Abstract
Purpose
Corneal injuries are associated with significant impairment in vision. Mesenchymal stem cells (MSCs) have been shown to limit inflammation and promote tissue repair at the ocular surface. Here, we evaluate the efficacies of different modes of MSC delivery (topical, subconjunctival, intraperitoneal [IP] and intravenous [IV]) to promote tissue repair and restore corneal transparency in a murine model of corneal injury.
Methods
MSCs were purified from the bone marrow of C57BL/6J mice and expanded using plastic adherence in vitro. Corneal injury was created using an Algerbrush, and 0.5×106 MSCs/mouse were administered via topical, subconjunctival, IP or IV routes. Qdot-labeled MSCs were employed to determine the effect of route of administration on corneal and conjunctival MSC frequencies. Corneal opacity scores were calculated using ImageJ. Expression of inflammatory cytokines was quantified by qPCR, and infiltration of CD45+ cells was evaluated by flow cytometry.
Results
Subconjunctival or IV administration results in increased frequencies of MSCs in ocular surface tissues following corneal injury, relative to topical or intraperitoneal delivery. Subconjunctival or IV administration reduces: (i) corneal opacity, (ii) tissue fibrosis as quantified by α-Sma expression, (iii) the expression of inflammatory cytokines (Il-1β and Tnf-α) and (iv) CD45+ inflammatory cell infiltration relative to untreated injured control animals. Administration via subconjunctival or IV routes was observed to accelerate corneal repair by restoring tissue architecture and epithelial integrity.
Conclusions
Our data suggest that subconjunctival or IV delivery of MSCs have superior therapeutic efficacy compared to topical or IP delivery following corneal injury.
Keywords: Mesenchymal stem cells, corneal injury, wound healing, inflammation, homing
Introduction
Corneal blindness due to ocular trauma is a major cause of visual impairment worldwide [1,2]. Opacification results from the complex cross-talk between a variety of cytokines, chemokines and growth factors produced by corneal epithelial cells, keratocytes, immune cells, nerves and lacrimal tissues [3]. There is substantial research interest in the employment of mesenchymal stem cell (MSC)-based therapies to treat corneal inflammatory disease and limit opacification [4–11].
MSCs are multipotent stromal cells that can differentiate into adipocytes, osteocytes and chondrocytes (i.e. cells of mesodermal lineage) but can also transdifferentiate into cells of other embryonic lineages [12]. MSCs have been shown to accelerate wound healing through cellular differentiation [13]. MSCs also have considerable immunoregulatory capacity, and have been shown to modulate effector functions of T cells, B cells, natural kills cells and dendritic cells [14]. These properties, coupled with their potent capacity for self-renewal and limited immunogenicity [14,15], have led to numerous investigations of the potential of MSCs to resolve inflammation and promote corneal transparency following injury [4–11,16]. However, there is a lack of consensus regarding the optimum route of MSC administration in these studies, with some groups administering MSCs topically [9,17–19], some intravenously (IV) [4–8], and others via subconjunctival [11] or intraperitoneal (IP) injection [8].
The purpose of our study was to evaluate the efficacy of different routes of MSC administration in suppressing the inflammatory response, and promoting corneal repair, following injury. Using a well-established murine model of corneal injury, we assessed the therapeutic effects of administering MSCs by various routes (topical, subconjunctival, IV or IP) on corneal opacity, inflammatory cell infiltration, expression of inflammatory cytokines and tissue architecture. Our data demonstrate that subconjunctival and intravenous administration of MSCs are most effective in suppressing corneal opacity and inflammation, and promoting tissue repair, following corneal injury.
Materials and Methods
Animals
Six- to eight-week-old male and female C57BL/6 wild-type mice (Charles River Laboratories, Wilmington, MA) were used in our experiments. The study protocol was approved by the Schepens Eye Research Institute Animal Care and Use Committee. All mice were treated according to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Isolation, expansion, and characterization of MSCs
Bone marrow was isolated from the harvested femurs of C57BL/6 wild-type mice. MSCs were phenotypically and functionally characterized as per the criteria detailed by The International Society for Cellular Therapy [20]. The cells derived from bone marrow (2×106cells/ml) were cultured in vitro at 37°C in mouse mesenchymal stem cell medium (Stem Cell Technologies, Inc., Vancouver, Canada) using the previously described adherence method of MSC cultivation [4,5,7,21]. The bone marrow-derived MSCs were passaged every 3 days, and were used in experiments at passage 3 following characterization. The cultured MSCs were phenotypically characterized for expression of MSC markers by flow cytometry as CD45−CD34−SCA1+CD29+. Their functional characterization was performed by differentiation into adipocytes, as previously described [4,5,7,21].
Corneal injury and administration of MSCs
Mice were subjected to general anesthesia and corneal injury was performed in the right eye using a hand-held motor brush (Algerbrush II, Alger Company Inc., Lago Vista, 75 TX) as described previously [4,22]. In brief, a 2mm trephine was used to mark the central cornea, and the corneal epithelium and anterior stroma in this region were removed mechanically (approximately 1/3 of total corneal thickness) using the Algerbrush. Following the procedure triple antibiotic ointment (Neomycin and Polymyxin B Sulfates and Bacitracin Zinc Ophthalmic Ointment USP, Bausch + Lomb, Wilmington, MA) was applied to the injured eyes, followed by a subcutaneous injection of buprenorphine to minimize pain. The in vitro cultured and characterized MSCs were stimulated with IL-1β for 6 hours and then administered (5×105 cells per mouse) to mice at one hour following injury. Cells were delivered in 10 μl of PBS topically and via subconjunctival injection, and in 100 μl of PBS via intraperitoneal or intravenous injection. For topical administration, mice received general anesthesia and were laid on their left flank. A Q-tip was used to remove tears from the ocular surface of the right eye, and 10μl of MSC-containing PBS was delivered onto the cornea and allowed to remain in position for 45 minutes.
Slit lamp biomicroscopy
To assess corneal opacity, the injured eyes were evaluated by slit lamp biomicroscopy with photographs captured, as previously described [4,23]. The injured PBS-treated eyes were used as controls (untreated). Corneal fluorescein staining (CFS) was performed by placing 1 μl of 1% sodium fluorescein on the ocular surface of injured eye, and corneal epitheliopathy was evaluated 3 minutes later by slit lamp biomicroscopy with images captured using cobalt blue light [4,23]. Both corneal opacity and epitheliopathy were evaluated immediately following injury (day 0) as well as on days 2 and 4 post-injury.
RNA isolation and real-time qPCR
Corneas were harvested at day 4 post-injury and RNA was isolated using the RNeasy® Micro Kit (Qiagen) as per the manufacturer’s instructions. Isolated RNA was quantified using the NanoDrop® ND-1000 spectrophotometer (ThermoScientific) and reverse transcribed into cDNA using oligo(dT) primer and SuperScript III First-Strand Synthesis System (Invitrogen). Quantitative real-time PCR (qPCR) was performed using TaqMan® Universal PCR Mastermix and pre-formulated murine TaqMan® primers for α-Sma (α-smooth muscle actin), Il-1β (Interleukin-1β), Tgf-β1 (Transforming growth factor-β1), Tnf-α (Tumor necrosis factor-α) and Gapdh (Glyceraldehyde-3-phosphate dehydrogenase) (Life Technologies, ThermoFisher Scientific) on the Mastercycler® RealPlex2 platform (Eppendorf). The results were normalized to Gapdh as an internal control and analyzed through comparative threshold cycle method.
Histology and immunofluorescence
Formalin-fixed paraffin-embedded sections of the whole eyeball were stained with hematoxylin and eosin (H&E) and examined using bright-field microscopy, as described previously [5,6]. The sections were de-paraffinized, blocked with 2% BSA and anti-FcR antibodies (catalog #14–0161-86, eBioscience, ThermoFisher Scientific), and immunostained with Alexa Fluor 488-conjugated anti-α-smooth muscle actin (anti-α-Sma) or isotype-matched control antibodies (#53–6496-80, eBioscience, ThermoFisher Scientific) overnight at 4°C, as performed previously [4]. Slides were then washed four times (15 minutes each) with washing buffer (0.5% Triton-X-100 and 2% FBS in PBS), mounted using DAPI-containing VECTASHIELD® mounting medium (Vector Laboratories), and examined under a fluorescence microscope (Nikon® Eclipse E800).
For evaluating MSC homing to the ocular surface, MSCs were labeled using a Q-Tracker 625 Cell Labeling kit (ThermoFisher, Waltham, MA) according to the manufacturer’s protocol, and administered as described above. Freshly excised corneas and conjunctivae were washed in PBS and fixed with 4% paraformaldehyde for a duration of 15 minutes. Subsequently, whole corneas and conjunctivae were mounted on slides, as described above, and visualized using a confocal microscope (Leica TCS-SP5; Buffalo Grove, IL, USA) at ×63 magnification for the detection of Qdot–labeled MSCs.
Flow cytometry
MSCs were suspended to form a single cell suspension and stained with fluorochrome-conjugated monoclonal antibodies and appropriate isotype controls [4,5,7,21]. Antibodies (BioLegend®) against CD34 (#119310), SCA-1 (#108105), CD45 (catalog #103133), CD29 (#102207) and CD105 (#120407) were used for the phenotypic characterization of MSCs. To assess the post-injury infiltration of CD45+ cells into cornea, harvested corneas were digested with 2mg/ml Collagenase IV (Sigma-Aldrich, St. Louis, MO) and 2mg/ml DNase I (Roche, 88 Basel, Switzerland) to form a single-cell suspension, as described previously [4,5,7,21,22]. The cells were stained with anti-CD45 fluorochrome conjugated antibody (#103133). Stained cells were analyzed on LSR-II flow cytometer (BD Biosciences) and data analysis was performed using with Summit™ software (Dako Colorado, Inc., Fort Collins, CO, USA).
Statistical analysis
Student’s t tests were performed for determination of statistical significance (p<0.05). Results are presented as the mean ± SD of three independent experiments. Quantification of images of corneal injury and opacity, as well as in vivo evaluations, were performed in a masked fashion. Sample sizes were estimated based on previous reports on corneal injury and inflammation [4,5,7,23].
Results
Effect of route of administration of MSCs on homing to ocular surface tissues
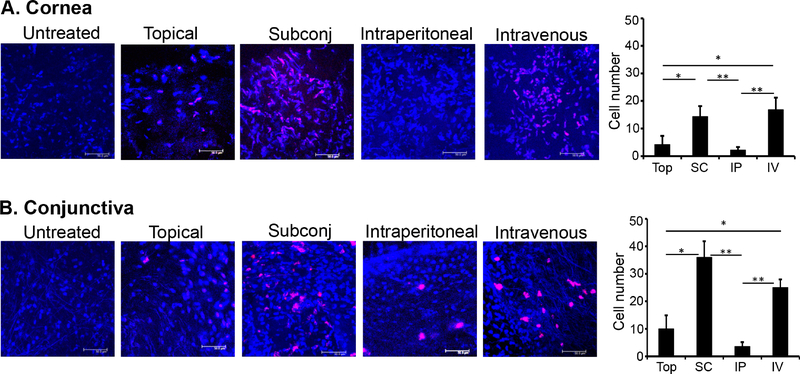
To investigate the migration of MSCs to ocular surface tissues, MSCs (0.5×106 cells) were labeled ex vivo with Qdots and administered topically or via subconjunctival, IP or IV injection at the time of corneal injury. Corneas and conjunctivae were harvested at 4 days post-injury. Our immunohistochemical analyses demonstrate increased frequencies of Qdot-labeled MSCs at both the cornea (Fig.1A) and conjunctivae (Fig.1B) following either subconjunctival or IV injection of MSCs, relative to topical application or IP injection.
Figure 1. Frequencies of exogenously-administered MSCs at the ocular surface following topical, subconjunctival, intraperitoneal and intravenous administration.
Corneal injury was performed by mechanical removal of the corneal epithelium and anterior stroma in C57BL/6 mice. In vitro cultured and characterized MSCs (0.5×106 cells) that had been labeled with Qdots were subsequently administered via topical, subconjunctival (Subconj), intraperitoneal (IP), and intravenous (IV) routes. At 4 days following injury, corneas and conjunctivae were harvested, fixed, and visualized using confocal microscopy. (A) Representative immunohistochemical images of corneas and quantitative bar chart of MSCs (cell number per microscopic field) demonstrating increased frequencies of Qdot-labeled MSCs following subconjunctival and intravenous administration, relative to topical application and intraperitoneal injection. (B) Representative immunohistochemical images of conjunctivae and quantitative bar chart of MSCs (cell number per microscopic field) demonstrating increased frequencies of Qdot-labeled MSCs following subconjunctival and intravenous administration, relative to topical application and intraperitoneal injection. Untreated injured mice were used as controls. Data from two independent experiments are shown, and each experiment consisted of 4 mice/group. Scale bar: 50μm. The values are shown as mean ± SEM. *p<0.05.
Effect of route of administration of MSCs on corneal opacification following injury
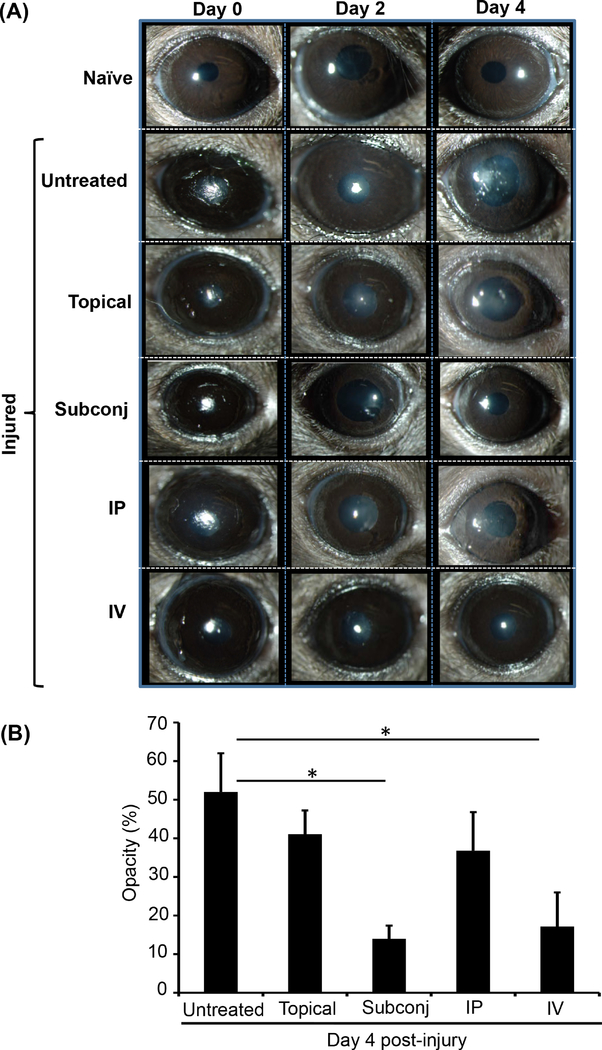
To study the efficacy of different routes of MSC administration on limiting corneal opacification following injury, MSCs (0.5×106 cells) were administered topically or by subconjunctival, IP or IV injection. Injured corneas were evaluated by slit lamp microscopy, and photographs were captured at Days 0, 2 and 4 post-injury (Fig. 2A). The area of opacification was quantified using ImageJ software, and scores were expressed as percentage of total area. Relative to corneal opacity in the untreated injured control (52.0±10.1%) our results indicate that both subconjunctival (14.0±3.4%, p=0.039) and intravenous (17.2±8.8%, p=0.041) administration of MSCs significantly suppress injury-induced corneal opacification (Fig. 2B).
Figure 2. MSCs administered via subconjunctival or intravenous injection reduce corneal opacity following injury.
Corneal injury was performed by mechanical removal of the corneal epithelium and anterior stroma in C57BL/6 mice. In vitro cultured and characterized MSCs (0.5×106 cells) were administered via topical, subconjunctival (Subconj), intraperitoneal (IP), and intravenous (IV) routes and mice were followed for 4 days. Corneas were visualized and photographed by slit lamp biomicroscopy immediately following injury (day 0) as well as days 2 and 4 post-injury. Percentage opacity was analyzed using ImageJ software. (A) Representative images of naïve, untreated and MSC-treated injured eyes. (B) Bar chart showing percentage corneal opacity in MSC-treated mice at day 4 post-injury. Untreated injured mice were used as control. Data from three independent experiments are shown, and each experiment consisted of 4–6 animals/group. The values are shown as mean ± SD. *p<0.05.
Impact of route of administration of MSCs on expression of markers of corneal fibrosis following injury
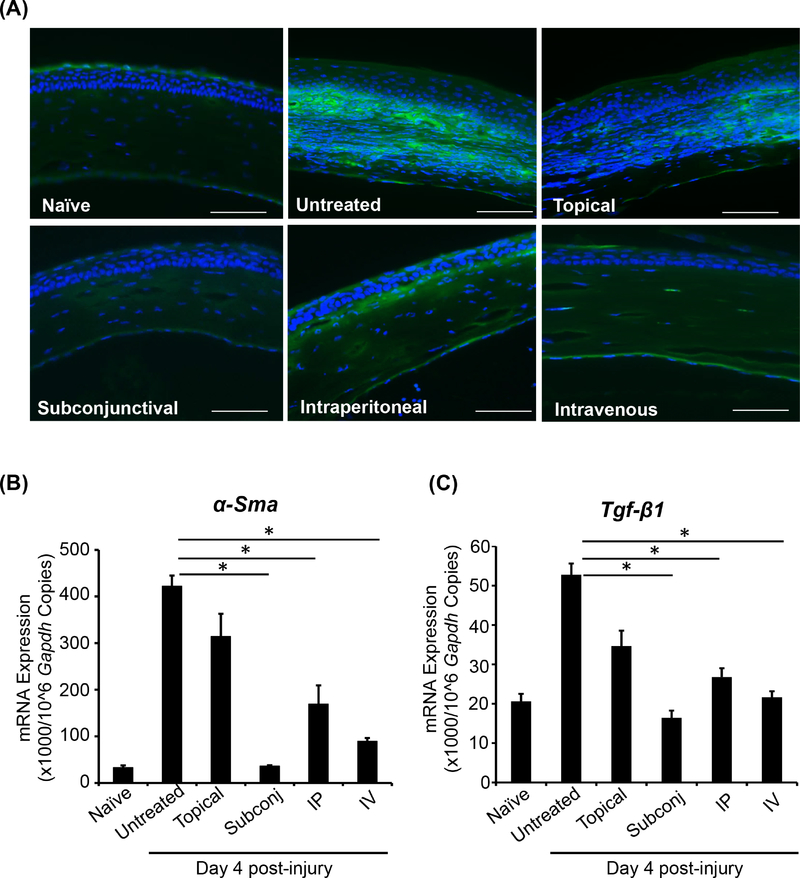
Upregulated expression of transforming growth factor-β (TGF-β) following corneal injury promotes the conversion of quiescent stromal keratocytes into α-smooth muscle actin (α-Sma)-containing myofibroblasts [24]. The excessive accumulation and activity of myofibroblasts gives rise to disorganized extracellular matrix, resulting in development of corneal opacity and scarring [25,26]. To determine the effect of different routes of MSC administration on injury-induced corneal fibrosis, we evaluated the expression of α-Sma in corneal cross sections by immunohistochemistry (Fig. 3A). Furthermore, we quantified expression of α-Sma (Fig. 3B) and Tgf-β1 (Fig. 3C) at the mRNA level using real-time PCR. Of the various routes of administration of MSCs, subconjunctival injection was observed to reduce α-Sma and Tgf-β1 expression to the greatest extent relative to untreated injured controls (α-Sma: ~90% decrease, p=0.024; Tgf-β1: ~70% decrease, p=0.038). In addition, both intravenous (α-Sma: ~80% decrease, p=0.021; Tgf-β1: ~60% decrease, p=0.043) and intraperitoneal administration (α-Sma: ~60% decrease, p=0.030; Tgf-β1: ~50% decrease, p=0.047) were observed to significantly reduce mRNA expression of α-Sma and Tgf-β1, suggesting diminished corneal fibrosis in these groups.
Figure 3. MSCs administered via subconjunctival, intravenous or intraperitoneal injection limit injury-induced corneal fibrosis.
(A) Corneas were harvested at day 4 post-injury and protein expression of α-smooth muscle actin (α-Sma; green) was analyzed by immunofluorescence studies of paraffin-embedded cross-sections. mRNA expression of (B) α-Sma and (C) Tgf-β1 was analyzed through real-time PCR. The injured-untreated mice were used as control. Data are representative of one of three independent experiments performed. Each experiment consisted of 3 animals/group. The values are shown as mean ± SD. *p<0.05. Scale bar: 50 μm.
Influence of route of administration of MSCs on restoration of corneal architecture, CD45+ cell infiltration and levels of inflammatory cytokines following injury
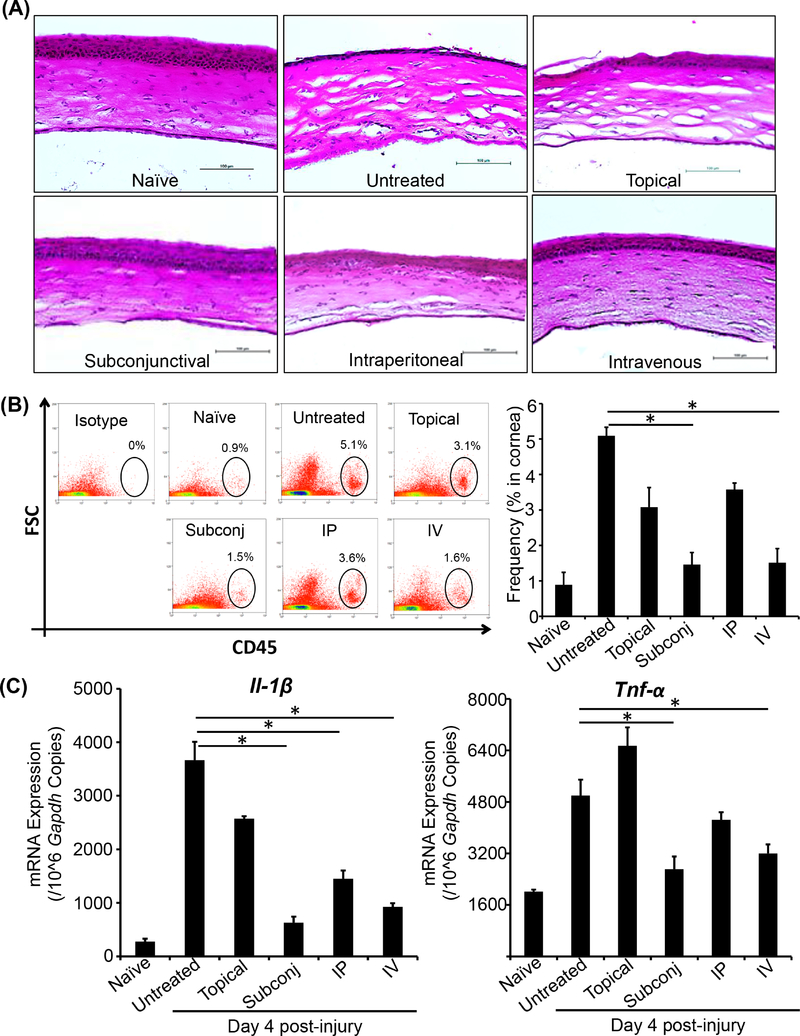
To determine the efficacy of different routes of MSC administration in restoring normal corneal architecture following injury, mice were sacrificed at day 4 post-injury and H&E staining was performed on corneal cross-sections (Fig. 4A). Naïve and untreated injured corneas served as controls. Both subconjunctival and intravenous delivery of MSCs were observed to restore normal cellular architecture. Notably, stromal disorganization was refractive to MSCs administered via intraperitoneal injection or topically.
Figure 4. MSCs administered via subconjunctival or intravenous injection promote normalization of corneal architecture and reduce corneal inflammation.
Corneas were harvested at day 4 post-injury. (A) Corneal cross-sections were stained with H&E to visualize tissue structure and inflammatory cell infiltration. Harvested corneas were digested with collagenase IV and DNase I (2mg/ml each) to form a single cell suspension. The cells were stained with anti-CD45 fluorophore conjugated antibody and analyzed using flow cytometry. (B) Representative dot plots (left) and bar graph (right) showing frequencies of CD45+ cells in the corneas of mice treated with MSCs by different routes of administration, compared to untreated injured controls. (C) mRNA expression of the inflammatory cytokines Il-1β and Tnf-α were evaluated using real-time PCR. Data are representative of one of three independent experiments performed. Each experiment consisted of 3–4 animals/group. The values are shown as mean ± SD. *p<0.05. Scale bar: 100 μm.
The capacity of MSCs delivered via different routes to limit tissue infiltration of CD45+ inflammatory cells was evaluated by harvesting corneas at day 4 post-injury, preparing single cell suspensions and performing flow cytometry (Fig. 4B). Naïve and untreated injured corneas served as controls. The untreated injured corneas demonstrated the highest frequencies of CD45+ cells. Relative to untreated injured controls, our data indicate that the infiltration of CD45+ inflammatory cells following corneal injury was significantly reduced by both subconjunctival (~70% decrease, p=0.042) and intravenous (~70% decrease, p=0.044) delivery of MSCs.
To evaluate the extent to which injury-induced inflammation was reduced by various routes of MSC administration, mRNA expression of the inflammatory cytokines Il-1β and Tnf-α was quantified in corneas harvested at day 4 post-injury by real-time PCR (Fig. 4C). Subconjunctival and intravenous administration of MSCs reduced expression of Il-1β and Tnf-α compared to untreated injured control. Subconjunctival administration resulted in an ~80% decrease in Il-1β expression (p=0.003), and an ~50% decrease in Tnf-α expression (p=0.005). Intravenous administration of MSCs resulted in an ~70% decrease in Il-1β expression (p=0.003), and an ~50% decrease in Tnf-α expression (p=0.003). Intraperitoneal injection of MSCs significantly reduced Il-1β expression (~60% decrease, p=0.001) but not Tnf-α expression. Topical administration of MSCs did not significantly decrease mRNA expression of either Il-1β or Tnf-α in corneal tissue.
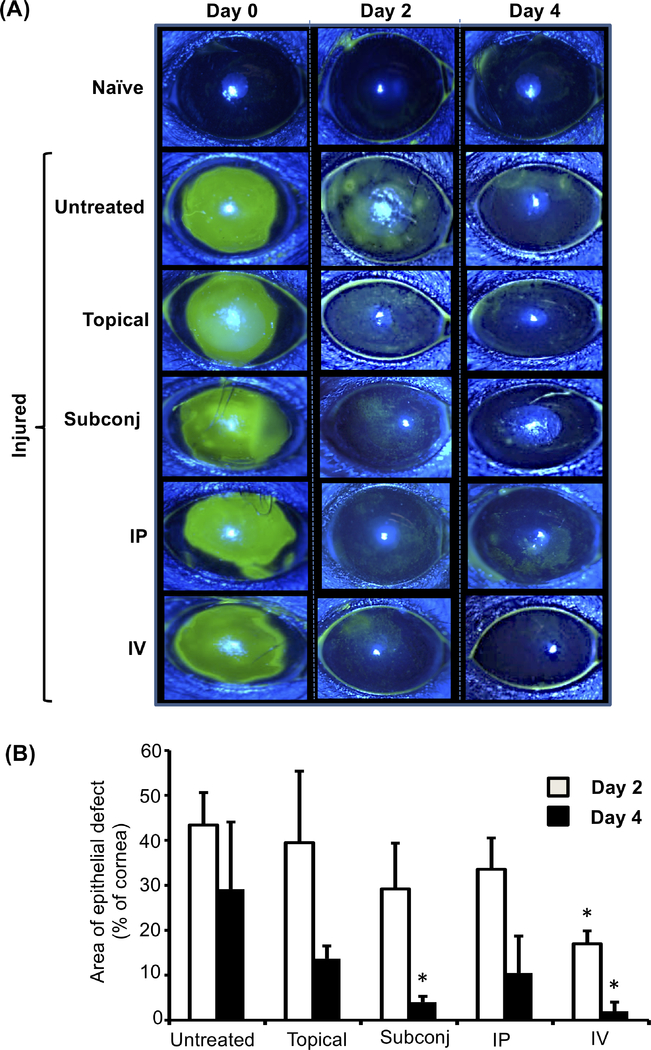
Effect of route of administration of MSCs on rate of corneal re-epithelialization.
To evaluate rates of re-epithelialization in injured eyes treated with MSCs by different routes, corneal fluorescein staining was performed immediately following injury (day 0) as well as at days 2 and 4 post-injury, and images were captured (representative images shown in Fig.5A). At day 0, the injured cornea exhibits green staining covering the entire wounded area, which decreases over the ensuing days as re-epithelialization occurs. Our data (Fig. 5B) demonstrate that intravenous delivery of MSCs significantly reduces the size of the epithelial defect by day 2 post-injury relative to untreated injured control. By day 4, we observed that subconjunctival delivery of MSCs had also significantly decreased the size of the epithelial defect relative to untreated injured control. These findings suggest that subconjunctival or intravenous administration of MSCs result in accelerated closure of corneal epithelial defects following ocular injury.
Figure 5. Subconjunctival or intravenous administration of MSCs accelerates corneal reepithelialization.
Corneal fluorescein staining of naïve and injured eyes was performed, and epithelial defects were evaluated by slit lamp biomicroscopy with cobalt blue light. (A) Representative images of fluorescein-stained corneas at days 0, 2, and 4 post-injury. The green areas represent epithelial defects. (B) Bar graph showing area of epithelial defect at days 2 and 4 post-injury (relative to day 0, set as 100%). Data from three independent experiments are shown, and each experiment consisted of 3–5 animals. The values are shown as mean ± SD. *p<0.05 as compared to untreated injured control.
Discussion
Due to their immunoregulatory and tissue regenerative properties, MSCs have attracted considerable interest for their potential application in ocular trauma and corneal inflammatory disease [4–11,16]. This study advances our understanding of how the route of administration of MSCs modulates their therapeutic activity. Our data show that following corneal injury, subconjunctival or intravenous delivery result in substantially higher frequencies of MSCs in ocular surface tissues compared to topical or intraperitoneal administration. Furthermore, our data indicate that subconjunctival or intravenous delivery (but not topical or intraperitoneal administration) significantly reduce corneal opacity, fibrosis and inflammation with restoration of normal tissue architecture and epithelial integrity.
The diversity of routes of MSC delivery in studies of ocular injury is notable; with some groups using topical administration [9,17–19], some intravenous [4–8], and others using subconjunctival [11] or intraperitoneal injection [8]. In view of reports that less than 1% of systemically infused MSCs reach the target tissue [27,28], we anticipated that local delivery of MSCs (topical or subconjunctival) would result in superior therapeutic efficacy relative to systemic delivery (intravenous or intraperitoneal). To our surprise, topical administration was the least effective mode of delivery of MSCs in reducing corneal opacity, decreasing fibrosis, limiting the inflammatory response and promoting re-epithelialization. It is important to note that the therapeutic efficacy of MSCs is not determined solely by cell frequencies. Indeed, the type of microenvironment that administered MSCs encounter influences their immunogenicity, their survival and their differentiation [29,30]. In contrast with topical application, one of the advantages of intravenous administration of MSCs is that delivered cells are in a nutrient- and oxygen-rich environment, and following extravasation MSCs remain in close proximity to the vasculature [31].
The cornea is easy to access, and thus lends itself to topical therapies. Indeed, numerous ocular pathologies including glaucoma [32], infections [33] and autoimmune disease [34] are treated with eye drops. When evaluating the efficacy of topical application of MSCs following corneal injury, it is important to note that protocols vary between investigators. Some groups suspend MSCs in fibrin gel that is subsequently grafted onto the cornea [9,10,35], while others use an approach in which MSCs are seeded onto amniotic membrane before grafting [18,19,36]. However, fibrin has previously been reported to alter the phenotype and functional characteristics of stem cells [37,38] and amniotic membrane has intrinsic immunosuppressive potential [39,40]. Thus, we used neither fibrin or amniotic membrane as MSC carriers, but rather applied MSCs directly to the ocular surface [17]. In order to permit extended contact of MSCs with the injured cornea following topical application, anesthetized mice were laid on the contralateral flank, tears were dried from the ocular surface using a Qtip, and 10μl of MSC-containing PBS was applied to the cornea and left in position for 45 minutes. We acknowledge that differences in the techniques of topical administration of MSCs employed by different investigators may limit the generalizability of our findings. Moreover, it may be possible to improve the efficacy of the topical route of administration by increasing either the frequency of application or the number of MSCs administered.
Systemically administered MSCs have been demonstrated to home to injured tissues following IV injection in models of cerebral ischemia [41,42], myocardial infarction [43,44] and pulmonary fibrosis [45]. Following ocular injury, GFP-labeled systemically administered MSCs have been shown to home to the injured cornea but not the normal cornea [7]. Our data corroborate these findings, showing substantially higher frequencies of Qdot-labeled MSCs in injured ocular surface tissues following subconjunctival or intravenous delivery, as compared to topical or intraperitoneal administration. In contrast, other studies have reported that systemically administered MSCs become trapped in the lungs and exert their immunoregulatory activities via secretion of anti-inflammatory factors [8,46]. In the context of this discussion, it is interesting to note that in our experiments subconjunctival and intravenous delivery of MSCs resulted not only in similar frequencies of MSCs in corneas and conjunctivae, but also in very similar immunologic and clinical outcomes. While subconjunctival delivery of MSCs resulted in marginally less opacification, fibrosis and inflammation relative to intravenous delivery, this difference was very slight.
In view of the similar frequencies of MSCs observed in ocular surface tissues following subconjunctival or intravenous delivery of MSCs, it is relevant to note the advantages and disadvantages of local delivery of cell-based therapies (i.e. subconjunctival) as compared to intravenous delivery. Local administration avoids the ‘first-pass’ accumulation of MSCs in the lungs and reduces the potential for off-target homing and immunomodulatory effects [47]. Furthermore, due to MSCs becoming trapped in the lungs, large numbers of cells are often necessary to obtain the desired therapeutic outcome, a factor that limits the feasibility of translating MSC-based therapies to the clinic [27,28,48]. In contrast, administering MSCs locally via subconjunctival injection permits targeted delivery of cells with bypass of the pulmonary first-pass effect. Both subconjunctival and intravenous administration of MSCs are minimally invasive techniques, with neither of these routes of administration resulting in significant tissue damage.
Our study elucidates the relative therapeutic efficacy of different routes of MSC administration following corneal injury. Our data demonstrate that subconjunctival and intravenous routes of MSC administration are effective in suppressing corneal inflammation and opacification following injury. Furthermore, subconjunctival and intravenous delivery of MSCs promoted normalization of tissue architecture and re-epithelialization. These findings have important implications for the route of administration selected in future studies of MSC-based therapies for ocular surface pathology.
Acknowledgments
Funding: This work was supported in part by the National Institutes of Health (EY024602 to S.K.C. and Core grant P30EY003790) and Department of Defense (W81XWH-15-1-0024) to S.K.C. Additional funding and support to S.S. was from Indo-US Science and Technology Forum (SERB-IUSSTF PDF 2017/159) and INSPIRE faculty grant (IFA-14-LSBM-104) from the Department of Science and Technology, Govt. of India.
Footnotes
Conflicts of interest: The authors have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Flaxman SR, Bourne RRA, Resnikoff S, Ackland P, Braithwaite T, Cicinelli MV, et al. Global causes of blindness and distance vision impairment 1990–2020: a systematic review and meta-analysis. Lancet Glob Heal 2017;5:e1221–34. doi: 10.1016/S2214-109X(17)30393-5. [DOI] [PubMed] [Google Scholar]
- [2].Mathews PM, Lindsley K, Aldave AJ, Akpek EK. Etiology of Global Corneal Blindness and Current Practices of Corneal Transplantation: A Focused Review. Cornea 2018;37:1198–203. doi: 10.1097/ICO.0000000000001666. [DOI] [PubMed] [Google Scholar]
- [3].Torricelli AAM, Wilson SE. Cellular and extracellular matrix modulation of corneal stromal opacity. Exp Eye Res 2014;129:151–60. doi: 10.1016/j.exer.2014.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Mittal SK, Omoto M, Amouzegar A, Sahu A, Rezazadeh A, Katikireddy KR, et al. Restoration of Corneal Transparency by Mesenchymal Stem Cells. Stem Cell Reports 2016;7:583–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Amouzegar A, Mittal SK, Sahu A, Sahu SK, Chauhan SK. Mesenchymal Stem Cells Modulate Differentiation of Myeloid Progenitor Cells During Inflammation. Stem Cells 2017;35:1532–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Mittal SK, Mashaghi A, Amouzegar A, Li M, Foulsham W, Sahu SK, et al. Mesenchymal Stromal Cells Inhibit Neutrophil Effector Functions in a Murine Model of Ocular Inflammation. Invest Ophthalmol Vis Sci 2018;59:1191–8. doi: 10.1167/iovs.17-23067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Lan Y, Kodati S, Lee HS, Omoto M, Jin Y, Chauhan SK. Kinetics and function of mesenchymal stem cells in corneal injury. Invest Ophthalmol Vis Sci 2012;53:3638–44. doi: 10.1167/iovs.11-9311. [DOI] [PubMed] [Google Scholar]
- [8].Roddy GW, Oh JY, Lee RH, Bartosh TJ, Ylostalo J, Coble K, et al. Action at a Distance: Systemically Administered Adult Stem/Progenitor Cells (MSCs) Reduce Inflammatory Damage to the Cornea Without Engraftment and Primarily by Secretion of TNF-α Stimulated Gene/Protein 6. Stem Cells 2011;29:1572–9. doi: 10.1002/stem.708. [DOI] [PubMed] [Google Scholar]
- [9].Hertsenberg AJ, Shojaati G, Funderburgh ML, Mann MM, Du Y, Funderburgh JL. Corneal stromal stem cells reduce corneal scarring by mediating neutrophil infiltration after wounding. PLoS One 2017;12:e0171712. doi: 10.1371/journal.pone.0171712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Basu S, Hertsenberg AJ, Funderburgh ML, Burrow MK, Mann MM, Du Y, et al. Human limbal biopsy-derived stromal stem cells prevent corneal scarring. Sci Transl Med 2014;6:266ra172. doi: 10.1126/scitranslmed.3009644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Yao L, Li Z, Su W, Li Y, Lin M, Zhang W, et al. Role of mesenchymal stem cells on cornea wound healing induced by acute alkali burn. PLoS One 2012;7:e30842. doi: 10.1371/journal.pone.0030842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol 2008;8:726–36. doi: 10.1038/nri2395. [DOI] [PubMed] [Google Scholar]
- [13].Saghizadeh M, Kramerov AA, Svendsen CN, Ljubimov AV. Concise Review: Stem Cells for Corneal Wound Healing. 2017;35:2105–14. doi: 10.1002/stem.2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Uccelli A, Moretta L, Pistoia V. Immunoregulatory function of mesenchymal stem cells. Eur J Immunol 2006;36:2566–73. doi: 10.1002/eji.200636416. [DOI] [PubMed] [Google Scholar]
- [15].Nombela-Arrieta C, Ritz J, Silberstein LE. The elusive nature and function of mesenchymal stem cells. Nat Rev Mol Cell Biol 2011;12:126–31. doi: 10.1038/nrm3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Eslani M, Putra I, Shen X, Hamouie J, Afsharkhamseh N, Besharat S, et al. Corneal Mesenchymal Stromal Cells Are Directly Antiangiogenic via PEDF and sFLT-1. Investig Opthalmology Vis Sci 2017;58:5507. doi: 10.1167/iovs.17-22680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Oh JY, Kim MK, Shin MS, Lee HJ, Ko JH, Wee WR, et al. The Anti-Inflammatory and Anti-Angiogenic Role of Mesenchymal Stem Cells in Corneal Wound Healing Following Chemical Injury. Stem Cells 2008;26:1047–55. doi: 10.1634/stemcells.2007-0737. [DOI] [PubMed] [Google Scholar]
- [18].Jiang T-S, Cai L, Ji W-Y, Hui Y-N, Wang Y-S, Hu D, et al. Reconstruction of the corneal epithelium with induced marrow mesenchymal stem cells in rats. Mol Vis 2010;16:1304–16. [PMC free article] [PubMed] [Google Scholar]
- [19].Galindo S, Herreras JM, López-Paniagua M, Rey E, de la Mata A, Plata-Cordero M, et al. Therapeutic Effect of Human Adipose Tissue-Derived Mesenchymal Stem Cells in Experimental Corneal Failure Due to Limbal Stem Cell Niche Damage. Stem Cells 2017;35:2160–74. doi: 10.1002/stem.2672. [DOI] [PubMed] [Google Scholar]
- [20].Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause DS, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006;8:315–7. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- [21].Omoto M, Katikireddy KR, Rezazadeh A, Dohlman TH, Chauhan SK. Mesenchymal stem cells home to inflamed ocular surface and suppress allosensitization in corneal transplantation. Invest Ophthalmol Vis Sci 2014;55:6631–8. doi: 10.1167/iovs.14-15413. [DOI] [PubMed] [Google Scholar]
- [22].Sahu SK, Mittal SK, Foulsham W, Li M, Sangwan VS, Chauhan SK. Mast cells initiate the recruitment of neutrophils following ocular surface injury. Investig Ophthalmol Vis Sci 2018;59. doi: 10.1167/iovs.17-23398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Omoto M, Suri K, Amouzegar A, Li M, Katikireddy KR, Mittal SK, et al. Hepatocyte Growth Factor Suppresses Inflammation and Promotes Epithelium Repair in Corneal Injury. Mol Ther 2017;25:1881–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Wilson SE. Corneal myofibroblast biology and pathobiology: generation, persistence, and transparency. Exp Eye Res 2012;99:78–88. doi: 10.1016/j.exer.2012.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Jester JV Corneal crystallins and the development of cellular transparency. Semin Cell Dev Biol 2008. doi: 10.1016/j.semcdb.2007.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Ljubimov AV, Saghizadeh M Progress in corneal wound healing. Prog Retin Eye Res 2015. doi: 10.1016/j.preteyeres.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Zhang M, Mal N, Kiedrowski M, Chacko M, Askari AT, Popovic ZB, et al. SDF-1 expression by mesenchymal stem cells results in trophic support of cardiac myocytes after myocardial infarction. FASEB J 2007;21:3197–207. doi: 10.1096/fj.06-6558com. [DOI] [PubMed] [Google Scholar]
- [28].Barbash IM, Chouraqui P, Baron J, Feinberg MS, Etzion S, Tessone A, et al. Systemic Delivery of Bone Marrow–Derived Mesenchymal Stem Cells to the Infarcted Myocardium. Circulation 2003;108:863–8. doi: 10.1161/01.CIR.0000084828.50310.6A. [DOI] [PubMed] [Google Scholar]
- [29].Nemeth K Mesenchymal stem cell therapy for immune-modulation: The donor, the recipient, and the drugs in-between. Exp Dermatol 2014. doi: 10.1111/exd.12459. [DOI] [PubMed] [Google Scholar]
- [30].Kurtz A Mesenchymal stem cell delivery routes and fate. Int J Stem Cells 2008;1:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Sarkar D, Spencer JA, Phillips JA, Zhao W, Schafer S, Spelke DP, et al. Engineered cell homing. Blood 2011. doi: 10.1182/blood-2010-10-311464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Sleath B, Robin AL, Covert D, Byrd JE, Tudor G, Svarstad B. Patient-Reported Behavior and Problems in Using Glaucoma Medications. Ophthalmology 2006;113:431–6. doi: 10.1016/J.OPHTHA.2005.10.034. [DOI] [PubMed] [Google Scholar]
- [33].Sheikh A, Hurwitz B. Topical antibiotics for acute bacterial conjunctivitis: Cochrane systematic review and meta-analysis update. Br J Gen Pract 2005;55:962–4. [PMC free article] [PubMed] [Google Scholar]
- [34].Marsh P, Pflugfelder SC. Topical nonpreserved methylprednisolone therapy for keratoconjunctivitis sicca in Sjögren syndrome. Ophthalmology 1999;106:811–6. doi: 10.1016/S0161-6420(99)90171-9. [DOI] [PubMed] [Google Scholar]
- [35].Gu S, Xing C, Han J, Tso MOM, Hong J. Differentiation of rabbit bone marrow mesenchymal stem cells into corneal epithelial cells in vivo and ex vivo. Mol Vis 2009;15:99–107. [PMC free article] [PubMed] [Google Scholar]
- [36].Ma Y, Xu Y, Xiao Z, Yang W, Zhang C, Song E, et al. Reconstruction of Chemically Burned Rat Corneal Surface by Bone Marrow-Derived Human Mesenchymal Stem Cells. Stem Cells 2006;24:315–21. doi: 10.1634/stemcells.2005-0046. [DOI] [PubMed] [Google Scholar]
- [37].Chung E, Rytlewski JA, Merchant AG, Dhada KS, Lewis EW, Suggs LJ. Fibrin-based 3D matrices induce angiogenic behavior of adipose-derived stem cells. Acta Biomater 2015;17:78–88. doi: 10.1016/j.actbio.2015.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Huang NF, Chu J, Lee RJ, Li S. Biophysical and chemical effects of fibrin on mesenchymal stromal cell gene expression. Acta Biomater 2010;6:3947–56. doi: 10.1016/j.actbio.2010.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Kubo M, Sonoda Y, Muramatsu R, Usui M. Immunogenicity of human amniotic membrane in experimental xenotransplantation. Invest Ophthalmol Vis Sci 2001;42:1539–46. [PubMed] [Google Scholar]
- [40].Ueta M, Kweon M-N, Sano Y, Sotozono C, Yamada J, Koizumi N, et al. Immunosuppressive properties of human amniotic membrane for mixed lymphocyte reaction. Clin Exp Immunol 2002;129:464–70. doi: 10.1046/J.1365-2249.2002.01945.X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Mahmood A, Lu D, Lu M, Chopp M. Treatment of traumatic brain injury in adult rats with intravenous administration of human bone marrow stromal cells. Neurosurgery 2003;53:697–702; discussion 702–3. [DOI] [PubMed] [Google Scholar]
- [42].Chen J, Li Y, Wang L, Zhang Z, Lu D, Lu M, et al. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke 2001;32:1005–11. [DOI] [PubMed] [Google Scholar]
- [43].Nagaya N, Fujii T, Iwase T, Ohgushi H, Itoh T, Uematsu M, et al. Intravenous administration of mesenchymal stem cells improves cardiac function in rats with acute myocardial infarction through angiogenesis and myogenesis. Am J Physiol Circ Physiol 2004;287:H2670–6. doi: 10.1152/ajpheart.01071.2003. [DOI] [PubMed] [Google Scholar]
- [44].Kawada H, Fujita J, Kinjo K, Matsuzaki Y, Tsuma M, Miyatake H, et al. Nonhematopoietic mesenchymal stem cells can be mobilized and differentiate into cardiomyocytes after myocardial infarction. Blood 2004;104:3581–7. doi: 10.1182/blood-2004-04-1488. [DOI] [PubMed] [Google Scholar]
- [45].Ortiz LA, Gambelli F, McBride C, Gaupp D, Baddoo M, Kaminski N, et al. Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects. Proc Natl Acad Sci 2003;100:8407–11. doi: 10.1073/pnas.1432929100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Oh JY, Lee RH, Yu JM, Ko JH, Lee HJ, Ko AY, et al. Intravenous mesenchymal stem cells prevented rejection of allogeneic corneal transplants by aborting the early inflammatory response. Mol Ther 2012;20:2143–52. doi: 10.1038/mt.2012.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Sohni A, Verfaillie CM. Mesenchymal stem cells migration homing and tracking. Stem Cells Int 2013;2013:130763. doi: 10.1155/2013/130763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Kean TJ, Lin P, Caplan AI, Dennis JE. MSCs: Delivery routes and engraftment, celltargeting strategies, and immune modulation. Stem Cells Int 2013. doi: 10.1155/2013/732742. [DOI] [PMC free article] [PubMed] [Google Scholar]