Abstract
Purpose:
To report safety and tolerability of a one-time administration of ophthalmic 5% povidone-iodine (5% PVP-I) in a double-masked randomized trial for the treatment of adenoviral conjunctivitis (Ad-Cs).
Methods:
Of 212 participants screened, 56 eligible participants with red eye symptoms ≤4 days and a positive adenoviral rapid immunoassay were randomized to a one-time administration of ophthalmic 5% PVP-I or preservative free artificial tears (AT). Safety was assessed by corneal fluorescein staining (baseline, immediate post-administration and Day 1) and visual acuity (VA) (baseline and Day 1). Tolerability was assessed using participant-rated overall ocular discomfort (baseline, immediately post-administration and on Day 1.
Results:
In the 5% PVP-I group, corneal staining increased immediately post-administration but returned to baseline levels by Day 1. There was no change in VA between baseline and Day 1 in either 5% PVP-I or AT groups (p=0.87). In the 5% PVPI group, there was no change in participant-rated overall discomfort immediately post-administration (p=0.78) or on day 1 (p=0.10) compared to baseline. In the AT group, participant-rated overall discomfort was lower immediately post- administration but returned to baseline levels by Day 1. One adverse event was reported in the 5% PVP-I group on Day 1–2 that was classified as not related to treatment.
Conclusion:
These results suggest ophthalmic 5% PVP-I used as a one-time treatment is safe and well tolerated by patients with Ad-Cs.
Keywords: Adenoviral conjunctivitis; Betadine, corneal staining; povidone-iodine; safety
Adenoviral conjunctivitis (Ad-Cs) is a highly contagious disease that can quickly spread through clinics, homes, schools and work places with significant morbidity. Both the American Academy of Ophthalmology preferred practice guidelines and American Optometric Association clinical practice guidelines recommend supportive care with artificial tears (AT), topical antihistamines, and cold compresses1, 2. There is no FDA-approved treatment for Ad-Cs; however, the use of several off-label treatments have been reported, including a one-time administration of ophthalmic 5% povidone-iodine (PVP-I)3–6. In a 2013 survey of eye care practitioners, one-third of respondents reported using off-label ophthalmic 5% PVP-I in the treatment of Ad-Cs7. There has been no systematic evaluation of the safety and tolerability of a one-time administration of ophthalmic 5% PVP-I for the treatment of Ad-Cs to date.
The ophthalmic formulation of 5% PVP-I (Betadine® 5%, Alcon, Fort Worth, TX) is approved for “prepping of the periocular region (lids, brow and cheek) and irrigation of the ocular surface (cornea, conjunctiva and palpebral fornices)”8 and is routinely used as a surgical ophthalmic scrub to prevent endophthalmitis9, 10. PVP-I is a highly effective antiseptic against bacteria including chlamydia (both intra- and extra-cellular), fungi, protozoa as well as viruses (including adeno, herpes simplex, and enteroviruses) without significant corneal or other ocular toxicity11. The efficacy and safety of PVP-I at various doses12–14 and in combination medications with steroids are being evaluated in randomized trials for the treatment of Ad-Cs15–18. The safety and tolerability of 5% ophthalmic PVP-I has not been systematically evaluated in eyes with AdCs. Therefore, it is important to evaluate the safety and tolerability of PVP-I in a double-masked randomized clinical trial with placebo control.
1.2. METHODS
1.2.1. Study Design
The Reducing Adenoviral Patient Infected Days (RAPID) study is a double-masked, randomized, planning trial to estimate parameters for designing a definitive clinical trial of the safety and efficacy of 5% PVP-I in the treatment of Ad-Cs. Participants were randomized 1:1 to a single in-office administration of either ophthalmic 5% PVP-I or preservative-free AT at the first visit. Institutional review board approval was obtained by each study site and the Coordinating Center at Washington University in St. Louis, MO. Informed consent was obtained from all participants after explanation of the nature and possible consequences of study participation. The study procedures were in compliance with the ethical standards of the Declaration of Helsinki and the study is registered at www.clinicaltrials.gov as #.
1.2.2. Study Participants
Patients were recruited at nine clinical sites in the United States between March 2015 and July 2018. Eligibility included ≥ 18 years of age with red eye symptoms ≤ 4 days were invited to be screened and, if fully eligible, to be randomized. Exclusion criteria included a history of thyroid disease, allergy to iodine or study medications, ocular surgery within the past 3 months, skin vesicles, corneal dendrites, conjunctival membrane or pseudomembrane, sub-epithelial corneal infiltrates, corneal ulceration, corneal abrasion, corneal foreign body, anterior chamber inflammation, or pregnancy/nursing. One eye was selected as the study eye. If both eyes were involved, the first affected eye was selected as the study eye. If both eyes became symptomatic at the same time, the study eye was randomly selected.
1.2.3. Study Protocol
Patients were informed of potential side effects of 5% PVP-I prior to consent including minor irritation, mild to moderate stinging, and temporary discoloration of the conjunctiva and eyelids. Patients screened for eligibility completed baseline tests and measures. Eligible patients were randomized and completed baseline/randomization visit and five follow-up visits with a masked clinician on days 1 to 2, 4(days 3–5), 7(days 6–10), 14 (days 11–17) and 21 (days 18–21). The baseline and follow-up visits included 10-item symptom survey including a patient-rated overall ocular discomfort question administered by a clinician or technician in compliance with the infection control protocol. The clinician or technician read the question and presented the response options in large print on a full size 8 1/2” × 11” sheet of paper. Overall ocular discomfort was rated from “0 - not at all bothersome” to “10- very bothersome”. Baseline and follow-up clinical examination included Snellen visual acuity (VA) (corrected, uncorrected or pinhole if less than 20/20), and corneal fluorescein staining (CFS). CFS was graded from 0 (none), 1 (micropunctate), 2 (macropunctate), 3 (coalescent macropunctate), or 4 (patch) in five corneal sectors using the Brien Holden Vision Institute visual grading system19.
The study eye was anesthetized with one drop of 0.5% proparacaine (Valeant Pharmaceuticals North America LLC, Bridgewater, NJ). After five minutes, the AdenoPlus rapid point-of-care, immunoassay for Ad-Cs (now named QuickVue Adenoviral conjunctivitis test, Quidel Corp., San Diego, CA) was performed according to the manufacturer’s instructions20. A clinically eligible eye was randomized if the eye tested positive for AdCs by AdenoPlus. After 5 minutes, conjunctival swab samples for qPCR for adenovirus were taken and stored in a −80 degree Celsius freezer within four hours of collection. Samples were shipped on dry ice to the Coordinating Center in St. Louis, MO for further molecular analysis.
1.2.4. Randomization
Eligible participants were randomized in a 1:1 ratio to receive either ophthalmic 5% PVP-I (Alcon, Fort Worth, TX) or AT. Randomization assignments were stored in sealed, numbered envelopes in sealed, coded boxes containing either 5% PVP-I or AT that had been sent to clinics by the Coordinating Center. Randomization was stratified by clinic using a permuted block design with small block sizes. The unmasked clinician instilled 1 drop of proparacaine followed by 4–5 drops of either 5% PVP-I or AT. Participants were instructed to close their eyes for 2 minutes during which time they moved their eyes in all directions while the clinician used a gloved finger to apply gentle pressure to the closed lids. Then, a 2×2 gauze pad moistened with the assigned solution was used to dab along the eyelid margins of the closed eye. After the 2 minutes, a non-preserved buffered sterile saline solution was used to generously lavage the eye and a 2×2 gauze pad moistened with sterile saline was used to wipe the eyelid margins to remove all traces of the study medication.
Immediately after administration of 5% PVP-I or AT, participants were asked to rate overall ocular discomfort from “0 - not at all bothersome” to “10- very bothersome”.
Participants were provided written instructions on infection control and dispensed single use, non-preserved AT to be used four times a day in the study eye. Follow-up visits were conducted by clinicians masked to randomization. Conjunctival swab samples were collected at each follow-up visit for molecular analysis and AdenoPlus testing was performed until two consecutive negative results were obtained.
1.2.5. Safety and Tolerability:
Safety of 5% PVP-I was assessed using CFS, VA, and adverse events. CFS was recorded at baseline, immediately post-administration of 5% PVP-I or AT and at Day 1. VA was measured at baseline and Day 1. Baseline measurements were used to assess changes immediately post- administration and at Day 1.
Tolerability was assessed with participant-rated overall ocular discomfort (Table 1). We compare participant-rated overall discomfort immediately post-treatment and on the Day 1 visit versus baseline.
Table 1.
Timeline of Study Visit Assessments
| Day 0 Baseline | Day 0 Immediate Post-Administration | Follow up Visits | |
|---|---|---|---|
| Participant-rated overall ocular discomfort | X | X | X |
| Corneal Fluorescein Staining | X | X | X |
| Snellen visual acuity | X | X |
1.2.6. Statistical Methods:
CFS scores in the five sectors were summed for a total CFS score19. All data are reported as sample mean ± standard deviation (SD). Visual acuity was measured using a standardized Snellen chart and converted to logarithm of the minimum angle of resolution (logMAR) for analysis. Repeated measures analysis of variance models were used to compare randomization groups over measurement periods. Separate repeated measures ANOVA models were used for each outcome: CFS, VA and participant-rated overall ocular discomfort (SAS software V9.4). Overall, 82% of study participants completed the Day 1 or Day 2 visit. Subgroup analysis was performed in the sample of participants who returned on Day 1 (n=29) to allow detection of any residual effect of 5% PVP-I, although return on Day 2 (n=17) was permitted based on participant availability.
1.3. Results:
Of 212 patients screened at nine US clinical centers, 56 eligible participants were randomized to a one-time administration of 5% PVP-I (n=30) or AT (n=26). The mean participant age was 34.1 years ± 14.4(Table 2).
Table 2.
Demographics of randomized participants
| 5% PVP-I | AT | ALL | |
|---|---|---|---|
| Gender | |||
| Male | 16 | 13 | 29 |
| Female | 14 | 13 | 27 |
| Age at screening | 34.6 ± 14.4 | 33.7 ± 13.6 | 34.1 ± 14.4 |
1.3.1. Corneal fluorescein staining
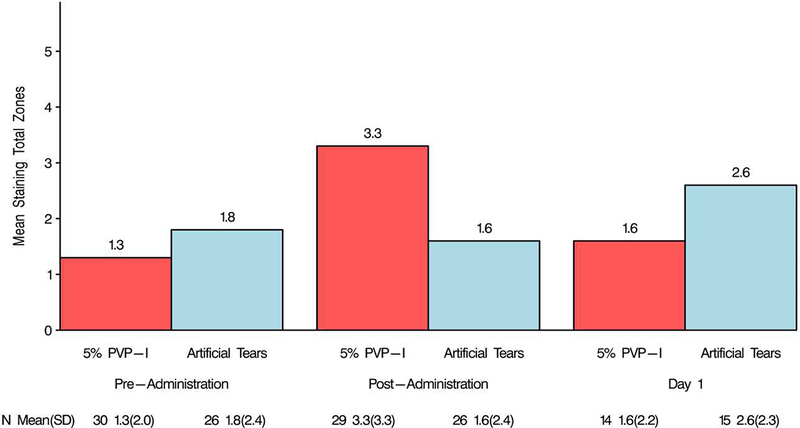
In the 5% PVP-I group, CFS baseline total was 1.3 ± 2.0 and the staining significantly increased (p=0.004) to 3.3 ± 3.3 immediate post-administration. In the AT group, baseline total CFS was 1.8 ± 2.4 and 1.6 ± 2.4 immediate post-administration (p=0.79). The difference between baseline and immediate post-administration total CFS score in the AT and 5% PVP-I groups was statistically significant (p=0.03; Figure 1).
Figure 1.
Total corneal fluorescein staining.
Mean corneal staining was significantly increased immediately post-administration in the 5% PVP-I group. There was no difference in mean staining in the AT group.
In the 5% PVP-I group, the mean Day 1 total CFS score was 1.6 ± 2.2 and did not differ from the baseline (p=0.63). In the AT group, the mean Day 1 total CFS score was 2.6 ±2.3 and also did not differ from baseline (p=0.25). There was no difference between randomization groups (p=0.16) at Day 1, no difference between baseline and Day 1 levels (p=0.25) in either the 5% PVP-I group or AT, and no difference in the direction of change between Day 1 and baseline between randomization group (p=0.63; Figure 1).
1.3.2. Visual Acuity
In the 5% PVP-I group, baseline logMAR VA was 0.08 ± 0.12 and 0.07 ± 0.15 at the Day 1 visit (p=0.88). In the AT group, baseline logMAR visual acuity was 0.11 ± 0.28 and 0.08 ± 0.11 at the Day 1 visit (p=0.71). There were no differences between the randomization groups (p=0.68), between time points (p=0.71) and no differences between time points between 5% PVP-I and AT groups at baseline compared to Day 1 (interaction p=0.87).
1.3.3. Adverse Events
Over the 21 days of follow up, no adverse events were reported in the AT group. One adverse event of “light sensitivity with mild anterior chamber reaction at the Day 1 visit” was reported in the 5% PVP-I group. The masked clinician classified it as “not related to treatment”.
1.3.4. Participant-rated overall ocular discomfort
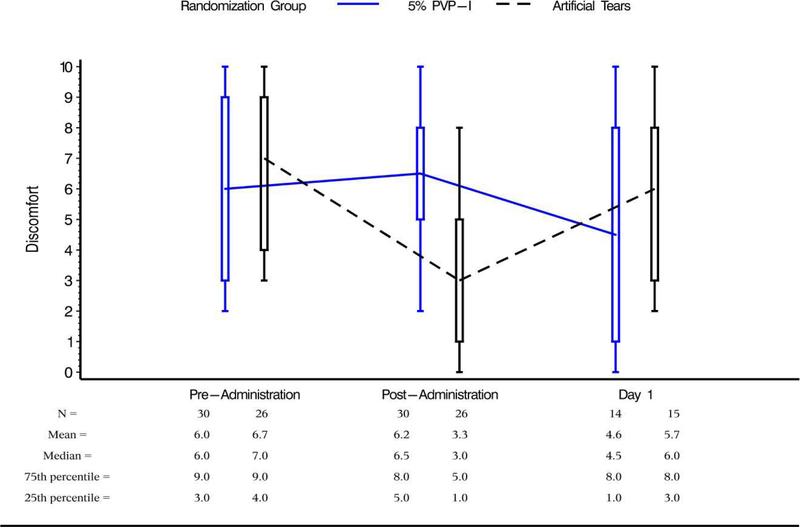
At the baseline visit prior to treatment, participants-rated overall ocular discomfort in the study eye (n=56) on a scale from “0- not at all bothersome” to “10-very bothersome”. There was no difference in mean baseline overall ocular discomfort between the 5% PVP-I group (6.0 ± 3.0 SD) and the AT group (6.7 ± 2.6; p=0.34; Figure 2).
Figure 2.
Participant-rated overall ocular discomfort
There was no difference in baseline and immediate post-administration overall discomfort in the 5% PVP-I group. In the AT group, overall discomfort immediately post-administration was lower than baseline rating. On Day 1, there was no difference in participant-rated overall discomfort compared to baseline levels.
In the 5% PVP-I group, the immediate post-administration overall discomfort (6.2 ± 2.8) did not differ from baseline (6.0 ± 3.0, p=0.78). In the AT group, immediate post-administration overall discomfort (3.3 ± 2.8, mean ± SD) was significantly lower than baseline overall discomfort (6.7 ± 2.6, p<0.0001; Figure 2). The difference between baseline and immediate post-administration overall ocular discomfort reported in AT and 5% PVP-I groups was statistically significant (p=0.0013) (Figure 2).
In both the AT and 5% PVP-I groups, participant-rated overall ocular discomfort on Day 1 did not differ from baseline (p=0.10). There was no difference between randomization groups (p=0.21) at Day 1, and no difference in the direction of change from baseline between randomization groups (p=0.83) (Figure 2).
1.4. Discussion:
For decades, PVP-I has been used as an ophthalmic surgical antiseptic and has been universally accepted as safe, however there are limited in vivo studies assessing patient tolerability to exposure. Peden et al reported sensitivity to standard ophthalmic 5% PVP-I in 16% of patients in a retrospective study of 1854 patients receiving intravitreal injections21. Saedon et al reported increased dry eye symptoms and corneal epithelial staining in eyes that received multiple intravitreal injections following 5% PVP-I antisepsis; however, the eyes were compared to fellow eyes receiving neither intravitreal injections nor PVP-I22. In a small study (n=10) of normal participants, Ridder et al. compared dry eye symptoms, corneal staining and visual acuity following a two-minute exposure to a small aliquot of 5% PVP-I versus AT. One drop of proparacaine was used to wash out the PVP-I or AT. Signs and symptoms were minimal with AT, but exposure to 5% PVP-I resulted in a transient increase in corneal staining, a reduction in VA and an increase in dry eye symptoms. After 24-hours, there was no difference between the eyes receiving AT or 5% PVP-I in terms of dry eye symptoms and VA. However, corneal staining remained mildly increased in eyes exposed to 5% PVP-I23.
These studies evaluated the safety and tolerability of PVP-I at various concentrations and exposure duration in eyes with no significant anterior segment disease. The purpose of the study reported here was to evaluate the safety and tolerability of a onetime, 2-minute exposure of ophthalmic 5% PVP-I in eyes with presumed AdCs. We hypothesized that individuals with active AdCs may have poorer tolerability to PVP-I due to the diseased state of the eye. Safety and tolerability were evaluated by assessing CFS, VA, adverse events and subjective discomfort at baseline, immediately after administration and again on Day 1. The signs and symptoms of toxicity to any ophthalmic solution are usually transient resolving within 24 hours. Therefore, although the study protocol allowed for the first follow-up visit to be 24–48 hours following baseline, only a subset of the patients (those who returned on the first day) were reported in this analysis.
This study found an increase in CFS immediately after instillation of 5% PVP-I but the amount of staining returned to baseline levels by Day 1 (Figure 1). We hypothesize that rapid recovery by Day 1 of corneal staining may be due to thorough lavage with saline after a 2-minute exposure to PVP-I. In addition, we instructed participants to use non-preserved AT four times a day. In the AT group, there was a statistically significant reduction in overall participant-rated discomfort immediately after administration with AT. This may provide additional support in recommending ocular lubricant use in the management of Ad-Cs with or without other therapeutic interventions. Also of note, corneal staining by Day 1 was minimal and was comparable to levels previously reported in successful daily wear and extended wear contact lens patients.24 Our study participants also had stable VA and were minimally symptomatic, providing evidence that supports the treatment with 5% PVP-I was safe in individuals with presumed AdCs. In the AT group, there was a trend for a slight increase from 1.8 mean CFS at baseline to 2.6 at Day 1. This could be indicative of Ad-Cs disease progression; however, vision was not reduced.
A phase 2, randomized trial of 0.6% PVP-I and 0.1% dexamethasone combination, 0.6% PVP-I alone or vehicle alone by Pepose et al reported safety in patients with AdCs. Participants rated comfort on a scale of 0 to 10 (0=very comfortable and 10=very uncomfortable) at the time of drop instillation as well as one minute and two minutes after instillation. Comfort ratings were similar among the three randomization groups upon instillation, with mean scores between 2 and 3, however, comfort at subsequent follow-up visits were not reported. The authors concluded that no difference in comfort between vehicle and study medication is noteworthy because comfort is an important part of ocular tolerability that can influence patient treatment adherence. Based on VA and slit-lamp biomicroscopy findings, there were no safety concerns. There were 281 treatment-emergent adverse events reports by 61.7% of the participants. All events were rated as mild to moderate and none was thought to be due to treatment. This investigational combination drug is now in Phase 3 clinical trials16. Another study of patients with AdCs using combination 0.1% dexamethasone/0.4% PVP-I versus AT four times a day for 7 days reported higher rates of stinging (22%) in patients using the combo medication compared to AT (2%)17.
The only contraindication listed in the package insert for ophthalmic 5% PVP-I is known iodine sensitivity. Caution is also advised in using 5% PVP-I in patients with thyroid disease8 due to the possibility of iodine absorption,25 and the safety is uncertain in individuals with thyroid dysfunction. For these reasons, we excluded all participants with a history of iodine allergy or history of thyroid disease from this study.
There are several study limitations. Because RAPID was designed as a pilot study, the sample size is small, and thus statistical power is low. In addition, our study population was limited to adult participants in the United States. As different populations across the globe have been shown to have vastly different proportions of subtypes of adenovirus infection,26 our results may not be applicable across all ages in all countries. In this study, non-preserved artificial tears were used as the control group as the study was originally designed to compare PVP-I treatment group to the current standard of care for adenoviral conjunctivitis. It is possible that there may have been differences in our results if the same vehicle of the 5% PVP-I was used instead. Finally, our protocol did not standardize an exact number of minutes after participants received treatment at which to record post-administration corneal staining and discomfort assessment, instead giving the general instruction to ask the participant to rate overall discomfort in the treated eye. In the 5% PVP-I group, the immediate post-administration overall discomfort (6.2 ± 2.8) did not differ from baseline (6.0 ± 3.0, p=0.78). In the AT group, immediate post-administration overall discomfort (3.3 ± 2.8, mean ± SD) was significantly lower than baseline overall discomfort (6.7 ± 2.6, p<0.0001; Figure 2). Despite the use of topical anesthetic, overall discomfort immediately post-administration was rated higher in the PVP-I group than the AT supporting the use of topical anesthetic with treatment. In future tolerability studies, timing should be recorded and standardized and measurements repeated to better assess discomfort/comfort after use of topical anesthetic.
In conclusion, our study provides support that ophthalmic 5% PVP-I was well tolerated in patients with presumed Ad-Cs. Although individuals may experience a temporary increase in corneal staining, the use of AT can help mitigate discomfort that may be associated with the temporary increase in epitheliopathy. By providing information regarding the transient and temporary effects of 5% PVP-I to patients who present with “red eye”, clinicians should not be deterred from a safety or tolerability perspective.
Acknowledgements:
The authors would like to thank DiaSorin Molecular LLC (Cypress, CA) for loaning the study their integrated cycler for qPCR analysis.
Funding:
This work was supported by the National Institutes of Health (EY023633–01A1, P30EY002687, EY01792) and an unrestricted grant from Research to Prevent Blindness.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures:
None: Mae Gordon, Andrew T. Hartwick, Julia Huecker, Spencer Johnson, Mathew Margolis, Mary Migneco, Christina Morettin, Christian K. Olson, Ellen Shorter, Tammy Than, Meredith Whiteside.
Jennifer Harthan: Consulting and research Alcon, Allergan, Contamac, Metro, SynergEyes, Takeda, Tangible Science
References:
- 1.Optometric Clinical Pratice Guideline: Care of the Patient with Conjunctivitis. American Optometric Association; 2002. [Google Scholar]
- 2.Preferred Pratice Pattern Guidelines: Conjunctivitis - Limited Revision. American Academy of Ophthalmology Corena/External Disease Panel; 2018. [Google Scholar]
- 3.Abelson MA S. A guide to understanding adenovirus, the disease it causes and the best ways to treat these conditions.: Review of Ophthalmology, 2010. [Google Scholar]
- 4.Shovlin JP. What’s the Buzz About Betadine? Review of Optometry 2011. [Google Scholar]
- 5.Pernicky J [Personal experience with the use of Betadine gtt. in the treatment of epidemic viral keratoconjunctivitis]. Cesk Oftalmol 1994;50(5):323–4. [PubMed] [Google Scholar]
- 6.Hutter H [Epidemic keratoconjunctivitis: treatm ent results during an epidemic]. Klin Monbl Augenheilkd 1990;197(3):214–7. [DOI] [PubMed] [Google Scholar]
- 7.Than TH AE; Shorter E; Lonsberry B; Gordon MO; Freddo T How to m anage adenoviral conjunctivitis. Optometry Times, 2014. [Google Scholar]
- 8.Chambers WA. Alcon New Drug Application for Betadine. In: FDA, ed. 2002. [Google Scholar]
- 9.Isenberg SJ, Apt L, Campeas D. Ocular applications of povidone-iodine. Dermatology 2002;204 Suppl 1:92–5. [DOI] [PubMed] [Google Scholar]
- 10.Speaker MG, Menikoff JA. Prophylaxis of endophthalmitis with topical povidone-iodine. Ophthalmology 1991;98(12):1769–75. [DOI] [PubMed] [Google Scholar]
- 11.Reimer K, Wichelhaus TA, Schafer V, et al. Antimicrobial effectiveness of povidone-iodine and consequences for new application areas. Dermatology 2002;204 Suppl 1:114–20. [DOI] [PubMed] [Google Scholar]
- 12.Yazar H, Yarbag A, Balci M, et al. The effects of povidone iodine (pH 4.2) on patients with adenoviral conjunctivitis. J Pak Med Assoc 2016;66(8):968–70. [PubMed] [Google Scholar]
- 13.Ozen Tunay Z, Ozdemir O, Petricli IS. Povidone iodine in the treatm ent of adenoviral conjunctivitis in infants. Cutan Ocul Toxicol 2015;34(1):12–5. [DOI] [PubMed] [Google Scholar]
- 14.Trinavarat A, Atchaneeyasakul LO. Treatment of epidemic keratoconjunctivitis with 2% povidone-iodine: a pilot study. J Ocul Pharmacol Ther 2012;28(1):53–8. [DOI] [PubMed] [Google Scholar]
- 15.Kovalyuk N, Kaiserman I, Mimouni M, et al. Treatment of adenoviral keratoconjunctivitis with a combination of povidone-iodine 1.0% and dexam ethasone 0.1% drops: a clinical prospective controlled randomized study. Acta Ophthalmol 2017. [DOI] [PubMed] [Google Scholar]
- 16.Pepose JS, Ahuja A, Liu W, et al. Randomized, Controlled, Phase 2 Trial of Povidone- Iodine/Dexam ethasone Ophthalmic Suspension for Treatment of Adenoviral Conjunctivitis. Am J Ophthalmol 2018;194:7–15. [DOI] [PubMed] [Google Scholar]
- 17.Pinto RD, Lira RP, Abe RY, et al. Dexamethasone/Povidone Eye Drops versus Artificial Tears for Treatment of Presumed Viral Conjunctivitis: A Randomized Clinical Trial. Curr Eye Res 2015;40(9):870–7. [DOI] [PubMed] [Google Scholar]
- 18.Pelletier JS, Stewart K, Trattler W, et al. A combination povidone-iodine 0.4%/dexam ethasone 0.1% ophthalmic suspension in the treatment of adenoviral conjunctivitis. Adv Ther 2009;26(8):776–83. [DOI] [PubMed] [Google Scholar]
- 19.Terry RL, Schnider CM, Holden BA, et al. CCLRU standards for success of daily and extended wear contact lenses. Optom Vis Sci 1993;70(3):234–43. [DOI] [PubMed] [Google Scholar]
- 20.QuickVue Adenoviral Conjunctivitis Test: Manufacturer’s Instructions. Quidel Corporation; San Diego, CA: http://www.quidel.com/sites/default/files/product/documents/EF1361400EN00.pdf. [Google Scholar]
- 21.Peden MC, Hammer ME, Suner IJ. Dilute Povidone-Iodine Prophylaxis Maintains Safety While Improving Patient Comfort after Intravitreal Injections. Retina 2018. [DOI] [PubMed] [Google Scholar]
- 22.Saedon H, Nosek J, Phillips J, et al. Ocular surface effects of repeated application of povidone iodine in patients receiving frequent intravitreal injections. Cutan Ocul Toxicol 2017;36(4):343–6. [DOI] [PubMed] [Google Scholar]
- 23.Ridder WH 3rd. Effect of Povidone Iodine 5% on the Cornea, Vision, and Subjective Comfort. 2017;94(7):732–41. [DOI] [PubMed] [Google Scholar]
- 24.Jalbert I, Sw eeney DF, Holden BA. The characteristics of corneal staining in successful daily and extended disposable contact lens wearers. Clin Exp Optom 1999;82(1):4–10. [DOI] [PubMed] [Google Scholar]
- 25.Randomized Cinal A., Controlled Phase 2 Trial of Povidone-Iodine/Dexamethasone Ophthalmic Suspension for Treatment of Adenoviral Conjunctivitis. Am J Ophthalmol 2019;197:184. [DOI] [PubMed] [Google Scholar]
- 26.Lee CS. The Evaluation of Worldwide Distribution of Adenoviral Genotypes in Acute/Epidemic Keratoconjunctivitis and Adenoviral-negative Keratoconjunctivitis with Next Generation Sequencing. Seattle, Washington: Poster presented at The Association for Research in Vision and Ophthalmology, 2016. [Google Scholar]