Abstract
Genetic mutations in TAR DNA-binding protein 43 (TDP-43) cause amyotrophic lateral sclerosis (ALS) and frontotemporal dementia (FTD). Importantly, TDP-43 proteinopathy, characterized by aberrant phosphorylation, ubiquitination, cleavage or nuclear depletion of TDP-43 in neurons and glial cells, is a common prominent pathological feature of various major neurodegenerative diseases including ALS, FTD, and Alzheimer’s disease (AD). Although the pathomechanisms underlining TDP-43 proteinopathy remain elusive, pathologically relevant TDP-43 has been repeatedly shown to be present in either the inside or outside of mitochondria, and functionally involved in the regulation of mitochondrial morphology, trafficking, and function, suggesting mitochondria as likely targets of TDP-43 proteinopathy. In this review, we first describe the current knowledge of the association of TDP-43 with mitochondria. We then review in detail multiple mitochondrial pathways perturbed by pathological TDP-43, including mitochondrial fission and fusion dynamics, mitochondrial trafficking, bioenergetics, and mitochondrial quality control. Lastly, we briefly discuss how the study of TDP-43 proteinopathy and mitochondrial abnormalities may provide new avenues for neurodegeneration therapeutics.
Keywords: TDP-43, TDP-43 proteinopathy, mitochondria, neurodegeneration, Neurodegenerative diseases, amyotrophic lateral sclerosis, frontotemporal dementia, Alzheimer’s disease
Introduction
Neurodegenerative diseases are a group of clinically heterogeneous disorders characterized by progressive loss or dysfunction of neurons in the central nervous system (CNS) or peripheral nervous system (PNS) during aging, including Alzheimer’s disease (AD), Parkinson’s disease (PD), Huntington’s disease (HD), amyotrophic lateral sclerosis (ALS), and frontotemporal dementia (FTD) (Lin and Beal, 2006). Although considerable progress has been made to understand how pathological changes in the diseased brain cause neurodegeneration, effective treatments for these devastating diseases are very limited. As a common feature, extracellular or intracellular inclusions containing abnormal accumulation of aggregate-prone proteins characterize many neurodegenerative diseases. In the past decade, TDP-43, encoded by the TARDBP gene, has emerged as a key player in the pathogenesis of diverse neurodegenerative diseases.
TDP-43 has been identified as a major component of the ubiquitinated cytoplasmic inclusions deposited in neurons and glial cells in ALS and FTD (Arai et al., 2006; Neumann et al., 2006). Importantly, TDP-43 gene mutations can cause ALS, together establishing the direct link between TDP-43 and neurodegenerative diseases (Kabashi et al., 2008; Pesiridis et al., 2009; Rutherford et al., 2008; Sreedharan et al., 2008; Van Deerlin et al., 2008). Neurodegenerative diseases associated with aberrant TDP-43 aggregation have been collectively referred to as “TDP-43 proteinopathies”, the term of which is also used to described characteristic histopathological presence of detergent-resistant ubiquitinated, hyperphosphorylated, and truncated species of TDP-43, in addition to its redistribution from the nucleus to cytoplasm (Geser et al., 2009; Mackenzie et al., 2007). Although the formation of cytoplasmic inclusions suggests likely gain of toxic function (Lee et al., 2011), increasing evidence suggests that pathological TDP-43 mediates neurodegeneration through both gain and loss-of-function mechanisms by interrupting multiple pathways including RNA metabolism (Lagier-Tourenne et al., 2010; Polymenidou et al., 2011; Tollervey et al., 2011), protein translation (Buratti and Baralle, 2008; Freibaum et al., 2010), stress-induced response (Colombrita et al., 2009; Dewey et al., 2011; McDonald et al., 2011), autophagy (Bose et al., 2011; Xia et al., 2016), endocytosis (Liu et al., 2017; Schwenk et al., 2016), ubiquitin-proteasome system (UPS) (Hanson et al., 2010; Kim et al., 2009), and mitochondrial function (Wang et al., 2013; Wang et al., 2016; Xu et al., 2010).
TDP-43-linked neurodegenerative diseases are generally multifactorial and involve diverse pathogenic mechanisms such as glutamate excitotoxicity (Li et al., 1997; Sasaki et al., 2000), oxidative stress (Pedersen et al., 1998; Smith et al., 1994), neuroinflammation (Henkel et al., 2004), and mitochondrial dysfunction (Beal et al., 1997; Gibson et al., 1998) in addition to the widely studied TDP-43 proteinopathy. Among them, mitochondrial dysfunction has been extensively studied in the past decade. As prominent early pathological features, mitochondrial abnormalities are closely associated with pathologically related TDP-43 in ALS and FTD patients and experimental models (Izumikawa et al., 2017; Magrane et al., 2014; Salvatori et al., 2018; Wang et al., 2013; Wang et al., 2016). In this review, we first describe the association of TDP-43 with mitochondria, then review in detail the possible mechanisms by which pathological TDP-43 causes mitochondrial abnormalities, and finally discuss future perspectives of mitochondrial related research for TDP-43 proteinopathy.
TDP-43 mitochondrial association
As a member of heterogeneous ribonucleoproteins (hnRNPs) family, TDP-43 is composed of two DNA/RNA recognition and interaction motifs (RRM1, 106–177aa and RRM2, 192–259aa), an N-terminal domain (NTD, 1–102aa), and a carboxyl-terminal glycine-rich domain (CTD, 274–414aa) (Buratti and Baralle, 2001; Kuo et al., 2009). The nuclear localization sequence (NLS, 82–98aa) resides in NTD. Although TDP-43 bears a putative nuclear export sequence (NES, 235–250aa), most recent studies suggest that the nuclear export of TDP-43 is predominantly driven by passive diffusion (Pinarbasi et al., 2018; Winton et al., 2008). The CTD consisting of two prion-like regions flanking the middle hydrophobic fragment, has been demonstrated to undergo liquid-liquid phase separation (LLPS) to facilitate the formation of membrane-less organelles such as RNA and stress granules (Choi et al., 2018; Conicella et al., 2016; Lei et al., 2018; Li et al., 2018; McGurk et al., 2018; Molliex et al., 2015; Sun et al., 2019; Wang et al., 2018a).
Under the physiological condition, the majority of TDP-43 resides in the nucleus and is involved in a wide range of cellular processes such as RNA processing 2018, cryptic splicing (Humphrey et al., 2017; Jeong et al., 2017; Ling et al., 2015), RNA transport (Alami et al., 2014; Pesiridis et al., 2011), and microRNA biogenesis (Buratti et al., 2010; Kawahara and Mieda-Sato, 2012; King et al., 2014) through its DNA/RNA binding ability. Besides its nuclear localization, TDP-43 can also be present in the cytoplasm and co-localize with subcellular compartments such as endoplasmic reticulum (ER) (Li et al., 2015; Walker et al., 2013), mitochondria (Wang et al., 2013; Wang et al., 2016), mitochondria-associated membranes (MAMs) (Stoica et al., 2014), RNA granules (Alami et al., 2014), and stress granules (Colombrita et al., 2009; Liu-Yesucevitz et al., 2010) to regulate ER-mitochondrial tethering, mitochondrial protein translation, mRNA transport, and translation.
Studies by our group and others have independently demonstrated the association of TDP-43 with mitochondria (Fig. 1A). It was firstly reported that exogenously expressed wild type or ALS-associated mutant TDP-43 could be detected in mitochondrial-enriched fractions from NSC-34 motor neuron-like cells (Hong et al., 2012). Consistently, we provided evidence showing the presence of endogenous TDP-43 in highly purified mitochondria from NSC-34 cells without ER contamination (Wang et al., 2013). Using HEK293 cells, human and mouse brain and spinal cord tissues, we further showed that at least a portion of TDP-43 could localize in the inner membrane of mitochondria, and contains several putative mitochondrial import sequences (Wang et al., 2016). However, the following confirmatory studies are largely controversial. For example, TDP-43 was suggested only present in membranes associated with mitochondria in HEK293 or HeLa cells and mouse brains (Kawamata et al., 2017). In contrast, a most recent study using NSC-34 cells reported that full-length and truncated forms of TDP‐43 could differentially reside in the matrix and intermembrane space of mitochondria (Salvatori et al., 2018), while the study using mouse cortical and hippocampal tissue showed truncated but not full-length TDP-43 in mitochondria (Davis et al., 2018) (Fig.1A).
Fig.1. TDP-43 proteinopathy and mitochondrial abnormalities in neurodegeneration.
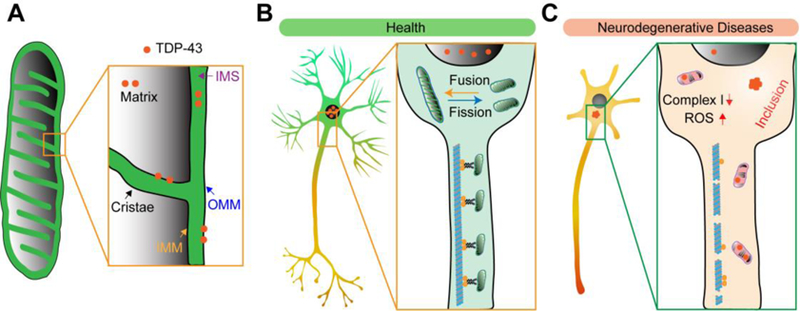
(A) TDP-43 mitochondrial association. OMM, outer mitochondrial membrane; IMM, inner mitochondrial membrane; IMS, intermembrane space. (B) Mitochondria are dynamic organelles that undergo continuous fission, fusion, and trafficking, which are critical for the maintenance of mitochondrial function in neurons. The majority of TDP-43 resides in the nuclei and involves in a wide range of cellular processes such as RNA processing, cryptic splicing, RNA transport, and microRNA biogenesis in healthy neurons. (C) In disease-affected neurons, TDP-43 forms inclusions in the cytoplasm, usually accompanied by depletion of nuclear TDP-43. Abnormal mitochondria with altered morphology, inner structure, and OXPHOS activity can be noted in neurons bearing TDP-43 proteinopathy.
There may be several possible reasons for these contradictory findings including methods used to isolate or enrich mitochondria, antibodies used to detect truncated or full-length TDP-43, and the likely variable expression of mitochondrial associated TDP-43. Along this line, it is worthwhile to note that mitochondria associated TDP-43 was found highly phosphorylated in ALS/FTD patient derived neurons or fibroblasts according to our and recent studies (Genin et al., 2018; Wang et al., 2016), together suggesting the likely crucial role of altered posttranslational modifications for TDP-43 mitochondrial accumulation in diseases. Nevertheless, while alternative or novel approaches are to be developed to determine the exact sub-mitochondrial localization of full-length or truncated TDP-43, all previously published studies unanimously support the direct association of TDP-43 with mitochondria. As the characteristic pathological features of TDP-43 proteinopathy include impaired nucleus-cytoplasm-trafficking and aberrant posttranslational modifications of TDP-43, future studies might investigate whether and how ubiquitin ligases, kinases, proteases, and other factors involved in TDP-43 posttranslational modifications and nucleus-cytoplasm-trafficking contribute to TDP-43 mitochondrial association.
TDP-43 and mitochondrial fission and fusion dynamics
Mitochondria are dynamic organelles that undergo continuous fission and fusion (Nunnari et al., 1997). Unopposed fission results in division, while unopposed fusion causes elongation (Bleazard et al., 1999; Sesaki and Jensen, 1999). Studies in the past decade have revealed that mitochondrial fission and fusion dynamics are essential for various aspects of mitochondrial function including respiratory complex assembly (Cogliati et al., 2013), ATP production (Benard et al., 2007), Ca2+ homeostasis (Frieden et al., 2004; Szabadkai et al., 2004), and reactive oxygen species (ROS) production (Yu et al., 2006). Mitochondria fission and fusion are tightly controlled by several key regulators including dynamin-like protein 1 (DLP1/Drp1) and its recruiting factors on mitochondria such as Mff and Fis1 (Loson et al., 2013), mitofusin 1 (Mfn1), mitofusin 2 (Mfn2), and optic atrophy protein 1(OPA1) (Detmer and Chan, 2007). Mitochondrial morphological alterations manifested as fragmented mitochondria with damaged inner membrane structures have been increasingly reported as prominent early features in various major neurodegenerative diseases including ALS (Sasaki et al., 2007) and AD (Wang et al., 2009) (Fig. 1B and 1C).
Xu et al. firstly reported mitochondria aggregation in transgenic mice overexpressing wild type TDP-43 (Xu et al., 2010). And, the subsequent studies observed swollen mitochondria with damaged cristae structures in cultured NSC34 cells expressing ALS-associated mutant TDP-43 (Hong et al., 2012; Lu et al., 2012). Consistently, we and others further demonstrated that mitochondria indeed became fragmented accompanied by loss of mitochondrial inner membrane structure in cultured primary motor neurons or transgenic mice expressing ALS-associated mutant TDP-43 (Gautam et al., 2019; Magrane et al., 2014; Wang et al., 2013). Although ALS patients-derived primary fibroblasts without TDP-43 mutation did not show any mitochondrial morphology changes (Codron et al., 2018), ALS patient-derived lymphoblastoid cell lines or fibroblasts bearing TDP-43 mutation also exhibited damaged and swollen mitochondria (Gautam et al., 2019; Onesto et al., 2016). Noteworthily, the morphology changes seen in TDP-43 experimental models are in good agreement with studies repeatedly reporting altered expression of mitochondria fission and fusion regulators such as Drp1, Fis1, MFN1, and OPA1 (Davis et al., 2018; Joshi et al., 2018; Xu et al., 2010). Although the mechanisms by which TDP-43 regulates mitochondrial dynamics remains elusive, our previous study reported that mutant TDP-43-induced mitochondrial fragmentation could be alleviated by the overexpression of Mfn2, suggesting the likely involvement of Mfn2 dependent fusion. This notion is indeed supported by one most recent study showing the possible physical interaction between TDP-43 and Mfn2 (Davis et al., 2018). However, it is worth noting a puzzling finding in this study: Mfn2 expression was increased rather than reduced by overexpression of wild type TDP-43 in brains, indicating that wild type and mutant TDP-43 may perturb mitochondrial dynamics through different mechanisms.
In addition to neurofibrillary tangles (NFTs) and senile plaques (SPs), cytoplasmic TDP-43 inclusions have been implied as the likely third proteinopathy in patients with AD (James et al., 2016; Uryu et al., 2008). While both Tau and Aβ, the major components of NFTs and SPs, have long been reported to disturb mitochondrial dynamics (Silva et al., 2011), there are limited studies about TDP-43 and mitochondrial dynamics in AD-related experimental models. The recent study reporting increased expression of Mfn2 by TDP-43 also demonstrated that overexpression of wild type TDP-43 caused giant and swollen-structured mitochondria in hippocampal neurons in APP/PS1 transgenic mice for AD (Davis et al., 2018). Although it is still unclear how these findings are reconciled with previous studies showing mitochondrial fragmentation and reduced expression of Mfn2 in AD patients and experimental models (Wang et al., 2009; Wang et al., 2008), the co-existence of multiple pathological features in AD and many other neurodegenerative diseases suggest the likely synergistic effects of TDP-43 and other proteinopathies on mitochondria dynamics, which are relatively unexplored and worthy of further detailed investigation.
TDP-43 and mitochondrial trafficking
In response to various physiological and pathological states, mitochondria are transported by motor-adaptor complexes to sites with bioenergetics requirements, which is vital for neuronal function and survival (Sheng and Cai, 2012). Failure of proper positioning of mitochondria in dendrites or axon terminals has long been implicated in neurodegenerative diseases and proposed as the potential cause of synaptic loss, a prominent early pathological feature well preceding neurodegeneration (Burte et al., 2015). In addition to changed mitochondrial morphology, impaired mitochondria transport was also consistently noted in cell and animal models expressing wild type or disease-associated mutant TDP-43 (Fig. 1B and 1C). In cultured primary motor neurons, overexpression of wild type TDP-43 resulted in impaired mitochondrial anterograde and retrograde transport in both axon and dendrites, which could be exacerbated by ALS-associated mutations (Wang et al., 2013). Unexpectedly, loss of TDP-43 also decreased mitochondrial trafficking in both axons and dendrites similar to TDP-43 overexpression (Wang et al., 2013), indicating the likely involvement of different pathways for TDP-43 mediated mitochondrial transport. Consistent with our findings, loss of fly TDP-43 caused an overall increase in stationary mitochondria in axon, which could be rescued by ectopic expression of fly TDP-43 or human TDP-43 (Baldwin et al., 2016), further suggesting that the mitochondrial trafficking related function of TDP-43 should be conserved between flies and mammals. Importantly, mitochondrial transport defects appeared to be early pathological features of TDP-43 transgenic mice, well proceeding the onset of symptoms and even morphological abnormalities (Magrane et al., 2014). Interestingly, human induced pluripotent stem cells (iPSCs)-derived motor neurons bearing TDP-43 mutation showed drastic slow moving speed at proximal and distal axons in an age-dependent manner without detectable cytoplasmic inclusions or phosphorylated TDP-43 accumulation, further indicating that mutant TDP-43 may cause mitochondrial toxicity independent of proteinopathy (Kreiter et al., 2018).
On the basis of the facts that cytoskeleton is essential for the intracellular transport and positioning of mitochondria, membrane vesicles, and membrane-less RNA granules from neuronal cell body to the distal axonal terminals (Chetta et al., 2015), and that aberrant aggregates of cytoskeletal proteins are neuropathological signatures of many neurodegenerative diseases (Baskaran et al., 2018; Schwenk et al., 2016), it could be anticipated that loss of cytoskeleton integrity caused by pathological TDP-43 might contribute to abnormal mitochondrial transport (Oberstadt et al., 2018). Along this line, by mediating the splicing and translation of mRNA targets, TDP-43 has also been reported to be functionally associated with other microtubule related proteins such as Futsch/MAP1B (Coyne et al., 2014; Godena et al., 2011), NFL (Strong et al., 2007), STMN2 (Klim et al., 2019; Melamed et al., 2019), and Tau (Gu et al., 2017a; Gu et al., 2017b; Lagier-Tourenne et al., 2012). In addition, cytoplasmic TDP-43 has been reported to regulate RNA granules trafficking through microtubule network (Alami et al., 2014). Therefore, although there is no evidence showing TDP-43-regulated mitochondrial transport directly through cytoskeleton, further studies will be interesting to characterize the functional role of reported TDP-43 targets in mediating mutant TDP-43 induced mitochondrial transport defects.
TDP-43 and mitochondrial function
The mitochondrial oxidative phosphorylation system (OXPHOS) utilizes substrates derived from glucose, fatty acids, and amino acids to produce reducing equivalents that are delivered to the respiratory chain to generate adenosine triphosphate (ATP). The respiratory chain consists of four protein complexes (complex I–IV), three of which (I, III, and IV) couple electron transfer to proton pumping across the mitochondrial inner membrane to generate a transmembrane electrochemical potential. The complex V, named ATP synthase, synthesizes ATP from ADP and inorganic phosphate using the energy provided by the proton electrochemical gradient. In addition to generate ATP, mitochondria are also required for a wide range of cellular processes such as the synthesis of key metabolites, Ca2+ hemostasis, inflammation and apoptosis. Thus, it is not surprising that mitochondrial functional abnormalities have been extensively studied in many neurodegenerative diseases, including ALS (Beal et al., 1997; Mattiazzi et al., 2002) and AD (Gibson et al., 1998; Ojaimi et al., 1999; Parker et al., 1990) (Fig. 1C).
Several groups have independently reported mitochondrial OXPHOS deficits in TDP-43-associated experimental models. For example, reduced mitochondrial complex I activity and mitochondrial transmembrane potential as well as increased expression of mitochondrial uncoupling protein 2 (UCP2) were firstly noted in NSC-34 cells overexpressing wild type or mutant TDP-43 (Lu et al., 2012). In support of these original findings, we and the recent study in HEK293 or NSC34 cells have independently found that the portion of full-length TDP-43 inside of mitochondria can bind mitochondria-transcribed messenger RNAs (mRNAs) encoding subunits (ND3/6) of OXPHOS complex I to specifically impair its assembly and function (Salvatori et al., 2018; Wang et al., 2019; Wang et al., 2016), whereas truncated TDP-43 lacking the M1 mitochondrial localization sequence is restricted to the intermembrane space and has no effect on ND3/6 expression or mitochondrial function (Salvatori et al., 2018). In addition, TDP-43 has also been reported to maintain mitochondrial function by stabilizing the processing intermediates of mitochondrial polycistronic transcripts encoding the components of electron transport and ribosomal RNAs (Izumikawa et al., 2017). Moreover, ALS patient-derived lymphoblastoid cell line with TDP-43 mutation exhibited perturbed mitochondrial function including increased basal oxygen consumption rate and decreased spare respiratory capacity (SRC), which refer to mitochondrial ability to generate energy (Pansarasa et al., 2018). The role of mitochondria for TDP-43 is further supported by observations that TDP-43 cellular toxicity in yeasts could be altered by manipulating mitochondrial function (Braun et al., 2011; Park et al., 2019). However, despite increasing evidence suggesting mitochondria as targets or mediators of TDP-43 toxicity (Davis et al., 2018; Genin et al., 2018; Hibiki Kawamata, 2017; Izumikawa et al., 2017; Salvatori et al., 2018; Wang et al., 2016; Woo et al., 2017), there are considerable discrepancies as to its impact on mitochondrial function. For example, although reduced mitochondrial membrane potential was observed, oxygen consumption, ATP production, and OXPHOS complex activity remained unchanged in human fibroblasts bearing TDP-43 (Onesto et al., 2016). Similarly, despite altered mitochondrial calcium capacity, oxygen consumption, ATP production, and mitochondrial membrane potential were all reported unchanged in HEK293 cells or transgenic mouse expressing mutant TDP-43 (Kawamata et al., 2017). To resolve these discrepancies, our future investigation might need novel tools or techniques to pinpoint the time-course change of mitochondrial function locally and systematically in different neuronal compartments in response to TDP-43 expression. In addition, as TDP-43 has been reported to interfere in ER–mitochondria associations (Stoica et al., 2014), which are important for calcium homeostasis, lipid metabolism, autophagy, and even protein transport (Pinton, 2018; Wang et al., 2018b), the possible indirect ability of TDP-43 to regulate mitochondrial function should also be considered.
TDP-43 and mitochondrial quality control
Mitochondrial AAA proteases are required for the maintenance of mitochondrial proteostasis and functional integrity (Quiros et al., 2015). And, genetic mutations in mitochondrial AAA proteases have been shown associated with neurodegeneration (Patron et al., 2018). Interestingly, mitochondrial proteases have recently been proposed as an alternative proteostasis mechanism to counteract cytosolic protein aggregates (Ruan et al., 2017). It was shown that during heat shock in yeast, TDP-43 could be imported into mitochondria for degradation by mitochondrial proteases (Ruan et al., 2017). While proteases responsible for mitochondria-imported TDP-43 remain unknown, one most recent study showed that DJ-1, a putative protease localized both in cytoplasm and mitochondria, protected against oxidative stress-induced cell death through the suppression of cytoplasmic TDP-43 aggregation, suggesting that DJ1 may alleviate TDP-43-caused toxicity through degrading both cytoplasmic and mitochondrial TDP-43 (Lei et al., 2018). In addition to the selective turnover of misfolded proteins by mitochondrial proteases, the general mitochondria quality control involves mitophagy, a mechanism by which damaged mitochondria are engulfed in autophagosomes to be degraded through the autophagic-lysosomal pathway (Chen and Chan, 2009). Similar to mitochondrial proteases, mutations in mitophagy-related genes are also closely associated with neurodegenerative disease (Pickrell and Youle, 2015; Youle and Narendra, 2011). Overexpression of TDP-43 has previously been shown to induce mitophagy in NSC34 cells (Hong et al., 2012). And, mutant TDP-43 transgenic mice exhibited mitochondrial accumulations fused with lysosomes, further supporting the possible link between TDP-43 and mitophagy (Gautam et al., 2019). In support of this notion, it has been reported that mitochondrial associated TDP-43 interacts with mitochondrial outer membrane protein prohibitin 2 (PHB2) and voltage-gated dependent anion channel 1 (VDAC1), which are crucial receptors for Parkin-mediated mitophagy (Davis et al., 2018). Although whether TDP-43 is involved in PINK1/Parkin mediated mitophagy remains elusive, Parkin has been reported to mediate poly-ubiquitination of TDP-43, and overexpression of Parkin could reverse TDP-43-induced cell toxicity (Hebron et al., 2014; Hebron et al., 2013; Wenqiang et al., 2014). Likewise, TDP-43 also modulates the expression of Parkin and PINK1, leading to compromised mitochondrial functions and mitophagy (Sun et al., 2018). Augmenting mitophagy by neuronal PINK1 overexpression reduces Aβ pathology and improves mitochondrial and synaptic dysfunction in APP mice (Du et al., 2017). Therefore, it may be anticipated that enhancing mitophagy may be a potential strategy to alleviate TDP-43-induced cytotoxicity.
Conclusions
Like mitochondrial dysfunction (Lin and Beal, 2006), TDP-43 proteinopathy is a common prominent pathological feature of various major neurodegenerative diseases including ALS, FTD, and AD. We have reported that the inhibition of TDP-43 mitochondrial localization is sufficient to alleviate mitochondrial dynamic abnormalities, neuronal loss, and behavioral deficits in different mutant TDP-43 transgenic mice (Wang et al., 2016; Wang et al., 2017). Therefore, targeting TDP-43 mitochondrial association may be a promising novel therapeutic approach for neurodegeneration. However, the pathogenic mechanisms linking mitochondrial abnormalities with TDP-43 proteinopathy, and related neurodegeneration are still poorly understood. Consdiering recent discrepant studies reporting the sub-mitochondrial localization of TDP-43 and the interaction of TDP-43 with different mitochondrial pathways, mitochondria-associted TDP-43 or TDP-43 fragments may synergistically mediate mitochondrial and neuronal function through multiple pathways involving but not limited to mitochondrial dynamics, trafficking, bioenergetics, and quality control. As mitochondria are increasingly implicated as critical targets of Aβ, tau, α-synuclein, and many other neurodegenerative disease-associated proteinopathies, mitochondria, therefore, possibly lie at the convergence of a diverse range of proteinopathies. Future research into TDP-43 mitochondrial association in the context of multiple proteinopathies may help us clarify whether and how TDP-43 is physically and functionally associated with mitochondria and contributes to disease progression, and importantly, provide new therapeutic targets for these devastating diseases.
Highlights.
This review describes our current knowledge of the physical and functional association of TDP-43 with mitochondria.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alami NH, Smith RB, Carrasco MA, Williams LA, Winborn CS, Han SSW, Kiskinis E, Winborn B, Freibaum BD, Kanagaraj A, Clare AJ, Badders NM, Bilican B, Chaum E, Chandran S, Shaw CE, Eggan KC, Maniatis T, Taylor JP, 2014. Axonal transport of TDP-43 mRNA granules is impaired by ALS-causing mutations. Neuron 81, 536–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai T, Hasegawa M, Akiyama H, Ikeda K, Nonaka T, Mori H, Mann D, Tsuchiya K, Yoshida M, Hashizume Y, Oda T, 2006. TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem Biophys Res Commun 351, 602–611. [DOI] [PubMed] [Google Scholar]
- Baldwin KR, Godena VK, Hewitt VL, Whitworth AJ, 2016. Axonal transport defects are a common phenotype in Drosophila models of ALS. Human molecular genetics 25, 2378–2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baskaran P, Shaw C, Guthrie S, 2018. TDP-43 causes neurotoxicity and cytoskeletal dysfunction in primary cortical neurons. PloS one 13, e0196528. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Beal MF, Ferrante RJ, Browne SE, Matthews RT, Kowall NW, Brown RH, 1997. Increased 3-nitrotyrosine in both sporadic and familial amyotrophic lateral sclerosis. Annals of neurology 42, 644–654. [DOI] [PubMed] [Google Scholar]
- Benard G, Bellance N, James D, Parrone P, Fernandez H, Letellier T, Rossignol R, 2007. Mitochondrial bioenergetics and structural network organization. Journal of cell science 120, 838–848. [DOI] [PubMed] [Google Scholar]
- Bleazard W, McCaffery JM, King EJ, Bale S, Mozdy A, Tieu Q, Nunnari J, Shaw JM, 1999. The dynamin-related GTPase Dnm1 regulates mitochondrial fission in yeast. Nature cell biology 1, 298–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bose JK, Huang CC, Shen CK, 2011. Regulation of autophagy by neuropathological protein TDP-43. The Journal of biological chemistry 286, 44441–44448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun RJ, Sommer C, Carmona-Gutierrez D, Khoury CM, Ring J, Buttner S, Madeo F, 2011. Neurotoxic 43-kDa TAR DNA-binding protein (TDP-43) triggers mitochondrion-dependent programmed cell death in yeast. The Journal of biological chemistry 286, 19958–19972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buratti E, Baralle FE, 2001. Characterization and functional implications of the RNA binding properties of nuclear factor TDP-43, a novel splicing regulator of CFTR exon 9. The Journal of biological chemistry 276, 36337–36343. [DOI] [PubMed] [Google Scholar]
- Buratti E, Baralle FE, 2008. Multiple roles of TDP-43 in gene expression, splicing regulation, and human disease. Frontiers in bioscience : a journal and virtual library 13, 867–878. [DOI] [PubMed] [Google Scholar]
- Buratti E, De Conti L, Stuani C, Romano M, Baralle M, Baralle F, 2010. Nuclear factor TDP-43 can affect selected microRNA levels. The FEBS journal 277, 2268–2281. [DOI] [PubMed] [Google Scholar]
- Burte F, Carelli V, Chinnery PF, Yu-Wai-Man P, 2015. Disturbed mitochondrial dynamics and neurodegenerative disorders. Nature reviews. Neurology 11, 11–24. [DOI] [PubMed] [Google Scholar]
- Chen H, Chan DC, 2009. Mitochondrial dynamics--fusion, fission, movement, and mitophagy--in neurodegenerative diseases. Human molecular genetics 18, R169–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chetta J, Love JM, Bober BG, Shah SB, 2015. Bidirectional actin transport is influenced by microtubule and actin stability. Cell Mol Life Sci 72, 4205–4220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi KJ, Tsoi PS, Moosa MM, Paulucci-Holthauzen A, Liao SJ, Ferreon JC, Ferreon ACM, 2018. A Chemical Chaperone Decouples TDP-43 Disordered Domain Phase Separation from Fibrillation. Biochemistry 57, 6822–6826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Codron P, Cassereau J, Vourc’h P, Veyrat-Durebex C, Blasco H, Kane S, Procaccio V, Letournel F, Verny C, Lenaers G, Reynier P, Chevrollier A, 2018. Primary fibroblasts derived from sporadic amyotrophic lateral sclerosis patients do not show ALS cytological lesions. Amyotrophic lateral sclerosis & frontotemporal degeneration 19, 446–456. [DOI] [PubMed] [Google Scholar]
- Cogliati S, Frezza C, Soriano ME, Varanita T, Quintana-Cabrera R, Corrado M, Cipolat S, Costa V, Casarin A, Gomes LC, Perales-Clemente E, Salviati L, Fernandez-Silva P, Enriquez JA, Scorrano L, 2013. Mitochondrial cristae shape determines respiratory chain supercomplexes assembly and respiratory efficiency. Cell 155, 160–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombrita C, Zennaro E, Fallini C, Weber M, Sommacal A, Buratti E, Silani V, Ratti A, 2009. TDP-43 is recruited to stress granules in conditions of oxidative insult. Journal of neurochemistry 111, 1051–1061. [DOI] [PubMed] [Google Scholar]
- Conicella AE, Zerze GH, Mittal J, Fawzi NL, 2016. ALS Mutations Disrupt Phase Separation Mediated by alpha-Helical Structure in the TDP-43 Low-Complexity C-Terminal Domain. Structure 24, 1537–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyne AN, Siddegowda BB, Estes PS, Johannesmeyer J, Kovalik T, Daniel SG, Pearson A, Bowser R, Zarnescu DC, 2014. Futsch/MAP1B mRNA is a translational target of TDP-43 and is neuroprotective in a Drosophila model of amyotrophic lateral sclerosis. J Neurosci 34, 15962–15974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis SA, Itaman S, Khalid-Janney CM, Sherard JA, Dowell JA, Cairns NJ, Gitcho MA, 2018. TDP-43 interacts with mitochondrial proteins critical for mitophagy and mitochondrial dynamics. Neurosci Lett 678, 8–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detmer SA, Chan DC, 2007. Functions and dysfunctions of mitochondrial dynamics. Nature reviews. Molecular cell biology 8, 870–879. [DOI] [PubMed] [Google Scholar]
- Dewey CM, Cenik B, Sephton CF, Dries DR, Mayer P 3rd, Good SK, Johnson BA, Herz J, Yu G, 2011. TDP-43 is directed to stress granules by sorbitol, a novel physiological osmotic and oxidative stressor. Molecular and cellular biology 31, 1098–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du F, Yu Q, Yan S, Hu G, Lue LF, Walker DG, Wu L, Yan SF, Tieu K, Yan SS, 2017. PINK1 signalling rescues amyloid pathology and mitochondrial dysfunction in Alzheimer’s disease. Brain 140, 3233–3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freibaum BD, Chitta RK, High AA, Taylor JP, 2010. Global analysis of TDP-43 interacting proteins reveals strong association with RNA splicing and translation machinery. Journal of proteome research 9, 1104–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frieden M, James D, Castelbou C, Danckaert A, Martinou JC, Demaurex N, 2004. Ca(2+) homeostasis during mitochondrial fragmentation and perinuclear clustering induced by hFis1. The Journal of biological chemistry 279, 22704–22714. [DOI] [PubMed] [Google Scholar]
- Gautam M, Jara JH, Kocak N, Rylaarsdam LE, Kim KD, Bigio EH, Hande Ozdinler P, 2019. Mitochondria, ER, and nuclear membrane defects reveal early mechanisms for upper motor neuron vulnerability with respect to TDP-43 pathology. Acta Neuropathol 137, 47–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genin EC, Bannwarth S, Lespinasse F, Ortega-Vila B, Fragaki K, Itoh K, Villa E, Lacas-Gervais S, Jokela M, Auranen M, Ylikallio E, Mauri-Crouzet A, Tyynismaa H, Vihola A, Auge G, Cochaud C, Sesaki H, Ricci JE, Udd B, Vives-Bauza C, Paquis-Flucklinger V, 2018. Loss of MICOS complex integrity and mitochondrial damage, but not TDP-43 mitochondrial localisation, are likely associated with severity of CHCHD10-related diseases. Neurobiol Dis 119, 159–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geser F, Martinez-Lage M, Robinson J, Uryu K, Neumann M, Brandmeir NJ, Xie SX, Kwong LK, Elman L, McCluskey L, Clark CM, Malunda J, Miller BL, Zimmerman EA, Qian J, Van Deerlin V, Grossman M, Lee VM, Trojanowski JQ, 2009. Clinical and pathological continuum of multisystem TDP-43 proteinopathies. Archives of neurology 66, 180–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson GE, Sheu KF, Blass JP, 1998. Abnormalities of mitochondrial enzymes in Alzheimer disease. J Neural Transm (Vienna) 105, 855–870. [DOI] [PubMed] [Google Scholar]
- Godena VK, Romano G, Romano M, Appocher C, Klima R, Buratti E, Baralle FE, Feiguin F, 2011. TDP-43 regulates Drosophila neuromuscular junctions growth by modulating Futsch/MAP1B levels and synaptic microtubules organization. PLoS One 6, e17808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu J, Chen F, Iqbal K, Gong CX, Wang X, Liu F, 2017a. Transactive response DNA-binding protein 43 (TDP-43) regulates alternative splicing of tau exon 10: Implications for the pathogenesis of tauopathies. The Journal of biological chemistry 292, 10600–10612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu J, Wu F, Xu W, Shi J, Hu W, Jin N, Qian W, Wang X, Iqbal K, Gong CX, Liu F, 2017b. TDP-43 suppresses tau expression via promoting its mRNA instability. Nucleic acids research 45, 6177–6193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson KA, Kim SH, Wassarman DA, Tibbetts RS, 2010. Ubiquilin modifies TDP-43 toxicity in a Drosophila model of amyotrophic lateral sclerosis (ALS). The Journal of biological chemistry 285, 11068–11072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebron M, Chen W, Miessau MJ, Lonskaya I, Moussa CE, 2014. Parkin reverses TDP-43-induced cell death and failure of amino acid homeostasis. J Neurochem 129, 350–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebron ML, Lonskaya I, Sharpe K, Weerasinghe PP, Algarzae NK, Shekoyan AR, Moussa CE, 2013. Parkin ubiquitinates Tar-DNA binding protein-43 (TDP-43) and promotes its cytosolic accumulation via interaction with histone deacetylase 6 (HDAC6). J Biol Chem 288, 4103–4115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henkel JS, Engelhardt JI, Siklos L, Simpson EP, Kim SH, Pan T, Goodman JC, Siddique T, Beers DR, Appel SH, 2004. Presence of dendritic cells, MCP-1, and activated microglia/macrophages in amyotrophic lateral sclerosis spinal cord tissue. Annals of neurology 55, 221–235. [DOI] [PubMed] [Google Scholar]
- Hibiki Kawamata PP, Konrad Csaba, Palomo Gloria, Bredvik Kirsten, Gerges Meri, Valsecchi Federica, Petrucelli Leonard, Ravits John M., Starkov Anatoly and Manfredi Giovanni, 2017. Mutant TDP-43 does not impair mitochondrial bioenergetics in vitro and in vivo. Molecular Neurodegeneration 12, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong K, Li Y, Duan W, Guo Y, Jiang H, Li W, Li C, 2012. Full-length TDP-43 and its C-terminal fragments activate mitophagy in NSC34 cell line. Neurosci Lett 530, 144–149. [DOI] [PubMed] [Google Scholar]
- Humphrey J, Emmett W, Fratta P, Isaacs AM, Plagnol V, 2017. Quantitative analysis of cryptic splicing associated with TDP-43 depletion. BMC medical genomics 10, 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumikawa K, Nobe Y, Yoshikawa H, Ishikawa H, Miura Y, Nakayama H, Nonaka T, Hasegawa M, Egawa N, Inoue H, Nishikawa K, Yamano K, Simpson RJ, Taoka M, Yamauchi Y, Isobe T, Takahashi N, 2017. TDP-43 stabilises the processing intermediates of mitochondrial transcripts. Sci Rep 7, 7709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James BD, Wilson RS, Boyle PA, Trojanowski JQ, Bennett DA, Schneider JA, 2016. TDP-43 stage, mixed pathologies, and clinical Alzheimer’s-type dementia. Brain : a journal of neurology 139, 2983–2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong YH, Ling JP, Lin SZ, Donde AN, Braunstein KE, Majounie E, Traynor BJ, LaClair KD, Lloyd TE, Wong PC, 2017. Tdp-43 cryptic exons are highly variable between cell types. Molecular neurodegeneration 12, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi AU, Saw NL, Vogel H, Cunnigham AD, Shamloo M, Mochly-Rosen D, 2018. Inhibition of Drp1/Fis1 interaction slows progression of amyotrophic lateral sclerosis. EMBO molecular medicine 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabashi E, Valdmanis PN, Dion P, Spiegelman D, McConkey BJ, Vande Velde C, Bouchard JP, Lacomblez L, Pochigaeva K, Salachas F, Pradat PF, Camu W, Meininger V, Dupre N, Rouleau GA, 2008. TARDBP mutations in individuals with sporadic and familial amyotrophic lateral sclerosis. Nature genetics 40, 572–574. [DOI] [PubMed] [Google Scholar]
- Kawahara Y, Mieda-Sato A, 2012. TDP-43 promotes microRNA biogenesis as a component of the Drosha and Dicer complexes. Proceedings of the National Academy of Sciences of the United States of America 109, 3347–3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamata H, Peixoto P, Konrad C, Palomo G, Bredvik K, Gerges M, Valsecchi F, Petrucelli L, Ravits JM, Starkov A, Manfredi G, 2017. Mutant TDP-43 does not impair mitochondrial bioenergetics in vitro and in vivo. Mol Neurodegener 12, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Shi Y, Hanson KA, Williams LM, Sakasai R, Bowler MJ, Tibbetts RS, 2009. Potentiation of amyotrophic lateral sclerosis (ALS)-associated TDP-43 aggregation by the proteasome-targeting factor, ubiquilin 1. The Journal of biological chemistry 284, 8083–8092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King IN, Yartseva V, Salas D, Kumar A, Heidersbach A, Ando DM, Stallings NR, Elliott JL, Srivastava D, Ivey KN, 2014. The RNA-binding protein TDP-43 selectively disrupts microRNA-½06 incorporation into the RNA-induced silencing complex. The Journal of biological chemistry 289, 14263–14271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klim JR, Williams LA, Limone F, Guerra San Juan I, Davis-Dusenbery BN, Mordes DA, Burberry A, Steinbaugh MJ, Gamage KK, Kirchner R, Moccia R, Cassel SH, Chen K, Wainger BJ, Woolf CJ, Eggan K, 2019. ALS-implicated protein TDP-43 sustains levels of STMN2, a mediator of motor neuron growth and repair. Nat Neurosci 22, 167–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreiter N, Pal A, Lojewski X, Corcia P, Naujock M, Reinhardt P, Sterneckert J, Petri S, Wegner F, Storch A, Hermann A, 2018. Age-dependent neurodegeneration and organelle transport deficiencies in mutant TDP43 patient-derived neurons are independent of TDP43 aggregation. Neurobiology of disease 115, 167–181. [DOI] [PubMed] [Google Scholar]
- Kuo PH, Doudeva LG, Wang YT, Shen CK, Yuan HS, 2009. Structural insights into TDP-43 in nucleic-acid binding and domain interactions. Nucleic acids research 37, 1799–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagier-Tourenne C, Polymenidou M, Cleveland DW, 2010. TDP-43 and FUS/TLS: emerging roles in RNA processing and neurodegeneration. Human molecular genetics 19, R46–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagier-Tourenne C, Polymenidou M, Hutt KR, Vu AQ, Baughn M, Huelga SC, Clutario KM, Ling SC, Liang TY, Mazur C, Wancewicz E, Kim AS, Watt A, Freier S, Hicks GG, Donohue JP, Shiue L, Bennett CF, Ravits J, Cleveland DW, Yeo GW, 2012. Divergent roles of ALS-linked proteins FUS/TLS and TDP-43 intersect in processing long pre-mRNAs. Nature neuroscience 15, 1488–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee EB, Lee VM, Trojanowski JQ, 2011. Gains or losses: molecular mechanisms of TDP43-mediated neurodegeneration. Nat Rev Neurosci 13, 38–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei Y, Zhang ZF, Lei RX, Wang S, Zhuang Y, Liu AC, Wu Y, Chen J, Tang JC, Pan MX, Liu R, Liao WJ, Feng YG, Wan Q, Zheng M, 2018. DJ-1 Suppresses Cytoplasmic TDP-43 Aggregation in Oxidative Stress-Induced Cell Injury. J Alzheimers Dis 66, 1001–1014. [DOI] [PubMed] [Google Scholar]
- Li HR, Chiang WC, Chou PC, Wang WJ, Huang JR, 2018. TAR DNA-binding protein 43 (TDP-43) liquid-liquid phase separation is mediated by just a few aromatic residues. The Journal of biological chemistry 293, 6090–6098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Yokoshi M, Okada H, Kawahara Y, 2015. The cleavage pattern of TDP-43 determines its rate of clearance and cytotoxicity. Nature communications 6, 6183. [DOI] [PubMed] [Google Scholar]
- Li S, Mallory M, Alford M, Tanaka S, Masliah E, 1997. Glutamate transporter alterations in Alzheimer disease are possibly associated with abnormal APP expression. J Neuropath Exp Neur 56, 901–911. [DOI] [PubMed] [Google Scholar]
- Lin MT, Beal MF, 2006. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 443, 787–795. [DOI] [PubMed] [Google Scholar]
- Ling JP, Pletnikova O, Troncoso JC, Wong PC, 2015. TDP-43 repression of nonconserved cryptic exons is compromised in ALS-FTD. Science 349, 650–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu-Yesucevitz L, Bilgutay A, Zhang YJ, Vanderweyde T, Citro A, Mehta T, Zaarur N, McKee A, Bowser R, Sherman M, Petrucelli L, Wolozin B, 2010. Tar DNA binding protein-43 (TDP-43) associates with stress granules: analysis of cultured cells and pathological brain tissue. PloS one 5, e13250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Coyne AN, Pei F, Vaughan S, Chaung M, Zarnescu DC, Buchan JR, 2017. Endocytosis regulates TDP-43 toxicity and turnover. Nature communications 8, 2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loson OC, Song Z, Chen H, Chan DC, 2013. Fis1, Mff, MiD49 and MiD51 mediate Drp1 recruitment in mitochondrial fission. Molecular biology of the cell [DOI] [PMC free article] [PubMed]
- Lu J, Duan W, Guo Y, Jiang H, Li Z, Huang J, Hong K, Li C, 2012. Mitochondrial dysfunction in human TDP-43 transfected NSC34 cell lines and the protective effect of dimethoxy curcumin. Brain Res Bull 89, 185–190. [DOI] [PubMed] [Google Scholar]
- Mackenzie IR, Bigio EH, Ince PG, Geser F, Neumann M, Cairns NJ, Kwong LK, Forman MS, Ravits J, Stewart H, Eisen A, McClusky L, Kretzschmar HA, Monoranu CM, Highley JR, Kirby J, Siddique T, Shaw PJ, Lee VM, Trojanowski JQ, 2007. Pathological TDP-43 distinguishes sporadic amyotrophic lateral sclerosis from amyotrophic lateral sclerosis with SOD1 mutations. Annals of neurology 61, 427–434. [DOI] [PubMed] [Google Scholar]
- Magrane J, Cortez C, Gan WB, Manfredi G, 2014. Abnormal mitochondrial transport and morphology are common pathological denominators in SOD1 and TDP43 ALS mouse models. Human molecular genetics 23, 1413–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattiazzi M, D’Aurelio M, Gajewski CD, Martushova K, Kiaei M, Beal MF, Manfredi G, 2002. Mutated human SOD1 causes dysfunction of oxidative phosphorylation in mitochondria of transgenic mice. The Journal of biological chemistry 277, 29626–29633. [DOI] [PubMed] [Google Scholar]
- McDonald KK, Aulas A, Destroismaisons L, Pickles S, Beleac E, Camu W, Rouleau GA, Vande Velde C, 2011. TAR DNA-binding protein 43 (TDP-43) regulates stress granule dynamics via differential regulation of G3BP and TIA-1. Human molecular genetics 20, 1400–1410. [DOI] [PubMed] [Google Scholar]
- McGurk L, Gomes E, Guo L, Mojsilovic-Petrovic J, Tran V, Kalb RG, Shorter J, Bonini NM, 2018. Poly(ADP-Ribose) Prevents Pathological Phase Separation of TDP-43 by Promoting Liquid Demixing and Stress Granule Localization. Molecular cell 71, 703–717 e709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melamed Z, Lopez-Erauskin J, Baughn MW, Zhang O, Drenner K, Sun Y, Freyermuth F, McMahon MA, Beccari MS, Artates JW, Ohkubo T, Rodriguez M, Lin N, Wu D, Bennett CF, Rigo F, Da Cruz S, Ravits J, Lagier-Tourenne C, Cleveland DW, 2019. Premature polyadenylation-mediated loss of stathmin-2 is a hallmark of TDP-43-dependent neurodegeneration. Nat Neurosci 22, 180–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molliex A, Temirov J, Lee J, Coughlin M, Kanagaraj AP, Kim HJ, Mittag T, Taylor JP, 2015. Phase separation by low complexity domains promotes stress granule assembly and drives pathological fibrillization. Cell 163, 123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann M, Sampathu DM, Kwong LK, Truax AC, Micsenyi MC, Chou TT, Bruce J, Schuck T, Grossman M, Clark CM, McCluskey LF, Miller BL, Masliah E, Mackenzie IR, Feldman H, Feiden W, Kretzschmar HA, Trojanowski JQ, Lee VM, 2006. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science 314, 130–133. [DOI] [PubMed] [Google Scholar]
- Nunnari J, Marshall WF, Straight A, Murray A, Sedat JW, Walter P, 1997. Mitochondrial transmission during mating in Saccharomyces cerevisiae is determined by mitochondrial fusion and fission and the intramitochondrial segregation of mitochondrial DNA. Molecular biology of the cell 8, 1233–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberstadt M, Classen J, Arendt T, Holzer M, 2018. TDP-43 and Cytoskeletal Proteins in ALS. Mol Neurobiol 55, 3143–3151. [DOI] [PubMed] [Google Scholar]
- Ojaimi J, Masters CL, McLean C, Opeskin K, McKelvie P, Byrne E, 1999. Irregular distribution of cytochrome c oxidase protein subunits in aging and Alzheimer’s disease. Annals of neurology 46, 656–660. [PubMed] [Google Scholar]
- Onesto E, Colombrita C, Gumina V, Borghi MO, Dusi S, Doretti A, Fagiolari G, Invernizzi F, Moggio M, Tiranti V, Silani V, Ratti A, 2016. Gene-specific mitochondria dysfunctions in human TARDBP and C9ORF72 fibroblasts. Acta neuropathologica communications 4, 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pansarasa O, Bordoni M, Drufuca L, Diamanti L, Sproviero D, Trotti R, Bernuzzi S, La Salvia S, Gagliardi S, Ceroni M, Cereda C, 2018. Lymphoblastoid cell lines as a model to understand amyotrophic lateral sclerosis disease mechanisms. Disease models & mechanisms 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SK, Park S, Liebman SW, 2019. Respiration Enhances TDP-43 Toxicity, but TDP-43 Retains Some Toxicity in the Absence of Respiration. Journal of Molecular Biology 431, 2050–2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker WD Jr., Filley CM, Parks JK, 1990. Cytochrome oxidase deficiency in Alzheimer’s disease. Neurology 40, 1302–1303. [DOI] [PubMed] [Google Scholar]
- Patron M, Sprenger HG, Langer T, 2018. m-AAA proteases, mitochondrial calcium homeostasis and neurodegeneration. Cell Res 28, 296–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen WA, Fu W, Keller JN, Markesbery WR, Appel S, Smith RG, Kasarskis E, Mattson MP, 1998. Protein modification by the lipid peroxidation product 4-hydroxynonenal in the spinal cords of amyotrophic lateral sclerosis patients. Annals of neurology 44, 819–824. [DOI] [PubMed] [Google Scholar]
- Pesiridis GS, Lee VM, Trojanowski JQ, 2009. Mutations in TDP-43 link glycine-rich domain functions to amyotrophic lateral sclerosis. Human molecular genetics 18, R156–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesiridis GS, Tripathy K, Tanik S, Trojanowski JQ, Lee VM, 2011. A “two-hit” hypothesis for inclusion formation by carboxyl-terminal fragments of TDP-43 protein linked to RNA depletion and impaired microtubule-dependent transport. The Journal of biological chemistry 286, 18845–18855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickrell AM, Youle RJ, 2015. The roles of PINK1, parkin, and mitochondrial fidelity in Parkinson’s disease. Neuron 85, 257–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinarbasi ES, Cagatay T, Fung HYJ, Li YC, Chook YM, Thomas PJ, 2018. Active nuclear import and passive nuclear export are the primary determinants of TDP-43 localization. Scientific reports 8, 7083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinton P, 2018. Mitochondria-associated membranes (MAMs) and pathologies. Cell death & disease 9, 413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polymenidou M, Lagier-Tourenne C, Hutt KR, Huelga SC, Moran J, Liang TY, Ling SC, Sun E, Wancewicz E, Mazur C, Kordasiewicz H, Sedaghat Y, Donohue JP, Shiue L, Bennett CF, Yeo GW, Cleveland DW, 2011. Long pre-mRNA depletion and RNA missplicing contribute to neuronal vulnerability from loss of TDP-43. Nat Neurosci 14, 459–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiros PM, Langer T, Lopez-Otin C, 2015. New roles for mitochondrial proteases in health, ageing and disease. Nat Rev Mol Cell Biol 16, 345–359. [DOI] [PubMed] [Google Scholar]
- Ruan LH, Zhou CK, Jin EL, Kucharavy A, Zhang Y, Wen ZH, Florens L, Li R, 2017. Cytosolic proteostasis through importing of misfolded proteins into mitochondria. Nature 543, 443–+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford NJ, Zhang YJ, Baker M, Gass JM, Finch NA, Xu YF, Stewart H, Kelley BJ, Kuntz K, Crook RJ, Sreedharan J, Vance C, Sorenson E, Lippa C, Bigio EH, Geschwind DH, Knopman DS, Mitsumoto H, Petersen RC, Cashman NR, Hutton M, Shaw CE, Boylan KB, Boeve B, Graff-Radford NR, Wszolek ZK, Caselli RJ, Dickson DW, Mackenzie IR, Petrucelli L, Rademakers R, 2008. Novel mutations in TARDBP (TDP-43) in patients with familial amyotrophic lateral sclerosis. PLoS genetics 4, e1000193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvatori I, Ferri A, Scaricamazza S, Giovannelli I, Serrano A, Rossi S, D’Ambrosi N, Cozzolino M, Giulio AD, Moreno S, Valle C, Carri MT, 2018. Differential toxicity of TAR DNA-binding protein 43 isoforms depends on their submitochondrial localization in neuronal cells. Journal of neurochemistry 146, 585–597. [DOI] [PubMed] [Google Scholar]
- Sasaki S, Horie Y, Iwata M, 2007. Mitochondrial alterations in dorsal root ganglion cells in sporadic amyotrophic lateral sclerosis. Acta Neuropathol 114, 633–639. [DOI] [PubMed] [Google Scholar]
- Sasaki S, Komori T, Iwata M, 2000. Excitatory amino acid transporter 1 and 2 immunoreactivity in the spinal cord in amyotrophic lateral sclerosis. Acta Neuropathol 100, 138–144. [DOI] [PubMed] [Google Scholar]
- Schwenk BM, Hartmann H, Serdaroglu A, Schludi MH, Hornburg D, Meissner F, Orozco D, Colombo A, Tahirovic S, Michaelsen M, Schreiber F, Haupt S, Peitz M, Brustle O, Kupper C, Klopstock T, Otto M, Ludolph AC, Arzberger T, Kuhn PH, Edbauer D, 2016. TDP-43 loss of function inhibits endosomal trafficking and alters trophic signaling in neurons. The EMBO journal 35, 2350–2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesaki H, Jensen RE, 1999. Division versus fusion: Dnm1p and Fzo1p antagonistically regulate mitochondrial shape. The Journal of cell biology 147, 699–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng ZH, Cai Q, 2012. Mitochondrial transport in neurons: impact on synaptic homeostasis and neurodegeneration. Nat Rev Neurosci 13, 77–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva DF, Esteves AR, Oliveira CR, Cardoso SM, 2011. Mitochondria: the common upstream driver of amyloid-beta and tau pathology in Alzheimer’s disease. Current Alzheimer research 8, 563–572. [DOI] [PubMed] [Google Scholar]
- Smith MA, Taneda S, Richey PL, Miyata S, Yan SD, Stern D, Sayre LM, Monnier VM, Perry G, 1994. Advanced Maillard reaction end products are associated with Alzheimer disease pathology. Proceedings of the National Academy of Sciences of the United States of America 91, 5710–5714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreedharan J, Blair IP, Tripathi VB, Hu X, Vance C, Rogelj B, Ackerley S, Durnall JC, Williams KL, Buratti E, Baralle F, de Belleroche J, Mitchell JD, Leigh PN, Al-Chalabi A, Miller CC, Nicholson G, Shaw CE, 2008. TDP-43 mutations in familial and sporadic amyotrophic lateral sclerosis. Science 319, 1668–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoica R, De Vos KJ, Paillusson S, Mueller S, Sancho RM, Lau KF, Vizcay-Barrena G, Lin WL, Xu YF, Lewis J, Dickson DW, Petrucelli L, Mitchell JC, Shaw CE, Miller CC, 2014. ER-mitochondria associations are regulated by the VAPB-PTPIP51 interaction and are disrupted by ALS/FTD-associated TDP-43. Nat Commun 5, 3996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strong MJ, Volkening K, Hammond R, Yang W, Strong W, Leystra-Lantz C, Shoesmith C, 2007. TDP43 is a human low molecular weight neurofilament (hNFL) mRNA-binding protein. Mol Cell Neurosci 35, 320–327. [DOI] [PubMed] [Google Scholar]
- Sun X, Duan Y, Qin C, Li JC, Duan G, Deng X, Ni J, Cao X, Xiang K, Tian K, Chen CH, Li A, Fang Y, 2018. Distinct multilevel misregulations of Parkin and PINK1 revealed in cell and animal models of TDP-43 proteinopathy. Cell death & disease 9, 953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Medina Cruz A, Hadley KC, Galant NJ, Law R, Vernon RM, Morris VK, Robertson J, Chakrabartty A, 2019. Physiologically Important Electrolytes as Regulators of TDP-43 Aggregation and Droplet-Phase Behavior. Biochemistry 58, 590–607. [DOI] [PubMed] [Google Scholar]
- Szabadkai G, Simoni AM, Chami M, Wieckowski MR, Youle RJ, Rizzuto R, 2004. Drp-1-dependent division of the mitochondrial network blocks intraorganellar Ca2+ waves and protects against Ca2+-mediated apoptosis. Mol Cell 16, 59–68. [DOI] [PubMed] [Google Scholar]
- Tollervey JR, Curk T, Rogelj B, Briese M, Cereda M, Kayikci M, Konig J, Hortobagyi T, Nishimura AL, Zupunski V, Patani R, Chandran S, Rot G, Zupan B, Shaw CE, Ule J, 2011. Characterizing the RNA targets and position-dependent splicing regulation by TDP-43. Nat Neurosci 14, 452–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uryu K, Nakashima-Yasuda H, Forman MS, Kwong LK, Clark CM, Grossman M, Miller BL, Kretzschmar HA, Lee VM, Trojanowski JQ, Neumann M, 2008. Concomitant TAR-DNA-binding protein 43 pathology is present in Alzheimer disease and corticobasal degeneration but not in other tauopathies. Journal of neuropathology and experimental neurology 67, 555–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Deerlin VM, Leverenz JB, Bekris LM, Bird TD, Yuan W, Elman LB, Clay D, Wood EM, Chen-Plotkin AS, Martinez-Lage M, Steinbart E, McCluskey L, Grossman M, Neumann M, Wu IL, Yang WS, Kalb R, Galasko DR, Montine TJ, Trojanowski JQ, Lee VM, Schellenberg GD, Yu CE, 2008. TARDBP mutations in amyotrophic lateral sclerosis with TDP-43 neuropathology: a genetic and histopathological analysis. The Lancet. Neurology 7, 409–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker AK, Soo KY, Sundaramoorthy V, Parakh S, Ma Y, Farg MA, Wallace RH, Crouch PJ, Turner BJ, Horne MK, Atkin JD, 2013. ALS-associated TDP-43 induces endoplasmic reticulum stress, which drives cytoplasmic TDP-43 accumulation and stress granule formation. PloS one 8, e81170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A, Conicella AE, Schmidt HB, Martin EW, Rhoads SN, Reeb AN, Nourse A, Ramirez Montero D, Ryan VH, Rohatgi R, Shewmaker F, Naik MT, Mittag T, Ayala YM, Fawzi NL, 2018a. A single N-terminal phosphomimic disrupts TDP-43 polymerization, phase separation, and RNA splicing. EMBO J 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Gao J, Liu J, Siedlak SL, Torres S, Fujioka H, Huntley ML, Jiang Y, Ji H, Yan T, Harland M, Termsarasab P, Zeng S, Jiang Z, Liang J, Perry G, Hoppel C, Zhang C, Li H, Wang X, 2018b. Mitofusin 2 Regulates Axonal Transport of Calpastatin to Prevent Neuromuscular Synaptic Elimination in Skeletal Muscles. Cell Metab 28, 400–414 e408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Deng J, Dong J, Liu J, Bigio EH, Mesulam M, Wang T, Sun L, Wang L, Lee AY, McGee WA, Chen X, Fushimi K, Zhu L, Wu JY, 2019. TDP-43 induces mitochondrial damage and activates the mitochondrial unfolded protein response. PLoS genetics 15, e1007947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Li L, Lin WL, Dickson DW, Petrucelli L, Zhang T, Wang X, 2013. The ALS disease-associated mutant TDP-43 impairs mitochondrial dynamics and function in motor neurons. Human molecular genetics 22, 4706–4719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Wang L, Lu J, Siedlak SL, Fujioka H, Liang J, Jiang S, Ma X, Jiang Z, da Rocha EL, Sheng M, Choi H, Lerou PH, Li H, Wang X, 2016. The inhibition of TDP-43 mitochondrial localization blocks its neuronal toxicity. Nat Med 22, 869–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang WZ, Arakawa H, Wang LW, Okolo O, Siedlak SL, Jiang YF, Gao J, Xie F, Petersen RB, Wang XL, 2017. Motor-Coordinative and Cognitive Dysfunction Caused by Mutant TDP-43 Could Be Reversed by Inhibiting Its Mitochondrial Localization. Mol Ther 25, 127–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Su B, Lee HG, Li X, Perry G, Smith MA, Zhu X, 2009. Impaired balance of mitochondrial fission and fusion in Alzheimer’s disease. J Neurosci 29, 9090–9103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Su B, Siedlak SL, Moreira PI, Fujioka H, Wang Y, Casadesus G, Zhu X, 2008. Amyloid-beta overproduction causes abnormal mitochondrial dynamics via differential modulation of mitochondrial fission/fusion proteins. Proceedings of the National Academy of Sciences of the United States of America 105, 19318–19323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenqiang C, Lonskaya I, Hebron ML, Ibrahim Z, Olszewski RT, Neale JH, Moussa CE, 2014. Parkin-mediated reduction of nuclear and soluble TDP-43 reverses behavioral decline in symptomatic mice. Human molecular genetics 23, 4960–4969. [DOI] [PubMed] [Google Scholar]
- Winton MJ, Igaz LM, Wong MM, Kwong LK, Trojanowski JQ, Lee VM, 2008. Disturbance of nuclear and cytoplasmic TAR DNA-binding protein (TDP-43) induces disease-like redistribution, sequestration, and aggregate formation. The Journal of biological chemistry 283, 13302–13309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo JA, Liu T, Trotter C, Fang CC, De Narvaez E, LePochat P, Maslar D, Bukhari A, Zhao X, Deonarine A, Westerheide SD, Kang DE, 2017. Loss of function CHCHD10 mutations in cytoplasmic TDP-43 accumulation and synaptic integrity. Nature communications 8, 15558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Q, Wang H, Hao Z, Fu C, Hu Q, Gao F, Ren H, Chen D, Han J, Ying Z, Wang G, 2016. TDP-43 loss of function increases TFEB activity and blocks autophagosome-lysosome fusion. The EMBO journal 35, 121–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu YF, Gendron TF, Zhang YJ, Lin WL, D’Alton S, Sheng H, Casey MC, Tong J, Knight J, Yu X, Rademakers R, Boylan K, Hutton M, McGowan E, Dickson DW, Lewis J, Petrucelli L, 2010. Wild-type human TDP-43 expression causes TDP-43 phosphorylation, mitochondrial aggregation, motor deficits, and early mortality in transgenic mice. J Neurosci 30, 10851–10859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youle RJ, Narendra DP, 2011. Mechanisms of mitophagy. Nature reviews. Molecular cell biology 12, 9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu T, Robotham JL, Yoon Y, 2006. Increased production of reactive oxygen species in hyperglycemic conditions requires dynamic change of mitochondrial morphology. Proceedings of the National Academy of Sciences of the United States of America 103, 2653–2658. [DOI] [PMC free article] [PubMed] [Google Scholar]


