Abstract
Gastroparesis is defined by delayed gastric emptying and symptoms of nausea, vomiting, bloating, postprandial fullness, early satiety and abdominal pain. Most common etiologies include diabetes, post-surgical and post-infectious, but in most cases it is idiopathic. Clinical presentation and natural history vary by the etiology. There is significant morbidity and health-care utilization associated with gastroparesis. Mechanistic studies from diabetic animal models of delayed gastric emptying as well as human full-thickness biopsies have significantly advanced our understanding of this disorder. An innate immune dysregulation and injury to the interstitial cells of Cajal and other components of the enteric nervous system through paracrine and oxidative stress mediators is likely central to the pathogenesis of gastroparesis. Scintigraphic and 13C breath testing provide the most validated assessment of gastric emptying. The current stagnant gastroparesis therapeutic landscape is likely to soon see significant changes. Relatively newer treatment strategies include antiemetics (aprepitant), prokinetics (prucalopride, relamorelin), and fundic relaxants (acotiamide, buspirone). Endoscopic pyloromyotomy appears promising over the short term, especially for symptoms of nausea and vomiting. Further controlled trials and identification of the appropriate subgroup with pyloric dysfunction and assessment of long-term outcomes are essential. This review highlights the clinical presentation, diagnosis, mechanisms and treatment advancements for gastroparesis.
INTRODUCTION
Gastroparesis is defined by a delay in gastric emptying (GE) in the absence of mechanical obstruction of the gastric outlet.1 Cardinal symptoms include post-prandial fullness/early satiety, nausea/vomiting and bloating. Abdominal pain is increasingly recognized to be one of the most common symptom in this disease2. Over the last decade, there has been a paradigm shift in our understanding of the clinical presentation, physiological and molecular alterations in gastroparesis.
Clinically, traditionally, diabetes has been seen as the prototypical cause of gastroparesis. However, diabetic gastroparesis (DG) constituted only about a third of gastroparesis patients in one tertiary-care study3 and population-based studies show that only 1-5% of diabetics develop gastroparesis.4, 5 In contrast, idiopathic gastroparesis (IG) constituted the most common etiology accounting for over 50% of the gastroparesis patients enrolled in the NIH Gastroparesis Clinical Research Consortium (GpCRC).6 A more recent US population-based cross sectional study showed that IG constituted 28% of the overall gastroparesis patients identified, while the remaining associated with diabetes. However, diagnostic code based determination may have resulted in greater association of gastroparesis with diabetes in this study.5 Another unexpected clinical observation has been the association of an overweight/obese status with gastroparesis7 which contrasts with the older observations of weight loss and malnutrition. This finding raises a potential link with the pro-inflammatory state and oxidative stress associated with obesity. Although not included in the three cardinal symptoms in the Gastroparesis Cardinal Symptom Index (GCSI), abdominal pain is observed in >80% patients with gastroparesis, particularly in those with associated bowel disturbances.2 More recently, opioid use was found to be common in gastroparesis patients, which can further exacerbate the symptoms and complicate the clinical presentation.8
From a physiological standpoint, a singular endpoint on the GE scintigraphy to define gastroparesis has allowed diffusion of the technique but has also been criticized because of a lack of correlation with symptoms in many but not all studies.9, 10 However, measurement of GE is important as patients with slow and rapid GE can have indistinguishable symptoms11 yet require different treatment approaches. Additionally, GE provides a tangible end-point in animal studies and in human clinical trials. The lack of a strong correlation may reflect dissociation between physiology and symptoms once the disease has established, or the fact that gut can only express a disease process with a limited set of symptoms which can be overlapping. It may also reflect a continuum between symptoms associated with mild/moderate GE delay to severe and ingrained symptoms with overlap from spinal and supra spinal mechanisms. A balanced appreciation of both physiology and clinical symptoms is necessary along with mechanistic studies to examine and clinical trials to address symptoms. Furthermore, refinement of and development of new techniques for studying the different aspects of gastric function is likely to provide better understanding of the clinical symptoms and develop targeted therapies.12
The molecular understanding of gastroparesis has significantly evolved over the last decade. We have gone from a disorder of unknown etiology to enteric neuropathy to myopathy to an established role for the loss or dysfunction of the Interstitial Cells of Cajal (ICC).13 The emerging animal model and human work suggests a role of an innate immune dysregulation in gastroparesis and putative immune interactions that may be driving the injury to the enteric nervous system (ENS) and ICC.14, 15 This has energized a new paradigm for targeting cellular and molecular dysfunction seen in gastroparesis. In this review, we will summarize recent literature on clinical presentation, epidemiology, natural history, pathophysiology, diagnosis and treatment considerations for gastroparesis. Additionally, we will provide future directions for research and discuss evolving landscape of therapeutic strategies for gastroparesis.
Clinical presentation, epidemiology and natural history
The term gastroparesis encompasses any clinical condition characterized by symptoms suggestive of a deranged digestive function of the proximal gastrointestinal tract that is associated with objective evidence of an abnormally prolonged retention of gastric contents in the absence of demonstrable mechanical obstruction. No stringent consensus exists on the symptoms that should be included in the definition of gastroparesis.10 Postprandial fullness, early satiety, nausea, vomiting and bloating are the cardinal symptoms originally proposed.1 Symptom profiling from patients enrolled in the GpCRC showed that severe early satiety and postprandial fullness was reported by 50-60% of gastroparesis patients and DG and IG had similar severity scores.16 Nausea was reported by 95% of the patients (predominant symptom in 29%). It was related to the meals in three-quarter of the patients and lasted most of day in over 40% of the patients. DG patients scored higher on the nausea/vomiting sub score of Patients Assessment of Upper Gastrointestinal Disorders-Symptoms (PAGI-SYM), with vomiting lasting several hours or most of the day in over 50% of DG patients compared to 24% in IG. DG patients also reported vomiting in the morning before eating.17 Severe bloating (GCSI ≥ 4 of 5) was reported by ~40% of gastroparetics and was associated with female sex, overweight status, altered bowel function and probiotic use.18 Bloating was not associated with disease etiology, smoking status or GE. In a large observational registry study, symptoms that prompted evaluation more often included vomiting for DG and abdominal pain for IG. DG had more severe retching and vomiting than those with IG, whereas, IG had more severe early satiety and postprandial fullness. Additionally, gastric retention was greater in patients with type 1 DG compared to IG.6 Upper abdominal pain of moderate-severe intensity was reported by two-third of gastroparesis patients. It was more prevalent in patients with non-acute onset of gastroparesis, those with bowel disturbances, opiate and antiemetic use. However, it was not associated with degree of GE delay or with presence of diabetic neuropathy in DG. Compared to nausea/vomiting predominant gastroparesis, pain predominant gastroparesis was associated with greater impairment in quality of life (QoL).2 Additionally, higher depression and anxiety scores associated with gastroparesis severity on both investigator- and patient-reported assessments. Notably, psychological dysfunction did not vary by the etiology or degree of GE delay.19 Another important aspect of gastroparesis that clinicians need to take into consideration is the presence of overlapping symptoms suggestive of involvement of other regions of the gastrointestinal tract including the small bowel and the colon.
Only limited information exists on the epidemiology and natural history of gastroparesis. In a population-based study in Minnesota20 epidemiological data were calculated by identifying: a) “true gastroparesis” defined as typical symptoms and scintigraphically proved delayed GE; b) “probable gastroparesis” defined as typical symptoms and food retention in the stomach at endoscopy; c) “possible gastroparesis” defined as either asymptomatic delayed GE or typical symptoms alone. Although as yet the best available study on gastroparesis, it was based on data collected by a symptom questionnaire that did not include postprandial fullness, one of the most frequent symptom of gastroparesis among patients seen both in Europe21 and in US.22 The age-adjusted incidence of gastroparesis in the period 1996-2006 was 6.3 per 100,000 person-years, ranging between 2.4 for men and 9.8 for women, with these figures increasing with advancing age with a peak incidence of 10.5 per 100,000 in patients 60 years of age or older. The age adjusted prevalence of definite gastroparesis per 100,000 persons in 2007 was 24.2, ranging between 37.8 for women and 9.6 for men, with a female-to-male ratio of 3.9:1. The vast majority of subjects with typical symptoms would never undergo GE tests, so that it was estimated that the actual prevalence of gastroparesis in the general population could approach 2%, but only one out of ten of these individuals is likely to be diagnosed.23 A different approach to estimating the prevalence of gastroparesis is by calculating the prevalence of dyspepsia in the general population and the relative proportion of postprandial distress syndrome and mixed dyspepsia; that is around 5-7% of the general population.24 Since GE is delayed in approximately 25-35% of these patients, one could predict a prevalence of gastroparesis ranging between 1.3 and 1.4% of the general population.
In a recent study looking at sex and ethnic distribution in a cohort of gastroparesis patients, a higher proportion of blacks presented with diabetes as the etiology, had more severe retching, vomiting, and hospitalization rates in the past year compared to whites. Hispanics had less-severe nausea, less early satiety, and lower proportion were using prokinetics than non-Hispanics.25 Another study also showed that non-white patients had worse symptoms, poorer quality of life and healthcare utilization as compared to whites.26 In a study from Type I diabetes exchange clinic registry, females are more likely to have gastroparesis than males27 but this was not found to be the case in the Olmsted county study.4 A greater proportion of women with gastroparesis was noted in the recent populated-based5 and also in a tertiary-care experience of patients with upper GI disorders.28 In the GpCRC registry, women were more likely to have IG, more severe symptoms of postprandial fullness, early satiety, bloating, and upper abdominal pain.25 They were also less likely to improve over 48 weeks of follow-up29 as compared to men.
Prospective follow-up of a large cohort of patients showed that two-thirds of gastroparesis patients did not improve over one year. Predictors of improvement (decrease in GCSI≥1) over 48 weeks included age ≥50 years, moderate/severe gastroparesis (>20% gastric retention at 4 hrs), and onset of gastroparesis following an infectious prodrome. Overweight/obese status, presence of severe abdominal pain, concomitant GERD and depression were associated with lower odds of improvement on GCSI over-time.29 Over the 48 weeks of follow-up, prokinetic, proton pump inhibitor, anxiolytic use and gastric stimulator implantation rates increased in type 1 diabetics, whereas, opiate use increased in type 2 diabetics.30 Additionally, type 1 diabetes patients had higher hemoglobin A1c, longer symptom duration, diabetes duration, greater gastric retention, and more hospitalisations. Type 2 diabetics were older, heavier, had more comorbidities, more bloating and fullness. Opioid use in gastroparesis, especially potent-opioids (morphine, hydrocodone, oxycodone, methadone, hydromorphone, etc.) is associated with worse symptoms, greater gastric retention, poorer quality of life and health-care utilization.8 Opioid users also had lower employment rates than non-users.31
As to the impact of gastroparesis upon general health status, the few data that are available suggest gastroparesis is a serious clinical condition. Patients with definite, probable and possible gastroparesis in Olmsted County were followed up for a median of five years and, compared to the general population, had increased rates of hospitalisations as well as a decreased life expectancy.20 On the contrary, delayed GE was not found to be associated with increased mortality rates in diabetic patients followed up for 25 years.32 Hospitalisation rates may increase in the future, since they are related to poor glycaemic control and infection rates in both type 1 and type 2 diabetes, two conditions that are rapidly increasing in industrialized countries.33 This may be balanced by and the trend even reversed by improved therapeutic approaches that have become available to larger proportions of affected individuals. Cardinal gastroparesis symptoms are reported by about 10% of patients with type 1 diabetes,34 but the majority never undergo a GE test. When GE was evaluated it was found to be delayed in over 40% of patients with insulin-dependent diabetes,35 but generally normal in uncomplicated type 2 diabetes.36 In fact DG seems to be associated with both poor glycaemic control and vascular and neurogenic complications that are more likely to occur in type 1 diabetes.34 Table 1 summarizes clinical features of gastroparesis using GpCRC studies.
Table 1:
Clinical features of Diabetic and Idiopathic gastroparesis from gastroparesis registries in the Gastroparesis Clinical Research Consortium
|
Physiology and pathophysiology of gastric emptying
Emptying of a meal from the stomach into the small bowel is the final result of a number of complex and highly coordinated motor and secretory events.37-39 Ingestion of a meal in humans of at least 250 Kcal converts fasting into fed motor and secretory activities. From a neuromuscular standpoint three separate areas are involved in GE: fundus, antrum and pylorus. Swallowing triggers active relaxation of the gastric fundus so that it can accept large volumes of ingesta without detectable increases of intragastric pressure. Subsequently, a steady increase in fundic tone pushes gastric contents that are captured by phasic contractions and pushed toward a rapidly closing pylorus so that digestible solids are ground together with gastric secretions and bounced backwards into the proximal part of the stomach. The maximal frequency of antral contractions is set by the ICC located at the upper part of the greater curvature generating a slow-wave basal electrical rhythm (pacesetter potential) with a frequency of three depolarisations per minute. This process continues until all digestible solids are reduced to particles of 2 millimetres or less and leave the stomach in small volumes of liquid and homogenized food (chyme) just before pyloric contractions. GE also requires normal small bowel function not only because antro-pyloro coordination is necessary to empty the stomach, but also because neuro-endocrine inhibitory signals arise from both the proximal and distal small bowel, based on the composition of the chyme, to modulate emptying rates, so that delivery to the absorbent mucosa matches liver and pancreas secretory activities.39-41 Recent data indicate that enteric dysmotility is more frequent than delayed GE in patients with symptoms suggestive of gastroparesis, and correlates with the severity of clinical manifestations42 suggesting the small bowel should be kept in mind when a patient presents with symptoms suggestive of gastroparesis. Once the caloric content of the meal has been completely emptied from the stomach, secretomotor activities switch back to the fasting state and the three phases of the interdigestive migrating motor complex (IDMMC) return.43 Gastric, biliary and pancreatic secretions also fluctuate during fasting with their peaks coincident with the late phase II preceding phase III which aborally sweeps gastrointestinal contents including the indigestible solid components of the meal (vegetable fibres) that had been left in the stomach. Unlike what happens in the postprandial state, the pylorus does not close on an oncoming phase III antral contraction. Since the frequency of IDMMCs varies extensively even in healthy individuals and not all phases III start from the stomach,44 emptying of indigestible solids is highly unpredictable, particularly in the disease state, since antral contractility has been found to be decreased in gastroparesis.45 Figure 1 provides an overview of physiological and molecular abnormalities in gastroparesis. Another entity to consider is rapid gastric emptying which can be due to increased amplitude of gastric contractions,46 and symptoms of which can be identical to those with gastroparesis, especially in the setting of diabetes.11 A study showed that patients with delayed gastric emptying had less frequent bloating than those with normal and accelerated gastric emptying.47
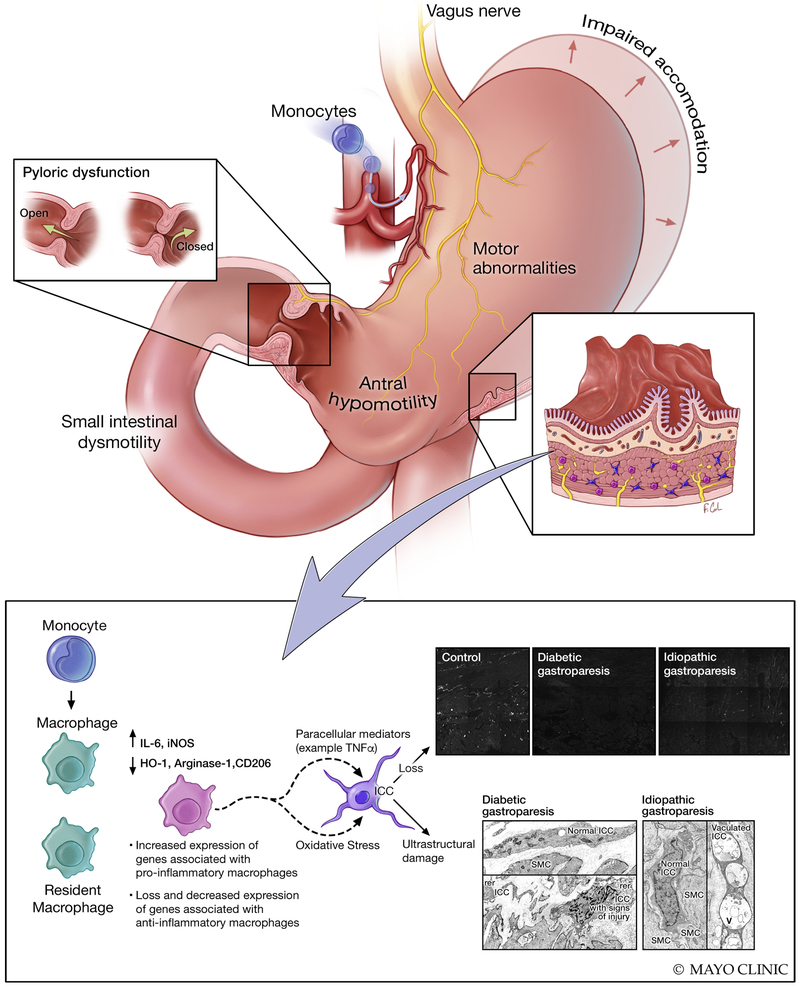
Figure 1: Gastrointestinal pathophysiological changes in human gastroparesis.
Physiological changes such as impaired accommodation of the gastric fundus/body, motor abnormalities including antral hypomotility, impaired pyloric relaxation and small intestinal dysmotility can result in impaired gastric emptying and clinical manifestations of gastroparesis. At a cellular level, loss of interstitial cells of Cajal (ICC) has been reported in over half of the gastroparesis patients and nearly all of the patients had signs of injury to ICC on ultrastructural studies. The loss of ICC correlated with delayed gastric emptying in diabetic gastroparesis. Ultrastructural studies also showed changes in nerves and smooth muscle cells which were less appreciable on immunohistochemistry. More recently, human studies have shown loss of macrophages with anti-inflammatory phenotype (CD206 positive, M2 or alternatively activated macrophages) and increased expression of genes associated with pro-inflammatory macrophages on transcriptomic analysis of full thickness biopsies. This is complemented by animal model studies of diabetic gastroparesis where an altered macrophage activation was shown to mediate injury to ICC likely through paracrine mediators. Additionally, CSF1op/op mice lacking macrophages were protected from development of gastroparesis in spite of having diabetes suggesting an essential role for immune cells in development of delayed gastric emptying. Immune mediated mechanisms likely play a critical role in pathogenesis of gastroparesis.
Pathogenesis
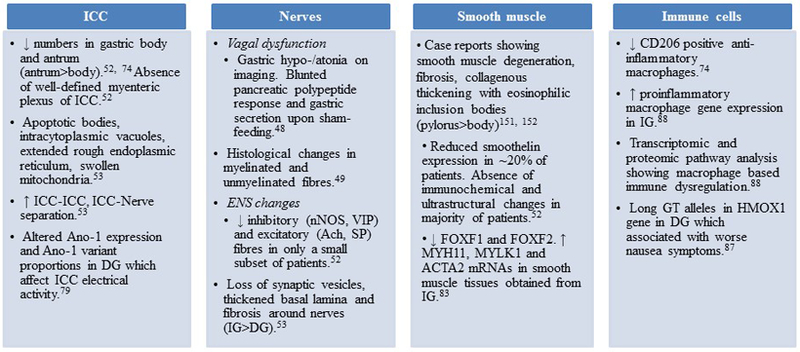
Normal gastric function relies on a neuro-muscular coordination by the extrinsic innervation, inhibitory and excitatory components of the ENS, ICC, fibroblast like cells and gastric smooth muscle. Regional abnormalities in motility patterns of the gastric fundus, body, antrum, pylorus and proximal small bowel can result in delayed GE as this is measured as a composite end-point. Vagal innervation to the stomach is important for accommodation and possibly mediating pyloric relaxation. Early observations in DG showed blunted pancreatic polypeptide response and gastric secretion upon sham-feeding suggesting vagal dysfunction.48 Histological changes in myelinated and unmyelinated vagal nerve fibers have been reported in patients with DG.49 The sympathetic component of the autonomic nervous system has also been shown to be affected in DG with axon-dendritic histological and pre vertebral ganglionic gene expression changes.50, 51 Abnormalities in both inhibitory (neuronal nitric oxide synthase, nNOS; vasoactive intestinal peptide, VIP) and excitatory (acetylcholine, Ach; substance P) components of the ENS have been described in humans with gastroparesis and in animal models of delayed GE although the findings are not always apparent on histology and may require electron microscopy. Indeed, in humans it appears that enteric neurons are preserved in gastroparesis with potential functional changes only seen on electron microscopy.52, 53 Streptozotocin induced diabetic rats demonstrated an increase in VIP immunoreactivity which was reversible with insulin administration.54 A non-insulin-dependent diabetic rat model showed attenuation of non-adrenergic, non-cholinergic inhibitory neurotransmission and reduced sensitivity of adrenoceptors to noradrenaline in the colon.55 Nitric oxide (NO) is a key physiological mediator of non-adrenergic non-cholinergic relaxation in the gastrointestinal smooth muscle. Sildenafil, an inhibitor of PDE 5 was shown to inhibit gastric and intestinal transit in high doses, an effect that was blocked by L-NAME, a non-selective NOS inhibitor and methylene blue, a guanylate cyclase inhibitor.56 Reduced NOS activity was seen in Streptozotocin induced diabetic rats and diabetic mice. One study has shown that dimerized nNOS expression correlates better with gastric relaxation suggesting that post translational modifications may be important in mediation of inhibitory neurotransmission.57 Diabetes related advanced glycation products can bind and inhibit nNOS in myenteric neurons,58 however, other factors like glucose, insulin, insulin like growth factor 59, lipoproteins and oxidative stress60, 61 molecules also play a role in nNOS regulation. In a study on nNOS−/− mice, a model of delayed GE,62 neural stem cell transplantation into the pylorus increased the smooth muscle relaxation and restored gastric emptying.63 Myenteric nitrergic neuropathy has been found in the jejunum of spontaneously diabetic BB-rats.64 Differences in nNOS signaling may explain increased predisposition for gastroparesis in women. In the gastric antrum of streptozotocin induced Sprague-Dawley rats, females had greater impairment in nNOS dimerization, nitrergic relaxation, increase in intragastric pressure and worse gastric emptying than males.57 Additionally, a diminished intracellular tetrahydrobiopterin:total biopterin ratio was shown to impair nNOS activity in diabetic female rats.65 Tetrahydrobiopterin supplementation has been proposed to be potentially effective in accelerating GE.66 The contribution of loss of nNOS expression towards GE delay in humans is unclear. In humans with DG and IG, only a small subset shows decrease in nNOS expression52 and efforts to increase NO using nitroglycerine and sildenafil did not result in clinical benefits.67, 68 Additionally, although nNOS expression was decreased in response to hyperglycemia in the NOD mice, it did not predict development of delayed GE.69 This suggests that nNOS may act as a cofactor or a post translational protein may be involved in mediating its role. Considering the increasing overlap between gastroparesis and obesity, animal models have also examined the effects of high-fat diet on the ENS. Duodenal myenteric density of neurons expressing VIP and nNOS was found to be decreased and ultrastructural studies showed axonal swelling and loss of neurofilaments.70 In ob/ob mouse model of obesity, lower inhibition of myosin light chain phosphatase genes was seen, but no clear effects on gastric antral smooth muscle contractility were noted.71 Although ob/ob mice are used as a model of delayed gastric emptying,72 the precise cellular injury in this model is not well established.
In addition to slow wave generation and setting of the smooth muscle membrane potential, ICC are also involved in cholinergic and nitrergic neurotransmission and mechanotransduction.73 Loss of ICC is one of the commonest cellular abnormalities reported in animal models of delayed GE as well as in humans. Full-thickness gastric body52 and antrum74 biopsies from both DG and IG have demonstrated a loss of ICC, with changes being more prominent in gastric antrum than the body. Other human studies have also shown a loss of ICC in patients with DG.75, 76 Upon ultrastructural examination, almost all patients with DG and IG had changes in ICC such as intracytoplasmic vacuoles, mitochondria with clear matrix, extended rough endoplasmic reticulum and apoptotic features (Figure 1).53 Two major animal models of DG (streptozotocin rats77 and NOD mice69) have shown a decrease in the number of ICC. In streptozotocin induced diabetic rats, antral ICC were depleted at 12 weeks of diabetes. NOD mice with delayed GE also showed loss of ICC networks in gastric corpus and antrum as demonstrated by c-Kit (receptor tyrosine kinase) expression. Ano-1, a calcium-activated chloride channel protein is expressed in ICC and is important for the electrical activity of the ICC.78 DG patients have altered Ano-1 expression and different proportions of Ano-1 variants as compared to diabetic controls which affect the electrical activity of the ICC.79 Loss of ICC has been associated with gastric dysrhythmias in patients with DG. Animal studies have shown that even patchy loss of ICC can result in reentrant tachy-arrhythmias and loss of slow waves resulting in brady-arrhythmias. A subset of patients with DG with severe ICC loss was found to have tachygastria patterns on the electrogastrogram.80
The key points in molecular pathogenesis of gastroparesis are summarized in Table 2. In DG, ICC loss correlates with the delay in GE.81 The underlying mechanisms for ICC loss were until recently, unclear. Smooth muscle atrophy/degeneration and fibrosis has been observed in severe diabetes. This can result in depletion of smooth muscle produced stem cell factor which plays a role in ICC survival.82 A recent human transcriptomic study showed changes in various smooth muscle genes in IG.83 Data obtained from NOD Type 1 diabetes model suggests a role of uncountered oxidative stress to play a role in ICC loss and development of delayed GE.69 One mechanism for the control of oxidative stress is expression of hemeoxygenase-1 (HO-1). HO-1 is produced by CD206 positive anti-inflammatory macrophages. Mice that develop delayed GE failed to upregulate HO-1 expression while upregulation of HO-1 by hemin increases expression of Kit and nNOS and reverses the delay in GE.84
Table 2:
Key points in molecular pathogenesis of gastroparesis
|
In the NOD mice, the development of delayed GE was associated with an increase in iNOS expression, a marker for macrophages with a “M1” phenotype (classically activated or pro-inflammatory).69 Macrophage-deficient CSF1op/op mice did not develop delayed GE despite the presence of severe hyperglycemia.85 Administration of CSF1 resulted in replenishment of macrophages and development of delayed GE and ICC damage in >75% of the mice. Conditioned media from macrophages exposed to oxidative stress resulted in damage to cultured ICC which could be prevented by neutralizing antibodies against IL6R and TNF.86 Decreased CD206+ (alternatively activated, anti-inflammatory M2 macrophages) have also been reported in the gastric antrum of patients with DG and IG which correlated with the loss of ICC.74 Additionally, patients with DG had long GT alleles in the HMOX1 gene and these associated with worse nausea symptoms.87 Recent transcriptomic analysis of full thickness (excluding mucosa) gastric body biopsies from DG and IG reveal macrophage driven immune dysregulation as the primary pathways affected. Additionally, genes associated with M1 (pro inflammatory) macrophages were enriched in tissues from IG compared to controls.88 These data taken together suggest a new paradigm for the development of gastroparesis with macrophage-driven immune dysregulation and oxidative stress central to driving injury to the ICC and subsequent delayed GE. Figure 2 provides a summary of cellular and molecular changes in human gastroparesis.
Figure 2:
Summary of human studies highlighting cellular and molecular changes in gastroparesis
Diagnostic considerations
Patients with cardinal gastroparesis symptoms and normal upper endoscopy in the absence of alarm features may be candidates for a short-term treatment with prokinetics. Non-responders and early relapsers could also try short term treatment with antisecretory drugs and antidepressants alone or in various combinations. If consistent response is not achieved or if country or state practice guidelines suggest ruling out mechanical obstruction first before targeting with prokinetics, patients should undergo traditional diagnostic testing to exclude mechanical obstruction or any organic disease. In case of negative findings patients should be referred to centres with specific interest in the field in order to undergo physiological testing of GE24. Therefore, the diagnosis of gastroparesis is based upon both recognition of cardinal symptoms and identification of delayed GE by appropriate instruments.
Symptom evaluation.
Cardinal gastroparesis symptoms include postprandial fullness, early satiety, nausea, vomiting and bloating. Validated questionnaires such as the GCSI based on the PAGI-SYM89 and the more recently revised GCSI-Daily Diary score90 have been developed to quantitate the severity of these and other digestive symptoms, but their use is mostly restricted to clinical trials. Self-administered questionnaires with simple descriptive definitions of individual symptoms enriched by explanatory cryptograms have been demonstrated to decrease patient-doctor misunderstanding91 and similar questionnaires validated in different languages might help clinical practice in the near future.
Gastric emptying measurement.
Numerous techniques are available for measuring GE, but scintigraphy is the gold standard. Stable isotope breath testing as well as WMC provide reasonable alternatives.9 Scintigraphic GE is the current gold standard.92 An antero-posterior gamma-camera recording of the gastric area for 4 hours after ingestion of a Tc-99m labelled low-fat EggBeaters (chicken egg white) meal is the standardized procedure that was internationally validated to obtain well-established normal values.93 The technique, however, has several limitations: a) caloric (255 KCal) and fat contents (2%) are low and do not mimic a normal meal even for symptomatic individuals, so that it probably underestimates the actual prevalence of gastroparesis, although, on the other hand, it has the advantage of reducing false positive cases; b) due to radiation exposure, it should not be used in women of child bearing potential which represent a large group of patients seeking medical help for symptoms suggestive of gastroparesis and, for the same reason, it is not an ideal technique for repeated measurements that would be necessary, to investigate changes in clinical manifestations over time and/or need of monitoring the effects of treatment; c) nuclear medicine departments are rare globally and certainly insufficient to evaluate all potential patients with gastroparesis; d) even if a nuclear medicine department is available, gamma-camera time is expensive and devoting it for diagnosing gastroparesis is not always possible, although this issue has been partly addressed by standardizing delayed GE as >10% at four hours,93 thus limiting the use of the dedicated equipment to a minimum and/or allowing investigating numerous patients in the same session.
Some, but not all of the above limitations have been overcome by the validation of GE breath tests. Different substrates including octanoic acid, a medium-chain fatty acid94, and Spirulina platensis, an edible algae,95 can be tagged with a stable (non-radioactive) carbon isotope (13C) and thereafter incorporated into the solid component of low caloric meals. After being emptied from the stomach, the organic substrates are digested and absorbed in the proximal small intestine and metabolized by the liver so that 13C is excreted by the lungs, and its rise over baseline in breath samples can be measured by mass-spectrometry. The rate limiting step is GE and therefore appearance in the breath correlates with GE. 13C breath testing GE shows a strong correlation with simultaneously obtained scintigraphy96 and the 13C spirulina technique has been approved by the U.S. Food and Drug Administration (FDA).97 The technique is noninvasive and therefore suitable for repeated testing, simple to perform at the point of care and relatively cheap, since it does not require any special equipment on-site, since breath samples remain stable for long periods of time and can be centralized. Its main limitations are represented by artefacts that can be encountered in patients with intestinal malabsorption and liver or lung diseases. One study has shown that up to 39% of patients with gastroparesis can have a positive lactulose breath test.98 Although, this represents an accelerated oro-cecal transit, small intestinal bacterial overgrowth can affect interpretation of the breath testing for GE, especially in the setting of diabetes and scleroderma which have been associated with small intestinal bacterial overgrowth. Furthermore, 13C spirulina is not available in Europe and has not been approved by European health authorities.
Similarly to scintigraphy and breath testing, the wireless motility capsule (WMC) has been approved by the FDA for measuring GE.97 WMC, also known as SmartPill®, is a non-invasive technique to indirectly measure GE and intestinal transit times, by an ingested capsule recording pH, pressure and temperature signals that are analyzed by an external receiver.99 As previously described, a large indigestible solid like the SmartPill, even if ingested with a test meal, does not reflect emptying of its caloric digestible components, since it is emptied separately during the fasting state. A study done to assess performance of WMC compared to 99mTc based scintigraphy showed a correlation of 0.73 between 4 hr GE on scintigraphy and breath testing based GE time. A 300-min cutoff for GE time provided a sensitivity of 65% and specificity of 87% for diagnosis of gastroparesis compared to a sensitivity of 44% and specificity of 93% for 4 hr GE scintigraphy.100 The WMC provided new diagnosis in >50% of patients with suspected gastroparesis or slow intestinal transit.101 This was again shown in a recent study where WMC detected delayed GE in 10% more individuals than scintigraphy and in almost twice as many diabetics got diagnosed with delayed GE than scintigraphy. As somewhat expected, diagnosis of rapid GE was made less often with WMC.102 It remains unclear if higher sensitivity of detecting delayed GE which is likely driven by emptying delay due to non-physiological nature of pill emptying is clinically meaningful. A clear advantage of WMC, however, is that it allows assessment of global transit abnormalities which can be helpful in evaluation and management of comorbidities like constipation that associate with gastroparesis.103
Other techniques for measuring GE are magnetic resonance imaging and ultrasonography, but they have been generally only used for research purposes. Magnetic resonance imaging can measure not only GE, but also gastric accommodation to meal ingestion and wall motion. It requires expensive equipment use and investigated patients must remain still in the supine position, thus limiting the clinical applicability of the technique.104 Ultrasound equipment is readily available and the technique can be used to measure GE of liquids105, 106 in non-obese individuals, but its use has also been restricted to research settings. Both GE and transpyloric liquid flow can be measured by transabdominal duplex ultrasonography, while three-dimensional ultrasonography can also measure intragastric volumes.107 Identification of intragastric bezoars or food residues at endoscopy or radiology, after an overnight fast also represents indirect confirmation of abnormal gastric motility.
Treatment – medical therapy
It remains reasonable to recommend patients with gastroparesis to avoid large, high-caloric, fatty meals, as well as dietary fibres and any food that is recognized by the individual patient to aggravate postprandial symptoms. Although these suggestions are based on pathophysiological considerations or good common sense they have never been validated in appropriate studies. A randomized, controlled study in patients with DG reported improvement of reflux symptoms, fullness, nausea, vomiting and bloating on a diet consisting of small particle sized solids.108 In one study, only a third of gastroparesis patients had nutritional counselling and only 2% were following any dietary suggestions. Despite the increasing percentage of patients with gastroparesis who are obese, over 60% of patients reported caloric-deficient diets (defined as <60% of estimated daily total energy requirement) and had deficiencies in several vitamins and minerals.109 Thus, a dedicated history for caloric intake and nutritional counselling is important for these patients.
The ideal therapy for gastroparesis should reverse the cellular defects, accelerate GE and improve cardinal symptoms. However, as previously discussed, the delay in GE is only partially related to the clinical manifestations of gastroparesis and other mechanisms are likely involved after the initial onset of the disease, including decreased gastric accommodation, small bowel motor abnormalities, visceral hypersensitivity, etc.10 Thus, current treatments are evaluated primarily based on their efficacy on symptoms.92 Since different pathogenic mechanisms may be concomitantly present in the same subject, combination therapies would be theoretically appropriate, but they have never been tested. Co-prescription of prokinetics, antiemetics and neuromodulators may be necessary to control symptoms in severe cases, but it may lead to drug-drug interactions, due to concomitant metabolism by liver cytochrome P-450 enzymes,110 thus increasing potential hazards typical of these pharmacological classes (see below).
Prokinetics.
A recent meta-analysis confirmed efficacy of prokinetics on clinical symptoms suggestive of gastroparesis, but most studies are old and their quality does not match the current requirements of American and European health authorities.111 They are classified into different pharmacological classes, including dopamine (D2) receptor antagonists, serotonin (5-HT4) receptor agonists, cholinesterase inhibitors, motilin-like agents, ghrelin-like agents, although many drugs have multiple mechanisms of action.
Metoclopramide is a D2-receptor antagonist with some 5-HT4 receptor agonism that exerts both prokinetic and antiemetic effects. It is the only medication available for the treatment of gastroparesis in the U.S., but, despite the chronicity of the condition, its use is restricted by the FDA to 12 weeks. It is worldwide available, unlike other compounds with similar mode of action and clinical effects that can be prescribed only in selected countries such as clebopride and cinitapride. Since they can cross the blood-brain barrier, they can exert central effects such as anxiety, depression, tremors and other more severe extrapyramidal side effects including, in rare cases, tardive dyskinesia. For this reason, metoclopramide received a black box warning from FDA.112
Domperidone does not cross the blood-brain barrier enough to cause similar neurologic side effects, but it still can exert the antiemetic effects typical of benzamide derivatives, since the vomiting centre is located in the floor of the 4th ventricle in the brainstem, outside the blood-brain barrier. Its use is under regulatory scrutiny in some of the countries where it is commercially available, since it bears a potential risk of cardiac arrhythmias and even sudden death, due to inhibition of hERG channel activity and relative prolongation of the QTc interval, typical of other pharmacological agents with 5-HT4 receptor agonistic activity.113 However, in other countries this can be obtained over-the-counter.
Prucalopride is a 5-HT4 receptor agonist devoid of cardiac adverse effects that exerts an enterokinetic effect and is approved for the treatment of chronic constipation in several countries including the U.S. It has been recently shown to exert also a gastrokinetic effect and to improve symptoms in a relatively small number of patients with IG.114 Velusetrag is another selective 5-HT4 receptor agonist with gastrointestinal prokinetic effects that was shown to induce both symptom relief and GE acceleration in a dose-finding, placebo controlled study in patients with DG or IG.115
Cholinesterase inhibitors exert a prokinetic activity throughout the alimentary canal and are effective in treating diffuse intestinal motor disorders including postoperative ileus, constipation and chronic intestinal pseudo-obstruction.116 Acotiamide is a recently developed cholinesterase inhibitor that also exerts a presynaptic muscarinic autoreceptor inhibitory activity. It was shown to enhance both contractile and accommodation activities of the stomach and, interestingly, to improve dyspeptic symptoms suggestive of gastroparesis, but not epigastric pain and burning symptoms.117, 118
Motilin and ghrelin are hormones secreted by the proximal portions of the alimentary canal that accelerate GE. Pharmacological analogues of these hormones are being investigated for the treatment of gastroparesis. Macrolide antibiotics are agonists at motilin receptors that induce a marked acceleration of GE. Both erythromycin and azithromycin are used in clinical practice, based on the results of small studies,111 but their use is limited by side effects including abdominal cramps, nausea, diarrhea, QT prolongation, as well as by tachyphylaxis.119 Furthermore, when a large study was conducted with a motilin-like agent devoid of antibiotic effect, no clinical benefit was obtained in patients with functional dyspepsia with or without gastroparesis.120 This negative result may be explained by the non-physiological effects of motilin analogues in the postprandial state, since motilin is a typical interdigestive hormone that activates phase III-like activity with a gastric component, thus emptying large undigested particles, unprepared to undergo digestion in the proximal small bowel. Since some patients with gastroparesis also present with impaired gastric accommodation, erythromycin should not be prescribed, as it increases fundic tone.121 The increase in fundic tone may have also contributed to the negative study result of the motilin-like agent. Relamorelin is a synthetic pentapeptide ghrelin receptor agonist that stimulates gastric contractions and has been shown to accelerate GE of solids and improve nausea, fullness, bloating and pain in patients with gastroparesis and type 2 diabetes.122 Two additional trials showed acceleration of GE, but mixed results on improvement in clinical symptoms.123, 124
Antiemetics.
As previously mentioned benzamide derivatives are prokinetics that exert an antiemetic effect by inhibiting dopamine receptors in the brainstem. 5HT-3 receptor antagonists such as granisetron and ondansetron are effective in controlling chemotherapy-induced nausea and vomiting125 and can be prescribed in patients with dysmotility whose clinical picture is dominated by nausea and vomiting but where prokinetics increase the cardiac risks. 5HT-3 receptor antagonists may also be administered via a patch reducing the variability in drug absorption in patients with frequent vomiting. Also, neurokinin antagonists are approved for the treatment of chemotherapy-induced emesis.126 Among these drugs, aprepitant, increases gastric accommodation127 and improves some digestive symptoms in patients with gastroparesis,128 but does not affect GE.127 Although the APRON trial failed to show positive results on the primary endpoint of nausea, several other secondary endpoints showed improvement with aprepitant.128 More recently, Tradipitant was shown to meet primary endpoint of improvement in nausea scores as well as nausea free days and other secondary endpoints on GCSI and PAGI-SYM in a female predominant population of gastroparesis patients (60% idiopathic, 40% diabetic) with moderate to severe nausea.129
Neuromodulators.
Levosulpiride is an antipsychotic agent which accelerates GE by exerting both antidopaminergic and 5-HT4 agonistic activities.130 It has been shown to improve symptoms in patients with both DG131 and IG,132 but this did not correlate with the acceleration of GE. The anxiolytic 5-HT1A agonist buspirone enhances gastric accommodation and improves postprandial symptoms independently from its anxiolytic effect.133 Contradictory results are reported for antidepressants. In a large, well designed study, patients with functional dyspepsia were randomized to amitriptyline or escitalopram. The former improved symptoms exclusively in patients with normal GE and predominant epigastric pain or burning, while the latter did not show any clinical effect.134 Nortriptyline also failed to prove superiority compared to placebo in another randomized controlled trial of gastroparesis patients.135 On the contrary, mirtazapine, an antidepressant with central adrenergic and serotonergic activity, improved nausea, vomiting and loss of appetite in an open-label study of gastroparesis patients.136 If these results are confirmed, mirtazapine would find an indication in patients in patients with weight loss and appetite loss. In severely ill patients with gastroparesis seen in the emergency department, intravenous haloperidol decreased abdominal pain and nausea.137
Treatment – Endoscopic and Surgical
Gastric electrical stimulation.
The precise mechanisms of action of gastric electrical stimulation (GES) are not known. Current stimulation settings do not alter GE. One study showed that loss of gastric ICC predicted poor response to GES.138 Another study showed better efficacy in DG, males and those with shorted duration of gastroparesis.139 The GES assembly consists of 2 leads that are placed in the muscularis propria of greater curvature of the stomach about 10 cm proximal to the pylorus and a subcutaneously placed pulse generator. A temporary GES may be tried to determine response before a permanent device is placed. The first double-blind RCT of 33 patients (17 DG, 16 IG), vomiting frequency decreased during the ON period. However, patients could not separate the ON and OFF periods over the course of the study140 In a retrospective study of 48 patients, using high frequency GES and longer follow up, upper GI symptoms, glycemic control and QoL improved with the use of GES.141 In another trial of diabetic gastroparesis patients, 6 weeks of gastric electrical stimulation reduced vomiting and other symptoms as well as improvement in quality of life.142 A trial by the same group in patients with idiopathic gastroparesis showed improvement in vomiting during the ON period. However, no significant reduction during the ON vs OFF period was noted which was the primary endpoint.143 Another study also documented improvement in abdominal pain as well as decreased use of prokinetics and antiemetic following GES.144 Patients who have malignancy-, drug-associated or those with severe comorbidities may benefit from a temporary GES. However, only three of all the published studies were controlled and of these, only one (see above) showed partially positive results.145 Thus, the evidence from controlled trials is not supportive of the use in the broader population of gastroparesis patients.
Botulinum toxin injections.
Mearin et al. made initial observations of pyloric dysfunction in a subset of patients with DG.146 Injection of botulinum toxin was first used for achalasia and subsequently extended to gastroparesis in open labelled studies. In spite of a widespread clinical use for this strategy, only 2 small randomized controlled trials have assessed efficacy.147, 148 Although underpowered, both of these showed no difference in improvement of symptoms between the placebo and active treatment arms. However, a larger open-labelled study of ~180 patients has suggested greater clinical response to 200 U as compared to 100 U and in women, IG patients and those younger than 50 years of age.149 A more recent study in patients who have 3 cycles per minute rhythm on EGG (thus suggestive of normal gastric body function), both Botox injections or pyloric balloon dilations resulted in 78% symptom improvement at 4 weeks.150 The conclusion in general is that there is no clinical evidence to support widespread use of Botox injections. However, resurgence of interest in assessment of pyloric function and in pyloric therapies may educate us more whether a specific subset of patients with gastroparesis who may respond to Botox injections.
Pyloromyotomy.
The data, albeit poor from the Botox studies, together with the prior manometry studies and advances in endoscopic techniques have led to a resurgence of pyloromyotomy as a treatment for gastroparesis. One study showed that ~70% of gastroparesis patients have loss of pyloric ICC and fibrosis.151 Another case series of 4 patients with Type 1 DG showed atrophy of smooth muscle fibers and collagenous thickening between bundles of muscle fibers. These changes were more prominent in pylorus than gastric body from the same subjects. Additionally, there was presence of eosinophilic inclusion bodies in the muscularis propria.152 The exact role of ICC in pylorus is not defined. Loss of inhibitory nitrergic neurotransmission may result in impaired pyloric relaxation.153 Most studies with Gastric Per Oral Endoscopic Pyloromyotomy (G-POEM) have presented 3-6 mo follow-up data with some studies documenting 12-18 mo follow-up.154 The clinical response rate has been reported to be between 70-80% with GE improvement ranging from 4-64%. Almost all studies showed improvement in nausea and vomiting. Improvement in abdominal pain has been reported in three studies, however, effects disappeared by 6 months. Gastric ulcer and related bleeding were the most commonly reported complications followed by tension capnoperitoneum. Most of the reported studies originate from tertiary care centers with endoscopic expertise and demonstrate 100% technical success. A recent study showed decline in ER visits and hospitalisation following G-POEM.155 The available data allows assessment of efficacy over short term. The long-term effects include possibility of pyloric scarring. Controlled studies are needed both to assess outcomes as well as help predict potential response to pyloromyotomy. Attempts to identify pylorospasm have been made using impedance planimetry (endoscopic functional luminal imaging probe, EndoFlip). In one study, early satiety and postprandial fullness correlated inversely with pyloric diameter and cross sectional area.156 Another approach used is response to intrapyloric Botox injection to triage patients for pyloromyotomy.157 Some groups have proposed use of GES along with pyloroplasty in those with refractory symptoms of DG.158 There is also no standardized technique currently for performing the G-POEM. Hook knife is favored by several endoscopists. Additionally, most studies have favored posterior gastric antral wall or greater curvature instead of anterior wall for submucosal tunneling.159 Correct identification of pyloric muscle ring and hence the extent of submucosal tunneling required is often technically challenging. Finally, there is a variability in the approach with some performing circular muscle whereas others full-thickness myotomy. Generally, a 2.5-3 cm long muscle incision is considered optimal. Further controlled clinical trials with patient selection using high-resolution antroduodenal manometry or EndoFlip assessment of pyloric in combination with long term follow up are awaited. Table 3 highlights the key points in treatment advancements for gastroparesis
Table 3:
Key points in treatment advances for gastroparesis
|
CONCLUSIONS AND FUTURE DIRECTIONS
The last decade has been a turning point in our clinical and molecular understanding of gastroparesis. We now have a good understanding of the cellular events that occur in the stomach of patients with gastroparesis and the central role of macrophages in the disease. The clinical features of once a poorly defined and elusive disease are now much better understood. Variations in clinical presentation by etiology as well as evolution of symptoms and GE over time have been described. From a diagnostic standpoint, 13C breath test and wireless motility capsule have been developed as non-invasive strategies for assessment of GE. Controlled clinical trials have helped evolve the treatment landscape of gastroparesis as well. For example, in a clinical trial of gastroparesis and another trial of functional dyspepsia patients some of whom had delayed GE, tricyclic antidepressants were not useful. Positive results on several parameters obtained with aprepitant and relamorelin have helped identify newer classes of antiemetics with potential for treatment of gastroparesis. Gastroenterokinetics have also been shown to exert positive effects in these patients. There is also an emerging interest in assessing and targeting regional motility disturbances of the stomach. For example, pharmacological fundic relaxants like acotiamide and buspirone and surgical or endoscopic pyloric relaxation using pyloromyotomy are now being investigated in gastroparesis.
However, the challenges still exist in expanding the molecular understanding and establishing approved treatment options. From a diagnostic standpoint and for targeting, future investigations will have to provide regional assessments of gastrointestinal functions and allow investigation of the pyloric anatomy and function. The mechanisms behind fundic and pyloric dysfunction in gastroparesis and their contribution towards GE and clinical symptoms associated with this disorder are still to be determined. Additionally, the role of duodenal and distal gut mechanisms in gastroparesis warrants further investigation. It is possible that a combination of symptoms, molecular and physiological testing may help us identify subsets of gastroparesis patients which can be then targeted using specific treatments. Additional work also needs to be done to understand the spinal and supraspinal mechanisms in severe and refractory end of the disease spectrum. Pain in gastroparesis is still poorly understood and one of the hardest symptom to treat often driving these patients towards opioids. Appropriate paradigms need to be developed for managing diabetes in patients with DG and for maintaining hydration and nutritional requirements. Lastly, identification of genetic, microbial and environmental triggers will help advance understanding of onset of gastroparesis and other associated upper gastrointestinal disorders. In summary, the progress made over the last decade has resulted in better understanding of gastroparesis and a promising outlook for management of this morbid disease. Future strategies will hopefully continue to build on this progress and help us reach a stage where gastroparesis can be prevented, assessed and treated with greater precision and success.
SIGNIFICANCE OF THE STUDY.
What is already known?
Gastroparesis is a morbid disease associated with diabetes, post-surgical, post-infectious or idiopathic in etiology.
Nausea, vomiting, bloating, post prandial fullness and early satiety are cardinal features of gastroparesis. Upper abdominal pain is highly prevalent.
Loss or injury to interstitial cells of Cajal (ICC) plays a central role in animal models of delayed gastric emptying and in humans with gastroparesis.
What is new?
Diabetic and idiopathic gastroparesis have varied clinical features. Idiopathic gastroparesis likely constitutes the biggest subset of gastroparesis patients.
Two-thirds of patients do not improve over 1 year of follow-up.
Small bowel motor abnormalities may also be found in patients with gastroparesis.
Macrophage based immune dysregulation mediates development of delayed gastric emptying by injuring ICC and other components of enteric nervous system.
Assessment of regional abnormalities in gastric function will likely provide a better correlation with symptoms and targeting therapy.
Pharmacological (NK1 antagonistic antiemetics, serotonergic prokinetics, and fundic relaxants) and surgical/endoscopic (pyloromyotomy) expand our treatment options.
How might it impact on clinical practice?
Understanding the clinical presentation, natural history, pathophysiology and new treatment options for gastroparesis will allow advancements in diagnosis, prevention and treatment of this disorder.
Acknowledgement:
The authors would like to acknowledge Mr. Frank Corl for help with the illustration and Ms. Kristy Zodrow and Ms. Lori Anderson for administrative assistance.
Funding: MG and GF are supported by NIH DK068055 and DK74008. MG is also supported by NIH K23 103911. GF is also supported by NIH DK057061.
Abbreviations:
- GE
gastric emptying
- DG
diabetic gastroparesis
- IG
idiopathic gastroparesis
- GCSI
Gastroparesis Cardinal Symptom Index
- ICC
interstitial cells of Cajal
- ENS
enteric nervous system
- GpCRC
NIH Gastroparesis Clinical Research Consortium
- PAGI-SYM
Patients Assessment of Upper Gastrointestinal Disorders-Symptoms
- QoL
quality of life
- IDMMC
interdigestive migrating motor complex
- nNOS
neuronal nitric oxide synthase
- VIP
vasoactive intestinal peptide
- NOD
non-obese diabetic
- FDA
U.S. Food and Drug Administration
- WMC
wireless motility capsule
- D2
dopamine
- 5-HT4
serotonin
- GES
gastric electrical stimulation
- G-POEM
gastric per oral endoscopic pyloromyotomy
Footnotes
Competing Interests: None to report for all authors
REFERENCES
- 1.Parkman HP, Hasler WL, Fisher RS, et al. American Gastroenterological Association medical position statement: diagnosis and treatment of gastroparesis. Gastroenterology 2004;127:1589–91. [DOI] [PubMed] [Google Scholar]
- 2.Hasler WL, Wilson LA, Parkman HP, et al. Factors related to abdominal pain in gastroparesis: contrast to patients with predominant nausea and vomiting. Neurogastroenterol Motil 2013;25:427–38, e300-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Soykan I, Sivri B, Sarosiek I, et al. Demography, clinical characteristics, psychological and abuse profiles, treatment, and long-term follow-up of patients with gastroparesis. Dig Dis Sci 1998;43:2398–404. [DOI] [PubMed] [Google Scholar]
- 4.Choung RS, Locke GR 3rd, Schleck CD, et al. Risk of gastroparesis in subjects with type 1 and 2 diabetes in the general population. Am J Gastroenterol 2012;107:82–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Syed AR, Wolfe MM, Calles-Escandon J. Epidemiology and diagnosis of gastroparesis in the United States: a population-based study. J Clin Gastroenterol 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parkman HP, Yates K, Hasler WL, et al. Similarities and differences between diabetic and idiopathic gastroparesis. Clin Gastroenterol Hepatol 2011;9:1056–64; quiz e133-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parkman HP, Yates K, Hasler WL, et al. Clinical features of idiopathic gastroparesis vary with sex, body mass, symptom onset, delay in gastric emptying, and gastroparesis severity. Gastroenterology 2011;140:101–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hasler WL, Wilson LA, Nguyen LA, et al. Opioid use and potency are associated with clinical features, quality of life, and use of resources in patients with gastroparesis. Clin Gastroenterol Hepatol 2019;17:1285–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vijayvargiya P, Jameie-Oskooei S, Camilleri M, et al. Association between delayed gastric emptying and upper gastrointestinal symptoms: a systematic review and meta-analysis. Gut 2018. [DOI] [PubMed] [Google Scholar]
- 10.Stanghellini V, Tack J. Gastroparesis: separate entity or just a part of dyspepsia? Gut 2014;63:1972–8. [DOI] [PubMed] [Google Scholar]
- 11.Bharucha AE, Camilleri M, Forstrom LA, et al. Relationship between clinical features and gastric emptying disturbances in diabetes mellitus. Clin Endocrinol (Oxf) 2009;70:415–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Orthey P, Dadparvar SM, Parkman HP, et al. Enhanced gastric emptying scintigraphy to assess fundic accommodation using intragastric meal distribution and antral contractility. J Nucl Med Technol 2019;47:138–43. [DOI] [PubMed] [Google Scholar]
- 13.Farrugia G Histologic changes in diabetic gastroparesis. Gastroenterol Clin North Am 2015;44:31–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cipriani G, Gibbons SJ, Kashyap PC, et al. Intrinsic gastrointestinal macrophages: their phenotype and role in gastrointestinal motility. Cell Mol Gastroenterol Hepatol 2016;2:120–30 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cipriani G, Terhaar ML, Eisenman ST, et al. Muscularis propria macrophages alter the proportion of nitrergic but not cholinergic gastric myenteric neurons. Cell Mol Gastroenterol Hepatol 2019;7:689–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parkman HP, Hallinan EK, Hasler WL, et al. Early satiety and postprandial fullness in gastroparesis correlate with gastroparesis severity, gastric emptying, and water load testing. Neurogastroenterol Motil 2017;29:doi: 10.1111/nmo.12981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parkman HP, Hallinan EK, Hasler WL, et al. Nausea and vomiting in gastroparesis: similarities and differences in idiopathic and diabetic gastroparesis. Neurogastroenterol Motil 2016;28:1902–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hasler WL, Wilson LA, Parkman HP, et al. Bloating in gastroparesis: severity, impact, and associated factors. Am J Gastroenterol 2011;106:1492–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hasler WL, Parkman HP, Wilson LA, et al. Psychological dysfunction is associated with symptom severity but not disease etiology or degree of gastric retention in patients with gastroparesis. Am J Gastroenterol 2010;105:2357–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jung HK, Choung RS, Locke GR 3rd, et al. The incidence, prevalence, and outcomes of patients with gastroparesis in Olmsted County, Minnesota, from 1996 to 2006. Gastroenterology 2009;136:1225–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sarnelli G, Caenepeel P, Geypens B, et al. Symptoms associated with impaired gastric emptying of solids and liquids in functional dyspepsia. Am J Gastroenterol 2003;98:783–8. [DOI] [PubMed] [Google Scholar]
- 22.Camilleri M, Dubois D, Coulie B, et al. Prevalence and socioeconomic impact of upper gastrointestinal disorders in the United States: results of the US Upper Gastrointestinal Study. Clin Gastroenterol Hepatol 2005;3:543–52. [DOI] [PubMed] [Google Scholar]
- 23.Rey E, Choung RS, Schleck CD, et al. Prevalence of hidden gastroparesis in the community: the gastroparesis “iceberg”. J Neurogastroenterol Motil 2012;18:34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stanghellini V, Chan FK, Hasler WL, et al. Gastroduodenal disorders. Gastroenterology 2016;150:1380–92. [DOI] [PubMed] [Google Scholar]
- 25.Parkman HP, Yamada G, Van Natta ML, et al. Ethnic, racial, and sex differences in etiology, symptoms, treatment, and symptom outcomes of patients with gastroparesis. Clin Gastroenterol Hepatol 2019;17:1489–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Friedenberg FK, Kowalczyk M, Parkman HP. The influence of race on symptom severity and quality of life in gastroparesis. J Clin Gastroenterol 2013;47:757–61. [DOI] [PubMed] [Google Scholar]
- 27.Aleppo G, Calhoun P, Foster NC, et al. Reported gastroparesis in adults with type 1 diabetes (T1D) from the T1D Exchange clinic registry. J Diabetes Complications 2017;31:1669–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chedid V, Brandler J, Vijayvargiya P, et al. Characterization of upper gastrointestinal symptoms, gastric motor functions, and associations in patients with diabetes at a referral center. Am J Gastroenterol 2019;114:143–54. [DOI] [PubMed] [Google Scholar]
- 29.Pasricha PJ, Yates KP, Nguyen L, et al. Outcomes and factors associated with reduced symptoms in patients with gastroparesis. Gastroenterology 2015;149:1762–74 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koch KL, Hasler WL, Yates KP, et al. Baseline features and differences in 48 week clinical outcomes in patients with gastroparesis and type 1 vs type 2 diabetes. Neurogastroenterol Motil 2016;28:1001–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jehangir A, Parkman HP. Chronic opioids in gastroparesis: Relationship with gastrointestinal symptoms, healthcare utilization and employment. World J Gastroenterol 2017;23:7310–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chang J, Rayner CK, Jones KL, et al. Prognosis of diabetic gastroparesis--a 25-year evaluation. Diabet Med 2013;30:e185–8. [DOI] [PubMed] [Google Scholar]
- 33.Nusrat S, Bielefeldt K. Gastroparesis on the rise: incidence vs awareness? Neurogastroenterol Motil 2013;25:16–22. [DOI] [PubMed] [Google Scholar]
- 34.Kofod-Andersen K, Tarnow L. Prevalence of gastroparesis-related symptoms in an unselected cohort of patients with Type 1 diabetes. J Diabetes Complications 2012;26:89–93. [DOI] [PubMed] [Google Scholar]
- 35.Phillips LK, Deane AM, Jones KL, et al. Gastric emptying and glycaemia in health and diabetes mellitus. Nat Rev Endocrinol 2015;11:112–28. [DOI] [PubMed] [Google Scholar]
- 36.Boronikolos GC, Menge BA, Schenker N, et al. Upper gastrointestinal motility and symptoms in individuals with diabetes, prediabetes and normal glucose tolerance. Diabetologia 2015;58:1175–82. [DOI] [PubMed] [Google Scholar]
- 37.Kelly KA. Gastric emptying of liquids and solids: roles of proximal and distal stomach. Am J Physiol 1980;239:G71–6. [DOI] [PubMed] [Google Scholar]
- 38.Malagelada JR. Gastric, pancreatic and biliary responses to a meal. In: Johnson LR, ed. Physiology of the Gastrointestinal Tract. New York: Raven Press, 1981:893–924. [Google Scholar]
- 39.Malagelada JR, Camilleri M, Stanghellini V. Manometric diagnosis of gastrointestinal motility disorders. New York: Thieme, 1986. [Google Scholar]
- 40.Pironi L, Stanghellini V, Miglioli M, et al. Fat-induced ileal brake in humans: a dose-dependent phenomenon correlated to the plasma levels of peptide YY. Gastroenterology 1993;105:733–9. [DOI] [PubMed] [Google Scholar]
- 41.Vella A, Camilleri M. The Gastrointestinal Tract as an Integrator of Mechanical and Hormonal Response to Nutrient Ingestion. Diabetes 2017;66:2729–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cogliandro RF, Rizzoli G, Bellacosa L, et al. Is gastroparesis a gastric disease? Neurogastroenterol Motil 2019:e13562. [DOI] [PubMed] [Google Scholar]
- 43.Szurszewski JH. A migrating electric complex of canine small intestine. Am J Physiol 1969;217:1757–63. [DOI] [PubMed] [Google Scholar]
- 44.Kellow JE, Borody TJ, Phillips SF, et al. Human interdigestive motility: variations in patterns from esophagus to colon. Gastroenterology 1986;91:386–95. [DOI] [PubMed] [Google Scholar]
- 45.Stanghellini V, Ghidini C, Maccarini MR, et al. Fasting and postprandial gastrointestinal motility in ulcer and non-ulcer dyspepsia. Gut 1992;33:184–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bharucha AE, Manduca A, Lake DS, et al. Gastric motor disturbances in patients with idiopathic rapid gastric emptying. Neurogastroenterol Motil 2011;23:617–e252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Park SY, Acosta A, Camilleri M, et al. Gastric motor dysfunction in patients with functional gastroduodenal symptoms. Am J Gastroenterol 2017;112:1689–99. [DOI] [PubMed] [Google Scholar]
- 48.Buysschaert M, Donckier J, Dive A, et al. Gastric acid and pancreatic polypeptide responses to sham feeding are impaired in diabetic subjects with autonomic neuropathy. Diabetes 1985;34:1181–5. [DOI] [PubMed] [Google Scholar]
- 49.Guy RJ, Dawson JL, Garrett JR, et al. Diabetic gastroparesis from autonomic neuropathy: surgical considerations and changes in vagus nerve morphology. J Neurol Neurosurg Psychiatry 1984;47:686–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schmidt RE, Green KG, Snipes LL, et al. Neuritic dystrophy and neuronopathy in Akita (Ins2(Akita)) diabetic mouse sympathetic ganglia. Exp Neurol 2009;216:207–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Carroll SL, Byer SJ, Dorsey DA, et al. Ganglion-specific patterns of diabetes-modulated gene expression are established in prevertebral and paravertebral sympathetic ganglia prior to the development of neuroaxonal dystrophy. J Neuropathol Exp Neurol 2004;63:1144–54. [DOI] [PubMed] [Google Scholar]
- 52.Grover M, Farrugia G, Lurken MS, et al. Cellular changes in diabetic and idiopathic gastroparesis. Gastroenterology 2011;140:1575–85 e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Faussone-Pellegrini MS, Grover M, Pasricha PJ, et al. Ultrastructural differences between diabetic and idiopathic gastroparesis. J Cell Mol Med 2012;16:1573–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Burnstock G, Mirsky R, Belai A. Reversal of nerve damage in streptozotocin-diabetic rats by acute application of insulin in vitro. Clin Sci (Lond) 1988;75:629–35. [DOI] [PubMed] [Google Scholar]
- 55.Imaeda K, Takano H, Koshita M, et al. Electrical properties of colonic smooth muscle in spontaneously non-insulin-dependent diabetic rats. J Smooth Muscle Res 1998;34:1–11. [DOI] [PubMed] [Google Scholar]
- 56.Patil CS, Singh VP, Jain NK, et al. Inhibitory effect of sildenafil on gastrointestinal smooth muscle: role of NO-cGMP transduction pathway. Indian J Exp Biol 2005;43:167–71. [PubMed] [Google Scholar]
- 57.Gangula PR, Maner WL, Micci MA, et al. Diabetes induces sex-dependent changes in neuronal nitric oxide synthase dimerization and function in the rat gastric antrum. Am J Physiol Gastrointest Liver Physiol 2007;292:G725–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Korenaga K, Micci MA, Taglialatela G, et al. Suppression of nNOS expression in rat enteric neurones by the receptor for advanced glycation end-products. Neurogastroenterol Motil 2006;18:392–400. [DOI] [PubMed] [Google Scholar]
- 59.Wang Y, Newton DC, Marsden PA. Neuronal NOS: gene structure, mRNA diversity, and functional relevance. Crit Rev Neurobiol 1999;13:21–43. [DOI] [PubMed] [Google Scholar]
- 60.Gangula PR, Chinnathambi V, Hale AB, et al. Impairment of nitrergic system and delayed gastric emptying in low density lipoprotein receptor deficient female mice. Neurogastroenterol Motil 2011;23:773–e335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ravella K, Yang H, Gangula PR. Impairment of gastric nitrergic and NRF2 system in apolipoprotein E knockout mice. Dig Dis Sci 2012;57:1504–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sivarao DV, Mashimo H, Goyal RK. Pyloric sphincter dysfunction in nNOS−/− and W/Wv mutant mice: animal models of gastroparesis and duodenogastric reflux. Gastroenterology 2008;135:1258–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Micci MA, Kahrig KM, Simmons RS, et al. Neural stem cell transplantation in the stomach rescues gastric function in neuronal nitric oxide synthase-deficient mice. Gastroenterology 2005;129:1817–24. [DOI] [PubMed] [Google Scholar]
- 64.Zandecki M, Vanden Berghe P, Depoortere I, et al. Characterization of myenteric neuropathy in the jejunum of spontaneously diabetic BB-rats. Neurogastroenterol Motil 2008;20:818–28. [DOI] [PubMed] [Google Scholar]
- 65.Gangula PR, Mukhopadhyay S, Ravella K, et al. Tetrahydrobiopterin (BH4), a cofactor for nNOS, restores gastric emptying and nNOS expression in female diabetic rats. Am J Physiol Gastrointest Liver Physiol 2010;298:G692–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gangula PR, Challagundla KB, Ravella K, et al. Sepiapterin alleviates impaired gastric nNOS function in spontaneous diabetic female rodents through NRF2 mRNA turnover and miRNA biogenesis pathway. Am J Physiol Gastrointest Liver Physiol 2018;315:G980–G90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dishy V, Cohen Pour M, Feldman L, et al. The effect of sildenafil on gastric emptying in patients with end-stage renal failure and symptoms of gastroparesis. Clin Pharmacol Ther 2004;76:281–6. [DOI] [PubMed] [Google Scholar]
- 68.Sun WM, Doran S, Jones KL, et al. Effects of nitroglycerin on liquid gastric emptying and antropyloroduodenal motility. Am J Physiol 1998;275:G1173–8. [DOI] [PubMed] [Google Scholar]
- 69.Choi KM, Kashyap PC, Dutta N, et al. CD206-positive M2 macrophages that express heme oxygenase-1 protect against diabetic gastroparesis in mice. Gastroenterology 2010;138:2399–409, 409 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Stenkamp-Strahm CM, Kappmeyer AJ, Schmalz JT, et al. High-fat diet ingestion correlates with neuropathy in the duodenum myenteric plexus of obese mice with symptoms of type 2 diabetes. Cell Tissue Res 2013;354:381–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bhetwal BP, An C, Baker SA, et al. Impaired contractile responses and altered expression and phosphorylation of Ca(2+) sensitization proteins in gastric antrum smooth muscles from ob/ob mice. J Muscle Res Cell Motil 2013;34:137–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Asakawa A, Inui A, Ueno N, et al. Ob/ob mice as a model of delayed gastric emptying. J Diabetes Complications 2003;17:27–8. [DOI] [PubMed] [Google Scholar]
- 73.Farrugia G Interstitial cells of Cajal in health and disease. Neurogastroenterol Motil 2008;20 Suppl 1:54–63. [DOI] [PubMed] [Google Scholar]
- 74.Grover M, Bernard CE, Pasricha PJ, et al. Diabetic and idiopathic gastroparesis is associated with loss of CD206-positive macrophages in the gastric antrum. Neurogastroenterol Motil 2017;29:doi: 10.1111/nmo.13018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Iwasaki H, Kajimura M, Osawa S, et al. A deficiency of gastric interstitial cells of Cajal accompanied by decreased expression of neuronal nitric oxide synthase and substance P in patients with type 2 diabetes mellitus. J Gastroenterol 2006;41:1076–87. [DOI] [PubMed] [Google Scholar]
- 76.Forster J, Damjanov I, Lin Z, et al. Absence of the interstitial cells of Cajal in patients with gastroparesis and correlation with clinical findings. J Gastrointest Surg 2005;9:102–8. [DOI] [PubMed] [Google Scholar]
- 77.Wang XY, Huizinga JD, Diamond J, et al. Loss of intramuscular and submuscular interstitial cells of Cajal and associated enteric nerves is related to decreased gastric emptying in streptozotocin-induced diabetes. Neurogastroenterol Motil 2009;21:1095–e92. [DOI] [PubMed] [Google Scholar]
- 78.Gomez-Pinilla PJ, Gibbons SJ, Bardsley MR, et al. Ano1 is a selective marker of interstitial cells of Cajal in the human and mouse gastrointestinal tract. Am J Physiol Gastrointest Liver Physiol 2009;296:G1370–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mazzone A, Bernard CE, Strege PR, et al. Altered expression of Ano1 variants in human diabetic gastroparesis. J Biol Chem 2011;286:13393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lin Z, Sarosiek I, Forster J, et al. Association of the status of interstitial cells of Cajal and electrogastrogram parameters, gastric emptying and symptoms in patients with gastroparesis. Neurogastroenterol Motil 2010;22:56–61, e10. [DOI] [PubMed] [Google Scholar]
- 81.Grover M, Bernard CE, Pasricha PJ, et al. Clinical-histological associations in gastroparesis: results from the Gastroparesis Clinical Research Consortium. Neurogastroenterol Motil 2012;24:531–9, e249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Horvath VJ, Vittal H, Lorincz A, et al. Reduced stem cell factor links smooth myopathy and loss of interstitial cells of cajal in murine diabetic gastroparesis. Gastroenterology 2006;130:759–70. [DOI] [PubMed] [Google Scholar]
- 83.Herring BP, Chen M, Mihaylov P, et al. Transcriptome profiling reveals significant changes in the gastric muscularis externa with obesity that partially overlap those that occur with idiopathic gastroparesis. BMC Med Genomics 2019;12:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bharucha AE, Kulkarni A, Choi KM, et al. First-in-human study demonstrating pharmacological activation of heme oxygenase-1 in humans. Clin Pharmacol Ther 2010;87:187–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cipriani G, Gibbons SJ, Verhulst PJ, et al. Diabetic Csf1(op/op) mice lacking macrophages are protected against the development of delayed gastric emptying. Cell Mol Gastroenterol Hepatol 2016;2:40–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cipriani G, Gibbons SJ, Miller KE, et al. Change in populations of macrophages promotes development of delayed gastric emptying in mice. Gastroenterology 2018;154:2122–36 e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gibbons SJ, Grover M, Choi KM, et al. Repeat polymorphisms in the Homo sapiens heme oxygenase-1 gene in diabetic and idiopathic gastroparesis. PLoS One 2017;12:e0187772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Grover M, Gibbons SJ, Nair AA, et al. Transcriptomic signatures reveal immune dysregulation in human diabetic and idiopathic gastroparesis. BMC Med Genomics 2018;11:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Revicki DA, Rentz AM, Dubois D, et al. Development and validation of a patient-assessed gastroparesis symptom severity measure: the Gastroparesis Cardinal Symptom Index. Aliment Pharmacol Ther 2003;18:141–50. [DOI] [PubMed] [Google Scholar]
- 90.Revicki DA, Camilleri M, Kuo B, et al. Evaluating symptom outcomes in gastroparesis clinical trials: validity and responsiveness of the Gastroparesis Cardinal Symptom Index-Daily Diary (GCSI-DD). Neurogastroenterol Motil 2012;24:456–63, e215-6. [DOI] [PubMed] [Google Scholar]
- 91.Tack J, Carbone F, Holvoet L, et al. The use of pictograms improves symptom evaluation by patients with functional dyspepsia. Aliment Pharmacol Ther 2014;40:523–30. [DOI] [PubMed] [Google Scholar]
- 92.Pasricha PJ, Camilleri M, Hasler WL, et al. White Paper AGA: Gastroparesis: clinical and regulatory insights for clinical trials. Clin Gastroenterol Hepatol 2017;15:1184–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tougas G, Eaker EY, Abell TL, et al. Assessment of gastric emptying using a low fat meal: establishment of international control values. Am J Gastroenterol 2000;95:1456–62. [DOI] [PubMed] [Google Scholar]
- 94.Ghoos YF, Maes BD, Geypens BJ, et al. Measurement of gastric emptying rate of solids by means of a carbon-labeled octanoic acid breath test. Gastroenterology 1993;104:1640–7. [DOI] [PubMed] [Google Scholar]
- 95.Szarka LA, Camilleri M, Vella A, et al. A stable isotope breath test with a standard meal for abnormal gastric emptying of solids in the clinic and in research. Clin Gastroenterol Hepatol 2008;6:635–43 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Viramontes BE, Kim DY, Camilleri M, et al. Validation of a stable isotope gastric emptying test for normal, accelerated or delayed gastric emptying. Neurogastroenterol Motil 2001;13:567–74. [DOI] [PubMed] [Google Scholar]
- 97.FDA approves breath test to aid in diagnosis of delayed gastric emptying.
- 98.George NS, Sankineni A, Parkman HP. Small intestinal bacterial overgrowth in gastroparesis. Dig Dis Sci 2014;59:645–52. [DOI] [PubMed] [Google Scholar]
- 99.Parkman HP. Assessment of gastric emptying and small-bowel motility: scintigraphy, breath tests, manometry, and SmartPill. Gastrointest Endosc Clin N Am 2009;19:49–55, vi. [DOI] [PubMed] [Google Scholar]
- 100.Kuo B, McCallum RW, Koch KL, et al. Comparison of gastric emptying of a nondigestible capsule to a radio-labelled meal in healthy and gastroparetic subjects. Aliment Pharmacol Ther 2008;27:186–96. [DOI] [PubMed] [Google Scholar]
- 101.Kuo B, Maneerattanaporn M, Lee AA, et al. Generalized transit delay on wireless motility capsule testing in patients with clinical suspicion of gastroparesis, small intestinal dysmotility, or slow transit constipation. Dig Dis Sci 2011;56:2928–38. [DOI] [PubMed] [Google Scholar]
- 102.Lee AA, Rao S, Nguyen LA, et al. Validation of diagnostic and performance characteristics of the wireless motility capsule in patients with suspected gastroparesis. Clin Gastroenterol Hepatol 2019:doi: 10.1016/j.cgh.2018.11.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zikos TA, Kamal AN, Neshatian L, et al. High prevalence of slow transit constipation in patients with gastroparesis. J Neurogastroenterol Motil 2019;25:267–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Fidler J, Bharucha AE, Camilleri M, et al. Application of magnetic resonance imaging to measure fasting and postprandial volumes in humans. Neurogastroenterol Motil 2009;21:42–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bolondi L, Bortolotti M, Santi V, et al. Measurement of gastric emptying time by real-time ultrasonography. Gastroenterology 1985;89:752–9. [DOI] [PubMed] [Google Scholar]
- 106.Jones KL, O’Donovan D, Horowitz M, et al. Effects of posture on gastric emptying, transpyloric flow, and hunger after a glucose drink in healthy humans. Dig Dis Sci 2006;51:1331–8. [DOI] [PubMed] [Google Scholar]
- 107.Gentilcore D, Hausken T, Horowitz M, et al. Measurements of gastric emptying of low- and high-nutrient liquids using 3D ultrasonography and scintigraphy in healthy subjects. Neurogastroenterol Motil 2006;18:1062–8. [DOI] [PubMed] [Google Scholar]
- 108.Olausson EA, Storsrud S, Grundin H, et al. A small particle size diet reduces upper gastrointestinal symptoms in patients with diabetic gastroparesis: a randomized controlled trial. Am J Gastroenterol 2014;109:375–85. [DOI] [PubMed] [Google Scholar]
- 109.Parkman HP, Yates KP, Hasler WL, et al. Dietary intake and nutritional deficiencies in patients with diabetic or idiopathic gastroparesis. Gastroenterology 2011;141:486–98, 98 e1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Youssef AS, Parkman HP, Nagar S. Drug-drug interactions in pharmacologic management of gastroparesis. Neurogastroenterol Motil 2015;27:1528–41. [DOI] [PubMed] [Google Scholar]
- 111.Pittayanon R, Yuan Y, Bollegala NP, et al. Prokinetics for functional dyspepsia: A systematic review and meta-analysis of randomized control trials. Am J Gastroenterol 2019. [DOI] [PubMed] [Google Scholar]
- 112.Rao AS, Camilleri M. Review article: metoclopramide and tardive dyskinesia. Aliment Pharmacol Ther 2010;31:11–9. [DOI] [PubMed] [Google Scholar]
- 113.Tack J, Camilleri M, Chang L, et al. Systematic review: cardiovascular safety profile of 5-HT(4) agonists developed for gastrointestinal disorders. Aliment Pharmacol Ther 2012;35:745–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Carbone F, Van den Houte K, Clevers E, et al. Prucalopride in gastroparesis: a randomized placebo-controlled cross-over study. Am J Gastroenterol 2019. (in press). [DOI] [PubMed] [Google Scholar]
- 115.Theravance Biopharma announces positive top-line results from phase 2b study of Velusetrag (TD-5108) in patients with gastroparesis.August 2, 2017.
- 116.Stanghellini V, Cogliandro RF, De Giorgio R, et al. Natural history of intestinal failure induced by chronic idiopathic intestinal pseudo-obstruction. Transplant Proc 2010;42:15–8. [DOI] [PubMed] [Google Scholar]
- 117.Altan E, Masaoka T, Farre R, et al. Acotiamide, a novel gastroprokinetic for the treatment of patients with functional dyspepsia: postprandial distress syndrome. Expert Rev Gastroenterol Hepatol 2012;6:533–44. [DOI] [PubMed] [Google Scholar]
- 118.Matsueda K, Hongo M, Tack J, et al. A placebo-controlled trial of acotiamide for meal-related symptoms of functional dyspepsia. Gut 2012;61:821–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Richards RD, Davenport K, McCallum RW. The treatment of idiopathic and diabetic gastroparesis with acute intravenous and chronic oral erythromycin. Am J Gastroenterol 1993;88:203–7. [PubMed] [Google Scholar]
- 120.Talley NJ, Verlinden M, Snape W, et al. Failure of a motilin receptor agonist (ABT-229) to relieve the symptoms of functional dyspepsia in patients with and without delayed gastric emptying: a randomized double-blind placebo-controlled trial. Aliment Pharmacol Ther 2000;14:1653–61. [DOI] [PubMed] [Google Scholar]
- 121.Yoon SB, Choi MG, Lim CH, et al. The effect of exenatide and erythromycin on postprandial symptoms and their relation to gastric functions. Digestion 2012;85:211–8. [DOI] [PubMed] [Google Scholar]
- 122.Shin A, Camilleri M, Busciglio I, et al. Randomized controlled phase Ib study of ghrelin agonist, RM-131, in type 2 diabetic women with delayed gastric emptying: pharmacokinetics and pharmacodynamics. Diabetes Care 2013;36:41–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Camilleri M, McCallum RW, Tack J, et al. Efficacy and safety of relamorelin in diabetics with symptoms of gastroparesis: A randomized, placebo-controlled study. Gastroenterology 2017;153:1240–50 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lembo A, Camilleri M, McCallum R, et al. Relamorelin reduces vomiting frequency and severity and accelerates gastric emptying in adults with diabetic gastroparesis. Gastroenterology 2016;151:87–96 e6. [DOI] [PubMed] [Google Scholar]
- 125.Gilmore J, D’Amato S, Griffith N, et al. Recent advances in antiemetics: new formulations of 5HT3-receptor antagonists. Cancer Manag Res 2018;10:1827–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yokoe T, Hayashida T, Nagayama A, et al. Effectiveness of antiemetic regimens for highly emetogenic chemotherapy-induced nausea and vomiting: A systematic review and network meta-analysis. The oncologist 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Jacob D, Busciglio I, Burton D, et al. Effects of NK1 receptors on gastric motor functions and satiation in healthy humans: results from a controlled trial with the NK1 antagonist aprepitant. Am J Physiol Gastrointest Liver Physiol 2017;313:G505–G10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Pasricha PJ, Yates KP, Sarosiek I, et al. Aprepitant has mixed effects on nausea and reduces other symptoms in patients with gastroparesis and related disorders. Gastroenterology 2018;154:65–76 e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Carlin JL, Lieberman VR, Dahal A, et al. Tradipitant, a novel NK-1 receptor antagonist, significantly improved nausea and other symptoms of gastroparesis: results of a multicenter, randomized, double-blind, placebo-controlled phase II trial. Gastroenterol 2019;156:S-1510-1. [Google Scholar]
- 130.Tonini M, Cipollina L, Poluzzi E, et al. Review article: clinical implications of enteric and central D2 receptor blockade by antidopaminergic gastrointestinal prokinetics. Aliment Pharmacol Ther 2004;19:379–90. [DOI] [PubMed] [Google Scholar]
- 131.Melga P, Mansi C, Ciuchi E, et al. Chronic administration of levosulpiride and glycemic control in IDDM patients with gastroparesis. Diabetes Care 1997;20:55–8. [DOI] [PubMed] [Google Scholar]
- 132.Mansi C, Borro P, Giacomini M, et al. Comparative effects of levosulpiride and cisapride on gastric emptying and symptoms in patients with functional dyspepsia and gastroparesis. Aliment Pharmacol Ther 2000;14:561–9. [DOI] [PubMed] [Google Scholar]
- 133.Tack J, Janssen P, Masaoka T, et al. Efficacy of buspirone, a fundus-relaxing drug, in patients with functional dyspepsia. Clin Gastroenterol Hepatol 2012;10:1239–45. [DOI] [PubMed] [Google Scholar]
- 134.Talley NJ, Locke GR, Saito YA, et al. Effect of amitriptyline and escitalopram on functional dyspepsia: a multicenter, randomized controlled study. Gastroenterology 2015;149:340–9 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Parkman HP, Van Natta ML, Abell TL, et al. Effect of nortriptyline on symptoms of idiopathic gastroparesis: the NORIG randomized clinical trial. JAMA 2013;310:2640–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Malamood M, Roberts A, Kataria R, et al. Mirtazapine for symptom control in refractory gastroparesis. Drug Des Devel Ther 2017;11:1035–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Roldan CJ, Chambers KA, Paniagua L, et al. Randomized controlled double-blind trial comparing haloperidol combined with conventional therapy to conventional therapy alone in patients with symptomatic gastroparesis. Acad Emerg Med 2017;24:1307–14. [DOI] [PubMed] [Google Scholar]
- 138.Omer E, Kedar A, Nagarajarao HS, et al. Cajal Cell Counts are Important Predictors of Outcomes in Drug Refractory Gastroparesis Patients With Neurostimulation. J Clin Gastroenterol 2018. [DOI] [PubMed] [Google Scholar]
- 139.Musunuru S, Beverstein G, Gould J. Preoperative predictors of significant symptomatic response after 1 year of gastric electrical stimulation for gastroparesis. World J Surg 2010;34:1853–8. [DOI] [PubMed] [Google Scholar]
- 140.Abell T, McCallum R, Hocking M, et al. Gastric electrical stimulation for medically refractory gastroparesis. Gastroenterology 2003;125:421–8. [DOI] [PubMed] [Google Scholar]
- 141.Lin Z, Forster J, Sarosiek I, et al. Treatment of diabetic gastroparesis by high-frequency gastric electrical stimulation. Diabetes Care 2004;27:1071–6. [DOI] [PubMed] [Google Scholar]
- 142.McCallum RW, Snape W, Brody F, et al. Gastric electrical stimulation with Enterra therapy improves symptoms from diabetic gastroparesis in a prospective study. Clin Gastroenterol Hepatol 2010;8:947–54; quiz e116. [DOI] [PubMed] [Google Scholar]
- 143.McCallum RW, Sarosiek I, Parkman HP, et al. Gastric electrical stimulation with Enterra therapy improves symptoms of idiopathic gastroparesis. Neurogastroenterol Motil 2013;25:815–e636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Lin Z, McElhinney C, Sarosiek I, et al. Chronic gastric electrical stimulation for gastroparesis reduces the use of prokinetic and/or antiemetic medications and the need for hospitalizations. Dig Dis Sci 2005;50:1328–34. [DOI] [PubMed] [Google Scholar]
- 145.Wo JM, Nowak TV, Waseem S, et al. Gastric Electrical Stimulation for Gastroparesis and Chronic Unexplained Nausea and Vomiting. Curr Treat Options Gastroenterol 2016;14:386–400. [DOI] [PubMed] [Google Scholar]
- 146.Mearin F, Camilleri M, Malagelada JR. Pyloric dysfunction in diabetics with recurrent nausea and vomiting. Gastroenterology 1986;90:1919–25. [DOI] [PubMed] [Google Scholar]
- 147.Arts J, Holvoet L, Caenepeel P, et al. Clinical trial: a randomized-controlled crossover study of intrapyloric injection of botulinum toxin in gastroparesis. Aliment Pharmacol Ther 2007;26:1251–8. [DOI] [PubMed] [Google Scholar]
- 148.Friedenberg FK, Palit A, Parkman HP, et al. Botulinum toxin A for the treatment of delayed gastric emptying. Am J Gastroenterol 2008;103:416–23. [DOI] [PubMed] [Google Scholar]
- 149.Coleski R, Anderson MA, Hasler WL. Factors associated with symptom response to pyloric injection of botulinum toxin in a large series of gastroparesis patients. Dig Dis Sci 2009;54:2634–42. [DOI] [PubMed] [Google Scholar]
- 150.Wellington J, Scott B, Kundu S, et al. Effect of endoscopic pyloric therapies for patients with nausea and vomiting and functional obstructive gastroparesis. Auton Neurosci 2017;202:56–61. [DOI] [PubMed] [Google Scholar]
- 151.Bashashati M, Moraveji S, Torabi A, et al. Pathological findings of the antral and pyloric smooth muscle in patients with gastroparesis-like syndrome compared to gastroparesis: similarities and differences. Dig Dis Sci 2017;62:2828–33. [DOI] [PubMed] [Google Scholar]
- 152.Ejskjaer NT, Bradley JL, Buxton-Thomas MS, et al. Novel surgical treatment and gastric pathology in diabetic gastroparesis. Diabet Med 1999;16:488–95. [DOI] [PubMed] [Google Scholar]
- 153.Ishiguchi T, Nakajima M, Sone H, et al. Gastric distension-induced pyloric relaxation: central nervous system regulation and effects of acute hyperglycaemia in the rat. J Physiol 2001;533:801–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Mekaroonkamol P, Dacha S, Patel V, et al. Outcomes of per oral endoscopic pyloromyotomy in the United States. Gastrointest Endosc Clin N Am 2019;29:151–60. [DOI] [PubMed] [Google Scholar]
- 155.Mekaroonkamol P, Dacha S, Wang L, et al. Gastric peroral endoscopic pyloromyotomy reduces symptoms, increases quality of life, and reduces health care use for patients with gastroparesis. Clin Gastroenterol Hepatol 2019;17:82–9. [DOI] [PubMed] [Google Scholar]
- 156.Malik Z, Sankineni A, Parkman HP. Assessing pyloric sphincter pathophysiology using EndoFLIP in patients with gastroparesis. Neurogastroenterol Motil 2015;27:524–31. [DOI] [PubMed] [Google Scholar]
- 157.Rodriguez JH, Haskins IN, Strong AT, et al. Per oral endoscopic pyloromyotomy for refractory gastroparesis: initial results from a single institution. Surg Endosc 2017;31:5381–8. [DOI] [PubMed] [Google Scholar]
- 158.Davis BR, Sarosiek I, Bashashati M, et al. The long-term efficacy and safety of pyloroplasty combined withgGastric electrical stimulation therapy in gastroparesis. J Gastrointest Surg 2017;21:222–7. [DOI] [PubMed] [Google Scholar]
- 159.Tao J, Patel V, Mekaroonkamol P, et al. Technical aspects of peroral endoscopic pyloromyotomy. Gastrointest Endosc Clin N Am 2019;29:117–26. [DOI] [PubMed] [Google Scholar]