Abstract
Background: A multi-institutional, randomized phase II trial of continuous dosing of dabrafenib with or without trametinib is ongoing in metastatic thyroid cancer. Preclinical evidence and emerging clinical experience in other cancers support evaluating intermittent dosing of these two agents to achieve more durable response, while being better tolerated and more cost effective.
Patients: Two consecutive patients with symptomatic, metastatic radioactive iodine–resistant BRAFV600E mutated papillary thyroid cancer and poor performance status were treated initially with dabrafenib 150 mg twice daily plus trametinib 2 mg once daily, first in continuous daily dosing, then in a five-week-on and three-week-off schedule.
Results: Both patients showed rapid clinical improvement upon starting the regimen. They also noted improved tolerance of treatment upon transitioning to the intermittent dosing schedule. They continue to show evidence of antitumor activity 27 and 18 months respectively from the start of treatment and 15 and 13 months respectively from the start of the first break using intermittent dosing.
Conclusions: Achieving durable palliation in these consecutive patients supports evaluating the intermittent dosing schedule of dabrafenib and trametinib in BRAFV600E mutated papillary thyroid cancer. Results of the ongoing phase 3 trial of continuous daily dosing and a subsequent trial of intermittent dosing, as is being tested in other cancers, will be needed to confirm that an intermittent dosing strategy in thyroid cancer can forestall resistant disease, improve tolerability, and decrease the cost of care.
Keywords: : papillary thyroid cancer, BRAF, dabrafenib, trametinib, intermittent dosing
Introduction
The american cancer society reports an increasing incidence of thyroid cancer in the United States, with estimates of 64,300 new cases and 1980 deaths in 2016 (1). About 10–20% of patients will develop metastatic disease, and at least a half of these patients will not respond to radioactive iodine. When refractory to radioactive iodine, the 10-year survival is 10% from the time of detection of metastasis (2), though patients with a BRAFV600E mutated thyroid cancer have a worse prognosis (3,4).
These patients are generally all treated with levothyroxine to suppress thyrotropin. Two oral agents targeting vascular endothelial growth factor receptors have recently been approved by the United States Food and Drug Administration (FDA), based on improved progression-free survival over placebo in phase III trials: Sorafenib (2013) (5), which also inhibits PDGFRβ, RAF-1, RET, CRAF, and BRAF; and Lenvatinib (2015) (6), which also inhibits FGFR 1–4, PDGFα, RET, and KIT. Both agents are contraindicated in patients having certain comorbidities or taking certain medications.
Papillary thyroid cancer frequently carries gene mutations and rearrangements that lead to activation of the mitogen-activated protein kinase that promotes cell division. The BRAFV600E mutation is the most common genetic alteration (29–69% of cases) and correlates with aggressive tumor characteristics. Vemurafenib and dabrafenib are both FDA approved agents for the treatment of advanced melanoma carrying the BRAFV600E mutation. These agents are well tolerated and have also shown encouraging activity in patients with radioiodine-refractory BRAFV600E–positive papillary thyroid cancer (7,8). While this is an important advance, drug resistance does become apparent after several months of treatment and most patients relapse with drug-resistant disease that can be lethal.
Two newer developments in advanced BRAFV600E mutated melanoma may also be relevant in thyroid cancer. Firstly, the combination of dabrafenib and a MEK inhibitor such as trametinib significantly improved response rates and nearly doubled the duration of response over dabrafenib alone (9). This resulted in the accelerated FDA approval in 2014 of this combination therapy in metastatic BRAF mutant melanoma. A similar trial in BRAFV600E mutated thyroid cancer has only recently completed accrual (NCT01723202).
The second potential advance comes from the observation that melanomas treated continuously with vemurafenib eventually develop resistance to BRAFV600E inhibition yet become dependent on vemurafenib for the continued proliferation of these resistant cells, such that cessation of vemurafenib administration causes regression of established drug-resistant tumors (10). In an effort to exploit the fitness disadvantage displayed by drug-resistant cells in the absence of these drugs, and with the aim to forestall the onset of lethal drug-resistant disease, an intermittent dosing strategy for dabrafenib and trametinib (five weeks on, three weeks off) has been established and is being tested in advanced melanoma (NCT02196181).
Since 2014, two consecutive patients presented to the medical oncology clinic at Boston Medical Center with life-threatening metastatic radioiodine-unresponsive BRAFV600E mutated thyroid cancer. Because of comorbidities and poor performance status, neither patient was eligible for enrollment in a clinical trial or could receive lenvatinib or sorafenib. Instead, both patients consented to and were treated with continuous dabrafenib plus trametinib. The first patient did not tolerate the daily treatment well and after dose reductions was converted to the intermittent dosing schedule as is being tested in melanoma patients. With objective evidence of continued improvement, the same strategy was also applied to the second patient, with similar outcome.
Patient 1
In 2009, an 80-year-old woman sustained an anterior wall myocardial infarction while taking warfarin for recurrent deep vein thromboses, while also undergoing work-up for a papillary thyroid cancer. After stent placement in the anterior descending artery, later requiring percutaneous transluminal coronary angioplasty, she underwent total thyroidectomy and bilateral neck dissection, revealing a primary papillary thyroid cancer with four positive level 4 lymph nodes. Positron emission tomography also revealed subcentimeter pulmonary metastases (confirmed histologically) and bilateral hilar adenopathy. Based on her American Joint Committee on Cancer stage IV disease (pT3pN1apM1), she was treated with 131I 215 mCi two months later. A follow-up 131I scan showed no residual uptake in the thyroid bed, nor in locoregional lymph nodes or the lungs. Serial thyroglobulin levels (Tg) remained under 10 ng/mL for 30 months, at which point the serum level of Tg began to rise with concurrent progression of bilateral perihilar masses. In November 2013 she developed hemoptysis which was attributed to the combination of warfarin use and pulmonary metastases. She underwent cyberknife treatment of the symptomatic left lung lesion. However, there was continued indolent progression of her disease.
Eight months later, she sustained another acute myocardial infarction with in-stent thrombosis and occlusion of her left circumflex artery, requiring urgent percutaneous coronary intervention and repeat stenting. A month later, in August 2014, she sustained a pathologic fracture of her left femur and plain film of the right femur showed an additional lytic lesion. She had open reduction and fixation of the fracture with an intramedullary rod, and surgical reamings from the lesion were consistent with papillary thyroid cancer. She underwent palliative radiation to the left femur. Sorafenib was felt to be relatively contraindicated in this patient because of her comorbidities and warfarin use; furthermore, sorafenib is not considered to be active in treating bone metastases from papillary thyroid cancer (5). The operative specimen was tested for presence of a BRAFV600E mutation and found to be positive in the tumor. She was progressively more symptomatic because of her lung and bone metastases despite optimized dosing of levothyroxine. In February 2015, she provided informed consent for compassionate, off-label use of oral dabrafenib 150 mg twice daily plus trametinib 2 mg daily. In the first two months, she required dose interruptions and two dose reductions of dabrafenib because of chills and intermittent fever, which are known side effects of this medication. She was able to tolerate dabrafenib 50 mg in the morning and 100 mg in the evening with trametinib 2 mg in the evening, together with prednisone 5–10 mg orally daily, but there was also cumulative fatigue that became dose limiting. In February 2016, after almost a full year of continuous daily dosing, she started a 3-week drug holiday before resuming treatment using an intermittent schedule of 5 weeks of treatment followed by a 3-week break, per 8-week cycle. This approach was associated with a considerable reduction in her fatigue and an improvement of her well-being. Subsequently, she has received seven cycles of combination therapy, with symptoms of fatigue and chills that were most pronounced in the first week of resuming treatment.
Her indicator lesions initially reduced and then stabilized with daily dosing. By Response Evaluation Criteria in Solid Tumors (RECIST) criteria, her disease remained stable over 27 months with no evidence of progression of disease. Measurement of her target lesions did not meet criteria by size for partial response (see Fig. 1). Of considerable interest and unusual for cancer treatments in general, her serum level of thyroglobulin continued to decline (see Fig. 2), even after instituting the intermittent dosing schedule for the last 15 months. Serum levels of thyroglobulin antibodies have been consistently undetectable.
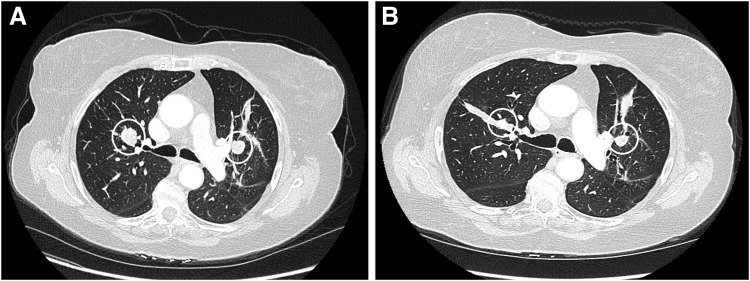
FIG. 1.
CT Indicator lesions in Patient 1. (A) Chest CT prior to initiation of treatment (February 2015) shows two indicator lesions: 2.2 cm × 1.5 cm in right upper lobe and 1.7 cm × 1.6 cm in left upper lobe. (B) These lung lesions (from December 2016) measure 1.2 cm × 1.1 cm and 1.3 cm × 1.1 cm, respectively. Evaluation of all target lesions using the Response Evaluation Criteria in Solid Tumors shows stable disease, with no new metastases.
FIG. 2.
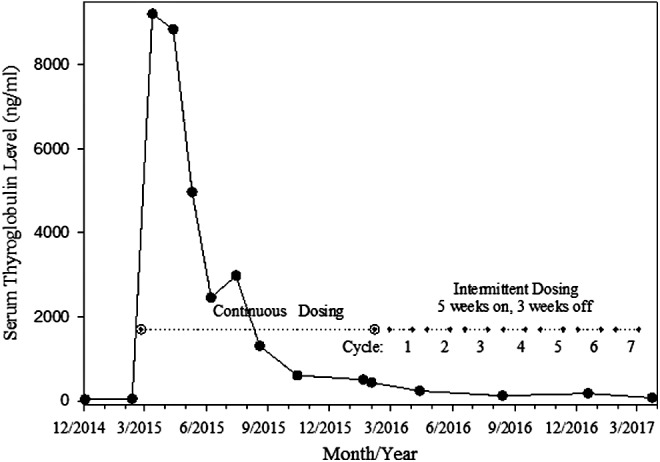
Dabrafenib and trametinib for advanced BRAFV600E radioiodine resistant papillary thyroid cancer: Serum level of thyroglobulin continues to decline in Patient 1 with intermittent dosing (15 months, ongoing) after continuous dosing for one year.
Patient 2
A 60-year-old woman presented in 2004 with hoarseness and dysphagia when swallowing solids for several months. She was found to have a three centimeter mass in the right lobe of the thyroid with invasion into surrounding muscle on computerized tomography (CT). Biopsy revealed a papillary thyroid cancer in June 2006. While her serum Tg level was undetectable, the serum anti-Tg antibodies were elevated at 20.7 U/mL. She underwent total thyroidectomy with right neck modified dissection revealing one involved lymph node. She was then treated with 131I and follow-up scans were negative for radioactive iodine uptake. She continued levothyroxine with no evidence of progression of disease until May 2012 when she was found to have an enlarged subcarinal node which was intensely fluorodeoxyglucose (18F)-avid but not radioactive iodine–avid. Biopsy confirmed metastatic papillary thyroid cancer that carried a BRAFV600E mutation. Subsequently, there was indolent progression of disease for three years, until she presented with acutely worsening dyspnea on exertion. Imaging at that time showed progressive enlargement of cervical, mediastinal and hilar lymphadenopathy encasing the right main bronchus and pulmonary artery with severe narrowing of the bronchus intermedius causing right middle lobe collapse, and extrinsic compression of the trachea. She also had a new pleural effusion. The patient initially required intubation for ventilation support. An endobronchial stent only partially alleviated her respiratory symptoms. In November 2015, she provided informed consent for compassionate off-label use of dabrafenib 150 mg orally twice a day with trametinib 2 mg once daily. Within two weeks, she was breathing normally and serial scans demonstrated stable disease by RECIST criteria.
In April 2016, treatment was suspended for three weeks while she was being managed for stent-related plugging, hemoptysis, and infection. With no histologic evidence of progression of disease, and based on the success of patient 1 and emerging data in metastatic melanoma, she was offered and accepted to continue with the intermittent dosing regimen of dabrafenib and trametinib, resuming therapy in April 2016. While serial scans showed stable disease on continuous therapy with the two drugs, a CT scan after three months of intermittent therapy (counting from the start of the first break) was associated with a slight decrease in the size of mediastinal adenopathy and pulmonary nodules, consistent with stable disease by RECIST criteria; this trend has persisted as such until the latest evaluation performed in May 2017. The serum level of thyroglobulin has been relatively stable through the course of her treatment, although in the setting of a high titer of thyroglobulin antibodies. The serum level of thyroglobulin antibodies (University of Southern California Endocrine Services Laboratory, Pasadena, CA) rose during the initial continuous dosing phase, but has since been declining linearly during the intermittent dosing phase, from a peak of 14,400 U/mL in February 2016 to 4,600 U/mL in May 2017 (see Fig. 3), supporting the possibility that the serum level of thyroglobulin antibody may be a surrogate tumor marker in this setting (11).
FIG. 3.
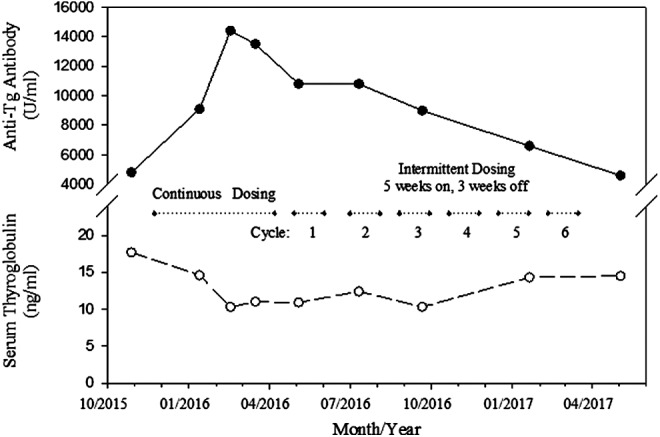
Dabrafenib and trametinib for advanced BRAFV600E radioiodine resistant papillary thyroid cancer: Anti-thyroglobulin antibody titer (solid line) continues to decline in Patient 2 with intermittent dosing for 13 months after peaking during the 4-month continuous daily dosing phase. Serum level of thyroglobulin (dashed line) is stable over this period.
Like patient 2, this patient noted considerable improvement in her sense of well-being after converting to an intermittent therapy regimen. On treatment for now 18 months (13 months on intermittent drug administration), this patient continues to tolerate dabrafenib and trametinib at full dose on the five-week-on, three-week-off schedule and has resumed work unencumbered by symptoms from her disease or from the treatment.
Discussion
Metastatic BRAFV600E mutated papillary thyroid cancer is sufficiently uncommon that development of new treatments requires multi-institutional enrollment. We report encouraging results in two consecutive patients with significant comorbidities treated at one community cancer center affiliated with an academic institution. Both patients presented with life-threatening metastatic papillary thyroid cancer and were not candidates for sorafenib or the more recently approved agent, lenvatinib. While not yet in the label, it is now common to treat these cancers with BRAF inhibitors such as dabrafenib if the tumor is BRAFV600E mutated—as is now common practice in the treatment of melanoma. Trametinib, an oral MEK inhibitor, is also approved as a single agent in the treatment of melanoma (12). We cannot assess the individual contributions of these two drugs in inducing responses in these two patients. The combination in melanoma, however, has been shown to be associated with superior overall survival, progression free survival, overall response rate, and mean duration of response, compared directly with dabrafenib alone (9) and compared with reported data for trametinib alone (12). We must await the results of a randomized trial (NCT01723202) that just completed accrual to confirm that the combination adds benefit in BRAFV600E mutated papillary thyroid cancer. The experiences in the two patients presented here illustrate that administering the two drugs is feasible and suggest that this approach is efficacious even in the instance of serious comorbidities. These two experiences go a step further, however, by demonstrating that taking a three-week break from treatment did not result in rampant progression of disease, as has been observed after stopping some tyrosine kinase inhibitors, including sorafenib (13). Instead, these patients demonstrate the contrary: ongoing evidence of measurable antitumor effects even while intermittently stopping dabrafenib and trametinib for three weeks in an eight-week cycle. These observations support the hypothesis that cells exposed to these agents may become dependent on them for their continued proliferation, and that stopping these agents intermittently may enhance efficacy. These findings are consistent with preclinical observations in BRAFV600E mutated advanced melanoma (10).
With intermittent dosing, prior dose reduction did not preclude continued efficacy, as was demonstrated in patient 1. Dose reductions and drug holidays are often used to manage adverse events. Both patients experienced considerably fewer adverse events and no cumulative toxicity with intermittent dosing, permitting them to resume active lifestyles largely unencumbered by the anticancer treatment.
The potential for preserved efficacy, better tolerance, and reduced drug procurement costs support further development of the intermittent dosing schedule of dabrafenib with or without trametinib in certain malignancies associated with BRAF mutations. The National Cancer Institute–sponsored randomized trial of intermittent versus continuous dosing of dabrafenib and trametinib in advanced melanoma (NCT02196181) can serve as a basis for designing an analogous study for BRAFV600E mutated thyroid cancer.
Acknowledgments
Special thanks are offered to Scott Gould, PharmD, Hristina Natcheva, MD, and J. Nicholas Tkacz, MD.
Author Disclosure Statement
The authors have nothing to disclose.
References
- 1.Siegel RL, Miller KD, Jemal A. 2016. Cancer statistics, 2016. CA Cancer J Clin 66:7–30 [DOI] [PubMed] [Google Scholar]
- 2.Durante C, Haddy N, Baudin E, Leboulleux S, Hartl D, Travagli JP, Caillou B, Ricard M, Lumbroso JD, De Vathaire F, Schlumberger M. 2006. Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: benefits and limits of radioiodine therapy. J Clin Endocrinol Metab 91:2892–2899 [DOI] [PubMed] [Google Scholar]
- 3.Kimura ET, Nikiforova MN, Zhu Z, Knauf JA, Nikiforov YE, Fagin JA. 2003. High prevalence of BRAF mutations in thyroid cancer: genetic evidence for constitutive activation of the RET/PTC-RAS-BRAF signaling pathway in papillary thyroid carcinoma. Cancer Res 63:1454. [PubMed] [Google Scholar]
- 4.Xing M. 2005. Braf mutation in thyroid cancer. Endocr Relat Cancer 12:245–262 [DOI] [PubMed] [Google Scholar]
- 5.Brose MS, Nutting CM, Jarzab B, Elisei R, Siena S, Bastholt L, de la Fouchardiere C, Pacini F, Paschke R, Shong YK, Sherman SI. 2014. Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, phase 3 trial. Lancet 384:319–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schlumberger M, Tahara M, Wirth LJ, Robinson B, Brose MS, Elisei R, Habra MA, Newbold K, Shah MH, Hoff AO, Gianoukakis AG, Kiyota N, Taylor MH, Kim SB, Krzyzanowska MK, Dutcus CE, de las Heras B, Zhu J, Sherman SI. 2015. Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N Engl J Med 372:621–630 [DOI] [PubMed] [Google Scholar]
- 7.Falchook GS, Long GV, Kurzrock R, Kim KB, Arkenau TH, Brown MP, Hamid O, Infante JR, Millward M, Pavlick AC, O'Day SJ. 2012. Dabrafenib in patients with melanoma, untreated brain metastases, and other solid tumours: a phase 1 dose-escalation trial. Lancet 379:1893–1901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dadu R, Shah K, Busaidy N, Waguespack S, Habra M, Ying A, Hu M, Bassett R, Jimenez C, Sherman S, Cabanillas M. 2015. Efficacy and tolerability of vemurafenib in patients with BRAFV600E-positive papillary thyroid cancer: M.D. Anderson Cancer Center Off Label Experience. J Clin Endocrinol Metab 100:E77–E81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Long GV, Stroyakovskiy D, Gogas H, Levchenko E, De Braud F, Larkin J, Garbe C, Jouary T, Hauschild A, Grob JJ, Chiarion-Sileni V. 2015. Dabrafenib and trametinib versus dabrafenib and placebo for Val600 BRAF-mutant melanoma: a multicentre, double-blind, phase 3 randomised controlled trial. Lancet 386:444–451 [DOI] [PubMed] [Google Scholar]
- 10.Thakur M, Salangsang F, Landman A, Sellers W, Pryer N, Levesque M, Dummer R, McMahon M, Stuart D. 2013. Modelling vemurafenib resistance in melanoma reveals a strategy to forestall drug resistance. Nature 494:251–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morbelli S, Ferrarazzo G, Pomposelli E, Pupo F, Pesce G, Calamia I, Fiz F, Clapasson A, Bauckneht M, Minuto M, Sambuceti G, Giusti M, Bagnasco M. 2017. Relationship between circulating anti-thyroglobulin antibodies (TgAb) and tumor metabolism in patients with differentiated thyroid cancer (DTC): prognostic implications. J Endocrinol Invest. 40:417–424 [DOI] [PubMed] [Google Scholar]
- 12.Flaherty KT, Robert C, Hersey P, Nathan P, Garbe C, Milhem M, Demidov LV, Hassel JC, Rutkowski P, Mohr P, Dummer R. 2012. Improved survival with MEK inhibition in BRAF-mutated melanoma. N Engl J Med 367:107–114 [DOI] [PubMed] [Google Scholar]
- 13.Yun KJ, Kim W, Kim EH, Kim MH, Lim DJ, Kang MI, Cha BY. 2014. Accelerated disease progression after discontinuation of sorafenib in a patient with metastatic papillary thyroid cancer. Endocrinol and Metab 29:388–93 [DOI] [PMC free article] [PubMed] [Google Scholar]