Abstract
INTRODUCTION:
Three CSF markers of neurodegeneration (N) (neurofilament light [NfL], total-tau [T-tau], and neurogranin [Ng]) have been proposed under the AT(N) scheme of the NIA-AA Research Framework.
METHODS:
We examined, in a community-based population (N=777, aged 50–95): 1) what variables were associated with each of the CSF (N) markers; and 2) whether the variables associated with each marker differed by elevated brain amyloid. CSF T-tau was measured with an automated electrochemiluminescence Elecsys immunoassay; NfL and Ng with in-house ELISAs.
RESULTS:
Multiple variables were differentially associated with CSF NfL and T-tau levels, but not Ng. Most associations were attenuated after adjustment for age and sex. T-tau had the strongest association with cognition in the presence of amyloidosis, followed by Ng. Variables associations with NfL did not differ by amyloid status.
DISCUSSION:
Understanding factors that influence CSF (N) markers will assist in the interpretation and utility of these markers in clinical practice.
Keywords: Neurofilament light chain, Neurogranin, Total tau, Cerebrospinal fluid, Community-based population, Neurodegeneration, Vascular factors
1. Introduction
Neurodegeneration is a key pathological feature of all neurodegenerative diseases and also occurs with normal aging. However, the causes, mechanisms, and brain regions of neurodegeneration differ. Under the AT(N) scheme of the new NIA-AA Research Framework, the proposed fluid-based measure of neuronal injury and neurodegeneration, (N), was high CSF total-tau (T-tau) [1]. However, a complication of using CSF T-tau as a marker of (N) is that levels are highly correlated with CSF phosphorylated tau (Spearman rho>0.90), a proposed CSF marker of ‘T’ [2–4]. There is a need to identify and understand other potential markers of (N). CSF neurofilament light (NfL) and neurogranin (Ng) are two potential options.
CSF NfL is a marker of large-caliber subcortical axonal degeneration whereas CSF Ng is a presumed marker of synaptic degeneration in Alzheimer’s disease (AD) (see [5,6]). Recent studies comparing CSF T-Tau, NfL, and Ng have shown differences in these markers for enhancing the diagnostic accuracy of AD dementia and for assessing the risk of MCI [2,3,7]. However, these CSF (N) markers, with the possible exception of Ng [8,9], are non-specific and elevated in other neurodegenerative diseases including multiple sclerosis, vascular dementia, and Lewy Body dementia [10–13].
Studies that have examined CSF T-tau, NfL, or Ng as markers of neurodegeneration have primarily focused on diagnostics, cognition, or their relationship to neuroimaging. As these CSF markers approach potential clinical use for neurodegenerative diseases, it is important to identify what variables might affect the levels of these markers for their clinical interpretation in the context of assessing neurodegeneration. For example, do all markers strongly associate with vascular risk factors and stroke or is one marker more specific to vascular-related neurodegeneration? Further, does this association differ by brain amyloid? What other variables are associated with the three potential CSF markers of neurodegeneration? To begin assessing these questions, we examined which variables (e.g., demographics, medical comorbidities, APOE genotype, anxiety and depressive symptoms, gaitspeed, cognition) were associated with CSF NfL, T-tau, and Ng in a community-based population. We also determined whether the variables associated with each CSF (N) marker differed by CSF amyloid-beta 42 (Aβ42) level.
2. Methods
2.1. Study participants
The Mayo Clinic Study on Aging (MCSA) is a prospective population-based study of residents living in Olmsted County, Minnesota [14]. In 2004, Olmsted County residents between the ages of 70 and 89 were enumerated using the Rochester Epidemiology Project (REP) medical records-linkage system [15]. An age- and sex-stratified random sampling design was utilized to ensure that men and women were equally represented in each 10-year age strata. The study was extended to include those aged 50 and older in 2012.
2.2. Protocol approvals standard, registrations and patient consents
The study was approved by Mayo Clinic and Olmsted Medical Center Institutional Review Boards. Written informed consent was obtained from all participants.
2.3. Participant assessment
MCSA visits include an interview by a study coordinator, physician examination, and neuropsychological testing [14]. The cognitive battery included 9 tests covering 4 domains: memory, language, executive function, and visuospatial. Sample-specific z-scores for all cognitive tests were calculated; domain-specific z-scores were created by averaging the z-scores for the individual tests within each domain. A global cognitive z-score was created by averaging the z-scores of the four domains.
2.4. Mild Cognitive Impairment (MCI) and dementia diagnostic determination
Clinical diagnoses were determined by a consensus committee of those who evaluated each participant. Cognitive performance was compared with the age-adjusted scores of cognitively unimpaired (CU) individuals previously obtained using Mayo’s Older American Normative Studies [16]. Participants with scores around 1.0 SD below the age-specific mean in the general population were considered for possible cognitive impairment. The operational definition of MCI was based on clinical judgment including a history from the patient and informant. Published criteria were used for the diagnosis: cognitive complaint, cognitive function not normal for age, essentially normal functional activities, no dementia [17]. A final decision was made after considering education, occupation, visual or hearing deficits, and reviewing all other participant information. The diagnosis of dementia was based on published criteria [18]. Participants who performed in the normal range and did not meet criteria for MCI or dementia were deemed (CU). The consensus committee was blinded to CSF results when determining the clinical diagnosis.
2.5. Lumbar punctures and CSF measurements
From November 2007 through August 2016, 777 MCSA participants underwent lumbar puncture for the collection of CSF and had assays of CSF NfL, Ng, T-tau, and amyloid-beta (Aβ)42. Compared to participants during this timeframe who did not undergo a lumbar puncture, those who did were younger, more likely to be male, and generally healthier (e.g., less likely to have coronary artery disease, hypertension, or stroke). Baseline clinical diagnosis (e.g., CU, MCI, dementia) was not associated with participation in a lumbar puncture.
Fasting lumbar punctures were performed early in the morning in the lateral decubitus position from the L3 and L4 intravertebral space using a 20 or 22 gauge Quincke needle. Two cc of CSF were used to evaluate routine markers (glucose, protein, cell count). The remainder was divided into 0.5cc aliquots and stored at −80°C. Samples had not undergone a freeze-thaw cycle prior to being pulled.
CSF Aβ42 and T-tau were measured with automated electrochemiluminescence Elecsys immunoassays (Roche Diagnostics). To determine the CSF Aβ42 cutpoint, 524 participants across the AD clinical spectrum from the MCSA and the Mayo Clinic Alzheimer’s Disease Research Center with 11C Pittsburgh Compound B (PiB) PET imaging within one year of CSF were included, as previously described [19]. Briefly, bivariate mixture modeling and the optimized Younden-index (PiB-PET SUVR reference value of 1.60) were used. A CSF Aβ42 level<893 pg/mL was considered amyloid positive (A+) and had 79% agreement with PiB-PET [19]. CSF NfL and Ng were measured using in-house ELISAs; their assay characteristics and methods have been described in detail [3,20–22].
2.6. Measurement of additional variables assessed in relation to CSF NfL, T-tau and Ng
Participant demographics (age, sex, and years of education) were ascertained at the in-clinic examination. Participants’ height (cm) and weight (kg) were measured and used to calculate body mass index (BMI) (kg/m2). Gaitspeed was assessed using the GAITRite® instrument [23]. Medical conditions and the Charlson Comorbidity Index [24] were determined for each participant by medical record abstraction using the REP medical records-linkage system [15]. Depressive symptoms were assessed using the Beck Depression Inventory-II (BDI-II) [25]; participants with a score of ≥13 were considered to have depression. Anxiety symptoms were assessed using the Beck Anxiety Inventory (BAI) [26]. Apolipoprotein E (APOE) ε4 genotyping was performed from a blood sample.
2.7. Statistical Analysis
CSF NfL, T-tau, and Ng were z-log transformed to normalize the distributions and to allow for comparison of associations. Linear regression models were used to examine the relationship between each characteristic and each CSF (N) marker, first in univariate analyses and then after adjustment for age and sex. For models examining global and domain-specific z-scores, education was also included as a covariate. In sensitivity analyses, the models were rerun, stratified by cognitive status (CU only and MCI/dementia only). An interaction between each variable and elevated amyloid was examined in both univariable and multivariable models. In all analyses, a 2-tailed, P < .05 was considered significant. Statistical analysis was completed using SAS, version 9.4 (SAS Institute Inc., Cary, NC), and R, version 3.4.2.
3. Results
The 777 participants were a median age of 72.9 (Interquartile range [IQR]: 64.0, 79.3), had a median education of 14.0 (IQR: 12, 16) and 57.0% were male (Table 1). The median number of comorbidities on the Charlson comorbidity index was 2 (IQR: 1, 4). There were 83 (10.7%) with a diagnosis of MCI, and 7 (0.9%) with a diagnosis of dementia. Of the 777 participants, 283 (36.4%) were determined to be A+: 235 of 687 CU (34.2%), 41 of 83 MCI (49.4%) MCI, and 7 of 7 dementia (100%) dementia.
Table 1.
Participant characteristics (N=777)
| Characteristics | Median (IQR)/N(%) |
|---|---|
| Age | 72.9 (64.0, 79.3) |
| Male | 443 (57.0%) |
| Education | 14.0 (12.0, 16.0) |
| Presence of APOE ε4 allele | 211 (27.2%) |
| Body Mass Index | 28.0 (25.1, 31.3) |
| Gaitspeed | 1.1 (1.0, 1.3) |
| Charlson comorbidity index | 2.0 (1.0, 4.0) |
| Hypertension | 487 (62.7%) |
| Dyslipidemia | 627 (80.7%) |
| Diabetes | 131 (16.9%) |
| Stroke | 21 (2.7%) |
| Head trauma | 112 (15.8%) |
| Myocardial infarction | 86 (11.1%) |
| Atrial Fibrillation | 50 (6.4%) |
| Cancer | 176 (22.7%) |
| Cancer with chemotherapy | 31 (4.0%) |
| Spinal Stenosis | 68 (8.8%) |
| Mild Cognitive Impairment | 83 (10.7%) |
| Dementia | 7 (0.9%) |
| Global Z-score | 0.0 (−0.6, 0.8) |
| Memory Z-score | 0.0 (−0.7, 0.7) |
| Language Z-score | 0.1 (−0.6, 0.7) |
| Attention Z-score | 0.1 (−0.6, 0.7) |
| Visuospatial Z-score | 0.1 (−0.6, 0.7) |
| CSF NfL (pg/ml) | 520.2 (374.3, 745.4) |
| CSF Total Tau (pg/ml) | 217.2 (167.5, 278.1) |
| CSF Neurogranin (pg/ml) | 166.6 (132.9, 220.8) |
| CSF Amyloid-beta 42 (pg/ml) | 1074.0 (761.5, 1527.0) |
Abbreviations: APOE, apolipoprotein E; IQR, interquartile range; NfL, neurofilament light.
The median (IQR) values for CSF NfL (CU: 497 [360, 722]; MCI: 725 [518, 985]; Dementia: 849 [482, 980], P < .0001) and CSF T-tau (CU: 213 [166, 270]; MCI: 245 [190, 337]; Dementia: 320 [150, 361], P = .002) increased with disease severity. However, there was no difference in median (IQR) CSF Ng levels by clinical diagnosis (CU: 165 [131, 218]; MCI: 177 [137, 238]; Dementia: 177 [99, 263], P = .223). CSF NfL had moderate correlations with Ng (Spearman’s rho = .321, P < .0001) and T-tau (Spearman’s rho = .477, P < .0001). CSF Ng was highly correlated with T-tau (Spearman’s rho = .831, P < .0001).
3.1. Univariable Associations between the variables and each CSF (N) marker
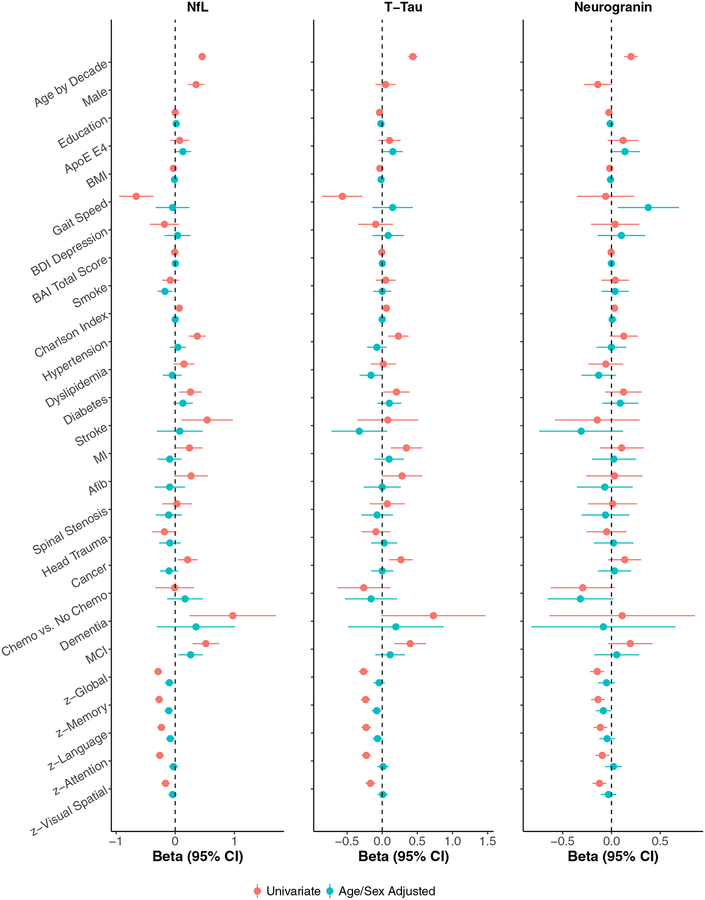
The univariable association between each demographic, medical, or cognitive variable and z-log transformed CSF NfL, T-Tau, or Ng is shown in Table 2 and Fig. 1. CSF NfL increased with increasing age, was higher for men, those with more comorbidities, and with a diagnosis of hypertension, diabetes, stroke, myocardial infarction, cancer, MCI, and dementia. Higher NfL was also associated with a lower BMI, slower gaitspeed, and worse performance in tests of all cognitive domains. CSF T-tau increased with increasing age, with more comorbidities, and with a diagnosis of hypertension, diabetes, myocardial infarction, cancer, and MCI. Higher T-tau was also associated with lower education, lower BMI, slower gaitspeed, and worse performance in tests of all cognitive domains. Fewer variables were associated with CSF Ng. Increasing Ng levels were associated with increasing age, and more comorbidities. Higher Ng was also associated with lower education, a lower BMI, and worse performance in tests of all cognitive domains. There was a trend for men to have lower levels of CSF Ng (b(se) = −0.14 (0.07), P = .054), which is in contrast to the significantly higher levels of CSF NfL for men (b(se)=0.35 (0.07), P < .0001). The higher NfL levels for men, compared to women, was equivalent to a 7.6-year increase in age.
Table 2.
Univariate associations between each characteristic and z-log transformed CSF neurofilament light chain, total tau, and neurogranin
| Neurofilament light | Total tau | Neurogranin | ||||
|---|---|---|---|---|---|---|
| Characteristics | estimate (se) | P value | estimate (se) | P value | estimate (se) | P value |
| Age, per decade | 0.46 (0.03) | <.0001 | 0.44 (0.03) | <.0001 | 0.20 (0.04) | <.0001 |
| Male | 0.35 (0.07) | <.0001 | 0.05 (0.07) | .518 | −0.14 (0.07) | .054 |
| Education, per year | 0.0002 (0.01) | .988 | −0.04 (0.01) | 0.004 | −0.03 (0.01) | .047 |
| Presence of APOE ε4 allele | 0.08 (0.08) | .350 | 0.10 (0.08) | .197 | 0.12 (0.08) | .140 |
| Body Mass Index | −0.03 (0.01) | <.0001 | −0.03 (0.01) | <.0001 | −0.02 (0.01) | .007 |
| Gaitspeed | −0.66 (0.15) | <.0001 | −0.57 (0.15) | <.001 | −0.06 (0.15) | .692 |
| Beck Depression Inventory | −0.18 (0.13) | .148 | −0.09 (0.13) | .459 | 0.04 (0.13) | .767 |
| Beck Anxiety Inventory | −0.01 (0.01) | .255 | −0.01 (0.01) | .417 | −0.004 (0.01) |
.621 |
| Ever smoker | −0.08 (0.07) | .254 | 0.05 (0.07) | .488 | 0.04 (0.07) | .589 |
| Charlson comorbidity index | 0.07 (0.01) | <.0001 | 0.06 (0.01) | <.0001 | 0.03 (0.01) | .012 |
| Hypertension | 0.37 (0.07) | <.0001 | 0.23 (0.07) | .002 | 0.13 (0.07) | .090 |
| Dyslipidemia | 0.15 (0.09) | .103 | 0.02 (0.09) | .865 | −0.06 (0.09) | .524 |
| Diabetes | 0.26 (0.10) | .007 | 0.20 (0.10) | .034 | 0.12 (0.10) | .198 |
| Stroke | 0.54 (0.22) | .014 | 0.08 (0.22) | .718 | −0.15 (0.22) | .509 |
| Myocardial infarction | 0.24 (0.11) | .035 | 0.34 (0.11) | .003 | 0.10 (0.11) | .364 |
| Atrial Fibrillation | 0.27 (0.15) | .066 | 0.28 (0.15) | .054 | 0.03 (0.15) | .832 |
| Spinal Stenosis | 0.03 (0.13) | .823 | 0.07 (0.13) | .574 | 0.01 (0.13) | .930 |
| Head trauma | −0.18 (0.10) | .079 | −0.09 (0.10) | .383 | −0.05 (0.10) | .635 |
| Cancer | 0.21 (0.09) | .014 | 0.27 (0.09) | .002 | 0.14 (0.09) | .114 |
| Cancer with chemotherapy vs. no chemotherapy | −0.01 (0.17) | .954 | −0.26 (0.19) | .169 | −0.30 (0.17) | .078 |
| Mild Cognitive Impairment | 0.52 (0.11) | <.0001 | 0.40 (0.12) | <.001 | 0.19 (0.12) | .098 |
| Dementia | 0.97 (0.37) | .009 | 0.73 (0.38) | .053 | 0.11 (0.38) | .773 |
| Global Z-score | −0.29 (0.04) | <.0001 | −0.27 (0.04) | <.0001 | −0.15 (0.04) | <.001 |
| Memory Z-score | −0.27 (0.03) | <.0001 | −0.24 (0.03) | <.0001 | −0.14 (0.04) | <.001 |
| Language Z-score | −0.23 (0.04) | <.0001 | −0.23 (0.04) | <.0001 | −0.11 (0.04) | .002 |
| Attention Z-score | −0.26 (0.04) | <.0001 | −0.23 (0.04) | <.0001 | −0.09 (0.04) | .010 |
| Visuospatial Z-score | −0.16 (0.04) | <.0001 | −0.17 (0.04) | <.0001 | −0.12 (0.04) | <.001 |
| CSF NfL | 0.41 (0.03) | <.0001 | 0.29 (0.03) | <.0001 | ||
| CSF Total Tau | 0.41 (0.03) | <.0001 | 0.74 (0.02) | <.0001 | ||
| CSF Neurogranin | 0.29 (0.03) | <.0001 | 0.74 (0.02) | <.0001 | ||
| CSF Amyloid-beta 42 | 0.06 (0.04) | .074 | 0.27 (0.03) | <.0001 | 0.29 (0.03) | <.0001 |
Abbreviations: NfL, neurofilament light; SE, standard error. CSF Neurofilament light chain, total tau, and neurogranin were log-transformed and z-scored in order to compare the coefficients.
Fig. 1.
Associations between each variable and CSF levels of neurofilament light chain (NfL), Total-tau (T-tau) and Neurogranin (Ng). Red (top line) represents beta estimates from univariable models. Blue (bottom line) represents beta estimates from multivariable models adjusting for age and sex. Models including cognitive variables also adjusted for education.
3.2. Multivariable Associations between the variables and each CSF (N) marker
In multivariable models adjusting for age and sex, the association between most variables and the CSF (N) measures was attenuated and no longer significant (Table 3 and Fig. 1). Examining cognitive variables, a diagnosis of MCI was only associated with higher levels of CSF NfL (b(se) = 0.26 (0.10), P = .013), but not with CSF T-tau or Ng. The effect sizes for associations between global- and domain-specific z-scores and each marker were similar.
Table 3.
Associations between each characteristic and z-log transformed CSF neurofilament light chain, total tau, and neurogranin after adjustment for age and sex
| Neurofilament light | Total tau | Neurogranin | ||||
|---|---|---|---|---|---|---|
| Characteristics | b (se) | P value | b (se) | P value | b (se) | P value |
| Education, per year | 0.02 (0.01) | .165 | −0.02 (0.01) | .134 | −0.01 (0.01) | .279 |
| Presence of APOE ε4 allele | 0.13 (0.07) | .067 | 0.15 (0.07) | .037 | 0.14 (0.08) | .079 |
| Body Mass Index | −0.01 (0.01) | .060 | −0.02 (0.01) | .012 | −0.01 (0.01) | .134 |
| Gait speed | −0.04 (0.15) | .766 | 0.15 (0.15) | .312 | 0.38 (0.16) | .018 |
| Beck Depression Inventory | 0.04 (0.11) | .725 | 0.08 (0.12) | .461 | 0.10 (0.12) | .416 |
| Beck Anxiety Inventory | 0.003 (0.01) | .703 | 0.001 (0.01) | .840 | −0.002 (0.01) | .796 |
| Ever smoker | −0.17 (0.06) | .006 | 0.001 (0.07) | .992 | 0.04 (0.07) | .605 |
| Charlson comorbidity index | 0.001 (0.01) | .939 | −0.001 (0.01) | .945 | 0.01 (0.01) | .558 |
| Hypertension | 0.05 (0.07) | .513 | −0.08 (0.07) | .283 | −0.001 (0.08) | .993 |
| Dyslipidemia | −0.05 (0.08) | .544 | −0.16 (0.08) | .054 | −0.13 (0.09) | .146 |
| Diabetes | 0.13 (0.08) | .124 | 0.10 (0.09) | .242 | 0.09 (0.09) | .340 |
| Stroke | 0.07 (0.20) | .698 | −0.33 (0.20) | .107 | −0.31 (0.22) | .157 |
| Myocardial infarction | −0.09 (0.10) | .372 | 0.10 (0.11) | .353 | 0.02 (0.11) | .833 |
| Atrial Fibrillation | −0.09 (0.13) | .486 | −0.001 (0.13) | .997 | −0.07 (0.15) | .637 |
| Spinal Stenosis | −0.11 (0.11) | .327 | −0.07 (0.12) | .529 | −0.06 (0.12) | .621 |
| Head trauma | −0.09 (0.09) | .332 | 0.03 (0.09) | .767 | 0.02 (0.10) | .834 |
| Cancer | −0.10 (0.08) | .184 | 0.0002 (0.08) | .998 | 0.03 (0.09) | .722 |
| Cancer with chemotherapy vs. no chemotherapy | 0.17 (0.15) | .278 | −0.16 (0.19) | .398 | −0.32 (0.17) | .063 |
| Mild Cognitive Impairment | 0.26 (0.10) | .013 | 0.11 (0.11) | .295 | 0.05 (0.12) | .645 |
| Dementia | 0.35 (0.34) | .298 | 0.19 (0.35) | .577 | −0.08 (0.38) | .825 |
| Global Z-score | −0.10 (0.04) | .014 | −0.04 (0.04) | .284 | −0.05 (0.04) | .252 |
| Memory Z-score | −0.11 (0.04) | .002 | −0.08 (0.04) | .029 | −0.08 (0.04) | .034 |
| Language Z-score | −0.08 (0.04) | .023 | −0.07 (0.04) | .077 | −0.04 (0.04) | .273 |
| Attention Z-score | −0.03 (0.04) | .444 | 0.01 (0.04) | .746 | 0.02 (0.04) | .645 |
| Visuospatial Z-score | −0.04 (0.04) | .244 | 0.01 (0.04) | .834 | −0.03 (0.04) | .444 |
| CSF NfL | 0.28 (0.04) | <.0001 | 0.28 (0.04) | <.0001 | ||
| CSF Total Tau | 0.27 (0.03) | <.0001 | 0.80 (0.03) | <.0001 | ||
| CSF Neurogranin | 0.23 (0.03) | <.0001 | 0.68 (0.02) | <.0001 | ||
| CSF Amyloid-beta 42 | 0.09 (0.03) | .004 | 0.30 (0.03) | <.0001 | 0.30 (0.03) | <.0001 |
Abbreviations: APOE, apolipoprotein E; NfL, neurofilament light; SE, standard error.
CSF Neurofilament light chain, total tau, and neurogranin were log-transformed and z-scored in order to compare the coefficients. Models including cognitive variables also adjusted for education.
3.3. Univariable and Multivariable Associations stratified by cognitive status
Supplementary Tables 1–4 replicate the above univariable and multivariable analyses but stratify the results by cognitive status (CU – Supplementary Tables 1–2; MCI/dementia – Supplementary Tables 3–4). The results of the CU only group were similar to the overall group, likely because the CU group comprises about 90% of the whole group. However, there were some differences. Although increasing age was associated with increasing levels of all CSF (N) markers for both the CU and MCI/Dementia groups, the association between age and either CSF NfL and T-tau were slightly stronger among the CU group (Supplementary Tables 1 and 3). Among the CU group, men had significantly higher levels of CSF NfL and lower levels of Ng. Among the MCI/Dementia group, the associations were in the same direction but the effect sizes were essentially halved.
There were also differences between some of the variables associated with the CSF (N) markers for the MCI/dementia group compared to the CU group in multivariable analyses (Supplementary Tables 2 and 4). First, the presence of an APOE ε4 allele was only associated with higher levels of CSF NfL, T-tau, and Ng among the MCI/dementia participants. Second, a higher BMI was associated with lower CSF T-tau and Ng levels only among the MCI/Dementia participants. Third, the associations between memory z-scores and all three CSF markers were stronger among the MCI/Dementia participants compared to the CU participants. Last, the associations between CSF Ng and T-tau were stronger among the MCI/Dementia participants compared to the CU participants.
3.4. Interactions between each variable and A+ for each CSF (N) marker
We next examined the interaction between the cognitive variables and CSF amyloid status (i.e., A+ vs. A−) for CSF NfL, T-tau, or Ng levels. Fig. 2 shows the univariable association between each variable and CSF (N) marker after stratification by amyloid status. Supplementary Tables 5, 6, and 7 show the univariable and multivariable models examining the interaction between each variable and CSF amyloid status for each CSF (N) level. When examining cognitive variables worse cognitive performance (i.e., global, memory, and visuospatial z-scores) was associated with higher CSF NfL among participants who were A+ compared to A− (Supplementary Table 5). Notably, the interactions of all global and domain-specific Z-scores and MCI with A+ were even more robust and stronger for CSF T-tau levels (all P-values for interaction <.001; Supplementary Table 6). The interactions between cognitive variables and A+ for CSF Ng levels (Supplementary Table 7) were not as strong as for CSF T-tau levels in multivariable models, but were still statistically significant (all P-values for interaction <.05 except for visuospatial z-score).
Fig. 2.
Associations between each variable and CSF levels of neurofilament light chain (NfL), Total-tau (T-tau) and Neurogranin (Ng) by amyloid status. Red (top line) shows associations for those who are amyloid positive (A+). Blue (bottom line) shows associations for those who are amyloid negative (A−). Models including cognitive variables also adjusted for education.
Lastly, we examined interactions between the other variables and A+ for each CSF (N) level. In multivariable analyses, none of the other variables interacted with A+ in relation to CSF NfL levels (Supplementary Table 5). In contrast faster gaitspeed and more depressive symptoms were associated with lower levels of CSF T-tau in A+ participants compared to A−; an increasing Charlson comorbidity index was associated with higher CSF T-tau among A+ versus A− participants (Supplementary Table 6). In addition, the presence of several comorbidities (stroke, myocardial infarction, hypertension and cancer) was associated with higher CSF T-tau among the A+ versus A− participants, but the results were not statistically significant (P-values ranging from .050 to .091). Faster gaitspeed was associated with lower level of CSF Ng in A+ participants whereas an increasing Charlson comorbidity index was associated with higher CSF Ng (Supplementary Table 7).
4. Discussion
Three CSF markers (NfL, Total-tau, and Ng) have been proposed as potential markers of neurodegeneration (N) under the AT(N) scheme of the new NIA-AA Research Framework [1]. The objective of this analysis was to examine, in a community-based population, what variables were associated with each of the three CSF (N) markers. We also determined whether the variables associated with each CSF (N) marker differed by the presence of elevated brain amyloid as measure by CSF Aβ42. We found that multiple variables were associated with levels of CSF NfL and T-tau, but not Ng. Most relationships were attenuated, including cognitive measures, after adjustment for age and sex (and education when examining the cognitive variables). Worse performance in global and domain-specific z-scores were more robustly associated with higher T-tau levels than Ng levels for participants who were A+. The association between cognition and CSF NfL were less affected by A+ status.
In univariable analyses, several variables were similarly associated with CSF NfL and T-tau including gaitspeed, Charlson comorbidity index, cognition, and vascular related factors (e.g., hypertension, diabetes, myocardial infarction), but not with Ng. Most of these vascular factors were more strongly associated with elevations in NfL levels compared to T-tau, and stroke was only associated with higher NfL levels. These results suggest that CSF NfL may be a more informative measure of vascular-related neurodegeneration. Our results are congruent with previous studies showing elevated CSF NfL in those with a history of stroke [27,28]. In addition, one study reported that CSF NfL, but not T-tau, was associated with white matter hyperintensities [29]. Future studies are needed to better understand the relationship between NfL and cerebrovascular pathology, specifically to determine whether there are differential relationships by type of vascular pathology (e.g., white matter hyperintensities, microbleeds, infarcts), and location (e.g. subcortical vs cortical).
Participants with a history of cancer had higher CSF NfL and T-tau levels compared to those without. In addition, the association between cancer and CSF T-tau was stronger among A+ participants. Notably, among those with a history of cancer, chemotherapy was not associated with either NfL or T-tau levels. This suggests that another aspect of cancer may be related to neurodegeneration. Alternatively, those who had the most severe cancer and recurrent treatments of chemotherapy may have died prior to enrollment in the MCSA or were less likely to participate in a lumbar puncture for CSF. We also did not have information on type of chemotherapy, dose, or duration. Recent studies have reported increased levels of CSF T-tau or P-tau in survivors of children with acute lympoblastic leukemia either five or 20 years after diagnosis, and an association between CSF T-tau or P-tau and cognition at follow-up [30,31]. Given these associations, it is important to better understand the relationship between cancer and CSF T-tau, as well as CSF NfL.
As expected, age was strongly associated with increasing levels of all CSF (N) measures, although the increase in CSF NfL and T-tau was greater than for Ng, in both the CU and MCI/dementia groups. This finding replicates the Bridel et al. study which reported associations between age and CSF NfL among participants with cognitive complaints and most neurodegenerative conditions [13]. In addition, CSF NfL and Ng levels, but not T-tau, differed by sex with men having higher NfL levels and women having higher Ng levels. The increased NfL levels among men was equivalent to more than a 7-year increase in age. Higher CSF NfL levels among men have previously been reported [13,32], and the higher levels have been hypothesized to be related to a greater vascular burden among men. Although studies of CSF Ng have adjusted for sex, the studies have not examined whether levels differ by sex. Thus, reasons for the sex difference in Ng are not known but warrant further exploration.
In multivariable models adjusting for age and sex (and education for cognitive variables), most of the associations between the variables and each of the CSF (N) measures were attenuated and did not remain significant, including the associations with cognition. This finding is not surprising given the strong associations of age with both comorbidities and neurodegeneration. It is interesting to note, however, that after adjustment MCI was only associated with higher NfL levels and not with T-tau or Ng. When stratified by cognitive status, the association between cognition, especially memory, and each of the CSF (N) markers was stronger in the MCI/Dementia group, as would be expected. When examining the interaction between variables and A+, gaitspeed and the Charlson comorbidity index were more strongly associated with CSF T-tau and Ng in the A+ group compared to the A− group. In addition, there were trends for the presence of several comorbidities (stroke, myocardial infarction, hypertension and cancer) to be associated with higher CSF T-tau among the A+ versus A− participants. These results suggest that additional variables beyond age and sex may be important predictors of CSF T-tau, especially among individuals with elevated brain amyloid.
The importance of age and sex for CSF NfL levels across neurodegenerative diseases has recently been discussed [13]. Despite the cross-sectional increase of each CSF (N) level with age, however, it is debatable as to whether age-corrected cutpoints should be developed for these markers. The development of cutpoints of other disease-related measures, such as hemoglobin A1C levels or blood pressure, has not been defined by age. If age-related cutpoints are not used, the results from the univariable analyses become more important for clinical interpretation. The CSF (N) measures will most likely be used as measures of disease progression than for a clinical diagnosis. Therefore, longitudinal studies with serial CSF (N) measures are needed to quantify these aging-related changes and should also consider the impact of incident comorbidities.
When we examined interactions between the variables and elevated brain amyloid, we found differences for each of the CSF (N) measures. There were no interactions between any of the variables and amyloid in multivariable analyses for predicting NfL levels, with the exception that CSF Ng was more strongly associated with CSF NfL among A+ compared to A−participants. This finding is consistent with previous studies suggesting that CSF NfL is a non-specific marker of neurodegeneration and independent of brain amyloid [3,32,33].
In contrast to CSF NfL, there were interactions between all cognitive variables (MCI and global- and domain-specific z-scores) and amyloid status for CSF T-tau such that worse cognition was associated with higher CSF T-tau levels for A+ participants compared to A−. There was a similar association for CSF Ng, but the association was less robust. For example, a higher global z-score and a higher attention z-score, but not other domains, were associated with lower Ng levels for A+ versus A− individuals. Thus, CSF T-tau appears to be a better (N) marker related to AD pathology compared to CSF Ng, which is consistent with a recent study that included participants across the AD clinical spectrum [7]. A complication with this assertion, though, is that we and others have shown that CSF T-tau is very highly correlated with CSF P-tau [2–4] and CSF P-tau is thought to be specific to AD pathology. As a result, CSF Ng is likely to be more useful as a marker of AD-associated neurodegeneration. This finding is similar to three previous studies which reported that CSF Ng was associated with AD-related neurodegeneration [7,9,34]. One other study did not find a cross-sectional association between CSF Ng and CSF Aβ42, but did find that the association emerged over the follow-up [35]. Longitudinal studies with serial CSF measures are needed to better determine the relationships between the CSF measures, CSF Aβ42, and the variables examined over time. Additional research is also needed to better understand what CSF Ng represents and how it can best be utilized as a (N) marker.
Strengths of the study include the large sample size, community-based sample, and the characterization of the cohort. The medical comorbidities of all participants were abstracted from the medical records by nurse abstractors rather than relying on self-report, which is less accurate. A limitation of the study, as mentioned above, is that the MCSA participants who agreed to LP for the collection of CSF were generally younger, more likely to be male, and healthier than those who did not agree. Therefore, the associations between comorbidities and the CSF levels may be conservative. In addition, we had a small number of individuals with dementia and CSF.
The present results support the notion that CSF T-tau and Ng may reflect a neuronal response to amyloid pathology, and AD-specific neurodegeneration, whereas CSF NfL is a more general biomarker for neurodegeneration. As CSF NfL, T-tau, and Ng move towards use in the clinic, or for clinical trials as markers of disease progression and neurodegeneration, it is critical to better understand what these markers are measuring. Further, with a move to clinical use, the development of reference ranges is needed. Although reference ranges are typically developed in populations without disease, it is extremely difficult to find elderly individuals without any of these comorbidities. Thus, it is important to understand what comorbidities affect these CSF (N) measures. Further discussion will be needed on whether age-, and even sex-specific cutpoints, should be used. Longitudinal studies are needed to better estimate change over time, after accounting for medical comorbidities and demographic variables that can affect levels. In addition, future studies will need to compare multiple neuroimaging measures of neurodegeneration including cerebrovascular pathologies, diffusion tensor imaging, cortical atrophy, and cortical thickness in order to better understand how each potential CSF (N) marker reflects neurodegeneration.
Supplementary Material
Supplementary Table 1. Univariable associations between each characteristic and z-log transformed CSF neurofilament light chain, total tau, and neurogranin among cognitively unimpaired participants.
Supplementary Table 2. Associations between each characteristic and z-log transformed CSF neurofilament light chain, total tau, and neurogranin after adjustment for age and sex among cognitively unimpaired participants
Supplementary Table 3. Univariable associations between each characteristic and z-log transformed CSF neurofilament light chain, total tau, and neurogranin among MCI/Dementia participants.
Supplementary Table 4. Associations between each characteristic and z-log transformed CSF neurofilament light chain, total tau, and neurogranin after adjustment for age and sex among MCI/Dementia participants.
Supplementary Table 5. Cross-sectional interactions between each characteristic and CSF amyloid for CSF neurofilament Light level.
Supplementary Table 6. Cross-sectional interactions between each characteristic and CSF amyloid for CSF total tau level.
Supplementary Table 7. Cross-sectional interactions between each characteristic and CSF amyloid for CSF neurogranin level.
RESEARCH IN CONTEXT.
Systematic review: We reviewed the literature using traditional (e.g., PubMed) resources. Studies examining CSF T-tau, NfL, or Ng as markers of neurodegeneration have primarily focused on diagnostics, cognition, or their relationship to neuroimaging. Identifying the variables that affect the levels of these markers will be important for their clinical interpretation
Interpretation: Multiple variables were differentially associated with CSF NfL and T-tau levels, but not Ng. Most associations were attenuated after adjustment for age and sex. T-tau had the greatest sensitivity for predicting cognition in the presence of amyloidosis. Variables associations with NfL did not differ amyloid status.
Future directions: Future studies should compare multiple neuroimaging measures of neurodegeneration including cerebrovascular pathologies, diffusion tensor imaging, cortical atrophy, and cortical thickness in order to better understand how each potential CSF (N) marker reflects neurodegeneration. Longitudinal studies with serial assessments of the CSF (N) markers are also needed.
Highlights.
Multiple variables were associated with levels of CSF NfL and T-tau, but not Ng.
Most relationships were attenuated after adjustment for age and sex.
There was not an interaction between the variables and amyloid (A+) for NfL levels.
Among A+, CSF T-tau had greater sensitivity than Ng for predicting cognition.
Acknowledgments
Funding: This study was supported by funding from the National institutes of Health/National Institute on Aging grants U01 AG006786, R01 AG011378, R01 AG041851, R01 AG049704, and R01 AG034676. Additional funding came from the GHR Foundation, the Swedish Research Council (2015–02830), the European Research Council (681712), the Knut and Alice Wallenberg Foundation, Frimurarestiftelsen, the Olav Thon Foundation, Swedish State Support for Clinical Research (ALF Västra Götalandsregionen), the Swedish state under the agreement between the Swedish Government and the county councils, the ALF-agreement (ALFGBG-813921, ALFGBG-65930, ALF-GBG-716681,ALF GBG-771071), the Torsten Söderberg Foundation, The Swedish Alzheimer Foundation, Hjärnfonden Sweden, Stiftelsen Demensfonden, Stiftelsen Hjalmar Svenssons Forskningsfond, Stiftelsen Wilhelm och Martina Lundgrens Vetenskapsfond.
Conflict of Interest Disclosures: Dr. Mielke served as a consultant to Eli Lilly and receives research support from the National Institutes of Health (R01 AG49704, P50 AG44170, U01 AG06786, RF1 AG55151), Department of Defense (W81XWH-15–1), and unrestricted research grants from Biogen. Mr. Syrjanen has nothing to disclose. Dr. Blennow has served as a consultant or at advisory boards for Alzheon, BioArctic, Biogen, Eli Lilly, Fujirebio Europe, IBL International, Merck, Novartis, Pfizer, and Roche Diagnostics, and is a co-founder of Brain Biomarker Solutions in Gothenburg AB, a GU Venture-based platform company at the University of Gothenburg. Dr. Zetterberg has served at advisory boards for Eli Lilly, Roche Diagnostics and Wave, has received travel grants from Teva and is a co-founder of Brain Biomarker Solutions in Gothenburg AB, a GU Ventures-based platform company at the University of Gothenburg. Dr. Skoog has been an advisor and speaker for Takeda. Dr. Prashanthi Vemuri has nothing to disclose. Dr. Machulda has nothing to disclose. Dr. Graff-Radford has nothing to disclose. Dr. Knopman serves on a Data Safety Monitoring Board for Lundbeck Pharmaceuticals and for the DIAN study; is an investigator in clinical trials sponsored by Biogen, Lilly Pharmaceuticals, and the Alzheimer’s Disease Cooperative Study; and receives research support from the National Institutes of Health. Dr. Jack has provided consulting services for Eli Lilly. He receives research funding from the National Institutes of Health, and the Alexander Family Alzheimer’s Disease Research Professorship of the Mayo Clinic. Dr. Petersen is a consultant for Roche, Inc., Merck, Inc., Biogen, Inc., Genentech, Inc., and GE Healthcare. He receives research support from the National Institutes of Health. Dr. Kern has nothing to disclose.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Jack CR Jr., Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, et al. NIA-AA Research Framework: toward a biological definition of Alzheimer’s disease. Alzheimers Dement 2018;14:535–62. 10.1016/j.jalz.2018.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Mattsson N, Andreasson U, Zetterberg H, Blennow K. Association of plasma neurofilament light with neurodegeneration in patients with Alzheimer disease. JAMA Neurol 2017;74:557–66. 10.1001/jamaneurol.2016.6117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Kern S, Syrjanen JA, Blennow K, Zetterberg H, Skoog I, Waern M, et al. Association of cerebrospinal fluid neurofilament light protein with risk of mild cognitive impairment among individuals without cognitive impairment. JAMA Neurol 2019;76:187–93. 10.1001/jamaneurol.2018.3459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Schindler SE, Gray JD, Gordon BA, Xiong C, Batrla-Utermann R, Quan M, et al. Cerebrospinal fluid biomarkers measured by Elecsys assays compared to amyloid imaging. Alzheimers Dement 2018;14:1460–9. 10.1016/j.jalz.2018.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Khalil M, Teunissen CE, Otto M, Piehl F, Sormani MP, Gattringer T, et al. Neurofilaments as biomarkers in neurological disorders. Nat Rev Neurol 2018;14:577–89. 10.1038/s41582-018-0058-z [DOI] [PubMed] [Google Scholar]
- [6].Blennow K, Zetterberg H. The past and the future of Alzheimer’s disease fluid biomarkers. J Alzheimers Dis 2018;62:1125–40. 10.3233/JAD-170773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Bos I, Vos S, Verhey F, Scheltens P, Teunissen C, Engelborghs S, et al. Cerebrospinal fluid biomarkers of neurodegeneration, synaptic integrity, and astroglial activation across the clinical Alzheimer’s disease spectrum. Alzheimers Dement 2019;15:644–54. 10.1016/j.jalz.2019.01.004 [DOI] [PubMed] [Google Scholar]
- [8].Portelius E, Olsson B, Hoglund K, Cullen NC, Kvartsberg H, Andreasson U, et al. Cerebrospinal fluid neurogranin concentration in neurodegeneration: relation to clinical phenotypes and neuropathology. Acta Neuropathol 2018;136:363–76. 10.1007/s00401-018-1851-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Wellington H, Paterson RW, Portelius E, Tornqvist U, Magdalinou N, Fox NC, et al. Increased CSF neurogranin concentration is specific to Alzheimer disease. Neurology 2016;86:829–35. 10.1212/WNL.0000000000002423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Skillback T, Mattsson N, Blennow K, Zetterberg H. Cerebrospinal fluid neurofilament light concentration in motor neuron disease and frontotemporal dementia predicts survival. Amyotroph Lateral Scler Frontotemporal Degener 2017;18:397–403. 10.1080/21678421.2017.1281962 [DOI] [PubMed] [Google Scholar]
- [11].Skillback T, Farahmand B, Bartlett JW, Rosen C, Mattsson N, Nagga K, et al. CSF neurofilament light differs in neurodegenerative diseases and predicts severity and survival. Neurology 2014;83:1945–53. 10.1212/WNL.0000000000001015 [DOI] [PubMed] [Google Scholar]
- [12].Olsson B, Portelius E, Cullen NC, Sandelius A, Zetterberg H, Andreasson U, et al. Association of cerebrospinal fluid neurofilament light protein levels with cognition in patients with dementia, motor neuron disease, and movement disorders. JAMA Neurol 2019;76:318–25. 10.1001/jamaneurol.2018.3746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Bridel C, van Wieringen WN, Zetterberg H, Tijms BM, Teunissen CE, and the N. F. L. Group. Diagnostic value of cerebrospinal fluid neurofilament light protein in neurology: a systematic review and meta-analysis. JAMA Neurol 2019. 10.1001/jamaneurol.2019.1534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Roberts RO, Geda YE, Knopman DS, Cha RH, Pankratz VS, Boeve BF, et al. The Mayo Clinic Study of Aging: design and sampling, participation, baseline measures and sample characteristics. Neuroepidemiology 2008;30:58–69. 000115751 [pii] 10.1159/000115751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].St. Sauver JL, Grossardt BR, Yawn BP, Melton LJ 3rd, Pankratz JJ, Brue SM, et al. Data resource profile: the Rochester Epidemiology Project (REP) medical records-linkage system. Int J Epidemiol 2012;41:1614–24. 10.1093/ije/dys195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Ivnik RJ, Malec JF, Smith GE, Tangalos EG, Petersen RC, Kokmen E, et al. Mayo’s Older Americans Normative Studies: updated AVLT norms for ages 56 to 97. Clin Neuropsychol 1992;6:83–104. [Google Scholar]
- [17].Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med 2004;256:183–94. [DOI] [PubMed] [Google Scholar]
- [18].American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-IV). 4th ed Washington, D.C.: American Psychiatric Association; 1994. [Google Scholar]
- [19].Van Harten AC, Franke A, Wiste JH, Weigand SD, Mielke MM, et al. Detection of Alzheimer’s disease in CSF using automated assays for classical CSF biomarkers. Alzheimer’s Association International Conference, July 2019. [Google Scholar]
- [20].van Harten AC, Mielke MM, Swenson-Dravis DM, Hagen CE, Edwards KK, Roberts RO, et al. Subjective cognitive decline and risk of MCI: the Mayo Clinic Study of Aging. Neurology 2018;91:e300–e12. 10.1212/WNL.0000000000005863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Gaetani L, Hoglund K, Parnetti L, Pujol-Calderon F, Becker B, Eusebi P, et al. A new enzyme-linked immunosorbent assay for neurofilament light in cerebrospinal fluid: analytical validation and clinical evaluation. Alzheimers Res Ther 2018;10:8. 10.1186/s13195-018-0339-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kvartsberg H, Lashley T, Murray CE, Brinkmalm G, Cullen NC, Hoglund K, et al. The intact postsynaptic protein neurogranin is reduced in brain tissue from patients with familial and sporadic Alzheimer’s disease. Acta Neuropathol 2019;137:89–102. 10.1007/s00401-018-1910-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Savica R, Wennberg AM, Hagen C, Edwards K, Roberts RO, Hollman JH, et al. Comparison of gait parameters for predicting cognitive decline: the Mayo Clinic Study of Aging. J Alzheimers Dis 2017;55:559–67. 10.3233/JAD-160697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373–83. [DOI] [PubMed] [Google Scholar]
- [25].Beck AT, Steer RA, Brown GK. BDI-II, Beck Depression Inventory: Manual. 2nd ed San Antonio, TX; Boston, MA: Psychological Corp.; Harcourt Brace; 1996. [Google Scholar]
- [26].Beck AT, Steer RA. Beck Anxiety Inventory Manual. San Antonio: TX; 1993. [Google Scholar]
- [27].Nylen K, Csajbok LZ, Ost M, Rashid A, Karlsson JE, Blennow K, et al. CSF-neurofilament correlates with outcome after aneurysmal subarachnoid hemorrhage. Neurosci Lett 2006;404:132–6. 10.1016/j.neulet.2006.05.029 [DOI] [PubMed] [Google Scholar]
- [28].Zanier ER, Refai D, Zipfel GJ, Zoerle T, Longhi L, Esparza TJ, et al. Neurofilament light chain levels in ventricular cerebrospinal fluid after acute aneurysmal subarachnoid haemorrhage. J Neurol Neurosurg Psychiatry 2011;82:157–9. 10.1136/jnnp.2009.177667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Osborn KE, Liu D, Samuels LR, Moore EE, Cambronero FE, Acosta LMY, et al. Cerebrospinal fluid beta-amyloid42 and neurofilament light relate to white matter hyperintensities. Neurobiol Aging 2018;68:18–25. 10.1016/j.neurobiolaging.2018.03.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Cheung YT, Khan RB, Liu W, Brinkman TM, Edelmann MN, Reddick WE, et al. Association of cerebrospinal fluid niomarkers of central nervous aystem injury with neurocognitive and brain imaging outcomes in children receiving chemotherapy for acute lymphoblastic leukemia. JAMA Oncol 2018;4:e180089 10.1001/jamaoncol.2018.0089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Elens I, Deprez S, Danckaerts M, Bijttebier P, Labarque V, Uyttebroeck A, et al. Neurocognitive sequelae in adult childhood leukemia survivors related to levels of phosphorylated tau. J Natl Cancer Inst 2017;109 10.1093/jnci/djw321 [DOI] [PubMed] [Google Scholar]
- [32].Mattsson N, Insel PS, Palmqvist S, Portelius E, Zetterberg H, Weiner M, et al. Cerebrospinal fluid tau, neurogranin, and neurofilament light in Alzheimer’s disease. EMBO Mol Med 2016;8:1184–96. 10.15252/emmm.201606540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Mielke MM, Syrjanen JA, Blennow K, Zetterberg H, Vemuri P, Skoog I, et al. Plasma and CSF neurofilament light: relation to longitudinal neuroimaging and cognitive measures. Neurology 2019. 10.1212/WNL.0000000000007767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Pereira JB, Westman E, Hansson O. Association between cerebrospinal fluid and plasma neurodegeneration biomarkers with brain atrophy in Alzheimer’s disease. Neurobiol Aging 2017;58:14–29. 10.1016/j.neurobiolaging.2017.06.002 [DOI] [PubMed] [Google Scholar]
- [35].Kester MI, Teunissen CE, Crimmins DL, Herries EM, Ladenson JH, Scheltens P, et al. Neurogranin as a cerebrospinal fluid biomarker for synaptic loss in symptomatic Alzheimer disease. JAMA Neurol 2015;72:1275–80. 10.1001/jamaneurol.2015.1867 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1. Univariable associations between each characteristic and z-log transformed CSF neurofilament light chain, total tau, and neurogranin among cognitively unimpaired participants.
Supplementary Table 2. Associations between each characteristic and z-log transformed CSF neurofilament light chain, total tau, and neurogranin after adjustment for age and sex among cognitively unimpaired participants
Supplementary Table 3. Univariable associations between each characteristic and z-log transformed CSF neurofilament light chain, total tau, and neurogranin among MCI/Dementia participants.
Supplementary Table 4. Associations between each characteristic and z-log transformed CSF neurofilament light chain, total tau, and neurogranin after adjustment for age and sex among MCI/Dementia participants.
Supplementary Table 5. Cross-sectional interactions between each characteristic and CSF amyloid for CSF neurofilament Light level.
Supplementary Table 6. Cross-sectional interactions between each characteristic and CSF amyloid for CSF total tau level.
Supplementary Table 7. Cross-sectional interactions between each characteristic and CSF amyloid for CSF neurogranin level.