Abstract
BACKGROUND:
The implications of bacteremia in critically ill patients are uncertain. Some reports suggest bacteremia is linked to higher mortality whereas others do not. These differences may, in part, be because of differences in patient cohorts. To address the potential independent relationship between bacteremia and outcome, we focused on critically ill trauma patients with ventilator-associated pneumonia (VAP), of whom a significant proportion had concomitant bacteremia. We tested the hypothesis that bacteremia was associated with death in trauma patients who developed VAP.
METHODS:
In this retrospective cohort study, we measured the incidence of bacteremia associated with VAP and compared the case-fatality rates between patients with and without bacteremia. We adjusted for other mortality risk factors and possible confounders in two ways. First, using forward conditional logistic regression and second, we calculated propensity scores and then adjusted for this score in a regression model.
RESULTS:
There were 554 with VAP. Patients with bacteremia had a 26% case-fatality rate (19 of 74 patients). Patients with VAP alone had a case-fatality rate of 12% (56 of 480 patients). The unadjusted relative risk (RR) for death associated with bacteremia was 2.2 (95% confidence interval = 1.4–3.5). After adjusting for age, acute physiology score, and severity of injury, patients with bacteremia had an increased risk of death compared with patients with VAP alone (adjusted odds ratio = 2.65, 95% confidence interval = 1.36–5.17). Our propensity score analysis resulted in a similar association between bacteremia and mortality.
CONCLUSIONS:
VAP with bacteremia is associated with an increased mortality in comparison with VAP alone after severe traumatic injury.
Keywords: Ventilator-associated pneumonia, bacteremia, outcome
Ventilator-associated pneumonia (VAP) is the most common nosocomial infection in critically ill patients and is considered an important cause of prolonged hospitalization and mortality.1 With timely diagnosis and appropriate antimicrobial treatment, patients with VAP typically survive. This is particularly true in trauma victims who are generally young and have few comorbid conditions.2 Nevertheless, even in trauma patients, VAP may increase the risk of death. Despite early recognition, diagnosis, and treatment, some patients may be unable to control their infection and ultimately die. Bacteremia in conjunction with pneumonia may reflect a failure of the host’s innate immune response and may be a harbinger for death, prolonged hospitalization, or other poor outcomes. The reasons for failure to localize an infection such as pneumonia are uncertain, but it is likely that the inability to prevent bacterial dissemination reflects an impaired innate immune response. Thus, we sought to determine the incidence of bacteremia associated with VAP and investigate whether bacteremia is associated with death in critically ill trauma patients.
It is possible that bacteremia occurring in conjunction with VAP reflects a state of immune suppression or “paralysis” in which the host is unable to localize the infection to the lung. We examined a large cohort of injury victims with VAP to determine whether bacteremia is associated with mortality in this group of critically injured patients.
PATIENTS AND METHODS
Patients
The study subjects come from a prospectively enrolled cohort of severely injured trauma patients admitted to the intensive care units (ICUs) at Harborview Medical Center (Seattle, WA). The entry criteria, enrollment procedures, and details of data collection have been previously published and are briefly outlined here.3 The Institutional Review Board of the University of Washington approved the study. Clinical data were obtained from two different sources. Detailed information about injuries, including the Injury Severity Score and the Abbreviated Injury Scale score, were obtained from the institutional trauma registry. All additional data were obtained from the electronic medical record. All study procedures were approved by the University of Washington Human Subjects Division.
Patients were followed until hospital discharge or death. For the purpose of this study, VAP diagnosis required evidence of the systemic inflammatory response syndrome, clinical and radiologic findings of pneumonia, and positive bacteriologic culture obtained either invasively or noninvasively. For patients undergoing bronchoscopy, VAP diagnosis required a quantitative bronchoalveolar lavage specimen demonstrating at least 105 colony forming units/mL. VAP-associated bacteremia was defined by the presence of VAP in conjunction with the same organism isolated in blood cultures obtained within 24 hours of VAP diagnosis.
In general, patients are managed according to an established care pathway. Once the diagnosis of VAP is suspected based on clinical criteria and after appropriate specimens are obtained for culture, empiric antibiotics against gram-negative and gram-positive organisms are begun. A cephalosporin has typically been the agent of choice and is supplemented with vancomycin if the patient’s surveillance cultures identified methicillin-resistant Staphylococcus aureus. Antibiotics are narrowed based on the culture and susceptibility results, usually within 48 hours to 72 hours. Multidrug-resistant gram-negative organisms (typically Enterobacter, Acinetobacter, and Pseudomonas sp.) are treated for 14 days. Patients with other organisms are treated for 8 days. Patients with bacteremia are typically treated for 14 days regardless of the organism. However, we have no institutional guideline for the treatment of bacteremia.
Data Analysis
Data were analyzed with SPSS 14.0 statistical software (SPSS, Chicago, IL). Continuous data are presented as medians and interquartile range (25th to 75th percentile) and were analyzed using Mann-Whitney U test. Categorical variables were compared using Pearson’s χ2 test. For each comparison, the actual p values are reported.
We analyzed our data in two ways to determine whether there was an association between concomitant bacteremia and mortality in patients with VAP. First, we conducted logistic regression and adjusted for factors potentially associated with mortality. In a second, parallel analysis, we calculated propensity scores to estimate each study subject’s risk for bacteremia based on known clinical data. In brief, an individual’s propensity score is an estimate of their probability for developing bacteremia and is calculated using logistic regression. We stratified patients into quartiles, according to their probability of developing bacteremia, and adjusted for this variable in the model testing whether bacteremia was associated with death.4 ORs are presented with their associated 95% confidence intervals (CIs) and considered to estimates of the risk of death associated with each independent variable.
One author (G.E.O.) reviewed the records and assigned a cause of death for the 66 fatalities. In cases where the immediate cause of death or the primary underlying factor leading to death were not clear, these cases were reviewed by two other authors (J.C. and H.L.E.), and the immediate cause and underlying factor for each case was determined by consensus. Assignment was done blinded to whether the patient had VAP alone or VAP with bacteremia.
RESULTS
A total of 2,791 subjects were enrolled in the parent study over the period from August 2003 to July 2007. Table 1 includes the demographic data for this entire cohort. Of these 2,791 patients, 554 (20%) developed VAP. The remaining 2,237 (80%) did not and are not considered in the analyses that follow.
TABLE 1.
Demographics, Injury, and Outcome Characteristics for Entire Cohort (n = 2,791)
| Demographics | Injury Characteristics | ||
|---|---|---|---|
| Age (yr) | 37 (23–53) | Injury Severity Score | 27 (19–36) |
| Sex | Severe brain injury | 1,517 (54.4) | |
| Male | 1,995 (71.5) | Severe thoracic injury | 1,407 (50.4) |
| Female | 796 (28.5) | Severe abdominal injury | 714 (25.6) |
| Race/ethnicity | Severe lower extremity injury | 914 (32.8) | |
| Caucasian | 2,236 (80.1) | Severe upper extremity injury | 342 (12.3) |
| African American | 177 (6.3) | Severe spine injury | 810 (29.0) |
| Hispanic | 206 (7.4) | Injury mechanism* | |
| Asian | 120 (4.3) | Blunt | 2,498 (89.5) |
| American Indian | 52 (1.9) | Penetrating | 266 (9.5) |
| Chronic comorbidities | Initial base deficit | 4.7 (2.6–7.4) | |
| Diabetes | 192 (6.9) | ||
| Cardiovascular | 711 (25.5) | Outcomes | |
| Respiratory | 313 (11.2) | Died | 257 (9.2) |
| Hepatic | 137 (4.9) | ICU length of stay (d) | 5 (3–12) |
| Cancer | 100 (3.6) | Hospital length of stay (d) | 14 (8–24) |
| VAP | 554 (19.8) |
These data are for the entire cohort. Continuous data are presented as medians (25th–75th percentile), and categorical data are indicated as total for the entire cohort (percentages). Severe traumatic brain, thoracic, abdominal, and extremity injuries refer to the number of patients with Abbreviated Injury Scale scores ≥3 in the indicated body region.
Twenty-seven patients with other/combined mechanism of injury.
Of the 554 with VAP, 74 patients (14%) had associated bacteremia and 480 (86%) had VAP alone. Subjects with VAP-associated bacteremia had a 26% case-fatality rate (19 of 74 patients). Patients with VAP alone had a case-fatality rate of 12% (56 of 480 patients). The unadjusted RR for death, associated with bacteremia, was 2.2 (95% CI = 1.4–3.5). The absolute increase in mortality associated with bacteremia was 14% (95% CI = 3–25%). The causative microorganisms are shown in Table 2 and summarized briefly here. Gram-negative and gram-positive organisms were each responsible for ~50% of the pneumonias, and there was no difference in the distribution of organisms between patients with VAP alone and patients with bacteremia. In bacteremic patients, a single species was isolated from all the patients’ blood cultures. There was a difference in the frequency of polymicrobial lung cultures, which were more common in patients with bacteremia than in patients without bacteremia (29 of 74 vs. 116 of 480; p = 0.006).
TABLE 2.
Bacteriologic Culture Results
| VAP (n = 480) |
VAP and Bacteremia (n = 74) |
|
|---|---|---|
| Respiratory culture result, n (%) | ||
| Monomicrobial | 364 (75.8) | 45 (60.8) |
| Polymicrobial | 116 (24.2) | 29 (39.2) |
| Predominant organism, n (%) | ||
| Gram-negative | 240 (50.0) | 36 (48.6) |
| Acinetobacter sp. | 54 (11.3) | 15 (20.3) |
| Citrobacter sp. | 14 (2.9) | 0 (0.0) |
| Enterobacter sp. | 53 (11.0) | 5 (6.8) |
| Escherichia coli | 25 (5.2) | 6 (8.1) |
| Klebsiella sp. | 37 (7.7) | 3 (4.1) |
| Proteus mirabilis | 5 (1.0) | 0 (0.0) |
| Pseudomonas aeruginosa | 29 (6.0) | 5 (6.8) |
| Serratia marcescens | 14 (2.9) | 2 (2.7) |
| Other | 9 (1.9) | 0 (0.0) |
| Gram-positive | 240 (50.0) | 38 (51.4) |
| Enterococcus | 10 (2.1) | 0 (0.0) |
| Staphylococcus aureus | 200 (41.7) | 37 (50.0) |
| Methicillin-sensitive | 136 (28) | 14 (19) |
| Methicillin-resistant | 64 (13) | 23 (31) |
| Streptococcus pneumoniae | 30 (6.3) | 1 (1.4) |
Of these 554 patients with VAP, 62 patients (11%) were missing one or more pieces of information necessary for multivariate analyses, leaving 492 patients for detailed analyses. Table 3 includes the demographic features, clinical characteristics, and outcomes for these 492 patients. All remaining analyses are based on these patients for whom we have complete data.
TABLE 3.
Comparison of VAP and VAP With Bacteremia Patients (n = 492 Patients With Complete Data)
| VAP Alone (n = 427) |
VAP With Bacteremia (n = 65) |
p | |
|---|---|---|---|
| Demographic features | |||
| Age (yr) | 39 (23–54) | 48 (27–66) | 0.014 |
| Male sex, n (%) | 314 (73.5) | 50 (76.9) | 0.672 |
| Race/ethnicity, n (%) | 0.40 | ||
| White | 334 (78.2) | 56 (86.2) | |
| African American | 24 (5.6) | 1 (1.5) | |
| Asian | 16 (3.7) | 1 (1.5) | |
| Hispanic | 40 (9.4) | 4 (6.2) | |
| Native American | 13 (3.0) | 3 (4.6) | |
| Clinical features, n (%) | |||
| Chronic comorbidities | |||
| Diabetes | 27 (6.3) | 9 (13.8) | 0.057 |
| Cardiovascular disease | 119 (27.9) | 27 (41.5) | 0.035 |
| Respiratory disease | 54 (12.6) | 8 (12.3) | 0.901 |
| Renal disease | 9 (2.1) | 0 (0) | 0.494 |
| Hepatic disease | 28 (6.6) | 6 (9.2) | 0.597 |
| Cancer | 16 (3.7) | 2 (3.1) | 0.931 |
| Injury characteristics, n (%) | |||
| Injury Severity Score | 34 (26–43) | 34 (27–41) | 0.807 |
| Severe brain injury | 270 (63.2) | 36 (55.4) | 0.281 |
| Severe thoracic injury | 263 (61.6) | 44 (67.7) | 0.419 |
| Severe abdominal injury | 138 (32.3) | 23 (35.4) | 0.727 |
| Severe extremity injury | 196 (45.9) | 34 (52.3) | 0.406 |
| Severe spinal injury | 151 (35.4) | 30 (46.2) | 0.123 |
| Injury mechanism, n (%) | 0.573 | ||
| Blunt | 401 (93.7)* | 63 (96.9) | |
| Penetrating | 27 (6.1)* | 2 (3.1) | |
| Initial base deficit | 5.5 (4.9–5.9) | 6.5 (5.6–8.3) | 0.038 |
| Acute physiology score | 23 (17–26) | 24 (18–29) | 0.081 |
| Units of blood transfused | 3 (0–11) | 7 (0–18) | 0.03 |
| Outcomes | |||
| Died, n (%) | 48 (11.2) | 18 (27.7) | <0.001 |
| ICU length of stay (d) | 17 (12–25) | 25 (16–41) | <0.001 |
| Hospital length of stay (d) | 26 (20–39) | 31 (22–54) | 0.017 |
Continuous data are presented as medians (25th–75th percentile), and categorical data are indicated as number in each group (percentages). Severe traumatic brain, thoracic, abdominal, and extremity injuries refer to the number of patients with Abbreviated Injury Scale scores ≥3 in the indicated body region. Categorical data are compared by χ2 analysis and continuous data by the Mann-Whitney U test.
One patient with combined mechanism of injury.
In addition to having a higher case-fatality rate, the patients with bacteremia spent a longer period of time in the ICU than patients with VAP alone. The median ICU length of stay for patients with bacteremia was 25 (16–41) days compared with 17 (12–25) days for patients with VAP without bacteremia. The Kaplan-Meier analysis depicting the ICU length of stay is shown in Figure 1.
Figure 1.
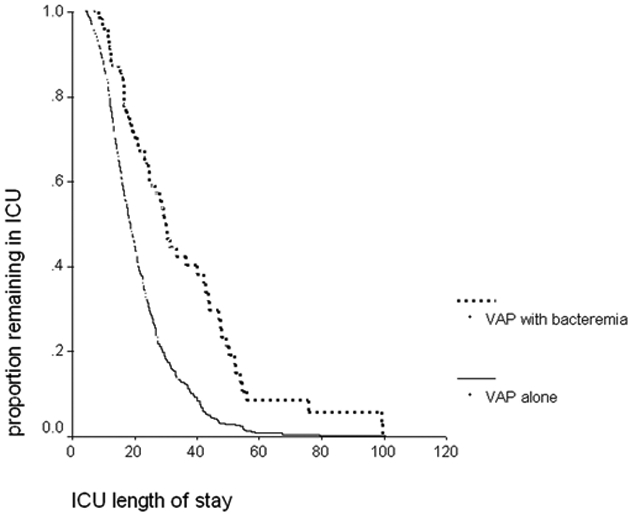
Kaplan-Meier analysis of ICU length of stay. This figure depicts the results of the Kaplan-Meier analysis of the duration of ICU stay for the 492 patients according to the presence or absence of bacteremia in association with VAP. Patients with concomitant bacteremia (n = 65) had a significantly longer ICU stay than the 427 patients with VAP alone (log rank p < 0.0001).
It was possible to clearly determine the timing antibiotic treatment was started relative to the diagnosis of VAP for 487 patients. Overall, appropriate antibiotics were started within 24 hours of diagnosis in 445 (91%) of patients. There was no difference in the number of patients in whom antibiotics were delayed beyond 24 hours between patients with or without bacteremia (Fig. 2).
Figure 2.
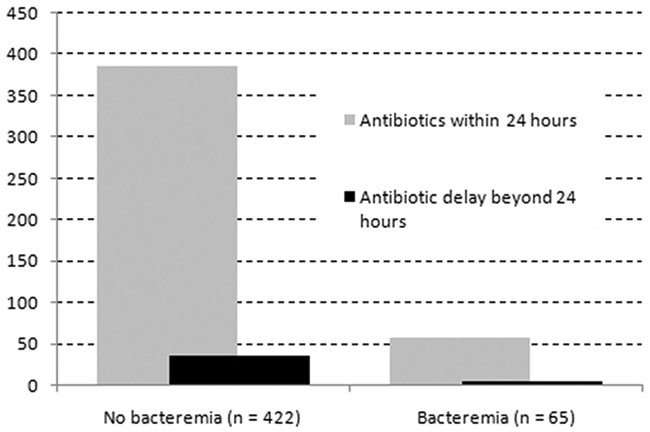
Timing of appropriate antibiotic therapy relative to VAP diagnosis. The majority of patients (91%) received appropriate antibiotics within 24 hours of the diagnosis of VAP. There was no difference in the percentage of patients with delayed initiation of antibiotic therapy between the patients with bacteremia and those without bacteremia. In this cohort, a delay in appropriate antibiotic treatment was not associated with mortality.
After Adjustment, VAP With Bacteremia Remains Associated With High Mortality
Patients who developed bacteremia were older, had a slighter higher initial base deficit, and were transfused slightly more blood than were VAP patients without bacteremia. Because of these and other potentially important differences between the two groups, we conducted two separate analyses to adjust for these differences and these analyses are discussed below.
First, we adjusted for factors potentially associated with mortality using forward stepwise logistic regression. Patients with bacteremia had an increased risk of death compared with patients with VAP alone (adjusted odds ratio [OR] = 2.65, 95% CI = 1.36–5.17). The results of the regression analysis are shown in Table 4.
TABLE 4.
Logistic Regression Analysis for Mortality
| Variable | OR | 95% CI |
|---|---|---|
| Age* (yr) | 1.91 | 1.46–2.49 |
| Acute physiology score* | 1.42 | 1.08–1.85 |
| Severe traumatic brain injury (Head AIS ≥3) | 2.14 | 1.17–3.94 |
| Severe upper extremity injury (upper extremity AIS ≥3) | 0.41 | 0.16–1.04 |
| VAP with bacteremia | 2.65 | 1.36–5.17 |
AIS, Abbreviated Injury Scale.
Data were analyzed using forward stepwise logistic regression, and the variables remaining in the final model are shown above. Variables included in the stepwise modeling were as follows: Injury Severity Score, body region AIS severity scores (0–2 or ≥3), units of transfused red blood cells, initial arterial base deficit, comorbid medical conditions, presence of a pulmonary contusion, VAP organism Gram stain (gram-positive or gram-negative), and blunt or penetrating injury mechanism.
Continuous variables were modeled in a variety of ways (raw data, dichotomized, categorized as quartiles, etc.). Each was ultimately entered into the final model in a format corresponding to the best fit with the mortality outcome according to the Hosmer-Lemeshow statistic. In the case of age and acute physiology score, both were included as quartiles modeled as a continuous (0, 1, 2, or 3) variable.
As a second, confirmatory analysis, we also calculated a propensity score for each subject which estimated their risk, based on clinical and demographic factors, for developing bacteremia. We then analyzed the risk for death in patients with bacteremia after including the calculated propensity score in the logistic model. The adjusted OR for death associated with bacteremia was 2.57 (95% CI = 1.36–4.89) (see Supplemental Digital Content 1, http://links.lww.com/TA/A90). Despite the modeling methods being different in this analysis, the result is essentially identical to that observed in the logistic analysis shown in Table 4.
Patients With Bacteremia Who Died Were More Likely to Die as a Consequence of Infection and Remote Organ Failure
A total of 66 patients died. We explored their clinical course, the immediate, and the underlying causes of death to understand what factors contributed to their deaths. First, as was seen in the comparisons involving the entire cohort, the patients with bacteremia who died had a relatively more prolonged hospital course compared with fatalities with VAP alone. Their ICU stay was considerably longer than the ICU stay for patients without bacteremia (median stay of 26 days vs. 15 days, p = 0.005). This was primarily because of a longer period of time between the diagnosis of the index episode of VAP and their death as shown in Figure 3. When considering both underlying and attributable (immediate) causes of death, multiple organ failure secondary to infection was more common in patients with bacteremia than in patients without bacteremia (13 of 18, [72%] vs. 15 of 48 [31%], p = 0.003).
Figure 3.
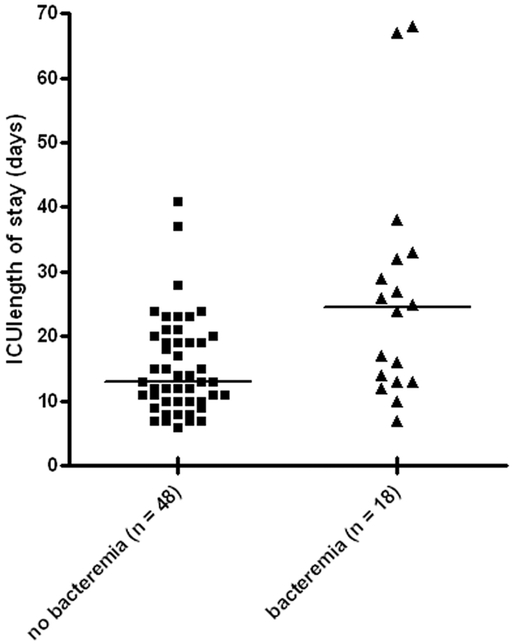
Time from VAP diagnosis to death. Patients with bacteremia who died, did so after a median of 12 days (interquartile range = 7–23 days) following the diagnosis of VAP. This was considerably longer than the time from diagnosis to death in patients who died with VAP alone (median 7 days, interquartile range = 4–10 days; p = 0.0067).
DISCUSSION
In patients admitted to the ICU after trauma, VAP is the most common infection. In 567 severely injured trauma patients enrolled in the “Inflammation and Host Response to Injury” Consortium, 99 patients (17%) developed VAP.5 In other studies of severely injured patients, such as those receiving a large blood volume resuscitation, the VAP rate is close to 40%.6 The incidence of VAP that we observed was ~20% and is therefore comparable with other reports of severely injured patients.
There are conflicting reports regarding the implications of bacteremia in critically ill patients. One group has reported that the presence of bacteremia is not associated with worse prognosis in infected surgical patients. First, in a cohort of patients with catheter-related infections, they observed that patients with associated bacteremia were not more likely to die than those with catheter infections without bacteremia.7 In a second report, the impact of bloodstream infections was examined in surgical patients with infection at other, but varied sites. The authors concluded that bloodstream infections were a marker of illness severity but not an independent predictor of death.8 Our results may differ from these reports because we focused on bacteremia associated with VAP, rather than all cases of bacteremia, regardless of the local site of infection (intra-abdominal abscess, intravascular catheter, etc.). Nevertheless, the conclusions that the authors of these two studies reached do not differ substantially from our observations and interpretation.7,8
Perhaps we observed an association between bacteremia and death because our cohort was more uniform. By focusing on a relatively homogeneous group of patients (severely injured patients who develop VAP), we were able to discern a relationship between bacteremia and death. This is consistent with other studies that have either included relatively few patients or have had an even more limited focus (patients with Staphylococcal pneumonia and bacteremia, for example).9,10 Agbaht et al. identified a twofold increased risk of death in VAP patients with bacteremia versus those without bacteremia. The risk factor and outcome comparison in that study was based on 32 patients with bacteremia who were matched to 57 patients without bacteremia. Although this was a smaller study, using different study design and inclusion criteria than ours, the observations were similar.9
Still other investigators have suggested that delayed diagnosis and treatment increases mortality attributable to VAP. We therefore considered this possibility and examined whether bacteremia reflects a delay in diagnosis or in treatment and whether this delay may have led to increased mortality. Our data suggest that this is not the case given that most patients in both groups received appropriate antibiotics within 24 hours of VAP diagnosis. The timing of both diagnosis and the institution of appropriate antibiotic treatment was not different between patients with and without bacteremia supporting the idea that bacteremia was not simply a consequence of a treatment delay. In addition to not being associated with bacteremia, a delay in starting appropriate antibiotics was not associated with death, regardless of whether the patient had bacteremia. The frequency of delay in appropriate antibiotic therapy in our patients is somewhat lower than in other reports.11 We attribute this relatively high rate of timely antibiotic therapy to our use of a clinical pathway for the prevention, diagnosis, and management of VAP in trauma patients.12 Causative microorganisms and antimicrobial resistance patterns were similar in patients with bacteremia and those with VAP alone, suggesting that pathogen virulence and resistance were not responsible for the development of bacteremia associated with VAP. Our data suggest that host factors, rather than suboptimal treatment or pathogen virulence, are primarily responsible for the development of bacteremia.
Despite suffering at least one nosocomial infection, most patients did not seem to die directly from VAP. In most fatalities, death occurred days to weeks after VAP diagnosis. Furthermore, the time between VAP diagnosis and death was considerably longer in patients with bacteremia than those without. These observations suggest that VAP-associated bacteremia is not an overwhelming infection but rather that bacteremia represents a failure to localize the infection, possibly because of immune suppression, and associated with progressive organ failure and death. Patients with bacteremia seemed to follow such a pattern more often than those with VAP alone. In fact, patients without bacteremia were more likely to die as a result of withdrawal of life-sustaining care in many cases because of severe brain injuries or underlying conditions, such as chronic liver failure. It is unknown whether any patients would have survived if care was not withdrawn.
The concept of “immunoparalysis” has been used to refer to a state of suppressed innate and acquired immune function after traumatic injury.13 Specifically, it has been observed that antigen presentation capacity, as defined by HLA-DR expression by alveolar macrophages and circulating monocytes is reduced after trauma.14,15 Other observed changes include lymphocyte apoptosis. These changes have been correlated with an increased risk for posttraumatic nosocomial infection and with death. We did not measure monocyte HLA-DR expression or other potential correlates of immune suppression and therefore cannot state with certainty whether bacteremia reflects failure of immunity. Studies to determine whether patients with bacteremia have reduced HLA-DR expression and increased lymphocyte apoptosis or other measures of immune dysfunction could provide insight into mechanisms behind our clinical observations. Although we have not demonstrated changes in immune function, our observations indicate that patients with bacteremia do follow a trajectory that includes prolonged respiratory failure, frequent organ failure and often death. All of which have been found by others to be associated with alterations in immune competence.15,16 Furthermore, our data are not definitive and do not necessarily support changing our therapy beyond what is presently recommended for the treatment of bacteremia. Given that bacteremia seems to be an important prognostic variable when occurring with VAP, perhaps it should be ruled out before limiting antibiotic therapy for VAP to less than 14 days.
We note the limitations of our study here. First, excluding patients from the adjusted analyses because of missing data may bias our observations in unanticipated ways. However, the adjusted odds of association between bacteremia and death (aOR = 2.5) did not differ substantially from the unadjusted RR (RR = 2.2). This supports the idea that missing data were random and unrelated to other risk factors or to mortality. It is possible that by using qualitative cultures we may have included patients who, in fact, did not have VAP. This might have led to bias if the culture technique was associated with bacteremia and with mortality. For example, if patients who truly did not have VAP were included based on a qualitative culture and these patients were both less likely to die and less likely to have bacteremia, we may have overstated the association between bacteremia and death in the context of VAP. We do not think this to be the case for the following reasons. First, when we limit our analysis to only patients with quantitative cultures, the OR for mortality is 3.1 (95% CI = 0.98–9.1). Second, only a slightly lower (rather than higher) proportion of patients with bacteremia had VAP based on quantitative culture, suggesting that we did not bias our results by erroneously including patients without VAP in the VAP alone group. Isolating similar organisms from the blood based on culture and sensitivity results does not definitively confirm that the lung was the primary source. However, in the absence of daily surveillance sampling, our approach to linking blood and lung cultures is a reasonable alternative definition. We also do not have data to confirm that the organisms isolated from the blood were genotypically identical to those isolated from the lung, and there is no published information to suggest the frequency of concomitant infection with genetically different organisms from the same species. On the other hand, we may be missing some instances of bloodstream infections by relying on blood culture results rather than mores sensitive molecular-based methods. Therefore, the incidence of bacteremia associated with VAP may be higher than we and other have reported.
Our interpretation of an association between bacteremia and death is based on a relatively small number of patients with bacteremia and it may be possible that we failed to identify and adjust for important confounders. The propensity score analysis attempts to address and seems to demonstrate a similar association than the stepwise logistic regression. Finally, our description of the ultimate causes of death and how they differ between the two groups is subjective. We cannot state with certainty that bacteremic patients died a pathophysiologically different death than nonbacteremic patients. Finally, our observations do not identify the molecular defects involved and only suggest a failure of host innate immunity.
In summary, VAP with bacteremia is associated with an increased mortality in comparison with VAP alone after severe traumatic injury. Further studies aimed at determining whether defects in host immunity are present in patients with bacteremia may help identify patients at higher risk for morbidity and death after traumatic injury.
Supplementary Material
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.jtrauma.com).
DISCLOSURE
The authors declare no conflicts of interest.
Contributor Information
Grant E. O’Keefe, Department of Surgery, University of Washington, Harborview Medical Center, Seattle, WA.
Ellen Caldwell, Division of Pulmonary and Critical Care Medicine, Department of Medicine, University of Washington, Harborview Medical Center, Seattle, WA..
Joseph Cuschieri, Department of Surgery, University of Washington, Harborview Medical Center, Seattle, WA.
Mark M. Wurfel, Division of Pulmonary and Critical Care Medicine, Department of Medicine, University of Washington, Harborview Medical Center, Seattle, WA.
Heather L. Evans, Department of Surgery, University of Washington, Harborview Medical Center, Seattle, WA.
REFERENCES
- 1.Safdar N, Dezfulian C, Collard HR, Saint S. Clinical and economic consequences of ventilator-associated pneumonia: a systematic review. Crit Care Med. 2005;33:2184–2193. [DOI] [PubMed] [Google Scholar]
- 2.Melsen WG, Rovers MM, Bonten MJ. Ventilator-associated pneumonia and mortality: a systematic review of observational studies. Crit Care Med. 2009;37:2709–2718. [DOI] [PubMed] [Google Scholar]
- 3.Shalhub S, Junker CE, Imahara SD, Mindrinos MN, Dissanaike S, O’Keefe GE. Variation in the TLR4 gene influences the risk of organ failure and shock posttrauma: a cohort study. J Trauma. 2009;66:115–122; discussion 122–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Austin PC. The performance of different propensity-score methods for estimating relative risks. J Clin Epidemiol. 2008;61:537–545. [DOI] [PubMed] [Google Scholar]
- 5.Sena MJ, Utter GH, Cuschieri J, et al. Early supplemental parenteral nutrition is associated with increased infectious complications in critically ill trauma patients. J Am Coll Surg. 2008;207:459–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cotton BA, Au BK, Nunez TC, Gunter OL, Robertson AM, Young PP. Predefined massive transfusion protocols are associated with a reduction in organ failure and postinjury complications. J Trauma. 2009;66:41–48; discussion 48–49. [DOI] [PubMed] [Google Scholar]
- 7.Pelletier SJ, Crabtree TD, Gleason TG, Pruett TL, Sawyer RG. Bacteremia associated with central venous catheter infection is not an independent predictor of outcomes. J Am Coll Surg. 2000;190:671–680. [DOI] [PubMed] [Google Scholar]
- 8.Raymond DP, Pelletier SJ, Crabtree TD, Gleason TG, Pruett TL, Sawyer RG. Impact of bloodstream infection on outcomes among infected surgical inpatients. Ann Surg. 2001;233:549–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Agbaht K, Diaz E, Muñoz E, et al. Bacteremia in patients with ventilator-associated pneumonia is associated with increased mortality: a study comparing bacteremic vs. nonbacteremic ventilator-associated pneumonia. Crit Care Med. 2007;35: 2064–2070. [DOI] [PubMed] [Google Scholar]
- 10.DeRyke CA, Lodise TP Jr, Rybak MJ, McKinnon PS. Epidemiology, treatment, and outcomes of nosocomial bacteremic Staphylococcus aureus pneumonia. Chest. 2005;128:1414–1422. [DOI] [PubMed] [Google Scholar]
- 11.Iregui M, Ward S, Sherman G, Fraser VJ, Kollef MH. Clinical importance of delays in the initiation of appropriate antibiotic treatment for ventilator-associated pneumonia. Chest. 2002;122:262–268. [DOI] [PubMed] [Google Scholar]
- 12.Minei JP, Nathens AB, West M, et al. ; Inflammation and the Host Response to Injury Large Scale Collaborative Research Program Investigators. Inflammation and the Host Response to Injury, a Large-Scale Collaborative Project: patient-oriented research core–standard operating procedures for clinical care. II. Guidelines for prevention, diagnosis and treatment of ventilator-associated pneumonia (VAP) in the trauma patient. J Trauma. 2006;60:1106–1113; discussion 1113. [DOI] [PubMed] [Google Scholar]
- 13.Nakos G, Malamou-Mitsi VD, Lachana A, et al. Immunoparalysis in patients with severe trauma and the effect of inhaled interferon-gamma. Crit Care Med. 2002;30:1488–1494. [DOI] [PubMed] [Google Scholar]
- 14.Anjos SM, Shao W, Marchand L, Polychronakos C. Allelic effects on gene regulation at the autoimmunity-predisposing CTLA4 locus: a re-evaluation of the 3′ +6230G>A polymorphism. Genes Immun. 2005;6:305–311. [DOI] [PubMed] [Google Scholar]
- 15.Livingston DH, Appel SH, Wellhausen SR, Sonnenfeld G, Polk HC Jr. Depressed interferon gamma production and monocyte HLA-DR expression after severe injury. Arch.Surg 1988;123:1309–1312. [DOI] [PubMed] [Google Scholar]
- 16.Weber SU, Schewe JC, Lehmann LE, et al. Induction of Bim and Bid gene expression during accelerated apoptosis in severe sepsis. Crit Care. 2008;12:R128. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


