Abstract
Objective:
To evaluate change in the incidence of FTT based on selected growth percentile criteria and diagnostic codes before and after a switch in growth curves.
Methods:
We performed a retrospective cohort study of children 2 to 24 months of age in a large primary care network that switched its default growth curve from the Centers for Disease Control (CDC) reference to the World Health Organization (WHO) standards in 2012. We compared the incidence of FTT defined by growth percentile criteria (using the default growth curve at the time of each measurement) and by ICD-9 codes in the three years before and after the CDC-WHO switch using an interrupted time series analysis. We performed these analyses stratified by age group (≤6 months and >6-24 months).
Results:
We evaluated 83,299 children. Among those ≤6 months, increases in FTT incidence were found in both growth-percentile and clinician-diagnosis criteria at the CDC-WHO switch (p<0.05). Among those >6-24 months, decreases in FTT incidence were found by growth-percentile criteria at the CDC-WHO switch (p<0.05), but no significant changes were found in FTT incidence by diagnostic codes.
Conclusions:
When switching from the CDC to the WHO growth curves, changes in the incidence of FTT by growth-percentile and clinician-diagnosis criteria differed for younger versus older infants. Factors beyond growth likely influence the decision to diagnose a child as having FTT and may differ in younger compared to older infants.
Keywords: growth charts, failure to thrive, malnutrition, diagnosis
Introduction
Ensuring that children grow adequately is fundamental to pediatric care. Failure to thrive (FTT) is the clinical sign of inadequate weight gain in infants and young children. Numerous criteria have been proposed for defining FTT, with minimal evidence to support any specific definition.1,2 Little is known about how clinicians currently distinguish between children who they consider to be small and healthy versus children who they expect would benefit from evaluation and treatment for inadequate weight gain.
Both under-identification and over-identification of FTT have the potential to cause harm. Delayed recognition of FTT in the subsets of children with treatable medical conditions3,4 or who are experiencing abuse or neglect5 may cause harm. Children with FTT have long-term cognitive deficits compared to unaffected children, with larger differences (approximately 12 intelligence quotient [IQ] points) seen in children with more severe FTT identified in inpatient and specialty settings, and smaller differences (approximately 3-4 IQ points) for children with FTT identified through screening or in primary care settings.6–8 Children with FTT are also smaller than unaffected children at long-term follow-up.8,9 Timely treatment may decrease these differences. Little published evidence is available regarding the harms of evaluation and treatment for FTT.2 Potential harms include the risks and costs associated with diagnostic testing, emotional distress for children and caregivers, discomfort associated with supplemental feeding, and rare but serious complications of enteral tube feeding, such as aspiration pneumonia.10
In 2010, the Centers for Disease Control (CDC) recommended that for children less than two years of age clinicians switch from using the CDC growth references based on U.S. data to the World Health Organization (WHO) growth standards based on data from six countries including the U.S.(Figure 1).11 The rationale for the recommendation included that the WHO curves were based on children without health problems, included more infant measurements, and were restricted to infants who were breastfed “in recognition that breastfeeding is the recommended standard for infant feeding.”
Figure 1.
Comparison of 5th and 95th weight-for-age (WFA) and weight-for-length (WFL) percentiles for Centers for Disease Control (CDC) and World Health Organization (WHO) growth curves for males.
For the weight-for-age (WFA) and weight-for-length (WFL) growth curves, the WHO 5th percentile line is higher than that of the CDC 5th percentile until approximately six months of age, when the CDC 5th percentile line becomes higher than that for WHO. (Figure 1) These differences suggest that more children younger than six months and fewer children older than six months will have a WFA or WFL <5th percentile using the WHO curves. Therefore, the change in growth curves could affect how often children are diagnosed with FTT.
In order to assess the public health impact of this growth curve change and to gain insight into the decision-making patterns of pediatric clinicians with respect to FTT identification, we sought to evaluate changes in the incidence of FTT based on commonly used growth percentile criteria and clinician-diagnosed FTT at the switch from the CDC to the WHO growth curves in a large pediatric primary care network.
Methods
Population
The study included children two to 24 months of age during the three years before and after July 2012, when the Children’s Hospital of Philadelphia (CHOP) switched from using the CDC to the WHO growth curves as the default in the electronic health record (EHR) for children under age two years. We included children born between July 1, 2007 and June 30, 2015. We restricted the analysis to children followed in the network for primary care, defined as ≥ 3 preventive care visits by age 24 months, with ≥ 1 preventive visit occurring between 0 and 12 months, ≥ 1 preventive visit occurring between 12 and 24 months, and ≥ 1 visit during the study period (July 2009 to June 2015).
Setting
The CHOP primary care network included a total of 33 practices that were open at any point during the study period. Five practices served as the primary teaching sites for residents (with two of an initial four sites consolidating into the fifth site in 2013). The sites varied in location type (urban, suburban, and semi-rural), payor mix, racial/ethnic background of patients, and involvement in teaching of trainees.
Data sources and preparation
We extracted the following data elements from the EHR (Epic™ [Verona, WI]): visit type (well child visit vs. other), payor (Medicaid vs. private), ICD-9 diagnostic codes, and growth parameters. We used an automated method to identify weight and length values that were implausible or that appeared to be carried forward from a previous value rather than representing an independent measurement.12 The method was validated in children over one year of age. We modified the method to increase the tolerance for large amounts of weight loss in infants <30 days of age, similar to modifications made for a prior published study.13 Further details of the modification are available upon request.
Growth-percentile FTT outcome measures
We chose growth-percentile outcome measures that are commonly used as FTT definitions in research, are referenced in textbooks and review articles as indications to consider a diagnosis of FTT, and are, in our experience, commonly used by clinicians as indications for concern about FTT.1,14,15 Our primary growth-percentile outcome measure was the proportion of patients with weight-for-age (WFA) < 5th percentile for age and sex using the growth curve that was the default in the EHR at the time of the measurement (CDC era July 2009 to June 2012; WHO era July 2012 to June 2015). Secondary growth-percentile FTT outcome measures were (1) a WFL <5th percentile for age and sex, (2) a WFA percentile that crossed two decreasing major percentile lines (MPL) and (3) a combined growth-percentile measure including any child who met the WFA, WFL, or MPL criteria in a given time period. Further details about these definitions and the rationale for the definitions are provided in the appendix (Supplemental Text).
Clinician-diagnosis FTT outcome measures
Our primary clinician-diagnosis FTT outcome measure was an incident ICD-9 code for FTT or a similar diagnosis (Table 1), referred to subsequently as the clinician-diagnosis criteria. We also evaluated an alternative outcome measure restricted to the single ICD-9 code for FTT (783.41), referred to as the narrow clinician-diagnosis criteria.
Table 1.
ICD-9 codes comprising the clinician-diagnosis criteria
| ICD-9 Code | Description |
|---|---|
| 263.9 | Unspecified protein-calorie malnutrition |
| 269.9 | Nutritional deficiency, unspecified |
| 783.21 | Loss of weight |
| 783.22 | Underweight |
| 783.41 | Failure to thrive |
We identified a large number of patients with typical weight gain meeting the clinician-diagnosis FTT criteria for a visit in the first two months of life with a diagnosis of the ICD-9 code for “loss of weight” or similar. The diagnostic code likely represented a “weight check.” This term is used in the network to describe a brief visit to ensure adequate growth and well-being in neonates, and it does not necessarily indicate that the weight gain at that visit was concerning. Therefore, we restricted evaluation of outcomes to children older than 2 months of age.
Exclusion criteria
We excluded children with a clinician-diagnosis FTT ICD-9 code prior to study eligibility and children who were diagnosed with a complex chronic condition (CCC) prior to study eligibility or prior to an FTT diagnosis. Additional details regarding exclusion criteria are available in the appendix (Supplemental Text and Supplemental Table 1).
Analysis
Our analyses were stratified by age into two groups: ≤6 months (the <6-month group) and >6-24 months (the 6-to-24-month group). We determined the proportion of children with incident FTT according to the growth-percentile and clinician-diagnosis criteria in the 12 quarters (3-month blocks) before and after the CDC-WHO switch. A child was eligible for analysis in a given quarter if the child had not previously met the FTT criteria in question and had at least one of the following recorded during the quarter: a weight measurement (WFA); a set of simultaneous weight and length measurements during a well visit (WFL); a weight measurement during the quarter and ≥1 weight measurements prior to the current visit (MPL); or a visit or problem list entry (clinician-diagnosis). A child who was eligible to be included in at least one of the growth analyses (WFA, WFL, or MPL) was eligible to be included in the combined growth analysis.
We used an interrupted time series analysis for each measure to evaluate change in FTT incidence at the CDC-WHO switch, which was designated as time zero (T0). We evaluated 12 quarters before and 12 quarters after T0, with the earliest quarter designated Q-12 and the latest designated Q+12. The outcome was binomial (number of children with incident FTT / number at risk) in each quarter, and thus logistic regression was used. To account for repeated measures for a given patient, generalized estimating equations were used to estimate parameters of the model; the working correlation was specified as unstructured. The model contained three parameters: time (quarter), an indicator for growth curve used during the quarter (CDC vs. WHO), and the number of quarters after change to the WHO growth curve (with 0 coded for quarters that occurred during the CDC growth chart). Conceptually, the model included a slope of FTT incidence across time for quarters in which the CDC chart was used (Q-12 to T0), a slope across time of FTT incidence for quarters in which the WHO chart was used (T0 to Q+12), and an immediate change in FTT incidence at the time point of the CDC-WHO switch (T0). The immediate change at the CDC-WHO switch (T0) was the primary outcome. Analyses were performed without adjustment for covariates.
Ethics
The study required use of a limited dataset that included dates of birth. It was designated not human subjects research by the CHOP Institutional Review Board (study number 16012658) and was approved by the Pennsylvania State University Institutional Review Board (Human Subjects Protection Office study number 6251). The funders had no role in the study.
Results
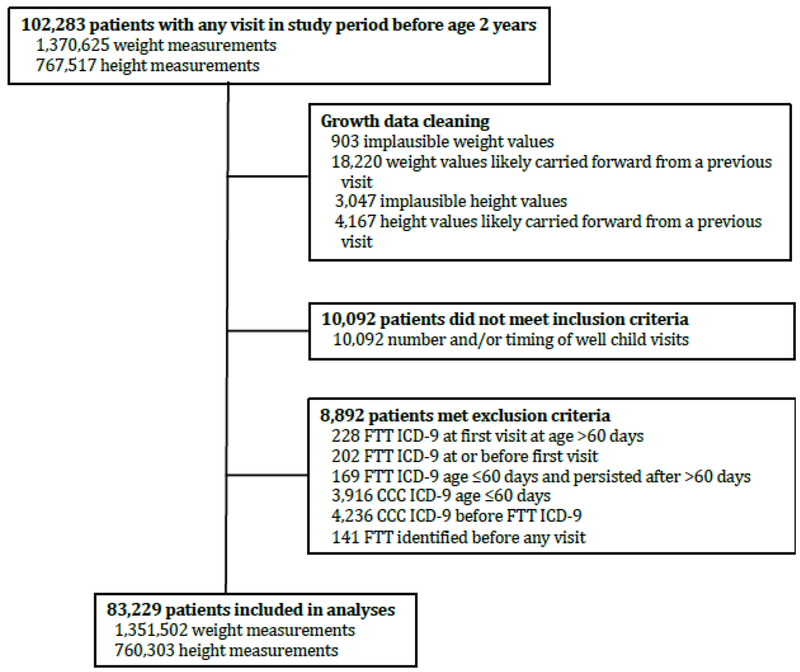
We identified 102,283 potentially eligible patients; 83,229 children were included in analyses (Figure 2). The patients were diverse in race, ethnicity, and payor (Table 2). The median number of visits per patient was 10 (interquartile range 6, 14). The incidence of clinician-diagnosis FTT was lower than the incidence of growth-percentile FTT (Figure 3).
Figure 2.
Flowchart of excluded patients and measurements.
Table 2.
Patient Demographics and Characteristics
| Number | % | |
|---|---|---|
| Total Patients | 83,229 | - |
| Race | ||
| American Indian or Alaska Native | 60 | 0.1% |
| Asian | 2,704 | 3.2% |
| Black or African American | 23,228 | 27.9% |
| Indian | 561 | 0.7% |
| Multiple Races | 1,691 | 2.0% |
| Native Hawaiian or Other Pacific Islander | 56 | 0.1% |
| Other | 12,816 | 15.4% |
| Refused | 75 | 0.1% |
| Unknown | 205 | 0.2% |
| White | 41,903 | 50.3% |
| Ethnicity | ||
| Hispanic or Latino | 6,070 | 7.5% |
| Not Hispanic or Latino | 74,804 | 92.3% |
| Missing or Refused | 2,272 | 2.9% |
| Sex | ||
| Female | 40,947 | 49.2% |
| Payor | ||
| Medicaid | 49,378 | 59.3% |
| Private | 33,921 | 40.7% |
| Practice Type | ||
| Resident teaching | 18,442 | 22.1% |
| Community | 64,857 | 77.9% |
| Met criteria for failure to thrive at any time | ||
| Weight-for-age <5th percentile (CDC) | 12,310 | 14.8% |
| Weight-for-length <5th percentile (CDC) | 10,955 | 13.2% |
| Crossed 2 decreasing major percentile linesa (CDC) | 33,508 | 40.2% |
| Weight-for-age <5th percentile (WHO) | 8,584 | 10.3% |
| Weight-for-length <5th percentile (WHO) | 7,471 | 9.0% |
| Crossed 2 decreasing major percentile linesa (WHO) | 11,157 | 13.4% |
| Clinician-diagnosis criteriab | 7,458 | 9.0% |
Defined using the following major percentile lines: 5, 10, 25, 50, 75, 90, 95
The clinician-diagnosis criteria was one or more of the following ICD-9 codes: 263.9, 269.9, 783.21, 783.22, 783.41.
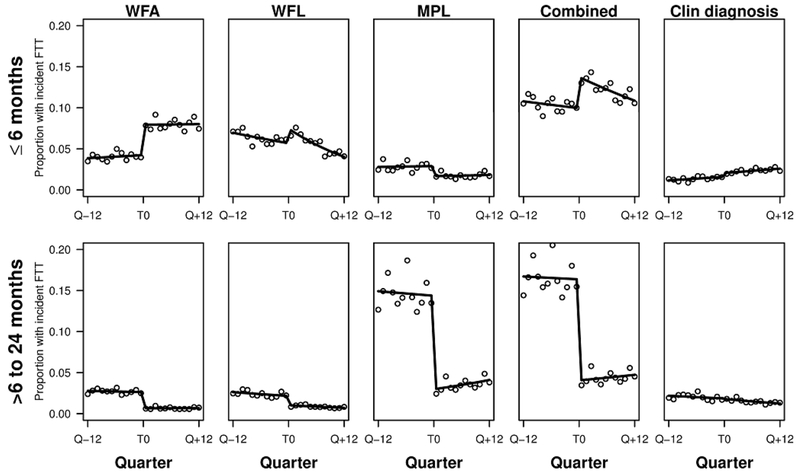
Figure 3.
Proportion of patients with incident FTT (points) over time in stratified analyses with fitted interrupted time series regression model overlaid (solid line) for each set of growth criteria and clinician diagnosis criteria in the <6 month age group (top row) and each set of growth criteria and clinician diagnosis in the 6-24 month age group (bottom row).
Change in FTT incidence at the CDC-WHO switch
In the <6-month age group, increases in FTT incidence occurred at the CDC-WHO switch for clinician-diagnosed FTT and for all growth-percentile criteria except MPL (Table 3) (Figure 3). In the 6-to-24-month age group, decreases in FTT incidence occurred at the CDC-WHO switch for all growth-chart criteria, but no statistically significant changes occurred in clinician-diagnosed FTT. Findings for the alternate growth-percentile outcome definitions and the narrow clinician-diagnosis outcome measure were similar (Supplemental Text and Supplemental Table 2).
Table 3.
Estimated odds ratios (ORs) and 95% confidence intervals (CIs) of parameters from interrupted time series logistic regression models for each outcome for age-stratified analyses
| Number of patients eligible for analysis | CDC era slope (Q-12 to T0) OR (95% CI) |
Change at CDC-WHO switch (T0) OR (95% CI) |
WHO era slope (T0 to Q+12) OR (95% CI) |
|
|---|---|---|---|---|
| <6 months | ||||
| Growth-WFA | 62,355 | 1.01 (0.99, 1.02) | 1.96 (1.73, 2.21)a | 1.00 (0.99, 1.01) |
| Growth-WFL | 60,826 | 0.98 (0.97, 0.99)a | 1.36 (1.21, 1.54)a | 0.94 (0.93, 0.96)a |
| Growth-MPL | 43,997 | 1.00 (0.98, 1.03) | 0.57 (0.44, 0.74)a | 1.01 (0.98, 1.04) |
| Growth-Combined | 62,353 | 0.99 (0.98, 1.00) | 1.45 (1.33, 1.59)a | 0.98 (0.97, 0.99)a |
| Clinician diagnosis | 63,153 | 1.03 (1.00, 1.05)a | 1.31 (1.06, 1.61)a | 1.02 (1.00, 1.04)a |
| 6-24 months | ||||
| Growth-WFA | 72,170 | 0.99 (0.98, 1.01) | 0.25 (0.21, 0.29)a | 1.00 (0.98, 1.02) |
| Growth-WFL | 71,145 | 0.98 (0.97, 0.99)a | 0.48 (0.41, 0.55)a | 0.97 (0.95, 0.98)a |
| Growth-MPL | 73,226 | 1.00 (0.99, 1.01) | 0.18 (0.16, 0.19)a | 1.03 (1.02, 1.04)a |
| Growth-Combined | 65,095 | 1.00 (0.99, 1.01) | 0.21 (0.19, 0.23)a | 1.01 (1.00, 1.02)a |
| Clinician diagnosis | 77,089 | 0.99 (0.98, 1.00)a | 0.97 (0.88, 1.08) | 0.97 (0.96, 0.98)a |
WFA (weigit-for-age percentile <5th); WFL (weight-for-length percentile <5th); MPL (WFA crossing two decreasing major percentile lines)
p<0.05 In the CDC era slope and WHO era slope columns, an OR significantly different from one indicates a decrease (OR <1) or increase (OR >1) in incidence within that era. In the change at CDC-WHO switch column, an OR significantly different from 1 indicates a decrease (OR <1) or increase (OR >1) in incidence at the time of the switch.
Change in FTT Incidence within the CDC and WHO eras
A decrease in the proportions of children meeting WFL criteria for FTT over time occurred for both CDC and WHO eras for both age groups (Table 3). We observed steady and statistically significant decreases in mean length (0.6 cm), mean CDC length-for-age z-score (0.16), and mean WHO length-for-age z-score (0.19) over the study period. An effort to improve measurement technique in the CHOP network occurred over the study period which may explain these findings.
For the WHO era, FTT incidence by the combined growth-percentile criteria significantly decreased over time for children <6 months and significantly increased over time for children >6 months. In contrast, for clinician-diagnosed FTT incidences in the WHO era for both age groups, we found a significant increase over time for children <6 months and a significant decrease over time for children >6 months.
Discussion
When a primary care network switched from using the CDC to the WHO growth curves as their default, increases in clinician-diagnosed FTT mirrored increases in the incidence of FTT by growth-percentile criteria in infants <6 months. However, in infants 6-24 months of age no change was observed in the overall incidence of clinician-diagnosed FTT at the time of the switch in growth curves despite decreases in FTT by growth-percentile criteria.
The differences we found in the incidence of FTT by growth-percentile criteria using the CDC and WHO growth curves are what we expected based on visual comparison of the two curves. For WFA (Figure 1) the WHO 5th percentile line is higher than that of the CDC 5th percentile until approximately six months of age, when the CDC 5th percentile is higher. The pattern is similar for WFL. Accordingly, we found an increase in FTT incidence for WFA, WFL, and the combined growth criteria from the CDC to the WHO era in those <6 months old, but conversely found a decrease in FTT incidence by WFA, WFL, and combined growth criteria from the CDC to the WHO era in those 6-24 months old. The MPL criteria (i.e. crossing multiple decreasing WFA percentile lines) are somewhat more complex. Initially, the WHO WFA percentiles rise faster than the CDC WFA percentiles, but by approximately three months of age the WHO WFA percentiles rise slower than the CDC percentiles. Even in our <6-month age group, most infants were older than three months. Therefore, we would expect to see a lower incidence of FTT by MPL criteria at the CDC-WHO switch in both age groups, which is indeed what we observed. When we evaluated the incidence of FTT by all growth criteria combined, the changes for the WFA and WFL criteria were larger than those for MPL criteria, leading to an overall increase in growth-percentile FTT incidence in the <6-month age group and an overall decrease in growth-percentile FTT incidence in the 6-to-24 month-age group.
The relationship between the change in incidence of FTT by growth-percentile criteria and clinician-diagnosis criteria at the CDC-WHO switch was different for our two age subgroups. Specifically, in the <6-month age group the increase in clinician-diagnosed FTT mirrored the increase in the incidence of combined growth-percentile FTT. However, in infants 6-24 months, the incidence of clinician-diagnosed FTT showed no change despite significant decreases in combined growth-percentile FTT.
The age groups also exhibited different changes in clinician-diagnosed FTT within each growth curve era that were not explained by changes in growth-percentile FTT within those eras. For those <6 months, the incidence of clinician-diagnosed FTT increased in both the CDC and WHO eras, although the incidence of combined growth-percentile FTT decreased within both eras (significant only in the WHO era). The opposite was true in infants 6-24 months of age; the incidence of clinician-diagnosed FTT decreased in both the CDC and WHO eras, although the incidence of combined growth-percentile FTT increased within the WHO era.
We therefore must consider why the relationship between FTT incidence by clinician diagnoses and FTT incidence by growth criteria was different for the two age groups. Our discussion of the reasons for these differences must be speculative because published data regarding clinician decision-making in FTT are lacking, and a qualitative evaluations of clinician decision-making were beyond the scope of our study.
For both eras and for both age subgroups the incidence of clinician-diagnosed FTT is substantially lower than the incidence of growth-percentile FTT. This is likely caused in part by clinicians using different, and generally more strict, growth criteria to define FTT than we used in this analysis. The lower incidence of clinician-diagnosed FTT compared to growth-chart-defined FTT is likely also partly explained by clinicians who, after seeing that an infant meets one or more growth criteria for FTT, use non-growth-criteria to identify children who meet growth criteria for FTT but who they believe are small and healthy rather than undernourished. Factors beyond growth percentiles likely strongly influence FTT diagnosis, including many factors that are not extractable from discrete EHR data, such as the size of parents and other family members, feeding habits, gastrointestinal symptoms, or concerns about the interaction between the child and caregiver. The role of non-growth criteria in FTT diagnosis could explain the lack of a decrease in clinician-diagnosed FTT from the CDC to WHO era in infants 6-24 months when there was a decrease in growth-chart defined FTT, but it does not explain the increase in clinician-diagnosed FTT in infants <6 months. One possibility is increased concern and a heightened sense of urgency when young infants have poor weight gain compared to older infants; clinicians may be more likely to defer a diagnosis of FTT in children >6 months than in younger infants. A difference in diagnostic thresholds for older and younger children, and a difference in the degree to which clinicians rely on non-growth criteria, may also contribute to the within-era increases in clinician-diagnosed FTT in the <6-month age group and the within-era decreases in clinician-diagnosed FTT in the 6-to-24-month age group.
Clinicians’ choice of growth curve may be influenced by patient age. The WHO growth curve became available in the EHR in approximately 2008, even though it was not made the default until 2012. The CDC growth curve always remained available. Clinicians may have switched to the WHO curve for young infants but continued to use the CDC growth curves for older infants under two years of age. Although this is a possible explanation for our findings, we are unaware of any colleagues who use the growth curves in this manner.
FTT may be analogous in some respects to pediatric hypertension, with clinical diagnosis only partly dependent on numeric criteria. Although there have long been published age-, sex-, and height-based criteria used to define pediatric hypertension, in a large retrospective cohort, most children who met criteria for hypertension did not receive an ICD-9 diagnosis of hypertension.30 There were differences in diagnosis frequency by sex, height, and obesity status. The criteria used to define pediatric hypertension were changed in 2017.31,32 Although the changes in the prevalence of children meeting numeric criteria for hypertension may be predictable,33 this may not translate predictably into changes in the prevalence of hypertension diagnoses in children, as in our analysis of FTT
The small decrease in mean length over the time course of the study may represent either a true decrease in length in the study population or differences in measurement, possibly associated with efforts to improve measurement technique in the network. The small absolute decrease in length (0.6 cm) was associated with a statistically and clinically significant decrease in the proportion of children below the WFL 5th percentile using either growth curve. Length measurements are difficult to perform correctly and may lack both precision and accuracy when measured in clinical settings.12, 20 Although evaluating weight in the context of length has benefits, our findings highlight the potential clinical impact of even small errors in length measurement.
Strengths of our study include the large sample size and the diversity of the cohort. We evaluated multiple sets of growth-percentile criteria, including one that was trajectory-based. We found similar results in sensitivity analyses using alternate growth and clinician-diagnosis outcome definitions. The interrupted time series design with three years of data preceding and following the CDC-WHO switch allowed us to account for within-era trends in FTT incidence.
Our study has several important limitations. We evaluated a large primary care network that is diverse in many respects and has a high level of practice variability,21–24 but all practices are in the same geographic region and share some clinical protocols (though none for FTT). We could not reliably evaluate FTT in infants <2 months of age due to coding practices in that age group. Although we used growth percentile criteria that have support from research and clinical recommendations, these criteria identify some children who are small but healthy and are not recommended for use by some experts.25 Additionally, we did not have extractable data on infant feeding practices that would permit evaluation of whether clinicians evaluated breastfed and formula-fed children differently, given that breastfeeding is known to be associated with rapid early weight gain and slower weight gain later in infancy.
FTT is a clinical sign rather than a specific condition.29 Our goal was to use ICD-9 codes for FTT and related conditions as a proxy for children for whom clinicians believed further investigation or treatment was warranted for inadequate growth. Clinicians likely vary in their use of these codes. Identifying downstream actions, such as follow-up visits, referrals, diagnostic testing, or supplementary feeding would be another way to identify children about whom clinicians are concerned. However, there is no standard evaluation or treatment plan for FTT. Furthermore, the presence of discrete EHR markers for some of these actions is likely to be differentially present based on patient and family factors. For example, families with more resources may purchase high-calorie oral supplements without a prescription, whereas a prescription for supplements would be written for children accessing the Women, Infants, and Children Food and Nutrition Service.
In summary, at the switch between the CDC and WHO growth curves, a concordant increase in growth-percentile and clinician-diagnosed FTT in infants <6 months was observed, but no change was observed in clinician-diagnosed FTT in children 6-24 months of age despite the expected decrease in the incidence of children meeting common growth-percentile criteria for FTT. Our research highlights how little is known about how clinicians make the consequential decision to diagnose FTT in young children. Qualitative and quantitative research is needed to understand how clinicians make decisions about poor weight gain, with specific attention to diagnostic considerations in younger and older infants, and to characterize the benefits and risks of strategies for evaluation and management of children with varying degrees of slow weight gain.
Supplementary Material
What’s New:
When switching from the CDC to the WHO growth curves, changes in the incidence of FTT by growth-percentile and clinician-diagnosis criteria differed for younger versus older infants.
Acknowledgments
This work was supported by funding from the Penn State College of Medicine Department of Pediatrics (Carrie Daymont) and NIH T32 Institutional Training Grant DK7740-18 (Noah Hoffman). We also want to thank the network of primary care clinicians, their patients and families for their contribution to this project and clinical research facilitated through the Pediatric Research Consortium (PeRC) at The Children’s Hospital of Philadelphia.
Funding Source: This work was supported by funding from the Penn State College of Medicine Department of Pediatrics (Carrie Daymont) and NIH T32 Institutional Training Grant DK7740-18 (Noah Hoffman). The funding sources had no role in the study design; collection, analysis, or interpretation of data; the writing of this article; or the decision to submit this article for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Potential Conflicts of Interest: The authors have no conflicts of interest relevant to this article to disclose.
References
- 1.Olsen EM. Failure to thrive: still a problem of definition. Clin Pediatr (Phila). 2006;45(1):1–6. [DOI] [PubMed] [Google Scholar]
- 2.National Guideline Alliance (UK). Faltering Growth – Recognition and Management. London: National Institute for Health and Care Excellence (UK); 2017. http://www.ncbi.nlm.nih.gov/books/NBK458459/. Accessed October 18, 2017. [PubMed] [Google Scholar]
- 3.Sills RH. Failure to thrive. The role of clinical and laboratory evaluation. Am J Dis Child. 1978;132(10):967–969. [DOI] [PubMed] [Google Scholar]
- 4.Berwick DM, Levy JC, Kleinerman R. Failure to thrive: diagnostic yield of hospitalisation. Arch Dis Child. 1982;57(5):347–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Block RW, Krebs NF, American Academy of Pediatrics Committee on Child Abuse and Neglect, American Academy of Pediatrics Committee on Nutrition. Failure to thrive as a manifestation of child neglect. Pediatrics. 2005;116(5):1234–1237. doi: 10.1542/peds.2005-2032 [DOI] [PubMed] [Google Scholar]
- 6.Corbett SS, Drewett RF. To what extent is failure to thrive in infancy associated with poorer cognitive development? A review and meta-analysis. J Child Psychol Psychiatry. 2004;45(3):641–654. [DOI] [PubMed] [Google Scholar]
- 7.Emond AM, Blair PS, Emmett PM, Drewett RF. Weight faltering in infancy and IQ levels at 8 years in the Avon Longitudinal Study of Parents and Children. Pediatrics. 2007;120(4):e1051–1058. doi: 10.1542/peds.2006-2295 [DOI] [PubMed] [Google Scholar]
- 8.Rudolf MCJ. What is the long term outcome for children who fail to thrive? A systematic review. Archives of Disease in Childhood. 2005;90(9):925–931. doi: 10.1136/adc.2004.050179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Drewett RF, Corbett SS, Wright CM. Physical and emotional development, appetite and body image in adolescents who failed to thrive as infants. J Child Psychol Psychiatry. 2006;47(5):524–531. doi: 10.1111/j.1469-7610.2005.01529.x [DOI] [PubMed] [Google Scholar]
- 10.Blumenstein I, Shastri YM, Stein J. Gastroenteric tube feeding: techniques, problems and solutions. World J Gastroenterol. 2014;20(26):8505–8524. doi: 10.3748/wjg.v20.i26.8505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grummer-Strawn LM, Reinold C, Krebs NF, Centers for Disease Control and Prevention (CDC). Use of World Health Organization and CDC growth charts for children aged 0-59 months in the United States. MMWR Recomm Rep. 2010;59(RR-9):1–15. [PubMed] [Google Scholar]
- 12.Daymont C, Ross ME, Russell Localio A, Fiks AG, Wasserman RC, Grundmeier RW. Automated identification of implausible values in growth data from pediatric electronic health records. J Am Med Inform Assoc. April 2017. doi: 10.1093/jamia/ocx037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Daymont C, Neal A, Prosnitz A, Cohen MS. Growth in children with congenital heart disease. Pediatrics. 2013;131(1):e236–242. doi: 10.1542/peds.2012-1157 [DOI] [PubMed] [Google Scholar]
- 14.Jaffe AC. Failure to thrive: current clinical concepts. Pediatr Rev. 2011;32(3):100–107; quiz 108. doi: 10.1542/pir.32-3-100 [DOI] [PubMed] [Google Scholar]
- 15.McLean HS, Price DT. Failure to Thrive. In: Nelson Textbook of Pediatrics. 20th ed Philadelphia, PA: Elsevier; 2016:249. [Google Scholar]
- 16.Paine CW, Wood JN. Skeletal surveys in young, injured children: A systematic review. Child Abuse Negl. 2017;76:237–249. doi: 10.1016/j.chiabu.2017.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gerber JS, Prasad PA, Localio AR, et al. Racial differences in antibiotic prescribing by primary care pediatricians. Pediatrics. 2013;131(4):677–684. doi: 10.1542/peds.2012-2500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goyal MK, Johnson TJ, Chamberlain JM, et al. Racial and Ethnic Differences in Antibiotic Use for Viral Illness in Emergency Departments. Pediatrics. 2017;140(4). doi: 10.1542/peds.2017-0203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goyal MK, Kuppermann N, Cleary SD, Teach SJ, Chamberlain JM. Racial Disparities in Pain Management of Children With Appendicitis in Emergency Departments. JAMA Pediatr. September 2015. doi: 10.1001/jamapediatrics.2015.1915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith N, Coleman KJ, Lawrence JM, et al. Body weight and height data in electronic medical records of children. Int J Pediatr Obes. 2010;5(3):237–242. doi: 10.3109/17477160903268308 [DOI] [PubMed] [Google Scholar]
- 21.Fiks AG, Ross ME, Mayne SL, et al. Preschool ADHD Diagnosis and Stimulant Use Before and After the 2011 AAP Practice Guideline. Pediatrics. 2016;138(6). doi: 10.1542/peds.2016-2025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mayne SL, Ross ME, Song L, et al. Variations in Mental Health Diagnosis and Prescribing Across Pediatric Primary Care Practices. Pediatrics. 2016;137(5). doi: 10.1542/peds.2015-2974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gerber JS, Prasad PA, Russell Localio A, et al. Variation in Antibiotic Prescribing Across a Pediatric Primary Care Network. J Pediatric Infect Dis Soc. 2015;4(4):297–304. doi: 10.1093/jpids/piu086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fiks AG, Hunter KF, Localio AR, et al. Impact of Electronic Health Record-Based Alerts on Influenza Vaccination for Children With Asthma. PEDIATRICS. 2009;124(1):159–169. doi: 10.1542/peds.2008-2823 [DOI] [PubMed] [Google Scholar]
- 25.Motil KJ, Duryea TK. Failure to thrive (undernutrition) in children younger than two years: Etiology and evaluation. In: UpToDate. Waltham, MA: UpToDate, Inc; www.uptodate.com. Accessed January 15, 2018. [Google Scholar]
- 26.Dewey KG. Growth characteristics of breast-fed compared to formula-fed infants. Biol Neonate. 1998;74(2):94–105. doi: 10.1159/000014016 [DOI] [PubMed] [Google Scholar]
- 27.Belanoff CM, McManus BM, Carle AC, McCormick MC, Subramanian SV. Racial/ethnic variation in breastfeeding across the US: a multilevel analysis from the National Survey of Children’s Health, 2007. Matern Child Health J. 2012;16 Suppl 1:S14–26. doi: 10.1007/s10995-012-0991-1 [DOI] [PubMed] [Google Scholar]
- 28.Centers for Disease Control and Prevention (CDC). Progress in increasing breastfeeding and reducing racial/ethnic differences - United States, 2000–2008 births. MMWR Morb Mortal Wkly Rep. 2013;62(5):77–80. [PMC free article] [PubMed] [Google Scholar]
- 29.Zenel JA. Failure to thrive: a general pediatrician’s perspective. Pediatr Rev. 1997;18(11):371–378. [PubMed] [Google Scholar]
- 30.Kaelber DC, Liu W, Ross M, et al. Diagnosis and Medication Treatment of Pediatric Hypertension: A Retrospective Cohort Study. PEDIATRICS. 2016;138(6):e20162195–e20162195. doi: 10.1542/peds.2016-2195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114(2 Suppl 4th Report):555–576. [PubMed] [Google Scholar]
- 32.Flynn JT, Kaelber DC, Baker-Smith CM, et al. Clinical Practice Guideline for Screening and Management of High Blood Pressure in Children and Adolescents. Pediatrics. 2017;140(3):e20171904. doi: 10.1542/peds.2017-1904 [DOI] [PubMed] [Google Scholar]
- 33.Sharma AK, Metzger DL, Rodd CJ. Prevalence and Severity of High Blood Pressure Among Children Based on the 2017 American Academy of Pediatrics Guidelines. JAMA Pediatrics. April 2018. doi: 10.1001/jamapediatrics.2018.0223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clinical Growth Charts. Centers for Disease Control https://www.cdc.gov/growthcharts/clinical_charts.htm. Accessed November 15, 2017.
- 35.The WHO Child Growth Standards. World Health Organization http://www.who.int/childgrowth/standards/en/. Accessed November 15, 2017.
- 36.Feudtner C, Feinstein JA, Zhong W, Hall M, Dai D. Pediatric complex chronic conditions classification system version 2: updated for ICD-10 and complex medical technology dependence and transplantation. BMC Pediatr. 2014;14:199. doi: 10.1186/1471-2431-14-199 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.