Abstract
Objective
Recent data suggest that increased levels of the HOTAIR long non-coding RNA (lncRNA) are involved in the development of various types of malignancy, including breast cancer. The aim of present study was to investigate HOTAIR lncRNA expression profile in breast cancer (BC) patients and cell lines.
Materials and Methods
In this experimental study, expression level of HOTAIR lncRNA was evaluated in BC and normal tissues of 15 patients as well as MDA-MB-231, MCF-7 and MCF-10A cell lines, using quantitative reverse- transcription polymerase chain reaction (qRT-PCR). HOTAIR lncRNA expression levels were estimated using 2-ΔΔCt method. Further, receiver operating characteristic (ROC) curve analysis was done to evaluate the selected lncRNA diagnostic potential. The Cox’s proportional hazards regression model was performed to evaluate the predictive value of this lncRNA level in BC patients.
Results
The results of present study demonstrated no significant difference in the expression of HOTAIR lncRNA in MCF7 and MDA-MB-231 cancer cell lines compared to MCF-10A as normal cell line (P>0.05). However, we observed a significantly increase in the expression of HOTAIR in BC patients compared to normal tissues (P<0.001). Significant associations were found between gene expression and tumour size and margin. We found 91.1% sensitivity and 95.7% specificity of circulating HOTAIR with an area under the ROC curve of 0.969. The Kaplan-Meier analysis indicated significant correlation between HOTAIR expression and overall survival.
Conclusion
This study demonstrated that expression of HOTAIR is increased in BC and might be associated with its progression. According to these findings, HOTAIR expression could be proposed as biomarkers for BC early diagnosis and prognosis.
Keywords: Breast Cancer, Cell Line, HOTAIR lncRNA, Quantitative Reverse-Transcription Polymerase Chain Reaction
Introduction
Long non-coding RNAs (lncRNAs) are a diverse and large class of non-coding RNA molecules with more than 200 nucleotides length which are mostly transcribed by RNA polymerase II (RNA pol II) (1). lncRNAs play essential role in regulation of different cellular processes. Recently, lncRNAs are reported as key regulators of gene expression and they can act as oncogenes or tumour suppressor genes. According to their oncogenic potential they may play a critical role in oncogenesis, migration, cell differentiation, angiogenesis, apoptosis and proliferation (2). Furthermore, lncRNAs associate with different cancers and malignant behaviour of cancer cells (3). One of the first described lncRNAs is the Hox (homeobox) transcript antisense intergenic lncRNA (HOTAIR lncRNA) and they play important role in development of breast cancer (BC) (4). Current studies suggest that HOTAIR lncRNA reprograms chromatin state which can promote cancer metastasis (5). HOTAIR coordinates in chromatin modification and through which it affects expression of multiple genes involved in various cellular functions (6). Recent studies indicated an association between circadian rhythm (CR) disruption and increased risk of BC development (7). Interestingly, CR disruption has been associated with decreased telomere length, where short telomere length, itself, is correlated with BC development (8).
HOTAIR expression levels are significantly high in breast tumours, and its measurement is a determinative indicator of primary breast tumours, possibility of metastasis and patient survival (9). The most commonly used cell line in BC research is MDA-MB-231 which provides essential tools for complex biological expression analysis (10). This cell line is originated from a pleural effusion with a metastatic mammary adenocarcinoma and it is a highly aggressive, used as a model of triple negative BC (TNBC) (11, 12). In the present study, we measured HOTAIR lncRNA expression level in BC and normal epithelial tissues, in addition to MCF7 and MDAMB- 231 BC cell lines.
Materials and Methods
Breast cancer and normal tissue sample collections
In this experimental study, BC and normal breast tissues from 15 patients (morphologically confirmed by a pathologist) were collected consecutively between July and November 2015 at Shiraz General Hospital (Shiraz, Iran). These 15 patients were included in the present study, according to the inclusion and exclusion criteria. The inclusion criteria were as follows: i. All patients hospitalized during 2015, those with diagnosis or procedure codes related to breast cancer, and ii. Adult patients (19 years of age or older) with cancer of the breast. The exclusion criteria include: i. History of any other malignancy, ii. History of previous relevant treatment, including chemotherapy, radiotherapy or endocrinotherapy, and iii. People with 18 years of age or younger. In this research, ethical considerations were approved based on the International Campus of Shahrekord University of Medical Sciences, Shahrekord, Iran.
Culture of the cell lines
MDA-MB-231 (ATCC® HTB-26™), MCF-7 (ATCC® HTB22™) and MCF-10A (ATCC® CRL-10317™) cell lines were cultured and maintained in RPMI-1640 medium (Sigma-Aldrich, USA) supplemented with L-Glutamine (Sigma-Aldrich, USA), 10% fetal bovine serum (FBS, Gibco, USA), 100 U/ml penicillin (Sigma-Aldrich, USA) and 100 μg/ml streptomycin (Sigma-Aldrich, USA) in the cell culture incubator at 37˚C, 5% CO2 and 95% humidity.
RNA extraction and cDNA synthesis
Total RNA was extracted from the tissue samples and cell lines, using the RNXTM-Plus solution (SinaClon, Iran) according to the manufacturer’s instructions except the additional step of extended treatment with DNaseI for one hour. Purity, concentration and quality of the extracted RNAs were analysed by Thermo Scientific NanoDrop™ 1000 Spectrophotometer (USA) and electrophoresis on 2% agarose gel. For complementary DNA (cDNA) synthesis, 1 μg RNA was added to PrimeScriptTM-RT reagent kit (TaKaRa, Japan) containing random hexamer priming mix. Concentration of the synthesized cDNA was measured by spectrophotometry.
Quantitative reverse transcription polymerase chain reaction
HOTAIR expression level in BC and normal breast epithelial tissues as well as MDA-MB-231, MCF7 and MCF-10A cell lines was evaluated by quantitative reverse transcription PCR (qRT-PCR) method, using a rotor gene 6000 Corbett detection system (Qiagen, Germany) and SYBR®Premix Ex TaqTM II kit (TaKaRa, Japan), according to the manufacturer’s instructions. Thermal cycling amplification was set up according to next protocol: initial activation at 95˚C for 5 minutes followed by 40 cycles at 95˚C for 15 seconds and 65˚C for 1 minute. As control sample nuclease free water was used without adding any template. Melting curve analysis was performed to verify specificity of PCR products. The size and specificity of PCR products were verified by electrophoresis on 2% agarose gel. For qRT-PCR analysis, all samples were normalized to Pumilio RNA Binding Family Member 1 gene (PUM1). The qRT-PCR assays were performed in triplicate and the data were presented as the mean ± standard error of mean (SEM). The mean value in each triplicate was used to calculate the relative lncRNA level (ΔCt=Ct mean lncRNAs-Ct mean PUM1). Expression fold changes were calculated using 2-ΔΔCt methods.
Statistical analysis
The relationship between expression of HOTAIR and clinical pathological parameters was determined using the chi-square test and Fisher’s exact test. Receiver operating characteristic (ROC) curves were used to assess diagnostic value of the marker. Area under the curve (AUC) was computed for ROC curve. Overall survivals (OS) were presented by the Kaplan-Meier curves, and the log-rank test was used to determine significance between gene expression levels and patient outcome. Data are shown as the means ± SEM and case (%) or number (%). A P value of 5% (*; P<0.05) was considered significant. Statistical analyses were performed by the Graph Pad Prism version 7.00 (Graph Pad Software, USA).
Results
Table 1 summarizes the available patients’ demographic and clinical data. All tissue samples at the time of resection were transferred into RNA-later solution (Sigma-Aldrich, USA) and stored at -20˚C for the further RNA extraction. Applicable international, national and institutional guidelines for the care of human were followed. Figure 1A shows relative expression of HOTAIR in MDAMB- 231 and MCF-7 cancer cell lines compared to MCF- 10A control cell line. There is no significant difference of HOTAIR expression in both of MCF-7 and MDAMB- 231 cancer cell lines compared to normal cell line MCF-10A (P>0.05). The expression level of HOTAIR was significantly increased in BC tissue of patients compared to normal tissues (P<0.001, Fig .1B). Correlation between HOTAIR expression and the clinical pathological variables of BC cases are shown in Table 2. Significant associations were found between gene expression and tumour size and margin.
Table 1.
Characteristics of the cancer specimens used in this study
| Variables | Frequency | Valid percent | Cumulative percent |
|---|---|---|---|
| Age (Y) | |||
| ≤47 | 15 | 65.2 | 65.2 |
| ˃47 | 8 | 34.8 | 100 |
| Histologic grade | |||
| Well differentiated | 1 | 6.7 | 6.7 |
| Moderate differentiated | 10 | 66.7 | 73.3 |
| Poor differentiated | 4 | 26.7 | 100 |
| Tumour side | |||
| Left | 7 | 46.7 | 46.7 |
| Right | 8 | 53.3 | 100 |
| Prevascular invasion | |||
| Negative | 4 | 26.7 | 26.7 |
| Positive | 10 | 66.7 | 93.3 |
| Other | 1 | 6.7 | 100 |
| Preneural invasion | |||
| Negative | 3 | 20 | 20 |
| Positive | 12 | 80 | 100 |
| Lymph-node involvement status | |||
| Free | 6 | 40 | 40 |
| Involved | 9 | 60 | 100 |
| Total | 15 | 100 | |
| Staging (TNM, Clinical) | |||
| I | 8 | 53.3 | 53.3 |
| II | 7 | 46.7 | 100 |
| Total | 15 | 100 | |
TNM; Tumour, nodes and metastases.
Fig 1.
Relative expression of HOTAIR in breast cancer cell lines, case and control samples. A. MDA-MB-231 and MCF-7 cancer cell lines compared to that in MCF-10A control cells. There is no significant difference in the expression of HOTAIR in both of MCF-7 and MDA-MB-231 cancer cell lines compared to normal cell line MCF-10A (P>0.05) and B. Breast cancer compared to normal breast tissues. There is a significant increase in the expression level of HOTAIR in patients with breast cancer compared to the controls (P<0.001). ***; Significant at the 0.0001 level.
Table 2.
Correlation of HOTAIR expression level and clinical pathological variables in the breast cancer cases
| Variables | Cases (%) | HOTAIR lncRNA | P value | ||
|---|---|---|---|---|---|
| Low | High | ||||
| Age (Y), 46.80 ± 2.57 (32-65) (mean ± SE) | 0.782 | ||||
| ≤47 | 53.3 | 26.7 | 26.7 | ||
| >47 | 46.7 | 26.7 | 20 | ||
| Tumour grade | 0.626 | ||||
| Ι | 6.7 | 6.7 | 0 | ||
| II | 66.7 | 33.3 | 33.3 | ||
| III | 26.7 | 13.3 | 13.3 | ||
| Nuclear grade | 0.394 | ||||
| Low | 7.1 | 7.1 | 0 | ||
| High and intermediate | 28.6 | 21.4 | 7.1 | ||
| High | 64.3 | 28.6 | 35.7 | ||
| Tumour stage | 0.232 | ||||
| T1 | 40 | 26.7 | 33.33 | ||
| T2 | 13.3 | 0 | 13.3 | ||
| T3 | 40 | 26.7 | 13.33 | ||
| T4 | 6.7 | 0 | 6.7 | ||
| Tumour size (cm) | 0.029 | ||||
| <2 | 73.3 | 26.7 | 46.7 | ||
| ≥2 | 26.7 | 26.7 | 0 | ||
| Area of invasive component, 4.09 ± 0.13 (0.7-9.5 cm2) (mean ± SE) | 0.464 | ||||
| <4 | 66.7 | 40 | 26.7 | ||
| ≥4 | 33.3 | 13.3 | 20 | ||
| Tumour side | 0.447 | ||||
| Right | 53.3 | 33.3 | 20 | ||
| Left | 46.7 | 20 | 26.7 | ||
| Margin | 0.029 | ||||
| Free | 73.3 | 26.7 | 46.7 | ||
| Involved | 26.7 | 26.7 | 0 | ||
| Prevascular Invasion | 0.512 | ||||
| Negative | 26.7 | 13.3 | 13.3 | ||
| Positive | 73.3 | 40 | 33.3 | ||
| Preneural Invasion | 0.506 | ||||
| Negative | 20 | 13.3 | 6.7 | ||
| Positive | 80 | 40 | 40 | ||
Bold values indicate P<0.05.
Diagnostic value of HOTAIR in breast cancer
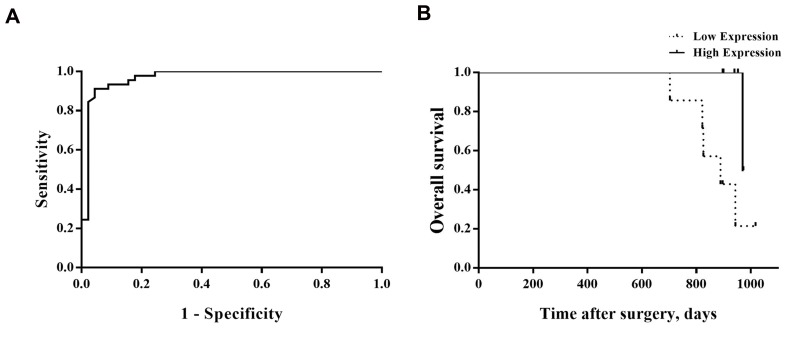
The ROC curve was created and the AUC was computed to determine capability of the HOTAIR expression and difference between cancer and control tissues by calculating sensitivity and specificity for possible cut-off point of HOTAIR. The ROC analysis distinguished the optimal cut-off value for HOTAIR. We found 91.1% sensitivity and 95.7% specificity for circulating HOTAIR with an area under the ROC curve of 0.969 (Fig .2A).
Fig 2.
Receiver operating characteristic (ROC) and Kaplan-Meier curves for HOTAIR expression. A. ROC curve analysis to determine cut-off point for high expression of HOTAIR. The area under curve (AUC) was 0.969 (95% CI, 0.933- 1.005) and B. Kaplan-Meier curve for BC patients in high-expression (46.7%) and low-expression (53.3%) groups segregated by the cut-off point. BC patients with a low expression level of HOTAIR had a poor prognosis (log-rank test, P=0.048).
Correlation of HOTAIR expression with patient survival
In order to assess prognostic value of HOTAIR as a BC biomarker, we investigated association of the HOTAIR expression levels with survival through Kaplan-Meier analysis. We used the log-rank test in BC patients. The Cox proportional hazards regression model was also used to evaluate predictive value of HOTAIR in BC patients. OS was defined as the time between date of surgery and date of death or last follow-up. Clinical pathological factors and OS were then analysed in the high and low level of HOTAIR expression groups. Results indicated that low levels of HOTAIR have shorter survival time (P>0.048, Table 3, Fig .2B).
Table 3.
Log rank test for all patients with breast cancer
| Variables | Overall survival | P value | |
|---|---|---|---|
| HR | 95% CI | ||
| HOTAIR (low vs. high) | 6.423 | 1.128-32.22 | 0.048 |
| Age (Y), (<47 vs. ≥47) | 1.749 | 0.3501-8.657 | 0.506 |
| Tumour grade (Ι-II vs. III) | 0.560 | 0.07296-3.501 | 0.493 |
| Nuclear grade (Low vs. high and intermediate-high) | 1.977 | 0.1596-40.67 | 0.517 |
| Tumour stage (T1-T2 vs. T3-T4) | 0.5745 | 0.1041-2.891 | 0.800 |
| Tumour size (<2 cm vs. ≥2 cm) | 0.460 | 0.04656-2.841 | 0.347 |
| Area of invasive component (<4 cm2 vs. ≥4 cm2) | 0.576 | 0.07625-3.827 | 0.378 |
| Tumour side (right vs. left) | 0.585 | 0.1185-2.937 | 0.525 |
| Margin (free vs. involved) | 0.507 | 0.05886-3.166 | 0.416 |
| Prevascular invasion (negative vs. positive) | 1.605 | 0.3264-8.170 | 0.566 |
| Preneural invasion (negative vs. positive) | 0.336 | 0.01890-1.809 | 0.166 |
HR; Hazard ratio. Bold values indicate P<0.05.
Discussion
A number of different genetic and environmental factors that can increase the likelihood of BC have been identified. Accumulated data suggests that lncRNAs play crucial roles in RNA processing, genomic reprogramming, apoptosis, cell proliferation, cell cycle and chromatin modification (13). HOTAIR is recognised as a risk factor of various types of tumourigenesis including BC (14). Overexpression of HOTAIR may influence tumour formation and induce invasion, migration and proliferation of BC. According to that, altered expression of HOTAIR may induce cell proliferation of BC. In this study we evaluated expression level of HOTAIR in BC patients and cell lines. Our result showed that HOTAIR was up-regulated in BC tissues, while it was not increased in MCF-7 and MDA-MB-231 cell lines.
Regarding the lncRNA functions, BC is one of the most studied malignancies. In has been demonstrated that HOTAIR might have a vital role in this regulation due to the interaction with a wide spectrum of miRNAs (15, 16). Recent studies indicated a significant association of HOTAIR overexpression with tumour size, advances and extensive metastasis in BC (16-19). HOTAIR might act as a gene expression regulator in the BC related to mutations of BRCA1. HOTAIR promoter contains several estrogen receptors (ERs), and it has been shown that estradiol regulated HOTAIR expression in ER positive BC cells. However, this regulation was abolished in BC cells with inactive ERs, indicating the critical role of these receptors in estradiol-mediated control of HOTAIR expression (20). HOTAIR and some other lncRNA expression analyses in 164 ER-positive primary BC cases demonstrated that these lncRNAs could be independent prognostic markers (21). Gökmen-Polar et al. (22) indicated that the utility of HOTAIR as a prognostic marker in BC is limited to ER-negative cases. Therefore, significant up-regulation of HOTAIR obtained from BC tumour samples compared to BC cell lines, in this study, may explain the molecular mechanism causing poor prognosis of this cancer. We found that level of HOTAIR expression was not associated with clinical characteristics of BC, while enhanced expression level of HOTAIR might associate with tumour size, margins and lower disease relapse. In a research, Lu et al. studied HOTAIR expression and methylation of its downstream intergenic CpG islands in 348 samples of primary BC (23). Their results indicated that increased methylation could associate with a worse prognosis in the patient.
According to the results of present study, patients with low level of HOTAIR showed shorter survival time. Bhan et al. (20) demonstrated that HOTAIR is critical for survival and proliferation of MCF-7 BC cells. Pádua Alves et al. (24) showed that HOTAIR in a BC cell line was a critical regulator of genes involved in epithelial to mesenchymal transition. Up-regulation of HOTAIR has been described as a useful predictor of survival and progression in several cancer types including pancreatic cancer (25), hepatocellular carcinoma (26) oesophageal cancer (27), gastrointestinal stromal (28), nasopharyngeal carcinoma (29) and colorectal cancer (30).
One major obstruction of this study was limited number of BC and control samples enrolled from the same hospital which might not fully substantiate accuracy of the results.
Conclusion
Taking into consideration that the major problem in early diagnostic and treatment of different types of cancer, including BC, is lack of highly sensitive and specific tumour biomarkers, altered expression levels of HOTAIR lncRNA can be a guide for investigation of cancer biomarkers. Thus, our results indicated that HOTAIR expression profiling and elucidating its function may be proposed as a useful biomarker for BC diagnosis as well as a therapeutic target in cancer gene therapy. However, further investigations and follow up studies on larger patient samples are required.
Acknowledgments
The authors are grateful to the collaboration and valuable help of Cellular and Molecular Research Centre staff, Shahrekord University of Medial Sciences (Shahrekord, Iran). This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors. The authors declare they have no conflict of interest.
Author’s Contributions
A.A., F.R., E.M.; Participated in design and evaluation experiments. F.M., H.K.; Responsible for Software, validation and formal analysis. R.F., M.Z.-L.; Responsible for investigation, resources and molecular experiments. A.J., A.A.; Writing-original draft preparation, writingreview and editing and supervision. All the authors read and approved the final manuscript.
References
- 1.Gibb EA, Brown CJ, Lam WL. The functional role of long non-coding RNA in human carcinomas. Mol Cancer. 2011;10:38–38. doi: 10.1186/1476-4598-10-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hajjari M, Salavaty A. HOTAIR: an oncogenic long non-coding RNA in different cancers. Cancer Biol Med. 2015;12(1):1–9. doi: 10.7497/j.issn.2095-3941.2015.0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kondo Y, Shinjo K, Katsushima K. Long non-coding RNAs as an epigenetic regulator in human cancers. Cancer Sci. 2017;108(10):1927–1933. doi: 10.1111/cas.13342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang Y, Zhou Y, Xu T, Tian W, Yang C, Wang X, et al. Clinical Value of long noncoding rna HOTAIR as a novel biomarker in digestive cancers : a meta-analysis. Technol Cancer Res Treat. 2018;17:1533034618756783–1533034618756783. doi: 10.1177/1533034618756783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kogo R, Shimamamura T, Mimori K, Kawahara K, Imoto S, Sudo T, et al. Long non-coding RNA HOTAIR regulates Polycomb-dependent chromatin modification and is associated with poor prognosis in colorectal cancers. Cancer Res. 2011;71(20):6320–6326. doi: 10.1158/0008-5472.CAN-11-1021. [DOI] [PubMed] [Google Scholar]
- 6.Tsai MC, Manor O, Wan Y, Mosammaparast N, Wang JK, Lan F, et al. Long noncoding RNA as modular scaffold of histone modification complexes. Science. 2010;329(5992):689–693. doi: 10.1126/science.1192002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Samulin Erdem J, Notø HØ, Skare Ø, Lie JS, Petersen-Øverleir M, Reszka E, et al. Mechanisms of breast cancer risk in shift workers: association of telomere shortening with the duration and intensity of night work. Cancer Med. 2017;6(8):1988–1997. doi: 10.1002/cam4.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Samulin Erdem J, Skare Ø, Petersen-Øverleir M, Notø HØ, Lie JS, Reszka E, et al. Mechanisms of Breast Cancer in Shift Workers: DNA Methylation in Five Core Circadian Genes in Nurses Working Night Shifts. J Cancer. 2017;8(15):2876–2884. doi: 10.7150/jca.21064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kong X, Liu W, Kong Y. Roles and expression profiles of long noncoding RNAs in triple-negative breast cancers. J Cell Mol Med. 2018;22(1):390–394. doi: 10.1111/jcmm.13327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shi Y, Ye P, Long X. Differential expression profiles of the transcriptome in breast cancer cell lines revealed by next generation sequencing. Cell Physiol Biochem. 2017;44(2):804–816. doi: 10.1159/000485344. [DOI] [PubMed] [Google Scholar]
- 11.Zhou Y, Wang C, Liu X, Wu C, Yin H. Long non-coding RNA HOTAIR enhances radioresistance in MDA-MB231 breast cancer cells. Oncol Lett. 2017;13(3):1143–1148. doi: 10.3892/ol.2017.5587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Q, Gao S, Li H, Lv M, Lu C. Long noncoding RNAs (lncRNAs) in triple negative breast cancer. J Cell Physiol. 2017;232(12):3226–3233. doi: 10.1002/jcp.25830. [DOI] [PubMed] [Google Scholar]
- 13.Ding W, Ren J, Ren H, Wang D4. Long noncoding RNA HOTAIR modulates MiR-206-mediated Bcl-w signaling to facilitate cell proliferation in breast cancer. Sci Rep. 2017;7(1):17261–17261. doi: 10.1038/s41598-017-17492-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao W, Geng D, Li S, Chen Z, Sun M. LcnRNA HOTAIR influences cell growth, migration, invasion, and apoptosis via the mi- 20a-5p/HMGA2 axis in breast cancer. Cancer Med. 2018;7(3):842–855. doi: 10.1002/cam4.1353. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 15.Yu Y, Lv F, Liang D, Yang Q, Zhang B, Lin H, et al. HOTAIR may regulate proliferation, apoptosis, migration and invasion of MCF- 7 cells through regulating the P53/Akt/JNK signaling pathway. Biomed Pharmacother. 2017;90:555–561. doi: 10.1016/j.biopha.2017.03.054. [DOI] [PubMed] [Google Scholar]
- 16.Nagini S. Breast cancer: current molecular therapeutic targets and new players. Anticancer Agents Med Chem. 2017;17(2):152–163. doi: 10.2174/1871520616666160502122724. [DOI] [PubMed] [Google Scholar]
- 17.Qiu JJ, Wang Y, Ding JX, Jin HY, Yang G, Hua KQ. The long noncoding RNA HOTAIR promotes the proliferation of serous ovarian cancer cells through the regulation of cell cycle arrest and apoptosis. Exp Cell Res. 2015;333(2):238–248. doi: 10.1016/j.yexcr.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 18.Yoon JH, Abdelmohsen K, Kim J, Yang X, Martindale JL, Tominaga- Yamanaka K, et al. Scaffold function of long non-coding RNA HOTAIR in protein ubiquitination. Nat Commun. 2013;4:2939–2939. doi: 10.1038/ncomms3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chiyomaru T, Fukuhara S, Saini S, Majid S, Deng G, Shahryari V, et al. Long non-coding RNA HOTAIR is targeted and regulated by MIR-141 in human cancer cells. J Biol Chem. 2014;289(18):12550–12565. doi: 10.1074/jbc.M113.488593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bhan A, Hussain I, Ansari KI, Kasiri S, Bashyal A, Mandal SS. Antisense transcript long noncoding RNA (lncRNA) HOTAIR is transcriptionally induced by estradiol. J Mol Biol. 2013;425(19):3707–3722. doi: 10.1016/j.jmb.2013.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sørensen KP, Thomassen M, Tan Q, Bak M, Cold S, Burton M, et al. Long non-coding RNA expression profiles predict metastasis in lymph node-negative breast cancer independently of traditional prognostic markers. Breast Cancer Res. 2015;17:55–55. doi: 10.1186/s13058-015-0557-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gökmen-Polar Y, Vladislav IT, Neelamraju Y, Janga SC, Badve S. Prognostic impact of HOTAIR expression is restricted to ER-negative breast cancers. Sci Rep. 2015;5:8765–8765. doi: 10.1038/srep08765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lu L, Zhu G, Zhang C, Deng Q, Katsaros D, Mayne ST, et al. Association of large noncoding RNA HOTAIR expression and its downstream intergenic CpG island methylation with survival in breast cancer. Breast Cancer Res Treat. 2012;136(3):875–883. doi: 10.1007/s10549-012-2314-z. [DOI] [PubMed] [Google Scholar]
- 24.Pádua Alves C, Fonseca AS, Muys BR, de Barros E, Lima Bueno R, Bürger MC, de Souza JE, et al. Brief report: The lincRNA Hotair is required for epithelial-to-mesenchymal transition and stemness maintenance of cancer cell lines. Stem Cells. 2013;31(12):2827–2832. doi: 10.1002/stem.1547. [DOI] [PubMed] [Google Scholar]
- 25.Chen FJ, Sun M, Li SQ, Wu QQ, Ji L, Liu ZL, et al. Upregulation of the long non-coding RNA HOTAIR promotes esophageal squamous cell carcinoma metastasis and poor prognosis. Mol Carcinog. 2013;52(11):908–915. doi: 10.1002/mc.21944. [DOI] [PubMed] [Google Scholar]
- 26.Nie Y, Liu X, Qu S, Song E, Zou H, Gong C. Long non-coding RNA HOTAIR is an independent prognostic marker for nasopharyngeal carcinoma progression and survival. Cancer Sci. 2013;104(4):458–464. doi: 10.1111/cas.12092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ishibashi M, Kogo R, Shibata K, Sawada G, Takahashi Y, Kurashige J, et al. Clinical significance of the expression of long non-coding RNA HOTAIR in primary hepatocellular carcinoma. Oncol Rep. 2013;29(3):946–950. doi: 10.3892/or.2012.2219. [DOI] [PubMed] [Google Scholar]
- 28.Kim K, Jutooru I, Chadalapaka G, Johnson G, Frank J, Burghardt R, et al. HOTAIR is a negative prognostic factor and exhibits prooncogenic activity in pancreatic cancer. Oncogene. 2013;32(13):1616–1625. doi: 10.1038/onc.2012.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tano K, Akimitsu N. Long non-coding RNAs in cancer progression. Front Genet. 2012;3:219–219. doi: 10.3389/fgene.2012.00219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu ZY, Yu QM, Du YA, Yang LT, Dong RZ, Huang L, et al. Knockdown of long non-coding RNA HOTAIR suppresses tumor invasion and reverses epithelial-mesenchymal transition in gastric cancer. Int J Biol Sci. 2013;9(6):587–597. doi: 10.7150/ijbs.6339. [DOI] [PMC free article] [PubMed] [Google Scholar]