Abstract
Although modern medical management has lowered overt stroke occurrence in patients with sickle cell disease (SCD), progressive white matter (WM) damage remains common. It is known that cerebral blood flow (CBF) increases to compensate for anemia, but sufficiency of cerebral oxygen delivery, especially in the WM, has not been systematically investigated. Cerebral perfusion was measured by arterial spin labeling in 32 SCD patients (age range: 10–42 years old, 14 males, 7 with HbSC, 25 HbSS) and 25 age and race-matched healthy controls (age range: 15–45 years old, 10 males, 12 with HbAS, 13 HbAA); 8/24 SCD patients were receiving regular blood transfusions and 14/24 non-transfused SCD patients were taking hydroxyurea. Imaging data from control subjects were used to calculate maps for CBF and oxygen delivery in SCD patients and their T-score maps. Whole brain CBF was increased in SCD patients with a mean T-score of 0.5 and correlated with lactate dehydrogenase (r2 = 0.58, P < 0.0001). When corrected for oxygen content and arterial saturation, whole brain and gray matter (GM) oxygen delivery were normal in SCD, but WM oxygen delivery was 35% lower than in controls. Age and hematocrit were the strongest predictors for WM CBF and oxygen delivery in patients with SCD. There was spatial co-localization between regions of low oxygen delivery and WM hyperintensities on T2 FLAIR imaging. To conclude, oxygen delivery is preserved in the GM of SCD patients, but is decreased throughout the WM, particularly in areas prone to WM silent strokes.
1 |. INTRODUCTION
Stroke is the most devastating complication in patients with sickle cell disease (SCD). It is not only a leading cause of death, but also impairs mobility, cognitive function and quality of life in these patients. The cooperative study of SCD demonstrated that 11% of patients had an overt stroke by age 20 and a cumulative lifetime stroke risk of 40%.1,2 Routine transcranial Doppler (TCD) screening and blood transfusion therapy have lowered this risk 10-fold.3–5 Despite this, risk of cerebral silent infarction (SCI) in SCD patients increases linearly with age, with a prevalence of 50% by the age of 30.6,7
Non-invasive neuroimaging has been a powerful tool to improve the selection of patients with high stroke risk for treatment.8 Nonspecific white matter (WM) atrophy, including volumetric decrease and microscopic derangement has been demonstrated in SCD using magnetic resonance imaging (MRI).9–11 Although recent studies have reported that WM, cerebral blood flow (CBF) is lowest in the regions of highest SCI density,12 and that SCI occur in regions of increased oxygen extraction fraction,13 no one has actually determined whether WM oxygen delivery is impaired to WM regions at risk. We reported that SCD and other anemic patients preserve resting whole brain O2 delivery, despite their impaired O2 carrying capacity, by increasing their CBF.14,15 However, it is unknown whether O2 delivery is preserved regionally, across the brain. Characterization of O2 delivery and its spatial association with WM lesions might help to identify brain regions that are most vulnerable to overt stroke.
Arterial spin labeling (ASL) is a non-invasive and contrast-free MRI imaging method to measure CBF, from which cerebral O2 delivery can be derived. In this work, we compare CBF and O2 delivery between 32 patients with SCD and 25 healthy age and race-matched control subjects. Imaging data from control subjects were used to calculate T-score maps of CBF and O2 delivery in SCD patients. We used regression analysis to identify clinical and laboratory predictors of impaired O2 delivery in white and gray matter (GM), and tested whether silent cerebral infarctions occurred in regions of poor O2 delivery.
2 |. METHODS
2.1 |. Population
The study was approved by the Institutional Review Board (CCI11–00083) at Children’s Hospital Los Angeles (CHLA). All participants were recruited from June 2016 to August 2017, with consent or assent. Two cohorts were studied: the first consisted of SCD subjects with hemoglobin SS (N = 25) and SC (N = 7) genotypes. Exclusion criteria included previous overt stroke, pregnancy and hospitalization within the month prior to the study visit. Children younger than 9 years of age were excluded because of inability to cooperate with study procedures but there was no upper limit for age. All the SCD patients at our institution are regularly screened by TCD ultrasonography to identify patients at risk of stroke. The patients who have a blood flow velocity > 200 cm/sec are placed on monthly blood transfusions to suppress hemoglobin S% to less than 30%, according to standard clinical practice. Patients with normal Doppler velocities are placed on hydroxyurea and titrated to maximum tolerated dose.16 The second cohort was composed of age-matched healthy controls, with or without sickle cell trait. Most were first or second-degree relatives of the patients studied or recruited from friends of patients’ families, so that two cohorts are ethnically and socioeconomically matched. Controls were excluded if they had developmental delay, a prior history of neurologic insult, or serious, uncontrolled, chronic illness.
2.2 |. Data acquisition
On the same day as the imaging study, complete blood count, hemoglobin electrophoresis, and cell free hemoglobin levels were analyzed in the clinical laboratory. Brain MRI was performed on the same day using a Philips Achieva 3 Tesla scanner with an 8-channel head coil. Arterial O2 saturation was measured from a fingertip pulse oximeter during the MRI scan. In the patients undergoing blood transfusion therapy, MRI was performed immediately prior to the blood transfusion. For each subject, a 3D T1-weighted image (echo time (TE) = 3.8 ms, repetition time (TR) = 8.3 ms, resolution 1 mm3 with a SENSE factor 2) and T2-weighted fluid attenuated inversion recovery (FLAIR) image (TE = 2.5 ms, TR = 4.8 ms, resolution = 1.3 × 1.0 × 1.0 mm3) were acquired for co-registration and screening for WM lesions. High resolution time of flight MR angiography (resolution = 0.5 × 0.5 × 1.5 mm3) was also performed at the Circle of Willis to screen for arterial stenosis. The field of view also spanned the supraclinoid, cavernous, petrous and 1 to 2 cm of the cervical portions of the internal carotid artery. Extracerebral vessels were also profiled by 3D angiography collected at a resolution of 1.5 × 1.7 × 3 mm3.
Pseudo continuous arterial spin labeling (PCASL) scans were performed using an unbalanced, Hanning shaped RF pules labeling train (mean gradient of 1 G/cm, interpulse interval of 1 ms and pulse duration of 0.5 ms), and a 3D GRASE two-shot readout (TE = 9.8 ms, TR = 3800 ms, resolution = 3.7 × 3.7 × 10 mm3, labeling duration of 2000 s, post labeling delay of 1600 s with 10 dynamics, EPI factor of 5). Two timed inversion pulses for background suppression following labeling were used, with an inversion efficiency of each background suppression pulse of 95%.
2.3 |. Pre-processing and WMH segmentation
We co-registered T1-weighted and T2-weighted images to the 2 mm3 Montreal Neurological Institute (MNI) atlas using FLIRT and FNIRT.17 The probability maps of WM and GM were obtained using FMRIB’s Automated Segmentation Tool from registered T1 images.18 The T2 FLAIR images were read for white matter hyperintensities (WMHs) by a licensed neuroradiologist who was blinded to disease status. A WMH was considered significant if it was greater or equal to three millimeters in two orthogonal planes.19 Although some WMH were observed in the control population, we limited WMH atlas generation to SCD patients. We used our in-house MATLAB toolbox published previously to semi-manually segment WMHs20 from several subjects. These lesions, and transformations of these lesions, were used to train a deep learning based method21 capable of detecting all of the WMH in the remaining patients. While the sensitivity of the deep learning method was excellent, all detected lesions were manually edited using ITK-SNAP22 to remove false alarms; final lesion morphometry was confirmed by a neuroradiologist (BT). WMH maps for each SCD subject were transformed to the MNI atlas, and fused to create a binary mask that localized WMHs across all the SCD subjects.
Regional CBF was calculated using the PCASL images, rigidly co-registered to the T1 images and then to MNI space using FLIRT.17 Voxel-wise gray and WM probability maps were derived at the same resolution as the PCASL images in order to quantify tissue CBF in native space as well as to perform partial volume effect correction.23 Finally, we applied the whole brain CBF to the cerebral arterial territory masks to measure regional CBF in three major cerebral arterial territories: anterior cerebral artery (ACA), middle cerebral artery (MCA) and posterior cerebral artery (PCA). The mask of major vascular territories was based on a published template of vascular territories in both hemispheres.24 The territories were drawn based on an extensive overview of anatomic studies of cerebral vascularization, and evaluated on the biocommissural plane.
2.4 |. CBF quantifications
CBF quantification was performed using a modified two-compartment kinetic quantification model proposed by Wang et al.25 The parameters in the equation for quantification used in this work were previously described in detail by Bush et al26 for a smaller cohort, using identical imaging protocols and quantification parameters.25 Briefly, we calculated labeling efficiency on a patient-by-patient basis, correcting for uneven radio-frequency excitation and differences in blood flow velocity.26,27 Flow quantitation also employed disease specific estimation of blood T1, and patient and tissue specific arterial transit time estimates.28,29 Complex difference was performed between tag and control images, so the norm of the signal difference was always positive. The median of 10 CBF dynamics was used for quantification and comparison. To alleviate the blurring due to the point spread function and mixing of tissue-specific signals, we performed partial volume effect correction, using a 3 × 3 × 3 regression model.23 During registration to common atlas space, rare peripheral voxels would be designated Not-a-Number (NaN) by FLIRT. We converted these flow values to zero.
2.5 |. Oxygen delivery maps
CBF maps were converted into cerebral O2 delivery maps to correct for patient-specific differences in hemoglobin and O2 saturation. The following equations show the relationship of hemoglobin, O2 content and O2 delivery:30
| (1) |
| (2) |
where SpO2 is the arterial O2 saturation, and pO2 is the partial pressure of O2, which is assumed to be 100 Torr for room air.
2.6 |. Statistical analysis
We used two-tailed, two sample t tests to compare CBF and O2 delivery in SCD and control subjects across the cerebral vascular territories. Predictors of whole brain, total GM and total WM CBF/O2 delivery were identified using univariate and stepwise multivariate regression. Test statistics with two-sided P < 0.05 were considered to be statistically significant. All statistical analyses were performed using JMP Pro 11 (SAS, Cary, NC).
Systematic spatial differences in CBF and O2 delivery were identified using T-maps. The voxelwise mean and SD, μ and σ, were first calculated from the control subjects. Voxel-wise 3D T-score maps were calculated for both CBF and O2 delivery by calculating t = (X-μ)/σ on a voxelwise basis where t is the T-score value of each voxel, and X is the group average of CBF (or O2 delivery) value in patients with SCD.
To determine the spatial concordance of CBF and O2 delivery between areas with white WMHs and normal appearing white matter (NAWM), we performed a permutation analysis of WM lesions on the CBF maps and O2 delivery maps. We created a WM lesion risk map by summating all the WMH segmented from T2-FLAIR images in 17 patients with SCD. The test distribution consisted of CBF values associated with each lesion, generating a distribution of CBF values in regions known to be at high risk from strokes. To create the corresponding null statistic, we randomly positioned each WM lesion 100 times within the NAWM regions and recalculated the CBF. The shape of the two distributions was compared by the Kolmogorov-Smirnov test, while the mean and variance of the distributions were compared by t test and ratio test, respectively. The permutation test on O2 delivery map followed the same procedure. This was done using our in-house MATLAB code.
3 |. RESULTS
3.1 |. Demographics
Patient demographic information is summarized in Supporting Information Table S1. There is no significant difference in age and sex between two cohorts. The healthy control (CTL) group included 12 participants with sickle cell trait (hemoglobin AS) and 13 with hemoglobin AA. The sickle trait subjects did not differ in any detectable way from the control subjects (hemoglobin AA) other than their hemoglobin electrophoresis. The SCD group consisted of seven patients with hemoglobin SC, and 25 hemoglobin SS. Eight of the SS patients were receiving chronic transfusion therapy for a history of abnormal TCD (N = 6) or recurrent acute chest syndrome (N = 2). Chronically transfused patients were maintained with a pre-transfusion hemoglobin S level below 30% and had relatively low hemoglobin F%, reflecting good suppression of endogenous erythropoiesis. Chronically transfused patients were studied at their hemoglobin nadir to better match their hemoglobin levels with non-transfused subjects. Hydroxyurea was being taken by 12/17 non-transfused SS patients and 2/7 SC patients. In addition to having decreased hemoglobin and hematocrit, SCD patients were mildly desaturated compared to healthy controls; three SCD patients has O2 saturations below 93% while the rest had O2 saturations from 97% to 95%.
MR angiography images were read by a licensed neuroradiologist for all participants. All subjects had normal MRA except one 37-yearold patient with bilateral ACA stenosis; this patient was excluded from the analysis. Small, WMH were found in 12/24 non-transfused SCD patients and 5/8 transfused SCD patients. Five of 17 WMH+ patients were HbSC phenotype.
3.2 |. CBF and oxygen delivery
The top panel of Supporting Information Figure S1 compares the spatial distribution of CBF in CTL and SCD patients. In both groups, CBF is greater in GM than WM, but appears to be particularly increased in SCD patients. To facilitate a comparison across groups, the top-right panel of Supporting Information Figure S1 demonstrates a T-score map of regional CBF between SCD and control subjects. CBF is equally elevated in GM structures (T-score > 2) but is normal or decreased in WM. The average T-score value for whole brain CBF is 0.5. Table 1 shows the quantitative summary of all whole brain and regional CBF measurements for two groups. CBF in both whole brain and three territories are significantly higher for SCD patients than healthy controls (P < 0.01). In GM, CBF is elevated across the brain, while in WM, there was no significant difference.
TABLE 1.
Global/regional CBF and oxygen delivery. Measurements presented as global (GM/WM) with mean ± SD, values in bold indicates significant difference with P < 0.01
| Regions | CTL (N = 25) | SCD (N = 32) |
P-value (adjusted) |
|
|---|---|---|---|---|
| CBF (mL/100 g/min) | ||||
| Whole brain | Total | 54.7 ± 12.9 | 72.2 ± 21.0 | 0.0006 |
| GM | 63.0 ± 14.7 | 84.0 ± 26.5 | 0.0006 | |
| WM | 41.2 ± 11.6 | 37.62 ± 9.6 | 0.2 | |
| ACA | Total | 46.5 ± 9.7 | 74.7 ± 27.3 | <0.0001 |
| GM | 53.4 ± 11.5 | 87.0 ± 33.8 | <0.0001 | |
| WM | 34.9 ± 8.4 | 38.8 ± 12.4 | 0.2 | |
| MCA | Total | 58.4 ± 14.0 | 79.1 ± 23.5 | 0.0003 |
| GM | 67.0 ± 16.7 | 92.1 ± 29.6 | 0.0004 | |
| WM | 44.0 ± 12.1 | 41.3 ± 11.0 | 0.4 | |
| PCA | Total | 56.4 ± 12.5 | 74.3 ± 22.9 | 0.0009 |
| GM | 64.7 ± 12.9 | 86.5 ± 28.9 | 0.001 | |
| WM | 42.5 ± 14.7 | 38.6 ± 9.7 | 0.2 | |
| Oxygen delivery (mL Oxygen/100 g/min) | ||||
| Whole brain | Total | 11.3 ± 3.0 | 10.4 ± 2.2 | 0.2 |
| GM | 12.9 ± 3.5 | 12.0 ± 2.8 | 0.3 | |
| WM | 8.5 ± 2.7 | 5.5 ± 1.3 | <0.0001 | |
| ACA | Total | 9.6 ± 2.2 | 10.6 ± 2.9 | 0.1 |
| GM | 11.0 ± 2.6 | 12.3 ± 3.6 | 0.1 | |
| WM | 7.2 ± 2.0 | 5.6 ± 1.5 | 0.001 | |
| MCA | Total | 12.1 ± 3.4 | 11.2 ± 2.4 | 0.3 |
| GM | 13.8 ± 4.0 | 13.1 ± 3.1 | 0.4 | |
| WM | 9.1 ± 2.9 | 5.9 ± 1.4 | <0.0001 | |
| PCA | Total | 11.6 ± 2.9 | 10.6 ± 2.3 | 0.1 |
| GM | 13.4 ± 3.5 | 12.3 ± 3.0 | 0.2 | |
| WM | 8.8 ± 2.7 | 5.5 ± 1.2 | <0.0001 | |
Abbreviations: CTL, controls; SCD, sickle cell disease; CBF, cerebral blood flow; ACA, anterior cerebral artery; MCA, middle cerebral artery; PCA, posterior cerebral artery.
To control for the significant differences in O2 content between the two groups, we converted CBF maps into O2 delivery maps following Equation 1. The bottom panel of Supplemental Figure S1 compares regional O2 delivery maps between the two groups; the rightmost panel depicts the corresponding T-score map. The WM areas exhibit cooler color on the T-score map, indicating decreased O2 delivery in SCD group; while in GM, the T-score is around zero, meaning the O2 delivery in GM is quite similar between SCD and CTL groups. The average value of the T-score for WM O2 delivery was −0.4 (0.4 standard deviations below the mean). The quantitative whole brain and regional O2 delivery results are summarized in the bottom panel of Table 1. Whole brain and GM O2 delivery in SCD was completely normal (P = 0.2 and 0.3, respectively, using one-way ANOVA test), but was 35% lower in the WM (P < 0.001, using one-way ANOVA test), consistent with what was observed on the T-score map.
To further investigate the relative contributions to CBF and O2 delivery, we performed univariate and multivariate regressions using demographic factors and blood test results (Table 2). In the SCD group, whole brain CBF and GM CBF were inversely correlated with O2 content, hemoglobin, and hematocrit, but positively correlated with lactate dehydrogenase (LDH), cell-free hemoglobin, reticulocytes, mean platelet volume (MPV) and heart rate on univariate analysis. On multivariate analysis, only LDH remained in the model for both whole brain (r2 = 0.58, P < 0.001) and GM CBF (r2 = 0.54, P < 0.001). The WM CBF had similar univariate correlates except that cell-free hemoglobin, reticulocytes, and MPV did not reach statistical significance, and WM CBF decreased significantly with age (r2 = 0.20, P = 0.01). On multivariate analysis, LDH, age, and hematocrit predicted WM CBF with a combined r2 of 0.45 (P < 0.001).
TABLE 2.
Univariate predictors of SCD brain perfusion and oxygen delivery
| Brain perfusion sickle cell disease patients | |||
|---|---|---|---|
| Predictor | Total perfusion | GM perfusion | WM perfusion |
| Plasma hemoglobin | r2 = 0.38, P = 0.0002 | r2 = 0.38, P = 0.0002 | - |
| Lactate dehydrogenase | r2 = 0.58, P < 0.0001 | r2 = 0.54, P = <0.0001 | r2 = 0.23, P = 0.006 |
| Reticulocyte count | r2 = 0.27, P = 0.002 | r2 = 0.27, P = 0.002 | - |
| Mean platelet volume | r2 = 0.23, P = 0.005 | r2 = 0.22, P = 0.007 | - |
| Heart rate | r2 = 0.29, P = 0.002 | r2 = 0.24, P = 0.004 | r2 = 0.19, P = 0.01 |
| Weight | - | - | r2 = 0.16, P = 0.02 |
| Age | - | - | r2 = 0.20, P = 0.01 |
| MCHC | r2 = 0.12, P = 0.05 | r2 = 0.12, P = 0.05 | - |
| Oxygen content | r2 = 0.37 P = 0.0002 | r2 = 0.34 P = 0.0004 | r2 = 0.14, P = 0.03 |
| Hemoglobin | r2 = 0.36, P = 0.0003 | r2 = 0.33, P = 0.0006 | r2 = 0.17, P = 0.03 |
| Hematocrit | r2 = 0.37, P = 0.0002 | r2 = 0.33, P = 0.0006 | r2 = 0.22, P = 0.007 |
| RDW | r2 = 0.09, P = 0.09 | r2 = 0.10, P = 0.07 | r2 = 0.14, P = 0.03 |
| White blood cell count | r2 = 0.12, P = 0.05 | r2 = 0.11, P = 0.06 | - |
| Oxygen delivery in sickle cell disease patients | |||
| Predictor | Total O2 delivery | GM O2 delivery | WM O2 delivery |
| Plasma hemoglobin | r2 = 0.30, P = 0.001 | r2 = 0.33, P = 0.0007 | - |
| Lactate dehydrogenase | r2 = 0.10, P = 0.07 | r2 = 0.11, P = 0.07 | - |
| Mean platelet volume | r2 = 0.17, P = 0.02 | r2 = 0.17, P = 0.02 | - |
| Hemoglobin S% | r2 = 0.10, P = 0.07 | r2 = 0.11, P = 0.06 | - |
| Age | - | - | r2 = 0.24, P = 0.005 |
| MCHC | - | - | r2 = 0.22, P = 0.007 |
| Oxygen content | - | - | r2 = 0.18, P = 0.02 |
| Hemoglobin | - | - | r2 = 0.15, P = 0.03 |
| Hematocrit | - | - | r2 = 0.09, P = 0.09 |
| RDW | - | - | r2 = 0.14, P = 0.03 |
| Reticulocyte count | - | - | r2 = 0.14, P = 0.04 |
Abbreviations: MCHC, mean corpuscular hemoglobin concentration; RDW: red cell distribution width; GM: gray matter; WM: white matter; O2: oxygen.Bold letter indicates retention on multivariate analysis.
When O2 delivery was examined instead of CBF, many factors related to anemia were no longer significant for whole brain and GM O2 delivery. Cell-free hemoglobin and MPV were the only significant predictors, with only cell-free hemoglobin surviving multivariate analysis (Table 2). WM O2 delivery was positively correlated with hemoglobin, hematocrit, O2 content, and mean corpuscular hemoglobin concentration (MCHC) and inversely correlated with age, red cell distribution width (RDW) and reticulocytes. Age, O2 content, and hematocrit remained in multivariate analysis with a combined r2 of 0.46.
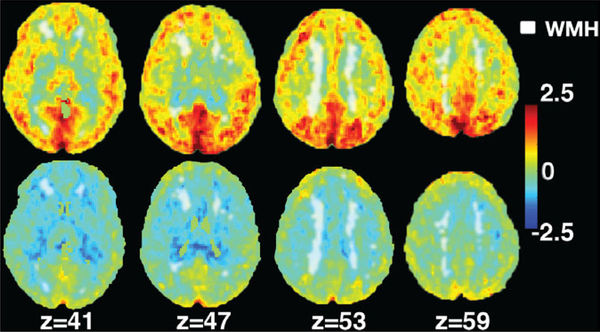
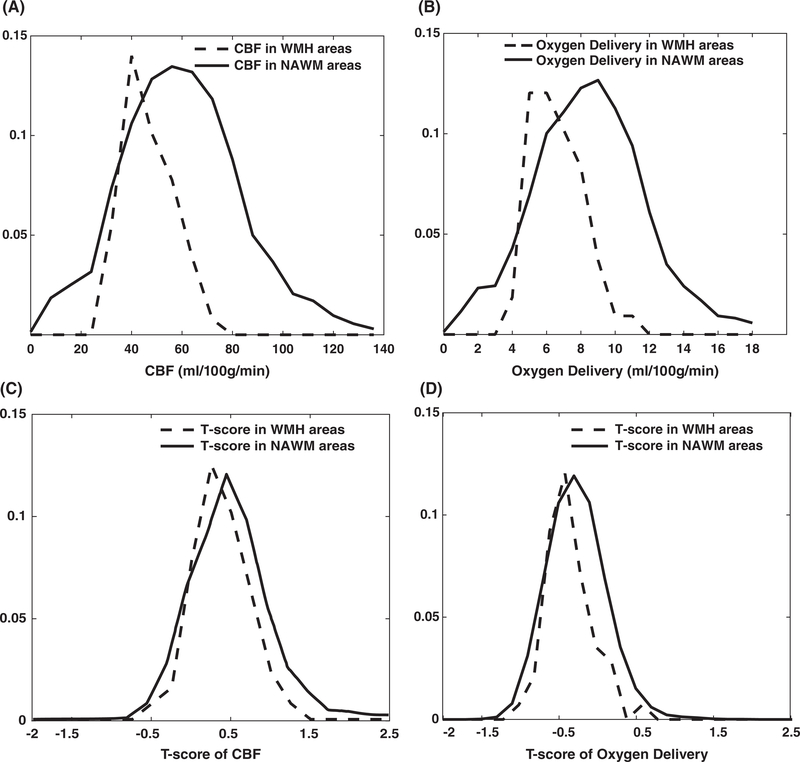
Figure 1 compares the spatial relationship between CBF, O2 delivery and WMH. The color-bar represents the T-score and Z indicates the position of the axial slice in the atlas. The lesion mask was gray-scaled and shown in white. WMH was all distributed in areas having negative T-values on the O2 delivery map. Figure 2A,B (dashed lines) summarizes the CBF and O2 delivery in the WMH prone regions, compared with values in NAWM (solid lines). The average value for CBF in WMH prone regions was 30.0 compared with 38.9 mL/100 g/min in NAWM (P < 0.001). The average value for O2 delivery in WMH prone regions was 4.3 mL/100 g/min compared with 5.9 mL/100 g/min in NAWM regions (P < 0.001). Figure 2C,D depicts the same relationships but converts them into T-scores. The mean CBF T-scores were 0.3 and 0.4 (P < 0.001) in WMH prone and NAWM respectively, while the corresponding mean T-scores for O2 delivery were −0.5 and −0.3 (P < 0.001), respectively.
FIGURE 1.
White matter lesion mask overlaid on T-score map. The scaled WM lesion mask in its axial view is overlaid on top of the T-score for CBF (top row) and O2 delivery (bottom row). The number z indicates the coordinates in the standard atlas
FIGURE 2.
WMH permutation results. The CBF and oxygen delivery distribution (top row) and T-score of CBF and oxygen delivery distribution (bottom row) in the normal appearing white matter (NAWM) and in the white matter hyperintensity (WMH) prone regions
4 |. DISCUSSION
There have been several studies focusing on quantifying CBF using ASL in SCD cohorts;26,31,32 however, we are the first to quantify O2 delivery in both healthy controls and SCD populations. We found that whole brain and GM CBF was increased in SCD patients, as a result of cerebral vasodilation to meet GM metabolic demands. Surprisingly, we did not observe a similar compensatory hyperemia in the WM. As a result, whole brain and GM O2 delivery were preserved in SCD patients, but WM O2 delivery was only 2/3 as high as for control subjects. Regions commonly associated with WMH had lower O2 delivery than NAWM. After performing univariate and multivariate regression, age and O2 content were the strongest predictors for WM O2 delivery in SCD patients.
Increased CBF in patients with SCD has also been described in other studies using MRI,15 PET,33 and Xenon Computed Tomography.34 The elevated CBF was found to be in part due to cerebral vasodilation in response of low hematocrit levels and high hemoglobin S concentration.35 The mechanism by which anemia mediates increased CBF is not entirely known. Although blood viscosity decreases linearly with hematocrit, CBF increase cannot be explained by passive alterations in arterial blood viscosity.36,37 Anemia, whether caused by SCD or acute hemodilution, produces cerebral tissue hypoxia.38,39 In turn, cerebral hypoxia triggers arterial vasodilation acutely, and promotes capillary proliferation in GM through hypoxia inducible factor mediated signaling to shorten O2 diffusion distances and lower cerebrovascular resistance.40,41
On a whole organ basis, the increased CBF completely compensates for the inadequate O2 carrying capacity.15,42 However, compensatory vasodilation was not observed in WM, causing WM O2 delivery to be directly proportional to hemoglobin concentration. Hematocrit-dependent cerebral O2 delivery has been described in subjects with cardiovascular disease, with optimal delivery for a hematocrit of 40 to 45%.43 WM O2 delivery also declined dramatically with age. The etiology of this decline is unclear, but likely reflects accelerated geometric and morphologic alterations, or even destruction of the microvascular network.44 Our observed decline in WM O2 delivery with age parallels the prevalence of WMH in SCD patients observed by Kassim et al7 and DeBaun et al.45
In contrast, GM O2 delivery is normal in patients with SCD, reflecting the powerful inverse relationship between CBF and O2 carrying capacity.15,45 The preserved GM O2 delivery we observed is consistent with the lack of GM atrophy we observed in our previous reports.11 GM O2 delivery was stable with age; however, the median age of our SCD cohort was only 21.4 years of age. Thus, we may have been underpowered to observe age-related reductions in GM O2 delivery.
Surprisingly, cell-free hemoglobin was the strongest predictor of GM O2 delivery. Cell free hemoglobin is a toxic byproduct of hemolysis and is associated with impaired nitric oxide metabolism46 and endothelial dysfunction.47 However, its impact on cerebral nitric oxide generation and cerebral endothelial function has not been reported. A healthy microvasculature is necessary for matching O2 supply and consumption. Bush et al have previously documented evidence of arterio-venous shunting and impaired O2 unloading in SCD patients,48,49 both suggestive O2 supply-demand mismatch. Alternatively, cell-free hemoglobin could have a more direct vasodilatory effect. Cell free hemoglobin is metabolized by heme oxygenase, producing free iron and carbon monoxide, a potent cerebral vasodilator.46,50 In a parallel vascular circuit, excessive vasodilation of one bed could produce ischemia in another. Whether the decreased WM O2 delivery represents vascular stealing by the GM, or simply inadequate neovascularization in response to chronic hypoxia could not be determined.
The WMHs and O2 delivery map overlay demonstrates two important points. First, CBF and O2 delivery were lower in regions prone to WMH, than in those with NAWM, independent of disease state (Figure 2C,D). It implies that WMHs are occurring in borderzone regions where perfusion is inherently decreased in anyone. Studies from Numaguchi, Baldeweg, Guilliams and Ford12,34,51,52 in SCD patients also support that conclusion. Our O2 delivery T-score maps agrees with the observations of Fields et al., who demonstrated increased O2 extraction fraction in watershed areas.53 However, the T-score for O2 delivery was lower in regions at risk for WM stroke (T = −0.5) than in NAWM (T = −0.3) as shown in Figure 2D. The use of a T-score between groups and permutation corrections for the expected reduction in O2 delivery based upon a watershed effect alone. Therefore, O2 delivery is disproportionately impaired with WMH prone regions, as exhibited in Figure 1.
Our study has several limitations. First, measurement of WM CBF and O2 delivery could be complicated by differences in blood transit time and longitudinal relaxation of blood, GM, and WM.23,54,55 While we used velocity-based corrections for transit time derived from previous studies in SCD patients,29 patient-wise measurement of transit times might improve the accuracy of the CBF estimation. Blood magnetic properties (T1 relaxation) are different in SS patients. While we used values appropriate for SCD patients, patient specific T1 blood measurements could the CBF accuracy in SCD patients.56 Blood T1 in SC patients has never been reported.
Second, the intrinsic low resolution of ASL images and the use of a 3D ASL acquisition, produce admixture of CBF values from gray and WM, known as partial volume effects. Our partial volume correction may not be sufficient performed to eliminate GM and WM cross-contamination. This will cause underestimation of GM CBF and overestimation of WM CBF, leading to a low GM/WM flow ratio; the thin, convoluted cerebral cortex (GM) is particularly vulnerable to CBF underestimation from this mixing effect. However, partial volume effects cannot explain low WM O2 delivery observed in SCD patients. Nor can it explain the dependence of WM O2 delivery on age and O2 content because those effects were not observed in GM. The use of T-scores for group comparison controls for most of the partial volume biases because both SCD patients and healthy controls suffer from comparable GM/WM admixture.
Third, hematocrit and O2 delivery at the microvascular level are different than in conduit vessels. It is possible that actual O2 delivery could be slightly better, or worse, than our calculations based on venous hematocrit values. However, use a global, venous hematocrit value nonetheless yields intuitive results that provide important insights into WM disease in SCD patients. Our sample size was too small to explore important covariates such as the role of blood transfusion therapy or the compliance with hydroxyurea, nor could we explore links between O2 delivery, brain volumes, and neurocognitive outcomes.
Lastly, our SCD population was heterogeneous with respect to genotype and treatment. The goal of the parent study (CCI-11–00083) was to examine the impact of hemoglobin level and hemoglobin S% on CBF and oxygenation, requiring a cohort having a broad range in those variables. We accomplished this objective, but at the cost of cohort homogeneity. While we were careful to control for possible group effects during statistical analysis, our subgroups were too small to make any inferences about the impact of transfusions, hydroxyurea, or genotype. Our study would have been strengthened if we could have studied chronically transfused patients prior to and following their transfusion visit but it was not feasible with our study budget.
In conclusion, we demonstrated that WM O2 delivery is impaired in SCD patients. Cerebral compensatory mechanisms appear optimized to protect GM O2 delivery, even while WM remains hypoxic. WMH occur in watershed areas, where CBF and O2 delivery are intrinsically low, but O2 delivery in these regions is even lower than uniquely predicted by watershed effects. Taken together, compensatory hyperemia preserves O2 delivery to the GM in SCD patients but is insufficient to maintain O2 delivery to the WM, explaining the distribution and progressive evolution of SCIs in SCD patients.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by the National Heart Lung and Blood Institute (1U01HL117718-01, Minority Supplement to 1U01HL117718-01 and 1RO1HL136484-A1), and the National Center for Clinical Research (UL1 TR001855-04). Phillips Healthcare kindly provided support for protocol development and applications engineering.
Funding information
National Center for Clinical Research, Grant/ Award Number: UL1 TR001855-04; National, Heart Lung and Blood Institute, Grant/Award, Numbers: 1RO1HL136484-A1, 1U01HL117718-01
Footnotes
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of the article.
CONFLICT OF INTEREST
JCW receives research support in kind from Philips Healthcare. None of the other authors have conflicts relevant to the study.
REFERENCES
- 1.Ohene-Frempong K, Weiner SJ, Sleeper LA, et al. Cerebrovascular accidents in sickle cell disease: rates and risk factors. Blood. 1998;91 (1):288–294. [PubMed] [Google Scholar]
- 2.Miller ST, Macklin EA, Pegelow CH, et al. Silent infarction as a risk factor for overt stroke in children with sickle cell anemia: A report from the Cooperative Study of Sickle Cell Disease. J Pediatr. 2001;139(3):385–390. [DOI] [PubMed] [Google Scholar]
- 3.DeBaun MR, Gordon M, McKinstry RC, et al. Controlled trial of transfusions for silent cerebral infarcts in sickle cell anemia. N Engl J Med. 2014;371(8):699–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thompson A, Sarnaik S, Woods GM, et al. Silent cerebral infarcts occur despite regular blood transfusion therapy after first stroke in children with sickle cell disease. Blood. 2011;117(3):772–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adams RJ, McKie VC, Hsu L, et al. Prevention of a first stroke by transfusions in children with sickle cell anemia and abnormal results on transcranial Doppler ultrasonography. N Engl J Med. 1998;339(1):5–11. [DOI] [PubMed] [Google Scholar]
- 6.Bernaudin F, Verlhac S, Arnaud C, et al. Chronic, acute anemia and eICA stenosis are independent risk factors for silent cerebral infarcts in sickle cell anemia. Blood. 2014;125(10):1653–1662. [DOI] [PubMed] [Google Scholar]
- 7.Kassim AA, Pruthi S, Day M, et al. Silent cerebral infarcts and cerebral aneurysms are prevalent in adults with sickle cell anemia. Blood. 2016; 127(16):2038–2041. [DOI] [PubMed] [Google Scholar]
- 8.Hoppe C. Defining stroke risk in children with sickle cell anaemia. Br J Haematol. 2005;128(6):751–766. [DOI] [PubMed] [Google Scholar]
- 9.Balci A, Karazincir S, Beyoglu Y, et al. Quantitative brain diffusiontensor MRI findings in patients with sickle cell disease. Am J Roentgenol. 2012;198(5):1167–1174. [DOI] [PubMed] [Google Scholar]
- 10.Chai Y, Coloigner J, Qu X, et al. Tract specific analysis in patients with sickle cell disease. In: 11th International Symposium on Medical Information Processing and Analysis; Vol 9681, 2015:968108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choi S, Bush AM, Borzage MT, et al. Hemoglobin and mean platelet volume predicts diffuse T1-MRI white matter volume decrease in sickle cell disease patients. NeuroImage Clin. 2017;15(04):239–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ford AL, Ragan DK, Fellah S, et al. Silent infarcts in sickle cell anemia occur in the borderzone region and are associated with low cerebral blood flow. Blood. 2018;132(16):1714–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guilliams KP, Fields ME, Ragan DK, et al. Red cell exchange transfusions lower cerebral blood flow and oxygen extraction fraction in pediatric sickle cell anemia. Blood. 2018;131(9):1012–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chai Y, Coloigner J, Bush AM, Wood JC, Lepore N. Regional cerebral blood flow measurement in patients with sickle cell disease using pseudo continuous arterial spin labeling. In: 39th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, 2016. https://www.researchgate.net/publication/317648194_Regional_Cerebral_Blood_Flow_Measurement_in_Patients_with_Sickle_Cell_Disease_Using_Pseudo_Continuous_Arterial_Spin_Labeling [Google Scholar]
- 15.Bush AM, Borzage MT, Choi S, et al. Determinants of resting cerebral blood flow in sickle cell disease. Am J Hematol. 2016;91(9):912–917. 10.1002/ajh.24441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adams RJ, Ataga KI, Ballard H, et al. The management of sickle cell disease. Natl Inst Heal. 2002. https://www.nhlbi.nih.gov/files/docs/guidelines/sc_mngt.pdf [Google Scholar]
- 17.Smith S, Jenkinson M, Woolrich M. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(November):S208–S219. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans Med Imaging. 2001;20(1):45–57. [DOI] [PubMed] [Google Scholar]
- 19.Casella JF, King AA, Barton B, et al. Design of the silent cerebral infarct transfusion (SIT) trial. Pediatr Hematol Oncol. 2010;27(2): 69–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vu CQ, Wood JC. White matter hyperintensities segmentation and stereological bias correction. In: International Society for Magnetic Resonance in Medicine (ISMRM), Vol 1, 2017. [Google Scholar]
- 21.Xu B, Chai Y, Galarza CM, et al. Orchestral fully convolutional networks for small lesion segmentation in brain MRI. In: Proceedings of the 2018 I.E. 15th International Symposium on Biomedical Imaging (ISBI 2018); IEEE; 2018:889–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yushkevich PA, Piven J, Hazlett HC, et al. User-guided 3D active contour segmentation of anatomical structures: Significantly improved efficiency and reliability. Neuroimage. 2006;31(3):1116–1128. [DOI] [PubMed] [Google Scholar]
- 23.Asllani I, Borogovac A, Brown TR. Regression algorithm correcting for partial volume effects in arterial spin labeling MRI. Magn Reson Med. 2008;60(6):1362–1371. [DOI] [PubMed] [Google Scholar]
- 24.Duvernoy HM. Arterial territories of the human brain. Neurology. 1999;30:99–110. [DOI] [PubMed] [Google Scholar]
- 25.Wang J, Alsop DC, Li L, et al. Comparison of quantitative perfusion imaging using arterial spin labeling at 1.5 and 4.0 Tesla. Magn Reson Med. 2002;48(2):242–254. [DOI] [PubMed] [Google Scholar]
- 26.Bush A, Chai Y, Choi SY, et al. Pseudo continuous arterial spin labeling quantification in anemic subjects with hyperemic cerebral blood flow. Magn Reson Imaging. 2018;47(2):137–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chai Y, Bush A, Coloigner J, et al. An experimental investigation of labeling efficiency for pseudo-continuous arterial spin labeling. In: Proceedings – International Symposium on Biomedical Imaging. Vol 2016; June; 2016:1101–1104. [Google Scholar]
- 28.Lu H, Clingman C, Golay X, Zijl PV. What is the longitudinal relaxation time (T1) of blood at 3.0 Tesla? Blood. 2003;11(3):21205–21205. [Google Scholar]
- 29.MacIntosh BJ, Filippini N, Chappell MA, Woolrich MW, Mackay CE, Jezzard P. Assessment of arterial arrival times derived from multiple inversion time pulsed arterial spin labeling MRI. Magn Reson Med. 2010;63(3):641–647. [DOI] [PubMed] [Google Scholar]
- 30.Wasserman K, Whipp BJ. Exercise physiology in health and disease. Am Rev Respir Dis. 1975;112:219–249. [DOI] [PubMed] [Google Scholar]
- 31.Oguz KK, Golay X, Pizzini FB, et al. Sickle cell disease: continuous arterial spin-labeling perfusion MR imaging in children. Radiology. 2003;227(2):567–574. [DOI] [PubMed] [Google Scholar]
- 32.Van Den Tweel XW, Nederveen AJ, Majoie CBLM, et al. Cerebral blood flow measurement in children with sickle cell disease using continuous arterial spin labeling at 3.0-tesla MRI. Stroke. 2009;40(3):795–800. [DOI] [PubMed] [Google Scholar]
- 33.Herold S, Brozovic M, Gibbs J, et al. Measurement of regional cerebral blood flow, blood volume and oxygen metabolism in patients with sickle cell disease using positron emission tomography. Stroke. 1986; 17(4):692–698. [DOI] [PubMed] [Google Scholar]
- 34.Numaguchi Y, Haller JS, Humbert JR, et al. Cerebral blood flow mapping using stable Xenon-enhanced CT in sickle cell cerebrovascular disease. Neuroradiology. 1990;32(4):289–295. [DOI] [PubMed] [Google Scholar]
- 35.Prohovnik I, Hurlet-Jensen A, Adams R, De Vivo D, Pavlakis SG. Hemodynamic etiology of elevated flow velocity and stroke in sicklecell disease. J Cereb Blood Flow Metab. 2009;29(4):803–810. [DOI] [PubMed] [Google Scholar]
- 36.Brown M, Marshall J. Regulation of cerebral blood flow in response to changes in blood viscosity. Lancet. 1985;325:604–609. [DOI] [PubMed] [Google Scholar]
- 37.Brown MM, Marshall J. Effect of plasma exchange on blood viscosity and cerebral blood flow. Br Med J. 1982;284(6331):1733–1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cahill LS, Gazdzinski LM, Tsui AK, et al. Functional and anatomical evidence of cerebral tissue hypoxia in young sickle cell anemia mice. J Cereb Blood Flow Metab. 2017;37(3):994–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hare GMT, Tsui AKY, Ozawa S, Shander A. Anaemia: Can we define haemoglobin thresholds for impaired oxygen homeostasis and suggest new strategies for treatment? Best Pract Res Clin Anaesthesiol. 2013;27(1): 85–98. [DOI] [PubMed] [Google Scholar]
- 40.Ni WW, Christen T, Rosenberg J, Zun Z, Moseley ME, Zaharchuk G. Imaging of cerebrovascular reserve and oxygenation in Moyamoya disease. J Cereb Blood Flow Metab. 2017;37(4):1213–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kedar A, Drane WE, Shaeffer D, Nicole M, Adams C. Measurement of cerebrovascular flow reserve in pediatric patients with sickle cell disease. Pediatr Blood Cancer. 2006;46(2):234–238. [DOI] [PubMed] [Google Scholar]
- 42.Borzage MT, Bush AM, Choi S, et al. Predictors of cerebral blood flow in patients with and without anemia. J Appl Physiol. 2016;120:976–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Blinman T, Maggard M. Rational manipulation of oxygen delivery. J Surg Res. 2000;92(1):120–141. [DOI] [PubMed] [Google Scholar]
- 44.Moeini M, Lu X, Avti PK, et al. Compromised microvascular oxygen delivery increases brain tissue vulnerability with age. Sci Rep. 2018;8(1):1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Debaun MR, Armstrong FD, Mckinstry RC, Ware RE, Vichinsky E, Kirkham FJ. Silent cerebral infarcts : a review on a prevalent and progressive cause of neurological injury in sickle cell anemia. Blood. 2012; 119(20):4587–4597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reiter CD, Wang X, Tanus-Santos JE, et al. Cell-free hemoglobin limits nitric oxide bioavailability in sickle-cell disease. Nat Med. 2002;8(12): 1383–1389. [DOI] [PubMed] [Google Scholar]
- 47.Belhassen L, Pelle G, Sediame S, et al. Endothelial dysfunction in patients with sickle cell disease is related to selective impairment of shear stress–mediated vasodilation. Blood. 2001;97(6):1584–1589. [DOI] [PubMed] [Google Scholar]
- 48.Hebbel RP, Hedlund BE. Sickle hemoglobin oxygen affinity-shifting strategies have unequal cerebrovascular risks. Am J Hematol. 2018;93 (3):321–325. [DOI] [PubMed] [Google Scholar]
- 49.Bush AM, Coates TD, Wood JC. Diminished cerebral oxygen extraction and metabolic rate in sickle cell disease using T2 relaxation under spin tagging MRI. Magn Reson Med. 2018;80(1):294–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rebel A, Ulatowski JA, Kwansa H, Bucci E, Koehler RC. Cerebrovascular response to decreased hematocrit: effect of cell-free hemoglobin, plasma viscosity, and CO2. Am J Physiol Heart Circ Physiol. 2003;285 (4):H1600–H1608. [DOI] [PubMed] [Google Scholar]
- 51.Guilliams KP, Fields ME, Ragan DK, et al. Large-vessel vasculopathy in children with sickle cell disease: a magnetic resonance imaging study of infarct topography and focal atrophy. Pediatr Neurol. 2017;69:49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Baldeweg T, Hogan AM, Saunders DE, et al. Detecting white matter injury in sickle cell disease using voxel-based morphometry. Ann Neurol. 2006;59(4):662–672. [DOI] [PubMed] [Google Scholar]
- 53.Fields ME, Guilliams KP, Ragan DK, et al. Regional oxygen extraction predicts border zone vulnerability to stroke in sickle cell disease. Neurology. 2018;90(13):10.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mutsaerts HJ, Richard E, Heijtel DF, van Osch MJ, Majoie CB, Nederveen AJ. Gray matter contamination in arterial spin labeling white matter perfusion measurements in patients with dementia. NeuroImage: Clin. 2014;4:139–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Petersen ET, Zimine I, Ho YCL, Golay X. Non-invasive measurement of perfusion: A critical review of arterial spin labelling techniques. Br J Radiol. 2006;79(944):688–701. [DOI] [PubMed] [Google Scholar]
- 56.Vaclavu L, Van Der Land V, Heijtel DFR, et al. In vivo T1 of blood measurements in children with sickle cell disease improve cerebral blood flow quantification from arterial spin-labeling MRI. Am J Neuroradiol. 2016;37(9):1727–1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.