Abstract
Antibodies are key to cutaneous host defense and inflammation. Despite their importance, the mechanisms by which skin antibodies are sustained are poorly described. Here, we identified that in addition to antibody production in lymphoid tissues, plasma cells reside in healthy mouse and human skin. In naïve mice, IgM was the predominant isotype produced in skin. Skin plasma cells developed independently of T cells and microbiota. Importantly, chronic skin inflammation promoted the massive accumulation of IgM-secreting cells, and cutaneous immunization directed both T cell-dependent and -independent antigen-specific IgM-secreting cells into skin. Unlike their counterparts in lymphoid tissues, cutaneous IgM-secreting cells were completely dependent on survival factors APRIL or BLyS/BAFF, which were constitutively expressed and upregulated during inflammation in skin. Our data support a model in which skin plasma cells supply natural and adaptive IgM to the cutaneous environment, thereby supporting homeostatic skin barrier functions and providing defense against pathogen intrusion. Our results are also of potential relevance for manipulation of cutaneous plasma cells in inflammatory skin diseases or cutaneous plasma cell malignancies.
INTRODUCTION
The skin is a large barrier organ that faces constant microbial and environmental threats, requiring the skin immune system to orchestrate appropriate responses that combat infection while limiting immunopathology. Antibodies are key to cutaneous host defense, as illustrated by the susceptibility to skin infections of individuals with immunodeficiencies that affect immunoglobulin production (Lehman, 2014). Antibodies have potent effector functions that include neutralization of toxins and pathogens, complement fixation, and activation of effector cells as well as promoting phagocytosis (Lu et al., 2018). Although most antibodies are protective, when they recognize cutaneous autoantigens or allergens, they can promote inflammatory disorders of the skin, such as pemphigus vulgaris or atopic dermatitis (Cipriani et al., 2014, Hammers and Stanley, 2016).
While most antibody is systemic, being produced in lymphoid tissues and reaching extralymphoid tissues via blood, there is a key role for localized antibody production in tissues. For example, intestinally produced IgA, and with increasing evidence IgM, regulate local microbiomes and prevent entry of toxins and pathogens (Bunker et al., 2015, Fadlallah et al., 2018, Mantis et al., 2011). In contrast, few studies address production of antibodies in mammalian skin. Specifically, two studies analyzed the origins of cutaneous IgA (Metze et al., 1989, Okada et al., 1988). The authors found that in healthy human skin, IgA ASCs localize to eccrine sweat glands and IgA is found in sweat and sebum, consistent with polymeric immunoglobulin receptor-mediated transport into excretions and subsequent reach of skin epithelia (Metze et al., 1989, Okada et al., 1988). In addition, ASCs of unknown isotype have been documented in healthy ovine skin (Geherin et al., 2012).
During inflammation, the existence of ASCs in skin is much better established. Moreover, there is recent evidence that pathogenic autoantibody production within lesional skin is part of the pathogenesis of pemphigus (Yuan et al., 2017) and likely other inflammatory skin disorders including IgG4-related disease (Hsiao and Wu, 2016, Tokura et al., 2014) and scleroderma (Bosello et al., 2018). Despite the importance of antibodies to skin health, there is a dearth of knowledge of how antibody titers are sustained in skin and if and how skin-localized antibody production can be regulated.
Antibody secreting cells (ASCs) differentiate from B cells and encompass proliferating plasmablasts and senescent plasma cells. Responses by conventional (follicular) B cells that involve T cell help and germinal center reactions give rise to potent isotype-switched antibodies of high affinity that develop over several weeks after primary antigen encounter (MacLennan, 1994). Innate-like B cells, which comprise B-1 B cells and marginal zone B cells, are enriched in B cell receptor specificities for conserved pathogen structures and respond rapidly without the need for T cell help, making them important early after infection (Baumgarth, 2011, Kearney, 2005). Consistent with an exposure to infectious agents, barrier sites such as the intestinal mucosa and the skin are enriched in B-1 B cells (Geherin et al., 2012, Geherin et al., 2016, Suzuki et al., 2010). Even in the absence of microbial stimulation (i.e in germ-free mice), B-1 B cells give rise to natural IgM, which is important in the defense against a number of pathogens and also enhances uptake of apoptotic cells and cell debris by macrophages, while limiting tissue inflammation (Grönwall and Silverman, 2014).
It is likely that multiple B cell subsets contribute to the development of skin ASCs during homeostasis and inflammation, and there are several factors that could potentially promote skin ASC generation and/or local survival. Potential candidates include TNF superfamily members B cell-activating factor (BAFF, also known as BLyS) and a proliferation-inducing ligand (APRIL). Interactions between BAFF/APRIL and their receptors on B lineage cells regulate B cell survival, differentiation, and antibody production, and they are critical for plasma cell survival (Schneider, 2005, Sindhava et al., 2013).
In this study, we revisited the question of antibody production in skin. We found that IgM is the predominant isotype produced by plasma cells in healthy murine skin and is also secreted in unperturbed human skin. In mice, cutaneous IgM plasma cells were independent of T cells and microbiota, suggesting that these cells provide natural antibody to the skin to support homeostatic functions. Importantly, chronic inflammation caused the massive accumulation of IgM ASCs that were dependent on APRIL or BAFF in skin but not in other sites. Thus, we reveal the skin as a niche for ASCs with important implications for the regulation of cutaneous antibodies in host defense and inflammation.
RESULTS
IgM plasma cells constitutively reside in skin
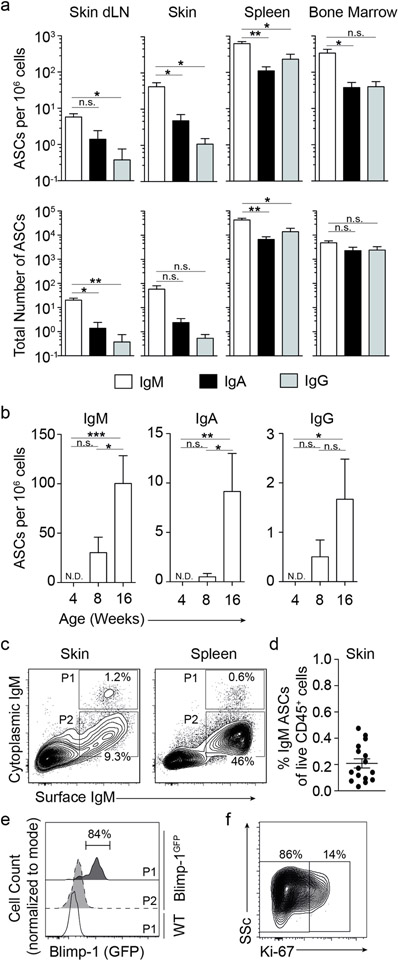
We isolated leukocytes from the abdominal and back skin regions and from lymphatic organs of naïve 8-week old wildtype mice and enumerated ASCs directly ex vivo by isotype-specific ELISPOT assay without exogenous cell stimulation. The skin of naïve mice harbored IgM ASCs at the highest frequency and number compared with IgA and IgG ASCs (Fig. 1a). Numbers and frequencies of IgM ASCs were about 10-fold higher than that of IgA ASCs (42 vs. 4.8 cells per million cells; P<0.05 for frequency; not significant for numbers), while individual IgG ASCs were only occasionally present (Fig. 1a), and IgE ASCs undetectable (data not shown). IgM ASCs also predominated in skin-draining lymph nodes, spleen, and bone marrow of naïve mice (Fig. 1a). ASCs were undetectable at 4 weeks of age, appeared at 8 weeks, and had markedly increased by 16 weeks of age (p<0.05, comparing 4 weeks relative to 16 weeks for IgM, IgA, and IgG ASCs; Fig. 1b). At both 8 and 16 weeks of age, IgM was the predominant isotype produced (Fig. 1b), and there was no significant further increase in IgM ASCs in the skin of 24-week old mice (not shown). Given the underrepresentation of other isotypes, we focused our studies on IgM. We performed intracellular staining of skin-isolated leukocytes for cytoplasmic IgM, which when combined with IgM surface staining, identifies ASC by flow cytometry (Reynolds et al., 2015). A cytoplasmic IgM+ ASC population was clearly identified in healthy skin (Fig. 1c), comprising 0.2±0.04% (mean±SEM) of live CD45+ cells isolated from naïve skin (Fig. 1d), corroborating our ELISPOT data showing IgM ASCs in skin (Fig 1a–b). Expression of transcription factor Blimp-1 (encoded by prdm1) in the B cell lineage marks ASCs and is part of their differentiation programming (Kallies et al., 2004, Savage et al., 2017, Shapiro-Shelef et al., 2003). We therefore examined the expression of Blimp-1 in skin IgM ACSs using Blimp-1GFP reporter mice (Kallies et al., 2004). As expected, skin IgM ASCs (cytoplasmatic IgM+) were largely GFP+ (Blimp-1+) compared with co-localizing surface IgM+ cytoplasmic IgM− cells or cytoplasmic IgM+ ASCs from the spleen of wildtype mice (Fig. 1e). To distinguish between proliferating plasmablasts and senescent plasma cells, we included staining for Ki-67, which marks proliferating cells including plasmablasts (Pracht et al., 2017). The majority (78.6 ± 11.9, mean ± SD) of IgM skin ASCs from wildtype mice were negative for Ki-67 (Fig. 1f). We conclude that healthy skin is home to ASCs that are mainly senescent IgM plasma cells.
Figure 1. IgM antibody secreting cells (ASCs) reside in healthy skin.
Cells were isolated from tissues of naïve mice. (a and b) ELISPOT assays were used to determine IgM, IgA, and IgG ASC frequencies among all isolated cells (a, top and b) and total ASC numbers (a, bottom) in 8-week old wildtype mice, or in skin cells from different age groups (b). (c) Flow cytometric analysis of intracellular and surface IgM to detect IgM ASCs in wildtype mice. Cells shown were gated on live CD45+CD3−F4/80−IgD− lymphocytes. (d) Summary of the results in (c) expressed as % IgM ASCs among live CD45+ skin cells. (e) Flow cytometric analysis of Blimp-1 expression in IgM ASCs (“P1”) and control cells (“P2”) from skin of Blimp-1GFP reporter mice or splenic IgM ASCs from wildtype mice. P1 and P2 gates correspond to those in Panel C. (f) Flow cytometric analysis of Ki-67 expression in skin IgM ASCs in wildtype mice, gated as in “P1” in Panel C. Data points show mean ± SEM (a, b, d), indicate individual mice (d), or one representative staining (c, e, f) of 2 (a, b, e, f) or 4 (c, d) independent experiments analyzing a total of 6–12 (a), 6–10 (b, f) and 16 (c, d) mice per group. Skin plots are concatenated. dLN, draining lymph node. ND, none detected. n.s., not significant; *, p<0.05, **, p<0.01, ***, p<0.001 using 1-way ANOVA followed by Bonferroni’s post-test.
Skin IgM ASCs are generated independently of T cells and microbiota
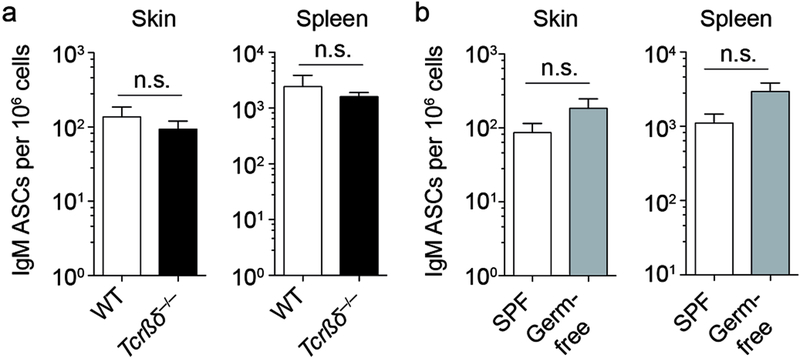
IgM ASCs can be generated as a result of B cell responses to thymus-dependent or -independent antigens and even without exogenous antigenic stimulation in case of ASCs that produce natural IgM (Ehrenstein and Notley, 2010). To test whether IgM ASC in healthy skin required T cell help, we analyzed naïve mice that lack all T cells (crosses between Tcrβ−/− and Tcrδ−/− mice, Tcrβδ−/− mice) and matched controls for the presence of cutaneous IgM by ELISPOT assay. Surprisingly, Tcrβδ−/− mice had the same frequency of skin IgM ASCs as control mice (Fig. 2a), indicating that cutaneous IgM ASCs do not require T cells for their generation. As skin commensals orchestrate skin-infiltrating T cell subsets (Naik et al., 2012), we aimed to determine whether microbial colonization was required to generate skin IgM ASCs. Unexpectedly, skin IgM ASCs were present in similar numbers in germ-free and specific pathogen-free mice (Fig. 2b). Thus, cutaneous IgM ASCs do not depend upon microbial stimulation of skin or other body sites and represent natural IgM secreting cells.
Figure 2. Skin IgM ASCs develop independently of T cells and microbial colonization.
(a and b) Skin and spleen cells were isolated from naïve mice. Frequency of IgM ASCs among all isolated cells was determined by ELISPOT assay analyzing wildtype and T cell-deficient Tcrβδ−/− mice (a) or specific pathogen free (SPF) and germ-free mice (b). Data points indicate the mean ± SEM of 15–23 mice per group from 3 independent experiments (a) or of 8–10 mice per group from 2 independent experiments (b). WT, wildtype; SPF, specific pathogen-free; n.s., not significant using the Mann Whitney test.
IgM ASCs accumulate in inflamed skin
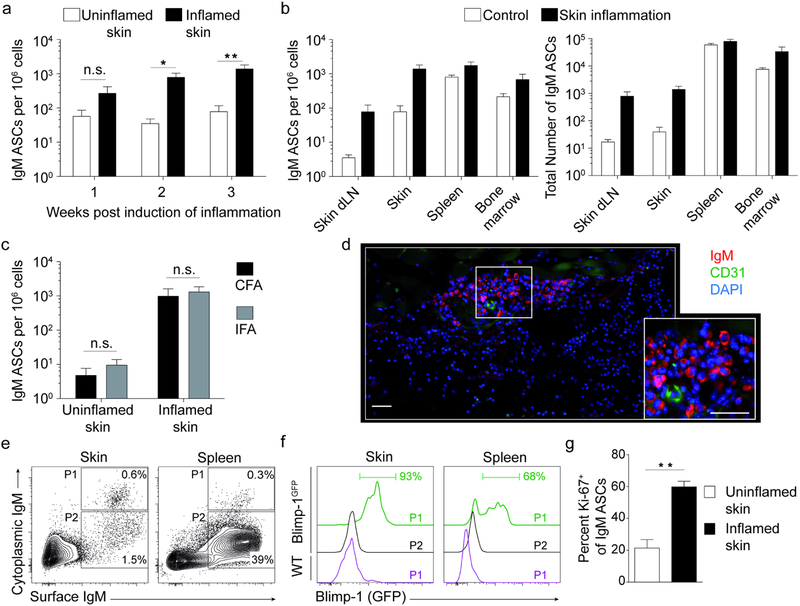
Having established the constitutive presence of IgM ASCs in healthy skin, we asked whether these cells would be affected by skin inflammation. Wildtype mice were injected subcutaneously with Complete Freund’s Adjuvant (CFA), a treatment that rapidly induces localized neutrophilic inflammation that advances into chronic granulomatous skin inflammation with mononuclear infiltrates (Brown et al., 2010) and B cell infiltration (Geherin et al., 2016). Skin IgM ASCs increased within one week after induction of inflammation, albeit not reaching statistical significance (P> 0.05 compared with uninflamed skin; Fig. 3a). After 2 and 3 weeks of inflammation, time points at which skin granulomas have formed (Brown et al., 2010), IgM ASC had significantly increased about 20-fold at the site of inflammation compared with nonlesional skin (P<0.05 and P<0.01, respectively; Fig. 3a). Importantly, IgM ASC frequencies in chronically inflamed skin (3 weeks post induction of inflammation) reached levels similar to that of typical lymphoid plasma cell niches, namely the bone marrow and spleen (Fig. 3b). However, total numbers of IgM ASCs in skin remained lower relative to bone marrow and spleen (Fig. 3b), reflecting the smaller relative size of the lymphoid compartment in inflamed skin. While the frequencies of IgM ASCs increased more than 20-fold in the skin-draining lymph node during inflammation, they stayed ~10-fold below the IgM frequencies in skin and spleen (P<0.01; Fig. 3b left).
Figure 3. Chronic skin inflammation promotes accumulation of IgM ASCs independent of microbial stimuli.
Skin inflammation in mice was induced by s.c. injection of CFA or IFA, as indicated. (a-c) Cells were isolated from tissues at indicated time points or 3 weeks after induction of skin inflammation and ELISPOT assays used to determine the frequency of IgM ASCs among all isolated cells (a, b left, c) and total ASC numbers per organ (b right). ASC frequencies were compared to those in tissues from separate control mice (a and b) or in uninflamed (non-lesional) skin of the same mice (c). (d) Immunofluorescence staining of frozen sections from 3-week old skin inflammation elicited with CFA. DAPI (4′,6-diamidino-2-phenylindole) was used to visualize nuclei. Scale bar, 50 μm. (e-g) Flow cytometric analysis of intracellular and surface IgM to detect IgM ASCs 3 weeks post induction of inflammation with CFA. Cells shown were gated on live CD45+CD3−F4/80−IgD− lymphocytes from indicated tissues of Blimp-1GFP reporter (f) and wildtype mice (e, g). Histograms (f) represent gates shown in Panel e. Summary of flow cytometric analysis of Ki-67+ IgM ASCs, gated as in “P1” in Panel e, in inflamed skin and uninflamed skin from separate controls (g). Data points indicate mean ± SEM for each group from 6–7 (a), 7–8 (b), or 5–10 (g) mice per group from two independent experiments, or for one representative experiment of three performed using 4–5 mice per group (c). One representative staining of a minimum of 10 (d) and 21 (e, f) analyzed mice from at least 3 independent experiments is shown. Skin flow plots are concatenated. dLN, draining lymph node; CFA, complete Freund’s Adjuvant; IFA, Incomplete Freund’s Adjuvant; n.s., not significant; *, p< 0.05; **, p<0.01 using the Kruskal-Wallis test followed by Dunn’s post-test (a-c) or the Mann Whitney test (g).
As ASCs accumulate in infected skin, we next addressed whether IgM ASC accumulation in inflamed skin required the presence of microbial signals by comparing granulomatous inflammation induced by CFA to that elicited by Incomplete Freund’s Adjuvant (IFA). While both contain granuloma-inducing mineral oil, only CFA, but not IFA, has a microbial component (inactivated mycobacteria). Notably, in both CFA and IFA-elicited inflammation, the frequency of skin IgM ASCs increased to the same extent (~50-fold compared to nonlesional skin; Fig. 3c). Thus, IgM ASCs accumulated in chronically inflamed skin also in the absence of mycobacterial signals. In chronically inflamed skin, IgM ASCs localized in irregularly distributed clusters within the dermis outside of CD31+ vessels, as assessed by immunofluorescence histology (Fig. 3d), validating our ELISPOT data (Fig. 3a–c). Using cytoplasmatic IgM staining as in Figure 1, a population of IgM ASCs was readily identified in chronically inflamed skin by flow cytometry (Fig. 3e). Employing Blimp-1GFP reporter mice, IgM ASCs (cytoplasmatic IgM+) from skin or spleen were GFP+ (Blimp-1+) compared with co-localizing surface IgM+ cytoplasmic IgM− cells or cytoplasmic IgM+ ASCs from wildtype mice (Fig. 3f). The percentage of proliferating Ki-67+ plasmablasts was significantly higher among ASCs in chronically inflamed relative to uninflamed skin (P=0.003; Fig. 3g). We conclude that chronic inflammation promotes accumulation of IgM+ plasma cells and plasmablasts in skin.
IgM ASCs reside in human skin
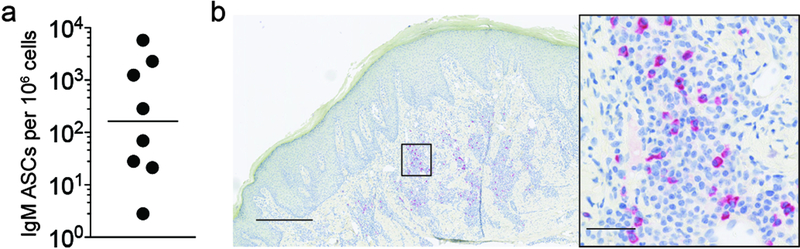
To determine whether skin IgM ASCs are also part of the skin immune system of humans, we analyzed leukocytes isolated from healthy human skin by ELISPOT assay. Importantly, IgM ASCs were present in all samples of healthy adult human skin (N=8) but frequencies varied widely between individual donors (range 3–5,800 IgM ASCs per million cells; geometric mean = 165; Fig. 4a) without an obvious association with skin site (not shown). We were unable to assess an influence by sex or age due to limited information available for the de-identified human skin samples. Next, we aimed to test whether IgM ASCs localize to chronically inflamed human skin. We assessed paraffin sections of acne keloidalis, a chronic inflammatory skin disorder featuring accumulation of plasma cells (Dinehart et al., 1989), for the presence of IgM+ plasma cells using immunohistochemistry. As in mouse skin granulomas, acne keloidalis harbored irregularly distributed clusters of IgM ASCs within the inflamed dermis (Fig. 4b). We conclude that healthy and chronically inflamed human skin represent niches for IgM ASCs.
Figure 4. Healthy and inflamed human skin harbor IgM ASCs.
(a) Cells from healthy human skin were isolated and the frequency of IgM ASCs among all isolated cells determined by ELISPOT assay. (b) Immunohistochemistry staining of paraffin sections for IgM in acne keloidalis skin biopsy specimens, Scale bar, 500 μm (overview) and 50 μm (magnified area). Data points indicate individual donors (N=8) and geometric mean (a) or a representative image from (N=7) individuals with acne keloidalis (b).
Inflammation induces the accumulation of both T-dependent and -independent antigen specific IgM ASCs in skin
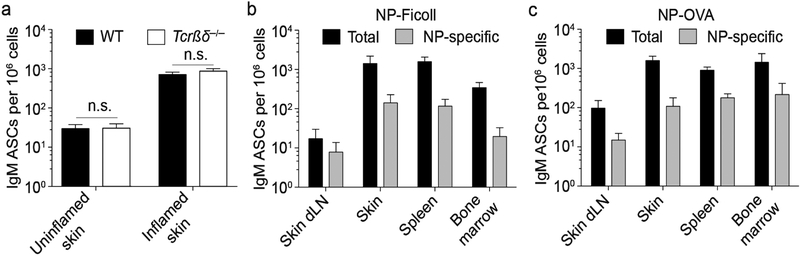
Skin-resident IgM ASCs were independent of T cells in unperturbed skin (Fig. 2). To address whether IgM ASC accumulation in inflamed skin requires T cells, we induced skin inflammation with CFA in T cell-deficient Tcrβδ−/− and wildtype mice and analyzed chronically inflamed skin for accumulation of skin IgM ASCs three weeks later. As in healthy skin (Fig. 2), Tcrβδ−/− mice contained IgM ASCs in inflamed skin at the same frequency as wildtype mice (Fig. 5a). Because the skin is an important entry point for various pathogens and IgM is potent in host defense, we wondered whether antigen-specific IgM ASCs induced by cutaneous immune responses would localize to skin. We immunized wildtype mice subcutaneously with standard experimental antigens 4-hydroxy-3-nitrophenylacetyl (NP)-Ficoll or NP-OVA, which are thymus-independent type II and T cell-dependent antigens, respectively. Consistent with the notion that skin IgM ASCs do not require T cells (Fig. 5a), NP-specific IgM ASCs were found in immunized skin, three weeks after immunization with NP-Ficoll in CFA (Fig. 5b). The data demonstrate that adaptive T cell-independent IgM ASCs localize to inflamed skin. Importantly, after immunization with NP-OVA in CFA, NP-specific IgM ASCs were also detected in the inflamed skin, showing that ASCs generated in T-cell dependent immune responses localize to immunized skin (Fig. 5c). Thus, inflamed skin supports the accumulation of both T cell-dependent and -independent IgM ASCs, and antigen-specific ASCs can be directed to the skin through vaccination.
Figure 5. T cell-independent and -dependent and antigen-specific IgM ASCs accumulate in inflamed skin.
(a) Skin inflammation was induced in wildtype and Tcrβδ−/− mice by s.c. injection of CFA. 3 weeks later, cells were isolated from inflamed and uninflamed (non-lesional) skin of the same mice and the frequency of IgM ASCs among all isolated cells was determined by ELISPOT assay. (b and c) Wildtype mice were immunized with CFA plus thymus-independent antigen NP-Ficoll (b) or thymus-dependent antigen NP-OVA (c). 3 weeks post immunization, cells were isolated and the frequency of total and NP-specific IgM ASCs determined by ELISPOT assay. Data indicate means ± SEM of 7–8 mice per group from 2 independent experiments (a) or from one representative experiment of a minimum of 3 performed with 4–5 mice per group each (b and c). WT, wildtype; dLN, draining lymph node; NP, 4-Hydroxy-3-nitrophenyacetyl; OVA, ovalbumin; n.s., not significant using the Kruskal-Wallis test followed by Dunn’s post-test.
Skin IgM ASCs require BAFF/BLyS or APRIL
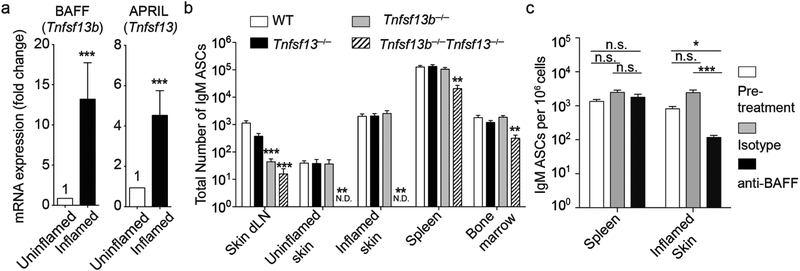
Having established that IgM ASCs reside in unperturbed and inflamed skin, we assessed whether the cytokines BAFF and APRIL played a role in creating an ASC survival niche in skin. Using quantitative RT-PCR, we examined chronically inflamed and control skin for expression of mRNA for BAFF and APRIL, encoded by Tnfs13b and Tnfs13, respectively. Both APRIL and BAFF mRNA were detected in healthy skin (Fig. 6a). Importantly, mirroring the local accumulation of IgM ASCs (Figs. 3a–c), in chronically inflamed skin both BAFF and APRIL transcripts mRNA were upregulated by 13.2- and 4.5-fold (p=0.0006 and p=0.0001), respectively, compared with skin from naïve mice (Fig. 6a). To test the roles of BAFF and APRIL in IgM ASCs localization in skin, we induced chronic skin inflammation with CFA in Tnfs13b−/− and Tnfs13−/− as well as double-deficient Tnfs13b−/−Tnfs13−/− mice. With exception of the inflamed skin-draining lymph node, IgM ASCs were only modestly and not significantly reduced in any of the organs of mice singly deficient in either APRIL or BAFF (p>0.05; Fig. 6b). Strikingly, while all analyzed lymphoid organs (skin-draining lymph node, spleen and bone marrow) showed a similar mild reduction in IgM ASCs in Tnfs13b−/−Tnfs13−/− mice, the inflamed and uninflamed skin were completely devoid of these cells (p<0.001 and p<0.01, respectively; Fig. 6b).
Figure 6. Cutaneous IgM ASCs require APRIL or BAFF.
(a) mRNA from skin of untreated wildtype mice or 3 weeks after induction of skin inflammation with CFA was analyzed by quantitative RT-PCR. Tnfsf13b and Tnfsf13 mRNA expression levels were quantified using gapdh as a standard and normalized to expression levels of uninflamed skin (set as 1). (b) Numbers of IgM ASCs from indicated tissues was determined by ELISPOT assay comparing wildtype to Tnfsf13−/−, Tnfsf13b−/−, and Tnfsf13b−/−Tnfsf13−/− mice 3 weeks post induction of skin inflammation with CFA. (c) Skin inflammation was induced in wildtype mice by s.c. injection of CFA. 2 weeks later, mice were given 2 doses of anti-BAFF (10F4) or isotype antibody. IgM ASCs were enumerated by ELISPOT assay prior to antibody treatment and on day 28 of inflammation. Data points indicate mean ± SEM of 3 (a) or 2 (b and c) combined experiments analyzing a total of 14 (a), 7–8 (b) and 9–10 (c) mice per group. n.s., not significant; *, p<0.05; **, p<0.01; ***, p<0.001using the Wilcoxon Signed-Rank test (a), the Kruskal-Wallis test followed by Dunn’s post-test (b and c); dLN, draining lymph node; WT, wildtype; N.D., none detected.
To test if anti-BAFF would reduce ASC accumulation in inflamed skin, we induced skin inflammation with CFA in wildtype mice and two weeks later treated with blocking anti-BAFF or isotype antibody. 2 weeks after initiation of antibody treatment (4 weeks post induction of inflammation), anti-BAFF treatment significantly reduced accumulation of IgM ASCs in inflamed skin relative to skin from both isotype-treated mice and the pre-treatment 2-week time point of inflammation on average by 94% (p<0.001) and 84% (p<0.05), respectively (Fig. 6c). In contrast, anti-BAFF had no effect on IgM ASCs in spleen (Fig. 6c). In conclusion, our data show that while presence of IgM ASCs at lymphoid sites is largely independent of BAFF or APRIL, IgM ASC development and/or survival in skin is absolutely dependent on presence of at least one of these survival cytokines.
DISCUSSION
In this study, we established the existence of IgM ASCs in skin of mice and humans. In mice, skin plasma cells developed independently of T cells and microbial-derived signals. Natural IgM is evolutionarily conserved and polyreactive antibody produced constitutively from birth even in the absence of microbial stimulation. Natural antibody secretion primarily occurs in the spleen and bone marrow with the majority of natural IgM coming from B-1 B lineage ASCs (Savage and Baumgarth, 2015, Savage et al., 2017). Therefore, our data are in line with our previous observation that healthy skin harbors B-1-like B cells (Geherin et al., 2016) and introduces the skin as a site for natural antibody production.
After immunization with TD or TI antigens, antigen-specific IgM secreting cells accumulated in skin, demonstrating that adaptive IgM, in addition to natural IgM, is produced in skin and that this pathway can be targeted by vaccination. A main function of IgM is host defense (Ehrenstein and Notley, 2010), and selective IgM deficiency, a rare immunodeficiency, is associated with recurring bacterial and viral skin infections including infection with the skin-untypical pathogen Streptoccoccus pneumoniae (Belgemen et al., 2009, Louis and Gupta, 2014). Human sweat contains IgM with reactivity for the skin pathogen Staphylococcus aureus and many skin microbes are covered in IgM, IgG and IgA (Metze et al., 1991), further supporting the notion of cutaneous host defense and barrier function enhancement by cutaneously produced IgM and other immunoglobulins. Recent studies extend the functions of IgM to the regulation of microbial colonization of intestinal barriers (Magri et al., 2017). Therefore, it will be interesting to further explore a role for IgM in shaping skin microbiomes.
Besides recognition of pathogens, natural IgM has important anti-inflammatory functions as demonstrated for atherosclerosis, arthritis, and type 2 diabetes (Chen et al., 2009a, Kyaw et al., 2012, Shen et al., 2015). This anti-inflammatory function is largely mediated by the ability of natural IgM to bind apoptotic cells and to facilitate their phagocytic uptake by macrophages (“efferocytosis”). For example, the T15 idiotype natural antibody (T15i) binds phosphorylcholine neo-determinants that become exposed on apoptotic cells, while sparing healthy cells (Chen et al., 2009a, Chen et al., 2009b). Complexes of T15 IgM with apoptotic cells amplify efferocytosis and suppress pro-inflammatory programming in macrophages (Chen et al., 2009a, Chen et al., 2009b). Given the various insults the skin is exposed to on a daily basis, ranging from UV radiation to thermal and other physical insults, clearance of damaged cells by efferocytosis before they release inflammatory substances and autoantigens seems of particular importance. Hence, natural antibody production within skin has likely roles in host defense, enhancement of apoptotic cell clearance, and suppression of inflammation.
B cells differentiate into ASCs in secondary lymphoid tissues and travel to survival niches within lymphoid tissues, such as the bone marrow, medullary cords of lymph nodes, and the splenic red pulp but also sites of chronic inflammation and the lamina propria mucosae (Tangye, 2011). In such niches, plasma cells make antibodies ranging from several days to a life time (Tangye, 2011, Tarlinton et al., 2008). It is well established that ASCs accumulate in skin during inflammation and infection. For examples, a plasma cell-rich perivascular infiltrate is a fundamental histopathologic change in syphilis (Engelkens et al., 1991), and plasma cell presence in inflammatory infiltrates facilitates dermatopathological diagnosis of several entities such as acne keloidalis (Dinehart et al., 1989). However, the factors that drive cutaneous accumulation of ASCs were unknown. Our study revealed that chronic skin inflammation upregulates cutaneous expression of BAFF and APRIL, thereby amplifying the cutaneous ASC niche. As a result, IgM ASCs accumulated at similar frequencies as in typical lymphoid plasma cell survival sites. As the uninflamed skin contained mostly Ki67− BLIMP-1+ senescent (terminally growth arrested) plasma cells, the observed accumulation of Ki67+ plasmablasts in inflamed skin was likely due to recruitment of newly formed ASCs into skin. This notion is further supported by the accumulation of antigen-specific ASCs that were newly generated in response to immunization with TD and TI antigens. In line with our results are studies that show increased BAFF expression in inflamed skin in discoid lupus lesions or reactive atopy test patches (Chen et al., 2011, Chong et al., 2014). Importantly, cutaneous IgM ASCs, unlike their counterparts in lymphoid tissues, required either APRIL or BAFF. A possible explanation for these tissue differences is that there are alternative ASC survival factors present in lymphoid tissues, including skin draining lymph nodes, that are absent from skin. For example, IL-6, CXCL12, and adhesive signals provided by specialized stromal cells promote ASC survival within specialized microenvironments of lymphoid tissues, e.g. within the medullary cords of lymph nodes and splenic red pulp (Tangye, 2011). Alternatively, it is conceivable that skin ASCs represent or are derived from a unique population of B-lineage cells that is reliant on survival factors different from those critical for lymphoid tissue ASCs.
Drugs that target B cell survival factors, such as the anti-BAFF antibody belimumab, may be attractive for the treatment of autoimmune diseases that affect skin. Four independent randomized, double-blind clinical trials in patients with systemic lupus erythematosus have each documented greater clinical response in patients treated with belimumab plus standard-of-care than in patients treated with placebo plus standard-of-care (Furie et al., 2011, Navarra et al., 2011, Stohl et al., 2017, Zhang et al., 2018). Of note, significantly greater improvement in mucocutaneous manifestations has been noted among belimumab-treated patients than among placebo-treated patients (Manzi et al., 2012). Other autoimmune disorders with skin manifestations may also be amenable to pharmacological targeting of B cell/ASC survival factors. For example, pemphigus and scleroderma are associated with autoantibody producing cells that reside in skin (Bosello et al., 2018, Yuan et al., 2017), and BAFF and BAFF receptor are potential drug targets for treatment (Gordon et al., 2018, Huang et al., 2016). Our study showed that BAFF inhibition in wildtype mice reduced accumulation of IgM ASCs in the inflamed skin. Moreover, the treatment was also able to ablate ASCs that had already accumulated in skin during the first two weeks of local inflammation (Fig. 6c). Of note, genetic BAFF deficiency did not have the same effect as the anti-BAFF treatment of wildtype mice (Figs. 6b and c), which is likely due to compensatory increase in BAFF-independent B cells and/or ASCs in skin of mutant mice. In support of this, genetic BAFF deficiency greatly reduces conventional B cells, but not B-1 B cells (Schneider, 2005, Sindhava et al., 2013). Non-cutaneous IgM ASC subsets have differential resistance to anti-BAFF treatment (Scholz et al., 2008). Thus, additional studies are needed to assess blockade of the BAFF and/or APRIL axes as a means of diminishing selective skin IgM ASC subsets, such as TD vs. TI and natural IgM ASCs. Because most pathogenic autoantibodies are IgGs, it will also be critical to test the dependence of cutaneous IgG vs. IgM ASCs on BAFF/APRIL.
Notably, malignant ASCs occasionally infiltrate the skin as primary or secondary cutaneous plasmacytomas, or in Waldenström macroglobulinemia, a lymphoproliferative disorder of IgM+ ASCs (Rongioletti et al., 2008, Tsang et al., 2016). Thus, the regulation of skin ASCs under physiological conditions by BAFF/APRIL raises the question whether the same pathways maintain malignant ASCs in extralymphoid sites, such as skin, and whether these pathways may be harnessed for novel therapeutic approaches.
In conclusion, we document the existence of IgM plasma cells in healthy mouse and human skin, and we shed light on the regulation of plasma cell accumulation during skin inflammation by TNF family members BAFF and APRIL. Understanding the skin as a specialized niche for natural and adaptive IgM producing ASCs opens the field for manipulation of cutaneous ASCs in inflammatory skin diseases, cutaneous plasma cell malignancies, and novel vaccine approaches aimed at increasing humoral skin immunity.
MATERIALS AND METHODS
Mice, immunizations, and induction of skin inflammation
All mice were on C57BL/6 background and bred and maintained under specific pathogen-free conditions at the University of Pennsylvania, Thomas Jefferson University and/or the University of Southern California. Breeder pairs of crosses between Tcrβ−/− and Tcrδ−/− mice (Tcrβd−/−) and Blimp-1GFP mice (Kallies et al., 2004) were kindly provided by Taku Kambayashi and David Allman (both at the University of Pennsylvania), respectively. APRIL-deficient (tnfsf13−/−), mice (Castigli et al., 2004) were obtained from Raif Geha (Childrens Hospital of Boston). BAFF-deficient (tnfsf13b−/−) mice (Schiemann et al., 2001) were obtained from Susan Kalled (Biogen Idec). These singly-deficient mice were crossed to generate doubly-deficient tnfsf13−/−tnfsf13b−/− mice. Tissues from germ-free and specific pathogen-free control mice were kindly provided by the lab of David Artis while at the University of Pennsylvania. Unless otherwise stated, sex- and age-matched groups of male and female mice were used in experiments without obvious differences between sexes. Unless otherwise indicated, mice were between 8 and 16 weeks of age when entering experiments with the exception of untreated Blimp-1GFP mice, which were up to 33 weeks of age. Skin inflammation in mice was induced by a single s.c. injection of 100 μl IFA or CFA (Sigma-Aldrich) emulsified with PBS into the area of the flank or back of 8–12-week-old mice. Unless otherwise indicated, the chronically inflamed skin was harvested and analyzed 3–3.5 weeks later. For antigen-specific immunizations, 50 μg of NP-Ficoll or NP-OVA (LGC Biosearch Technologies) was included in the s.c. CFA injections, and mice were analyzed 3 weeks later. For BAFF inhibition studies, CFA-mediated skin inflammation was induced in wildtype mice as above, and 15 days later a cohort of mice was analyzed for IgM ASCs by ELISPOT assay. The remaining mice were treated with 100 μg of anti-BAFF (clone 10F4, (Scholz et al., 2008)) or isotype Armenian hamster IgG (BioXCell) intraperitoneally on days 15 and 20 of inflammation, and IgM ASCs were enumerated on day 28 by ELISPOT assay. Efficacy of the anti-BAFF treatment (Scholz et al., 2008) was confirmed by a ≥90% reduction in follicular splenic B cells (not shown). All animal procedures were approved by the Institutional Animal Care and Use Committees of the University of Pennsylvania, Thomas Jefferson University, and/or the University of Southern California and conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals.
Human specimens
De-identified male and female healthy adult human skin specimens were obtained through the University of Pennsylvania Skin Biology and Diseases Research Center collected from skin surgery procedures (Burow’s triangles) with approval by the Institutional Review Board of the University of Pennsylvania. De-identified paraffin sections from skin with histopathologically confirmed acne keloidalis diagnosis were received from the dermatopathology tissue bank in the Department of Dermatology and Cutaneous Biology with approval from the Institutional Review Board of Thomas Jefferson University. The use of de-identified human tissues, originally collected for clinical purposes, did not require written patient consent.
Cell isolations and ELISPOT assays
Mouse skin from back and abdomen was collected after shaving. Leukocytes were isolated from human or mouse skin samples by tissue mincing and subsequent enzymatic digestion with 0.1 mg/ml DNase I (Roche) and 25 – 50 μg/ml Liberase TM (Roche) in HBSS containing Ca2+ and Mg2+ (Gibco) through two subsequent 30 minutes-incubations at 37°C. After each incubation, the suspension was immediately filtered through 100 μm cell strainers (Corning or VWR) and the released cells were washed. Subiliac lymph node and spleen cells were isolated by grinding through 40- and 70- μm cell strainers, respectively. To isolate mouse bone marrow cells, femurs and tibias were flushed with media using a 27-gauge needle and subsequently passed through 18-gauge needle. Finally, all samples were lysed using Red Blood Cell Lysing Buffer Hybri-Max (Sigma). Total cell counts were calculated for to the entire spleen and skin sample, bone marrow from one leg and one subiliac lymph node.
For ELISPOT assays, MultiScreen HTS 96-well filter plates (Millipore) were coated with isotype-specific polyclonal goat anti-mouse antibodies (KPL/Seracare) or NP (Biosearch Technologies). Plates were blocked with goat serum (Life Technologies), and cells isolated from tissues of individual mice were plated in RPMI 1640 containing 10% newborn calf serum (Invitrogen) and incubated for 12–14 h at 37°C and 5% CO2. After washing, plates were incubated with HRP-conjugated isotype-specific polyclonal goat anti-mouse antibodies (KPL). Next, the plates were washed and developed using an AEC Peroxidase Substrate Kit according to the manufacturer’s instructions (Vector Laboratories). Human ELISPOTs were performed without B cell stimulation following the manufacturer’s instructions (Mabtech). Spots were enumerated using an Olympus SZ51 dissecting microscope or an ImmunoSpot Reader and Image Acquisition software (both from Cellular Technology).
Flow cytometry, immunofluorescence and immunohistochemistry
Cells were stained with the following rat anti-mouse monoclonal antibodies specific for CD45-BV395 [clone 30-F11], CD138-PE [281–2] from BD Bioscience; CD19-AF700 [1D3], IgD-PE or FITC [11–26c], IgM-PECy7 and eF450 [II/41] from ThermoFisher; F4/80-PECy5 [BM8], Ki-67-APC [16A8], and Armenian hamster anti-mouse CD3-PECy5 [145–2C11] from Biolegend. Cells were pre-incubated with LIVE/DEAD Fixable Aqua Dead Cell Stain Kit (Life Technologies), blocking anti-FcγRII/III (clone 2.4G2; University of California San Francisco Monoclonal Antibody Core) and rat IgG (Jackson Immunoresearch). After surface staining, cells were fixed in 2% paraformaldehyde, followed by staining of cytoplasmic IgM in 0.5% saponin buffer (Sigma Aldrich). Ki-67 staining was performed using the Foxp3/Transcription Factor Staining kit (Ebioscience). Samples were acquired on a BD LSRFortessa and analyzed with FlowJo software v10 (Treestar). Doublets and dead cells were excluded by gating.
For histology in mice, inflamed skin was collected, fixed for 3–5h in 4% PFA, and incubated overnight in 20% sucrose prior to freezing in OCT. 7-μm tissue sections were blocked for 1h with 10% rat and 10% goat serum prior to incubation with rat anti-mouse CD31-FITC (MEC13.3; BD Biosciences) and IgM-PE-Dazzle594 (RMM-1; Biolegend). Nuclei were visualized with 4′,6-diamidino-2-phenylindole (Invitrogen). Sections were mounted with Prolong Gold Antifade (Invitrogen), and images acquired on a BZ-X710 microscope (KEYENCE). For human immunohistochemistry, paraffin sections were processed and stained with the Leica Bond Max automated slide stainer using mouse anti-human IgM (clone IM260, Abcam) and the Bond™ Polymer Refine Red Detection system (Leica Biosystems). Images were scanned with the NanoZoomer and analyzed with NDP.view2 software (Hamamatsu Photonics K.K.).
RT-quantitative PCR
Skin samples were harvested into RNAlater RNA Stabilization Reagent (Qiagen), incubated overnight at room temperature, and stored at −80°C. Total RNA was extracted using TRIzol (Ambion Life Technologies) and cDNA synthesized with High-Capacity cDNA Reverse Transcription Kit according to the manufacturer’s instructions (Applied Biosystems). Quantitative PCR was performed on a StepOnePlus™ Real-Time PCR System (Applied Biosystems™) using Taqman probes and primer pairs (Applied Biosystems). Transcripts were quantified using the housekeeping gene Gadph as a standard.
Statistical analysis
Statistical analyses to compare experimental groups were performed with GraphPad Prism (GraphPad Software) using the parametric 1-way ANOVA followed by Bonferroni’s post-test, the non-parametric Mann Whitney test or Kruskal Wallis test followed by Dunn’s post-test, and the Wilcoxon Signed Rank test comparing collected values to a hypothetical (normalized) value of 1. P<0.05 was considered statistically significant and tests used are indicated in the respective figure legends.
ACKNOWLEDGEMENTS
We thank John Riley and Carolina Lopez for protocols for RNA isolation, Stephen Nutt (Walter and Eliza Hall Institute of Medical Research, Australia) for Blimp-1GFP mice, the Penn Skin Biology and Diseases Resource-based Center for human skin samples, Amanda Schmidt for establishing ELISPOT assays, and Mufei Liu, Samantha Measner, Jack Ringe, and Michael Lee for excellent technical assistance, and Jean Scholz for sharing antibodies. We thank Justin Jesse (Leica Biosystems) for establishing human IHC staining. We are indebted to Aimee Payne, Dave Allman, Malissa Diehl, and Skye Geherin for helpful discussion and advice along the way. We thank Zia Rahman for critical comments on the manuscript. This work was supported in parts by NIH grants R01AR067751 and R01AI127389 to GFD, and an Ethel Brown Foerderer Foundation Fellowship from Thomas Jefferson University and T32AI134646 to SEM.
Abbreviations used:
- APRIL
a proliferation-inducing ligand
- ASC
antibody secreting cell
- BAFF
B-cell activating factor
- CFA
Complete Freund’s Adjuvant
- IFA
Incomplete Freund’s Adjuvant
- NP
4-Hydroxy-3-nitrophenyacetyl
- OVA
ovalbumin
- TD
thymus-dependent
- TI
thymus-independent
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DATA AVAILABILITY STATEMENT
Datasets related to this article are available upon request from Dr. Gudrun Debes at Thomas Jefferson University. Gudrun.debes@jefferson.edu.
CONFLICT OF INTEREST STATEMENT
The authors state no conflict of interest.
REFERENCES
- Baumgarth N The double life of a B-1 cell: self-reactivity selects for protective effector functions. Nat Rev Immunol 2011;11(1):34–46. [DOI] [PubMed] [Google Scholar]
- Belgemen T, Suskan E, Dogu F, Ikinciogullari A. Selective immunoglobulin M deficiency presenting with recurrent impetigo: a case report and review of the literature. Int Arch Allergy Immunol 2009;149(3):283–8. [DOI] [PubMed] [Google Scholar]
- Bosello S, Angelucci C, Lama G, Alivernini S, Proietti G, Tolusso B, et al. Characterization of inflammatory cell infiltrate of scleroderma skin: B cells and skin score progression. Arthritis Res Ther 2018;20(1):75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MN, Fintushel SR, Lee MH, Jennrich S, Geherin SA, Hay JB, et al. Chemoattractant receptors and lymphocyte egress from extralymphoid tissue: changing requirements during the course of inflammation. J Immunol 2010;185(8):4873–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunker JJ, Flynn TM, Koval JC, Shaw DG, Meisel M, McDonald BD, et al. Innate and Adaptive Humoral Responses Coat Distinct Commensal Bacteria with Immunoglobulin A. Immunity 2015;43(3):541–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castigli E, Scott S, Dedeoglu F, Bryce P, Jabara H, Bhan AK, et al. Impaired IgA class switching in APRIL-deficient mice. Proc Natl Acad Sci U S A 2004;101(11):3903–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Khanna S, Goodyear CS, Park YB, Raz E, Thiel S, et al. Regulation of dendritic cells and macrophages by an anti-apoptotic cell natural antibody that suppresses TLR responses and inhibits inflammatory arthritis. J Immunol 2009a;183(2):1346–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Lind Enoksson S, Johansson C, Karlsson MA, Lundeberg L, Nilsson G, et al. The expression of BAFF, APRIL and TWEAK is altered in eczema skin but not in the circulation of atopic and seborrheic eczema patients. PLoS One 2011;6(7):e22202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Park YB, Patel E, Silverman GJ. IgM antibodies to apoptosis-associated determinants recruit C1q and enhance dendritic cell phagocytosis of apoptotic cells. J Immunol 2009b;182(10):6031–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong BF, Tseng LC, Kim A, Miller RT, Yancey KB, Hosler GA. Differential expression of BAFF and its receptors in discoid lupus erythematosus patients. J Dermatol Sci 2014;73(3):216–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipriani F, Ricci G, Leoni MC, Capra L, Baviera G, Longo G, et al. Autoimmunity in atopic dermatitis: biomarker or simply epiphenomenon? J Dermatol 2014;41(7):569–76. [DOI] [PubMed] [Google Scholar]
- Dinehart SM, Herzberg AJ, Kerns BJ, Pollack SV. Acne keloidalis: a review. J Dermatol Surg Oncol 1989;15(6):642–7. [DOI] [PubMed] [Google Scholar]
- Ehrenstein MR, Notley CA. The importance of natural IgM: scavenger, protector and regulator. Nat Rev Immunol 2010;10(11):778–86. [DOI] [PubMed] [Google Scholar]
- Engelkens HJ, ten Kate FJ, Vuzevski VD, van der Sluis JJ, Stolz E. Primary and secondary syphilis: a histopathological study. Int J STD AIDS 1991;2(4):280–4. [DOI] [PubMed] [Google Scholar]
- Fadlallah J, El Kafsi H, Sterlin D, Juste C, Parizot C, Dorgham K, et al. Microbial ecology perturbation in human IgA deficiency. Sci Transl Med 2018;10(439). [DOI] [PubMed] [Google Scholar]
- Furie R, Petri M, Zamani O, Cervera R, Wallace DJ, Tegzova D, et al. A phase III, randomized, placebo-controlled study of belimumab, a monoclonal antibody that inhibits B lymphocyte stimulator, in patients with systemic lupus erythematosus. Arthritis Rheum 2011;63(12):3918–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geherin SA, Fintushel SR, Lee MH, Wilson RP, Patel RT, Alt C, et al. The skin, a novel niche for recirculating B cells. J Immunol 2012;188(12):6027–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geherin SA, Gomez D, Glabman RA, Ruthel G, Hamann A, Debes GF. IL-10+ Innate-like B Cells Are Part of the Skin Immune System and Require alpha4beta1 Integrin To Migrate between the Peritoneum and Inflamed Skin. J Immunol 2016;196(6):2514–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon JK, Martyanov V, Franks JM, Bernstein EJ, Szymonifka J, Magro C, et al. Belimumab for the Treatment of Early Diffuse Systemic Sclerosis: Results of a Randomized, Double-Blind, Placebo-Controlled, Pilot Trial. Arthritis Rheumatol 2018;70(2):308–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grönwall C, Silverman GJ. Natural IgM: beneficial autoantibodies for the control of inflammatory and autoimmune disease. J Clin Immunol 2014;34 Suppl 1:S12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammers CM, Stanley JR. Mechanisms of Disease: Pemphigus and Bullous Pemphigoid. Annu Rev Pathol 2016;11:175–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao PF, Wu YH. Characterization of Cutaneous Plasmacytosis at Different Disease Stages. Dermatology 2016;232(6):738–47. [DOI] [PubMed] [Google Scholar]
- Huang A, Madan RK, Levitt J. Future therapies for pemphigus vulgaris: Rituximab and beyond. J Am Acad Dermatol 2016;74(4):746–53. [DOI] [PubMed] [Google Scholar]
- Kallies A, Hasbold J, Tarlinton DM, Dietrich W, Corcoran LM, Hodgkin PD, et al. Plasma cell ontogeny defined by quantitative changes in blimp-1 expression. J Exp Med 2004;200(8):967–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearney JF. Innate-like B cells. Springer Semin Immunopathol 2005;26(4):377–83. [DOI] [PubMed] [Google Scholar]
- Kyaw T, Tipping P, Bobik A, Toh BH. Protective role of natural IgM-producing B1a cells in atherosclerosis. Trends Cardiovasc Med 2012;22(2):48–53. [DOI] [PubMed] [Google Scholar]
- Lehman H Skin manifestations of primary immune deficiency. Clin Rev Allergy Immunol 2014;46(2):112–9. [DOI] [PubMed] [Google Scholar]
- Louis AG, Gupta S. Primary selective IgM deficiency: an ignored immunodeficiency. Clin Rev Allergy Immunol 2014;46(2):104–11. [DOI] [PubMed] [Google Scholar]
- Lu LL, Suscovich TJ, Fortune SM, Alter G. Beyond binding: antibody effector functions in infectious diseases. Nat Rev Immunol 2018;18(1):46–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLennan IC. Germinal centers. Annu Rev Immunol 1994;12:117–39. [DOI] [PubMed] [Google Scholar]
- Magri G, Comerma L, Pybus M, Sintes J, Llige D, Segura-Garzon D, et al. Human Secretory IgM Emerges from Plasma Cells Clonally Related to Gut Memory B Cells and Targets Highly Diverse Commensals. Immunity 2017;47(1):118–34 e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantis NJ, Rol N, Corthesy B. Secretory IgA’s complex roles in immunity and mucosal homeostasis in the gut. Mucosal Immunol 2011;4(6):603–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzi S, Sanchez-Guerrero J, Merrill JT, Furie R, Gladman D, Navarra SV, et al. Effects of belimumab, a B lymphocyte stimulator-specific inhibitor, on disease activity across multiple organ domains in patients with systemic lupus erythematosus: combined results from two phase III trials. Ann Rheum Dis 2012;71(11):1833–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metze D, Jurecka W, Gebhart W, Schmidt J, Mainitz M, Niebauer G. Immunohistochemical demonstration of immunoglobulin A in human sebaceous and sweat glands. J Invest Dermatol 1989;92(1):13–7. [DOI] [PubMed] [Google Scholar]
- Metze D, Kersten A, Jurecka W, Gebhart W. Immunoglobulins coat microorganisms of skin surface: a comparative immunohistochemical and ultrastructural study of cutaneous and oral microbial symbionts. J Invest Dermatol 1991;96(4):439–45. [DOI] [PubMed] [Google Scholar]
- Naik S, Bouladoux N, Wilhelm C, Molloy MJ, Salcedo R, Kastenmuller W, et al. Compartmentalized control of skin immunity by resident commensals. Science 2012;337(6098):1115–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarra SV, Guzman RM, Gallacher AE, Hall S, Levy RA, Jimenez RE, et al. Efficacy and safety of belimumab in patients with active systemic lupus erythematosus: a randomised, placebo-controlled, phase 3 trial. Lancet 2011;377(9767):721–31. [DOI] [PubMed] [Google Scholar]
- Okada T, Konishi H, Ito M, Nagura H, Asai J. Identification of secretory immunoglobulin A in human sweat and sweat glands. J Invest Dermatol 1988;90(5):648–51. [DOI] [PubMed] [Google Scholar]
- Pracht K, Meinzinger J, Daum P, Schulz SR, Reimer D, Hauke M, et al. A new staining protocol for detection of murine antibody-secreting plasma cell subsets by flow cytometry. Eur J Immunol 2017;47(8):1389–92. [DOI] [PubMed] [Google Scholar]
- Reynolds AE, Kuraoka M, Kelsoe G. Natural IgM is produced by CD5- plasma cells that occupy a distinct survival niche in bone marrow. J Immunol 2015;194(1):231–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rongioletti F, Patterson JW, Rebora A. The histological and pathogenetic spectrum of cutaneous disease in monoclonal gammopathies. J Cutan Pathol 2008;35(8):705–21. [DOI] [PubMed] [Google Scholar]
- Savage HP, Baumgarth N. Characteristics of natural antibody-secreting cells. Ann N Y Acad Sci 2015;1362:132–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage HP, Yenson VM, Sawhney SS, Mousseau BJ, Lund FE, Baumgarth N. Blimp-1-dependent and -independent natural antibody production by B-1 and B-1-derived plasma cells. J Exp Med 2017;214(9):2777–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiemann B, Gommerman JL, Vora K, Cachero TG, Shulga-Morskaya S, Dobles M, et al. An essential role for BAFF in the normal development of B cells through a BCMA-independent pathway. Science 2001;293(5537):2111–4. [DOI] [PubMed] [Google Scholar]
- Schneider P The role of APRIL and BAFF in lymphocyte activation. Curr Opin Immunol 2005;17(3):282–9. [DOI] [PubMed] [Google Scholar]
- Scholz JL, Crowley JE, Tomayko MM, Steinel N, O’Neill PJ, Quinn WJ 3rd, et al. BLyS inhibition eliminates primary B cells but leaves natural and acquired humoral immunity intact. Proc Natl Acad Sci U S A 2008;105(40):15517–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro-Shelef M, Lin KI, McHeyzer-Williams LJ, Liao J, McHeyzer-Williams MG, Calame K. Blimp-1 is required for the formation of immunoglobulin secreting plasma cells and pre- plasma memory B cells. Immunity 2003;19(4):607–20. [DOI] [PubMed] [Google Scholar]
- Shen L, Chng MH, Alonso MN, Yuan R, Winer DA, Engleman EG. B-1a lymphocytes attenuate insulin resistance. Diabetes 2015;64(2):593–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sindhava VJ, Scholz JL, Cancro MP. Roles for BLyS family members in meeting the distinct homeostatic demands of innate and adaptive B cells. Front Immunol 2013;4:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stohl W, Schwarting A, Okada M, Scheinberg M, Doria A, Hammer AE, et al. Efficacy and Safety of Subcutaneous Belimumab in Systemic Lupus Erythematosus: A Fifty-Two-Week Randomized, Double-Blind, Placebo-Controlled Study. Arthritis Rheumatol 2017;69(5):1016–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K, Maruya M, Kawamoto S, Fagarasan S. Roles of B-1 and B-2 cells in innate and acquired IgA-mediated immunity. Immunol Rev 2010;237(1):180–90. [DOI] [PubMed] [Google Scholar]
- Tangye SG. Staying alive: regulation of plasma cell survival. Trends Immunol 2011;32(12):595–602. [DOI] [PubMed] [Google Scholar]
- Tarlinton D, Radbruch A, Hiepe F, Dorner T. Plasma cell differentiation and survival. Curr Opin Immunol 2008;20(2):162–9. [DOI] [PubMed] [Google Scholar]
- Tokura Y, Yagi H, Yanaguchi H, Majima Y, Kasuya A, Ito T, et al. IgG4-related skin disease. Br J Dermatol 2014;171(5):959–67. [DOI] [PubMed] [Google Scholar]
- Tsang DS, Le LW, Kukreti V, Sun A. Treatment and outcomes for primary cutaneous extramedullary plasmacytoma: a case series. Curr Oncol 2016;23(6):e630–e46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan H, Zhou S, Liu Z, Cong W, Fei X, Zeng W, et al. Pivotal Role of Lesional and Perilesional T/B Lymphocytes in Pemphigus Pathogenesis. J Invest Dermatol 2017;137(11):2362–70. [DOI] [PubMed] [Google Scholar]
- Zhang F, Bae SC, Bass D, Chu M, Egginton S, Gordon D, et al. A pivotal phase III, randomised, placebo-controlled study of belimumab in patients with systemic lupus erythematosus located in China, Japan and South Korea. Ann Rheum Dis 2018;77(3):355–63. [DOI] [PMC free article] [PubMed] [Google Scholar]