalamic-pituitary-adrenocortical axis: a review of animal models and human studies across development. Psychol Bull. 140: 256–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Teicher MH, Anderson CM, Polcari A (2012): Childhood maltreatment is associated with reduced volume in the hippocampal subfields CA3, dentate gyrus, and subiculum. Proc Natl Acad Sci U S A. 109: E563–E572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Iglesias JE, Augustinack JC, Nguyen K, Player CM, Player A, Wright M, et al. (2015): A computational atlas of the hippocampal formation using ex vivo, ultra-high resolution MRI: Application to adaptive segmentation of in vivo MRI. NeuroImage. 115: 117–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Whelan CD, Hibar DP, van Velzen LS, Zannas AS, Carrillo-Roa T, McMahon K, et al. (2016): Heritability and reliability of automatically segmented human hippocampal formation subregions. NeuroImage. 128: 125–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dale AM, Fischl B, Sereno MI (1999): Cortical surface-based analysis. I. Segmentation and surface reconstruction. NeuroImage. 9: 179–194. [DOI] [PubMed] [Google Scholar]
- 46.Fischl B, Dale AM (2000): Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A. 97: 11050–11055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, et al. (2006): An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage. 31: 968–980. [DOI] [PubMed] [Google Scholar]
- 48.Fischl B, van der Kouwe A, Destrieux C, Halgren E, Ségonne F, Salat DH, et al. (2004): Automatically parcellating the human cerebral cortex. Cereb Cortex. 14: 11–22. [DOI] [PubMed] [Google Scholar]
- 49.Ghosh SS, Kakunoori S, Augustinack J, Nieto-Castanon A, Kovelman I, Gaab N, et al. (2010): Evaluating the Validity of Volume-Based and Surface-Based Brain Image Registration for Developmental Cognitive Neuroscience Studies in Children 4-to-11 Years of Age. NeuroImage. 53: 85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Han X, Jovicich J, Salat D, van der Kouwe A, Quinn B, Czanner S, et al. (2006): Reliability of MRI-derived measurements of human cerebral cortical thickness: the effects of field strength, scanner upgrade and manufacturer. NeuroImage. 32: 180–194. [DOI] [PubMed] [Google Scholar]
- 51.Cohen S, Kamarck T, Mermelstein R (1983): A global measure of perceived stress. J Health Soc Behav. 24: 385–396. [PubMed] [Google Scholar]
- 52.Sarason IG, Johnson JH, Siegel JM (1978): Assessing the impact of life changes: development of the Life Experiences Survey. J Consult Clin Psychol. 46: 932–946. [DOI] [PubMed] [Google Scholar]
- 53.Pilkauskas NV, Currie J, Garfinkel I (2012): The Great Recession, Public Transfers, and Material Hardship. Soc Serv Rev. 86: 401–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Davenport MD, Tiefenbacher S, Lutz CK, Novak MA, Meyer JS (2006): Analysis of endogenous cortisol concentrations in the hair of rhesus macaques. Gen Comp Endocrinol. 147: 255–261. [DOI] [PubMed] [Google Scholar]
- 55.Meyer J, Novak M, Hamel A, Rosenberg K (2014): Extraction and Analysis of Cortisol from Human and Monkey Hair. J Vis Exp. . doi: 10.3791/50882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bauer PJ, Dikmen SS, Heaton RK, Mungas D, Slotkin J, Beaumont JL (2013): III. NIH TOOLBOX COGNITION BATTERY (CB): MEASURING EPISODIC MEMORY. Monogr Soc Res Child Dev. 78: 34–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tulsky DS, Carlozzi N, Chevalier N, Espy K, Beaumont J, Mungas D (2013): NIH Toolbox Cognitive Function Battery (NIHTB-CFB): Measuring Working Memory. Monogr Soc Res Child Dev. 78: 70–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zelazo PD, Anderson JE, Richler J, Wallner-Allen K, Beaumont JL, Weintraub S (2013): II. NIH Toolbox Cognition Battery (CB): measuring executive function and attention. Monogr Soc Res Child Dev. 78: 16–33. [DOI] [PubMed] [Google Scholar]
- 59.Akshoomoff N, Newman E, Thompson WK, McCabe C, Bloss CS, Chang L, et al. (2014): The NIH Toolbox Cognition Battery: results from a large normative developmental sample (PING). Neuropsychology. 28: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lupien SJ, Maheu F, Tu M, Fiocco A, Schramek TE (2007): The effects of stress and stress hormones on human cognition: Implications for the field of brain and cognition. Brain Cogn. 65: 209–237. [DOI] [PubMed] [Google Scholar]
- 61.Benjamini Y, Hochberg Y (1995): Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J R Stat Soc Ser B Methodol. 57: 289–300. [Google Scholar]
- 62.MacKinnon DP, Lockwood CM, Hoffman JM, West SG, Sheets V (2002): A comparison of methods to test mediation and other intervening variable effects. Psychol Methods. 7: 83–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hayes AF (2013): Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-Based Approach. Guilford Press. [Google Scholar]
- 64.Preacher KJ, Hayes AF (2008): Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav Res Methods. 40: 879–891. [DOI] [PubMed] [Google Scholar]
- 65.Lupien SJ, McEwen BS, Gunnar MR, Heim C (2009): Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 10: 434–445. [DOI] [PubMed] [Google Scholar]
- 66.McEwen BS, Gianaros PJ (2010): Central role of the brain in stress and adaptation: Links to socioeconomic status, health, and disease. Ann N Y Acad Sci. 1186: 190–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Diorio D, Viau V, Meaney MJ (1993): The role of the medial prefrontal cortex (cingulate gyrus) in the regulation of hypothalamic-pituitary-adrenal responses to stress. J Neurosci Off J Soc Neurosci. 13: 3839–3847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Eisenberger NI, Taylor SE, Gable SL, Hilmert CJ, Lieberman MD (2007): Neural pathways link social support to attenuated neuroendocrine stress responses. NeuroImage. 35: 1601–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bishop S, Duncan J, Brett M, Lawrence AD (2004): Prefrontal cortical function and anxiety: controlling attention to threat-related stimuli. Nat Neurosci. 7: 184–188. [DOI] [PubMed] [Google Scholar]
- 70.Milad MR, Quirk GJ, Pitman RK, Orr SP, Fischl B, Rauch SL (2007): A role for the human dorsal anterior cingulate cortex in fear expression. Biol Psychiatry. 62: 1191–1194. [DOI] [PubMed] [Google Scholar]
- 71.Milad MR, Rauch SL (2012): Obsessive Compulsive Disorder: Beyond Segregated Corticostriatal Pathways. Trends Cogn Sci. 16: 43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mills KL, Tamnes CK (2014): Methods and considerations for longitudinal structural brain imaging analysis across development. Dev Cogn Neurosci. 9: 172–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Whittle S, Simmons JG, Dennison M, Vijayakumar N, Schwartz O, Yap MBH, et al. (2014): Positive parenting predicts the development of adolescent brain structure: a longitudinal study. Dev Cogn Neurosci. 8: 7–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Whittle S, Vijayakumar N, Dennison M, Schwartz O, Simmons JG, Sheeber L, Allen NB (2016): Observed Measures of Negative Parenting Predict Brain Development during Adolescence. PLoS ONE. 11. doi: 10.1371/journal.pone.0147774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shaw P, Greenstein D, Lerch J, Clasen L, Lenroot R, Gogtay N, et al. (2006): Intellectual ability and cortical development in children and adolescents. Nature. 440: 676–679. [DOI] [PubMed] [Google Scholar]
- 76.Vijayakumar N, Whittle S, Yücel M, Dennison M, Simmons J, Allen NB (2014): Thinning of the lateral prefrontal cortex during adolescence predicts emotion regulation in females. Soc Cogn Affect Neurosci. 9: 1845–1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Brito NH, Piccolo LR, Noble KG (2017): Associations between cortical thickness and neurocognitive skills during childhood vary by family socioeconomic factors. Brain Cogn. 116: 54–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Duval ER, Garfinkel SN, Swain JE, Evans GW, Blackburn EK, Angstadt M, et al. (2017): Childhood Poverty is Associated with Altered Hippocampal Function and Visuospatial Memory in Adulthood. Dev Cogn Neurosci. 23: 39–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ofen N, Tang L, Yu Q, Johnson EL (2018): Memory and the developing brain: From description to explanation with innovation in methods. Dev Cogn Neurosci. . doi: 10.1016/j.dcn.2018.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sheline YI, Liston C, McEwen BS (2019): Parsing the Hippocampus in Depression: Chronic Stress, Hippocampal Volume, and Major Depressive Disorder. Biol Psychiatry. 85: 436–438. [DOI] [PubMed] [Google Scholar]
- 81.Grotzinger AD, Mann FD, Patterson MW, Tackett JL, Tucker-Drob EM, Harden KP (2018): Hair and Salivary Testosterone, Hair Cortisol, and Externalizing Behaviors in Adolescents. Psychol Sci. 29: 688–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Teicher MH, Anderson CM, Ohashi K, Khan A, McGreenery CE, Bolger EA, et al. (2018): Differential effects of childhood neglect and abuse during sensitive exposure periods on male and female hippocampus. NeuroImage. 169: 443–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Alexander-Bloch A, Clasen L, Stockman M, Ronan L, Lalonde F, Giedd J, Raznahan A (2016): Subtle In-Scanner Motion Biases Automated Measurement of Brain Anatomy From In Vivo MRI. Hum Brain Mapp. 37: 2385–2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Morey RA, Petty CM, Xu Y, Hayes JP, Wagner HR, Lewis DV, et al. (2009): A comparison of automated segmentation and manual tracing for quantifying hippocampal and amygdala volumes. NeuroImage. 45: 855–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Schmidt MF, Storrs JM, Freeman KB, Jack CR, Turner ST, Griswold ME, Mosley TH (2018): A comparison of manual tracing and FreeSurfer for estimating hippocampal volume over the adult lifespan. Hum Brain Mapp. 39: 2500–2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.