Abstract
BACKGROUND CONTEXT
Radiological observations of soft-tissue changes that may relate to clinical symptoms in patients with traumatic and non-traumatic spinal disorders are highly controversial. Studies are often of poor quality and findings are inconsistent. A plethora of evidence suggests some pathoanatomical findings from traditional imaging applications are common in asymptomatic participants across the life span, which further questions the diagnostic, prognostic, and theranostic value of traditional imaging. Although we do not dispute the limited evidence for the clinical importance of most imaging findings, we contend that the disparate findings across studies may in part be due to limitations in the approaches used in assessment and analysis of imaging findings.
PURPOSE
This clinical commentary aimed to (1) briefly detail available imaging guidelines, (2) detail research-based evidence around the clinical use of findings from advanced, but available, imaging applications (eg, fat and water magnetic resonance imaging and magnetization transfer imaging), and (3) introduce how evolving imaging technologies may improve our mechanistic understanding of pain and disability, leading to improved treatments and outcomes.
STUDY DESIGN/SETTING
A non-systematic review of the literature is carried out.
METHODS
A narrative summary (including studies from the authors’ own work in whiplash injuries) of the available literature is provided.
RESULTS
An emerging body of evidence suggests that the combination of existing imaging sequences or the use of developing imaging technologies in tandem with a good clinical assessment of modifiable risk factors may provide important diagnostic information toward the exploration and development of more informed and effective treatment options for some patients with traumatic neck pain.
CONCLUSIONS
Advancing imaging technologies may help to explain the seemingly disconnected spectrum of biopsychosocial signs and symptoms of traumatic neck pain.
Keywords: Biopsychosocial, Imaging, Low back pain, MRI, Neck pain, Whiplash
Introduction
With an increasingly aging population, health-care spending is expected to increase dramatically [1,2]. In the United States, dollars spent on health care is greater than any other country in the world [2], with the largest increase in spending between 1996 and 2013 for musculoskeletal disorders such as neck and low back pain [2]. Despite the rising expenditures, little appreciable change in neck and low back pain prevalence has occurred either in the United States or across the globe [3–6]. Efforts to control spending and improve outcomes must consider the expense associated with delivery of interventions and diagnostic tests with little evidential support. Unnecessary imaging for patients with low back and neck pain has rightly received wide criticism [7–9], and triggered important work examining behaviors in physicians (and patients), aimed at reducing imaging overuse [8–10].
Routine use of early diagnostic imaging tests is challenged for multiple reasons. Numerous studies demonstrate abnormal or variant morphology of the cervical [11] and lumbar [9,12–16] spines of asymptomatic participants (false positives) [17], and other studies highlight the lack of imaging findings in some patients injured from whiplash [18–21] or suffering from low back pain (potential false negatives) [8,13,22]. Few studies have investigated the longitudinal predictive value of imaging findings in the lumbar [23] and cervical spine [18,21], and most importantly, there is currently little evidence that magnetic resonance imaging (MRI) findings help identify those who respond best to specific interventions [24].
On the other hand, although some imaging findings are common in those without pain, several findings (eg, disc degeneration, Modic change, annular tear, disc herniation) have been shown to be substantially more common in those with low back pain [17,25] and traumatic neck pain (eg, muscle fatty infiltrates [MFIs]) [26–32] than those without. Such discrepant findings have created a clinical (and research) dilemma that we believe is due partly to a lack of high-quality studies and many perhaps misguided attempts to investigate the usefulness of imaging in understanding spinal pathology.
In this clinical commentary, we draw from existing and emerging research to (1) briefly detail available imaging guidelines, (2) present research-based evidence around the potential clinical use of findings from advanced but accessible imaging applications (eg, fat and water MRI and magnetization transfer imaging [MTI]), and (3) introduce evolving imaging technologies that may improve our mechanistic understanding of pain and disability, ultimately leading to improved treatment outcomes.
Imaging guidelines
We do not dispute the universal guideline recommendations to avoid routine, non-indicated imaging for spinal pain, and we further endorse that routine imaging should not be conducted once the patient has been medically screened and determined to not have serious pathology. Furthermore, we agree with Chou et al. [14] who state
…addressing inefficiencies in diagnostic testing could minimize potential harms to patients and have a large effect on use of resources by reducing both direct and downstream costs. In this area, more testing does not equate to better care. Implementing a selective approach to [spinal imaging] as suggested by the American College of Physicians and American Pain Society guideline on low back pain, would provide better care to patients, improve outcomes, and reduce costs. [page 181]
The primary evidence-derived imaging guideline for healthcare providers in the United States is the American College of Radiology Appropriateness Criteria (ACR-AC). Relevant to this paper are the ACR-AC clinical conditions of (1) Chronic Neck Pain [33], (2) Suspected Spine Trauma [34], and (c) Low Back Pain [35]. Readers are encouraged to revisit the “clinical conditions” and subcategories (or variants) of the ACR-AC guidelines detailed above.
The authors support the value of these well-established and expert-derived guidelines that imaging is appropriately not recommended for the majority of patients with spinal pain. However, despite the proposed benefits of following the guidelines (cost-savings, reductions in exposure to ionizing radiation, avoiding the identification of pathology that may simply represent normal variants, and potentially misinforming clinical decision making), adherence to guidelines is quite variable [36–38], and it is largely unknown if adherence results in improved outcomes. Furthermore, there remains a lack of a gold standard quantitative metric for diagnosing low back and neck pain. Without a gold standard against which to compare, it is impossible to investigate whether diagnosis improves outcomes in our current landscape of care. Secondly, the presence of pathology in some people with low back and neck pain should not be dismissed as a normal variant on grounds they are also present in some without these conditions. Accordingly, there is an urgent need to perform high quality prospective imaging studies with quantitative measures using existing (T1-, T2-weighting) and other developed, but not an exhaustive list of, techniques (fat and water MRI or MTI) to better understand which imaging findings are and are not important.
A potential outcome of ongoing research and development could be that emerging technologies and research findings afford the opportunity to interrogate our own clinical instincts when managing patients with more complex, and seemingly unexplainable, signs and symptoms. Moreover, such knowledge would provide for the judicious use of carefully selected quantitative imaging sequences in tandem with known psychosocial risk factors that improve diagnostics, and hopefully improve outcomes.
Not forgetting the bio in the bio-psycho-social model of spinal pain
A potential risk of the strong push to reduce inappropriate imaging in clinical practice is to “forget” a biological component of spinal pain and to stifle important research that aims to better understand the contribution of local lumbar and cervical pathology to spinal pain. It is widely accepted that low back and neck pain are complex multifactorial conditions with both spinal (eg, local biological contributors) and extraspinal contributors (eg, psychosocial factors); however, much research [39–41] has focused on the extraspinal domains and, with some exceptions [42–45], largely ignores the potential contribution of local pathology. We argue that high-quality imaging research (especially those using new technology and advanced standardized analysis approaches) investigating the potential biological contributors to spinal pain form an important part of this inquiry. Without a better mechanistic understanding of the many biological contributors, it is likely the personal, societal, and economic burden of spinal pain will remain unchanged and enormous.
A fundamental difficulty underlying almost all spinal imaging studies is the lack of a gold standard test to identify sources of spinal pain. Importantly, spinal pain, similar to abdominal pain or headache pain, is a symptom. Differentiating a painful structural change (eg, disc degeneration) from a non-painful structural change remains a key challenge for the research community. Ultimately, the value of imaging findings from investigations of the spinal column [30,46,47] (and the brain [48–56]) will be demonstrated if such findings strongly predict important outcomes or identify phenotypes of patients who respond best to specific interventions.
Muscle fat infiltration as a biological marker of disease
The observation and description of MFIs has become increasingly common in the literature spanning acute and chronic whiplash [26,27,31,57,58], low back pain [59–62], spondylytic myelopathy [63], rotator cuff injury [64–68], osteoarthritis [69,70], and spinal cord injury [71,72].
Whereas some early studies suggest this finding may be associated with development of persistent pain and poor recovery in whiplash [26,27,29,30,32], others report no association between measures of muscle structure (eg, size without measuring fat) and symptoms [19,20]. Accordingly, the causal relationships between changes in muscle structure, symptoms, and the mechanisms underlying their generation following whiplash are largely unknown. Irrespective of the condition, current theories behind the expression of MFI could include the result of trauma, age-related changes [73,74], ethnic differences [75], spinal phenotypes [42–45], disuse [59,60], or degeneration [15].
Imaging of whiplash injury—potential pathology
Here we examine whiplash injury from a motor vehicle collision on grounds it is a common, yet enigmatic, condition whereby the role of imaging in clinical practice remains controversial.
Radiculopathy or myelopathy have their own distinctive clinical features, and accompanying abnormalities on radiography and MRI [76], yet the identification of salient pathologies of discs, ligaments, vertebral and carotid arteries, and facet joints that are related to the signs and symptoms of acute or chronic whiplash remains obscure [18,77–83].Accordingly, whiplash continues to be conceptualized as an almost purely psychosocial phenomenon [84].
Yet, it is possible that the lack of consistent imaging findings that are related to whiplash-related symptoms [19,20,27,30,32,85] are the result of study limitations and differences in methodological approaches (eg, ultrasound imaging, fat and water imaging, T1-, T2-weighted, proton-density, or gradient echo sequences).Another limitation of existing studies of imaging findings using longitudinal research designs (within and beyond whiplash) is that few, if any, use more quantitative measurement tools. Rather, they have tended to rely on qualitative grades or scores. Although qualitative grading is shown to be adequate and with acceptable use in the clinical environment, they may be prone to more variability [86–90]. Few investigators report using even simple but critical methodological controls such as co-registration and how the slices were aligned in plane to reduce noise, and discrepant findings from repeated measures [91]. We argue a way forward is to explore and develop consensus-driven standardized measurement approaches similar to what has been proposed for measuring the structure and composition of lumbar paravertebral muscles [92] and for quantifying the patient’s pain experience using functional magnetic resonance imaging [93].
The progression toward fat and water MRI (muscle fat infiltration)
In traumatic whiplash, MFI is a potentially interesting marker as it is more common than in patients with non-traumatic neck pain [28,29], suggesting that traumatic factors may play a role in their development [30] on standard T1-weighted images [27]. Considering a growing body of evidence around muscle degeneration [58], these changes may represent one physiological contributor to poor functional recovery in a discrete number of patients with poor functional recovery following whiplash injury.
Imaging techniques such as fat and water MRI (detailed below) could help quantify the rapid onset of compositional changes in muscle, which may precede macroscopic muscle changes on standard T1-weighted sequences. A preliminary study [30], case-series [94], and interdisciplinary lines of work [95] suggest this may be the case for a subset of patients with whiplash, meaning these advances in imaging techniques could lead to more timely and effective intervention trials and thus, informed clinical decision making.
Several approaches for quantitatively measuring the water and fat composition on an MRI exist. These include T1-weighted imaging and a dual acquisition method, where one image is fat suppressed [96] (water image) and a standard image (fat and water combined) is collected [97]. By removing the water from the co-registered combined image, muscle fat can be identified with high sensitivity and specificity [30]. A challenge with such an acquisition is its reliance on the uniform frequency difference between water and fat, and this can be difficult to obtain when using higher magnetic fields (3 Tesla and above) where chemical shift may feature. A fat-suppressed inversion recovery sequence (eg, short tau inversion recovery, or STIR) is promising, but as STIR nulls signal from fat species, the quantity of fat will be estimated rather than quantified, and this may vary across ethnicities [75], age [73,74], phenotypes [42–45], and conditions whereby the composition of and temporal changes in muscle fat may differ [91,98].
A well-known alternative is the Dixon method [99] where data are collected at echo times when water and fat are in- and out-of-phase. The data can be used to generate a fat and water image, but this is not without potential image distortions from field inhomogeneities [100,101]. Current methods collect multiple echo time data to improve the estimation of the fat and water images, and this has been applied successfully [102,103]. The methods [32,74,104,105] have been tested and used in animal- and human-based studies of the appendicular and axial muscle system collecting different echo times for generating a quantitative measure for fat and water composition [97,106].
Although previous research across the globe has identified changes in the size, shape, and spatial distribution of MFI in paraspinal muscle following whiplash [26,27,30–32,85] and in low back pain (and asymptomatic participants) [74,75,107], they are not typically reported in clinical practice, likely because radiologists are neither looking for them nor using the techniques that would enable them to observe and measure such changes. We are of the opinion, based on basic [105] and clinical research [30,68,74,85,104], that fat and water imaging is the preferred imaging method for quantifying MFI. We further expect that a richer investigative landscape for musculoskeletal conditions will result in diagnostic imaging standards based on sound biological, psychological, and social parameters [108,109] resulting in improved outcomes.
Magnetization transfer imaging of the spinal cord
Magnetization transfer imaging and Spinal Cord Toolbox briefly detail new imaging techniques and mechanistic measurement tools that pertain to patients with suspected spine trauma or cervical cord involvement (eg, whiplash, spinal cord injury, myelopathy) but, as yet, not patients with low back pain, shoulder dysfunction, or osteoarthritis where mechanistic origins are less grounded in trauma.
Magnetization transfer imaging has been used to provide a semiquantitative metric for traumatic brain injury [110,111], peripheral neuropathies [112], and is used clinically in diagnostic studies of neuronal degeneration in multiple sclerosis [113], Alzheimer disease [114–118], and Parkinson disease [119,120]. Magnetization transfer imaging provides an indirect measure of tissue integrity, relying on the exchange between saturated hydrogen molecules (protons associated with free water) and another pool of protons that belong to bound water residing on hydrophilic macromolecular surfaces (eg, lipids and proteins) [121,122].
Magnetization transfer imaging has demonstrated predictive value in determining sensory and motor disability levels following spinal cord injury, suggesting that a non-invasive MTI measure of the cord and determination of impairment is possible [123]. It is our contention that MTI could provide a more sensitive measure of cellular level changes in the spinal cord and brain in a discrete number of patients without radiological abnormalities following whiplash [94] and, possibly, concussion [124].
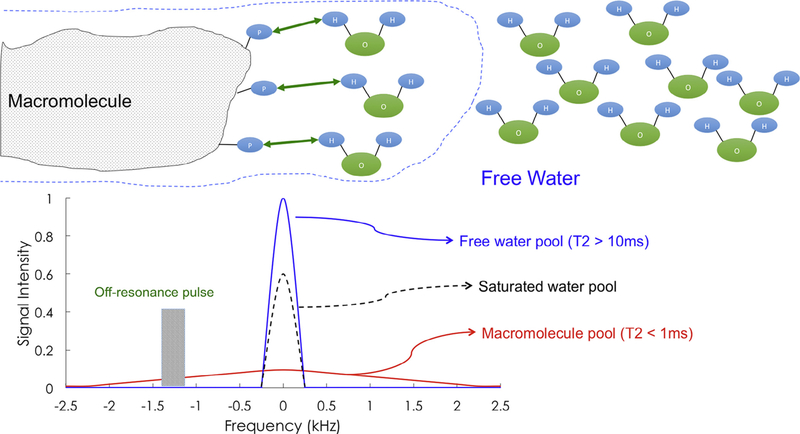
Positive findings could inform the prognostic picture of, and expected response to, functional rehabilitation schemas by acutely characterizing the structure of white matter spinal pathways following head and neck trauma. Larger scaled prospective investigations involving patients with varying levels of condition-related disability and impairment are required before definitive conclusions can be drawn. Fig. 1 details the basic physics underlying MTI.
Fig. 1.
Basic physics underlying magnetization transfer imaging. Typical magnetic resonance imaging (MRI) draws its signal from protons associated with free water. There is also a pool of protons bound to macromolecules—such as the myelin surrounding an axon. If one compares the resonance spectra of these two pools, free water has a sharp resonance peak and long T2, whereas macromolecular protons have a broad spectrum and an ultra-short T2 (~100 μs), making imaging of this group difficult. By use of an off-resonance radiofrequency pulse before imaging, one can selectively saturate the macromolecular pool of protons. Although the relaxation will not be visible, magnetization of the bound pool will partially exchange with the surrounding free water, degrading the local free water signal in proximity to macromolecules, as shown by the dashed line. This exchange between pools of magnetization allows for the indirect study of the bound protons, and thus the density and stability of macromolecular content of a given imaging voxel. This technique is often reported as the magnetization transfer ratio or MTR, the signal change in free water caused by magnetization exchange.
Tools for imaging spinal cord pathways
The Spinal Cord Toolbox, an open-source image processing software, has been developed to facilitate the advancement of spinal cord imaging [125]. One key component of this software is the MNI-Poly-AMU T2-weighted template, which allows for a fitting of spinal cord imaging data from anatomically varied participants into a standardized anatomical template of the spinal cord [126]. This important registration step in image processing permits researchers the opportunity to analyze precise anatomical locations of the cord, including gray matter, cerebrospinal fluid, and specific white matter tracts, which can then be compared within- and between-subjects in a standardized manner [127]. In 2016, the Spinal Cord Toolbox was used to study spinal cord changes in patients with degenerative cervical myelopathy, using diffusion tensor imaging, MT, and T2-weighted MRI [128]. Significant relationships between white matter injury and specific motor deficits, in an ipsilesional manner (ie, rightsided white matter damage correlated with right-sided motor deficits) were observed [128]. Using the Spinal Cord Toolbox, and in accordance with the findings of Martin et al., preliminary work coming out of the Neuromuscular Imaging Research Laboratory at Northwestern University observed damage involving the lateral corticospinal tract that was associated with ipsilesional motor deficits in patients with incomplete spinal cord injury (A. Smith, PhD, written communication, May 2017). The Spinal Cord Toolbox represents an innovative program with great potential to improve the segmentation, registration, and calculation of spinal cord anatomical metrics (Fig. 2) across a spectrum of patients with persistent spine-related disability (eg, whiplash, known spinal cord injury, or myelopathy). Any indication for its use in patients with other musculoskeletal conditions whose mechanistic origins are less ground in trauma (eg, low back pain or joint-related conditions) is, at this stage, unknown.
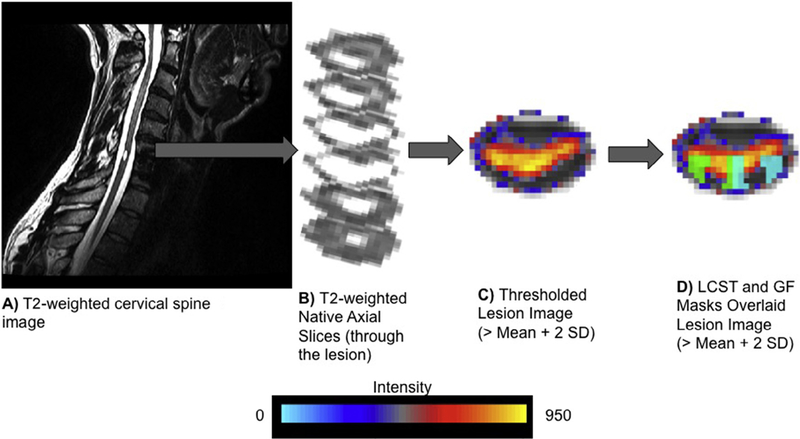
Fig. 2.
(A) A native sagittal T2-weighted image of a participant with spinal cord injury. (B) Native axial T2-weighted images through the spinal cord lesion. (C) The lesion filled image was then straightened along the spinal cord and registered to the MNI-Poly-AMU spinal cord template. The mean and standard deviation (SD) of the voxel intensities were then calculated within a non-lesioned 1-cm axial cross section of the spinal cord immediately superior to the lesion. The maximum intensity projection image was then thresholded at two SDs above the mean to define the lesion. (D) The extent of spinal cord damage was then quantified in the axial plane as the ratio of the spinal cord that was lesioned across the total cord and within the right and left lateral corticospinal tracts (LCST) and gracile fasciculi (GF). One representative participant is shown. The right and left LCST and GF are shown in green and light blue, respectively.
Where to go from here
The current climate of rejecting imaging as a viable modality for spinal pain or disability appears to have been borne largely from a series of studies that found positive spinal imaging findings in asymptomatic cohorts [11,17,129] and the appropriate desire to reduce some unnecessary imaging. Although we do not dispute the value of this research, we see several clear reasons why high-quality research into MRI findings remains important. Given the recurrent nature of most spinal pain and clear evidence that many MRI findings are more common in those who have spinal pain than those who do not [25,130], we believe future research should focus on understanding the link between imaging findings and future spinal pain (eg, the course of a current episode, development of recurrences, or persistent pain-related disability), rather than focusing on imaging findings in asymptomatic people that would not be sent for imaging in clinical practice.
Conclusion
Our intention is not to throw darts at our peers, nor is it to endorse imaging for all, or even most, people with traumatic or non-traumatic spinal pain. On the contrary, our intention is to refocus research and clinical efforts toward identifying the right evaluation, for the right patient, at the right time (acute, subacute, chronic stages). Although we are not there yet, advancing imaging technologies, and pathologic findings (or processes) may explain the seemingly disconnected spectrum of biopsychosocial signs and symptoms of chronic traumatic and non-traumatic neck and low back pain. The sequences and measures described are not meant to be exhaustive, rather they offer an encouraging preview of imaging findings that could eventually guide clinical treatment decisions by identifying spinal phenotypes with a target to determine which patients respond best to specific interventions. Current and future research investigations should aim to enhance tomorrow’s imaging guidelines toward providing appropriate directives for the timely performance of imaging in tandem with consideration of the psychosocial factors that are unique to the individual person seeking our care.
Acknowledgments
Author disclosure: JME: Consulting: Relevant activities outside the body of work—medical consulting start-up Pain ID, LLC (None); Grants: NIH R01 R01HD079076 (H, Paid directly to institution/employer), outside the submitted work. MJH: Nothing to disclose. RJC: Nothing to disclose. ACS: Nothing to disclose. DMW: Speaking and/or Teaching Arrangements: David Walton Rehabilitation Education (B); Scientific Advisory Board/Other Office: Elsevier Associate Editor for Musculoskeletal Science and Practice (B); Grants: Canadian Institutes of Health Research, Canadian Pain Society (E, Paid directly to institution/employer), outside the submitted work.
Footnotes
FDA device/drug status: Not applicable.
The disclosure key can be found on the Table of Contents and at www.TheSpineJournalOnline.com.
References
- [1].GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015;385:117–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Dieleman JL, Baral R, Birger M, et al. US spending on personal health care and public health, 1996–2013. JAMA 2016;316:2627–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Manchikanti L, Singh V, Falco FJ, Benyamin RM, Hirsch JA. Epidemiology of low back pain in adults. Neuromodulation 2014;17(Suppl. 2):3–10. [DOI] [PubMed] [Google Scholar]
- [4].Hoy D, Brooks P, Blyth F, Buchbinder R. The Epidemiology of low back pain. Best Pract Res Clin Rheumatol 2010;24:769–81. [DOI] [PubMed] [Google Scholar]
- [5].Hoy D, March L, Brooks P, et al. The global burden of low back pain: estimates from the Global Burden of Disease 2010 study. Ann Rheum Dis 2014;73:968–74. [DOI] [PubMed] [Google Scholar]
- [6].Hoy D, March L, Woolf A, et al. The global burden of neck pain: estimates from the Global Burden of Disease 2010 study. Ann Rheum Dis 2014;73:1309–15. [DOI] [PubMed] [Google Scholar]
- [7].Chou R, Fu R, Carrino JA, Deyo RA. Imaging strategies for low-back pain: systematic review and meta-analysis. Lancet 2009;373:463–72. [DOI] [PubMed] [Google Scholar]
- [8].Jarvik JG, Gold LS, Comstock BA, et al. Association of early imaging for back pain with clinical outcomes in older adults. JAMA 2015;313:1143–53. [DOI] [PubMed] [Google Scholar]
- [9].Jarvik JG, Hollingworth W, Martin B, et al. Rapid magnetic resonance imaging vs radiographs for patients with low back pain: a randomized controlled trial. JAMA 2003;289:2810–18. [DOI] [PubMed] [Google Scholar]
- [10].Jenkins HJ, Hancock MJ, French SD, Maher CG, Engel RM, Magnussen J. Effectiveness of interventions designed to reduce the use of imaging for low-back pain: a systematic review. CMAJ 2015;187:401–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Nakashima H, Yukawa Y, Suda K, Yamagata M, Ueta T, Kato F. Abnormal findings on magnetic resonance images of the cervical spines in 1211 asymptomatic subjects. Spine 2015;40:392–8. [DOI] [PubMed] [Google Scholar]
- [12].Chou R, Deyo RA, Jarvik J. Appropriate use of lumbar imaging for evaluation of low back pain. Radiol Clin North Am 2012;50:569–85. [DOI] [PubMed] [Google Scholar]
- [13].Chou R, Fu R, Carrino JA, Deyo RA. Imaging strategies for low-back pain: systematic review and meta-analysis. Lancet 2009;373:463–72. [DOI] [PubMed] [Google Scholar]
- [14].Chou R, Qaseem A, Owens DK, Shekelle P. Diagnostic imaging for low back pain: advice for high-value health care from the American College of Physicians. Ann Intern Med 2011;154:181–9. [DOI] [PubMed] [Google Scholar]
- [15].Haig AJ. Paraspinal denervation and the spinal degenerative cascade. Spine J 2002;2:372–80. [DOI] [PubMed] [Google Scholar]
- [16].Kirkaldy-Willis WH, Wedge JH, Yong-Hing K, Reilly J. Pathology and pathogenesis of lumbar spondylosis and stenosis. Spine 1978;3:319–28. [DOI] [PubMed] [Google Scholar]
- [17].Brinjikji W, Luetmer PH, Comstock B, et al. Systematic literature review of imaging features of spinal degeneration in asymptomatic populations. AJNR Am J Neuroradiol 2015;36:811–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Anderson SE, Boesch C, Zimmermann H, et al. Are there cervical spine findings at MR imaging that are specific to acute symptomatic whiplash injury?Aprospective controlled study with four experienced blinded readers. Radiology 2012;262:567–75. [DOI] [PubMed] [Google Scholar]
- [19].Ulbrich EJ, Aeberhard R, Wetli S, et al. Cervical muscle area measurements in whiplash patients: acute, 3, and 6 months of follow-up. J Magn Reson Imaging 2012;36:1413–20. [DOI] [PubMed] [Google Scholar]
- [20].Matsumoto M, Ichihara D, Okada E, et al. Cross-sectional area of the posterior extensor muscles of the cervical spine in whiplash injury patients versus healthy volunteers—10-year follow-up MR study. Injury 2012;43:912–16. [DOI] [PubMed] [Google Scholar]
- [21].Matsumoto M, Okada E, Ichihara D, et al. Prospective ten-year follow-up study comparing patients with whiplash-associated disorders and asymptomatic subjects using magnetic resonance imaging. Spine 2010;35:1684–90. [DOI] [PubMed] [Google Scholar]
- [22].Hebert JJ, Kjaer P, Fritz JM, Walker BF. The relationship of lumbar multifidus muscle morphology to previous, current, and future low back pain: a 9-year population-based prospective cohort study. Spine 2014;39:1417–25. [DOI] [PubMed] [Google Scholar]
- [23].Steffens D, Hancock MJ, Maher CG, Williams C, Jensen TS, Latimer J. Does magnetic resonance imaging predict future low back pain? A systematic review. Eur J Pain 2014;18:755–65. [DOI] [PubMed] [Google Scholar]
- [24].Panagopoulos J, Hush J, Steffens D, Hancock M, Do MRI. Findings change over a period of up to one year in patients with low back pain and/or sciatica? A systematic review. Spine 2017;42:504–12. [DOI] [PubMed] [Google Scholar]
- [25].Brinjikji W, Diehn FE, Jarvik JG, et al. MRI findings of disc degeneration are more prevalent in adults with low back pain than in asymptomatic controls: a systematic review and meta-analysis. Am J Neuroradiol 2015;36:2394–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Elliott J, Jull G, Noteboom JT, Darnell R, Galloway G, Gibbon WW. Fatty infiltration in the cervical extensor muscles in persistent whiplash-associated disorders: a magnetic resonance imaging analysis. Spine 2006;31:E847–55. [DOI] [PubMed] [Google Scholar]
- [27].Elliott J, Pedler A, Kenardy J, Galloway G, Jull G, Sterling M. The temporal development of fatty infiltrates in the neck muscles following whiplash injury: an association with pain and posttraumatic stress. PLoS ONE 2011;6:e21194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Elliott J, Sterling M, Noteboom JT, Darnell R, Galloway G, Jull G. Fatty infiltrate in the cervical extensor muscles is not a feature of chronic, insidious-onset neck pain. Clin Radiol 2008;63:681–7. [DOI] [PubMed] [Google Scholar]
- [29].Elliott JM, Pedler AR, Jull GA, Van Wyk L, Galloway GG, O’Leary S. Differential changes in muscle composition exist in traumatic and non-traumatic neck pain. Spine 2014;39:39–47. [DOI] [PubMed] [Google Scholar]
- [30].Elliott JM, Courtney DM, Rademaker A, Pinto D, Sterling MM, Parrish T. The rapid and progressive degeneration of the cervical multifidus in whiplash: an MRI study of fatty infiltration. Spine 2015;40:E694–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Elliott JM, O’Leary S, Sterling M, Hendrikz J, Pedler A, Jull G. Magnetic resonance imaging findings of fatty infiltrate in the cervical flexors in chronic whiplash. Spine 2010;35:948–54. [DOI] [PubMed] [Google Scholar]
- [32].Karlsson A, Dahlqvist Leinhard O, West J, et al. An investigation of fat infiltration of the multifidus muscle in patients with severe neck symptoms associated with chronic whiplash associated disorder. J Orthop Sports Phys Ther 2016;46:886–93. [DOI] [PubMed] [Google Scholar]
- [33].Newman J, Weissman B, Angevine P, et al. Chronic neck pain. Appropriateness criteria 2013. 2013. Available at: https://acsearch.acr.org/docs/69426/Narrative/. Accessed December 21, 2015. [Google Scholar]
- [34].Daffner R, Weissman B, Wippold F, et al. Suspected spine trauma. Appropriateness criteria 2012. 2012. Available at: https://acsearch.acr.org/docs/69359/Narrative/. Accessed December 20, 2015. [Google Scholar]
- [35].Davis PC, Wippold FJN, Brunberg JA, et al. ACR appropriateness criteria on low back pain. J Am Coll Radiol 2009;6:401–7. [DOI] [PubMed] [Google Scholar]
- [36].Michaleff ZA, Harrison C, Britt H, Lin CW, Maher CG. Ten-year survey reveals differences in GP management of neck and back pain. Eur Spine J 2012;21:1283–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Fullen BM, Maher T, Bury G, Tynan A, Daly LE, Hurley DA. Adherence of Irish general practitioners to European guidelines for acute low back pain: a prospective pilot study. Eur J Pain 2007;11:614–23. [DOI] [PubMed] [Google Scholar]
- [38].Piccoliori G, Engl A, Gatterer D, Sessa E, der Schmitten JI, Abholz HH. Management of low back pain in general practice—is it of acceptable quality: an observational study among 25 general practices in South Tyrol (Italy). BMC Fam Pract 2013;14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Kent PM, Keating J . Can we predict poor recovery from recent-onset nonspecific low back pain? A systematic review. Man Ther 2008; 13:12–28. [DOI] [PubMed] [Google Scholar]
- [40].Henschke N, Maher CG, Refshauge KM, et al. Prognosis in patients with recent onset low back pain in Australian primary care: inception cohort study. BMJ 2008;337:a171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Grotle M, Foster NE, Dunn KM, Croft P. Are prognostic indicators for poor outcome different for acute and chronic low back pain consulters in primary care? Pain 2010;151:790–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Li Y, Samartzis D, Campbell DD, et al. Two subtypes of intervertebral disc degeneration distinguished by large-scale population-based study. Spine J 2016;16:1079–89. [DOI] [PubMed] [Google Scholar]
- [43].Maatta JH, Karppinen JI, Luk KD, Cheung KM, Samartzis D. Phenotype profiling of Modic changes of the lumbar spine and its association with other MRI phenotypes: a large-scale population-based study. Spine J 2015;15:1933–42. [DOI] [PubMed] [Google Scholar]
- [44].Mok FP, Samartzis D, Karppinen J, Fong DY, Luk KD, Cheung KM. Modic changes of the lumbar spine: prevalence, risk factors, and association with disc degeneration and low back pain in a large-scale population-based cohort. Spine J 2016;16:32–41. [DOI] [PubMed] [Google Scholar]
- [45].Teraguchi M, Samartzis D, Hashizume H, et al. Classification of high intensity zones of the lumbar spine and their association with other spinal MRI phenotypes: the Wakayama Spine Study. PLoS ONE 2016;11:e0160111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Farrell SF, Osmotherly PG, Cornwall J, Lau P, Rivett DA. Morphology of cervical spine meniscoids in individuals with chronic whiplash-associated disorder: a case-control study. J Orthop Sports Phys Ther 2016;46:902–10. [DOI] [PubMed] [Google Scholar]
- [47].Elliott J Are there implications for morphological changes in neck muscles after whiplash injury? Spine 2011;36(25 Suppl):S205–10. Review. [DOI] [PubMed] [Google Scholar]
- [48].Apkarian AV, Krauss BR, Fredrickson BE, Szeverenyi NM. Imaging the pain of low back pain: functional magnetic resonance imaging in combination with monitoring subjective pain perception allows the study of clinical pain states. Neurosci Lett 2001;299:57–60. [DOI] [PubMed] [Google Scholar]
- [49].Apkarian AV, Sosa Y, Krauss BR, et al. Chronic pain patients are impaired on an emotional decision-making task. Pain 2004;108:129–36. [DOI] [PubMed] [Google Scholar]
- [50].Apkarian AV, Sosa Y, Sonty S, et al. Chronic back pain is associated with decreased prefrontal and thalamic gray matter density. J Neurosci 2004;24:10410–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Baliki MN, Geha PY, Jabakhanji R, Harden N, Schnitzer TJ, Apkarian AV. A preliminary fMRI study of analgesic treatment in chronic back pain and knee osteoarthritis. Mol Pain 2008;4:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Baliki MN, Petre B, Torbey S, et al. Corticostriatal functional connectivity predicts transition to chronic back pain. Nat Neurosci 2012;15:1117–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Meier ML, Stampfli P, Vrana A, Humphreys BK, Seifritz E, Hotz-Boendermaker S. Fear avoidance beliefs in back pain-free subjects are reflected by amygdala-cingulate responses. Front Hum Neurosci 2015;9:424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Vrana A, Hotz-Boendermaker S, Stampfli P, et al. Differential neural processing during motor imagery of daily activities in chronic low back pain patients. PLoS ONE 2015;10:e0142391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Hotz-Boendermaker S, Marcar VL, Meier ML, Boendermaker B, Humphreys BK. Reorganization in secondary somatosensory cortex in chronic low back pain patients. Spine 2016;41:E667–73. [DOI] [PubMed] [Google Scholar]
- [56].Meier ML, Stampfli P, Vrana A, Humphreys BK, Seifritz E, Hotz-Boendermaker S. Neural correlates of fear of movement in patients with chronic low back pain vs. pain-free individuals. Front Hum Neurosci 2016;10:386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Elliott J, Sterling M, Noteboom JT, Treleaven J, Galloway G, Jull G. The clinical presentation of chronic whiplash and the relationship to findings of MRI fatty infiltrates in the cervical extensor musculature: a preliminary investigation. Eur Spine J 2009;18:1371–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].De Pauw R, Coppieters I, Kregel J, De Meulemeester K, Danneels L, Cagnie B. Does muscle morphology change in chronic neck pain patients? A systematic review. Man Ther 2016;22:42–9. [DOI] [PubMed] [Google Scholar]
- [59].Hodges P, Holm AK, Hansson T, Holm S. Rapid atrophy of the lumbar multifidus follows experimental disc or nerve root injury. Spine 2006;31:2926–33. [DOI] [PubMed] [Google Scholar]
- [60].Hodges PW, James G, Blomster L, et al. Multifidus muscle changes after back injury are characterized by structural remodeling of muscle, adipose and connective tissue, but not muscle atrophy: molecular and morphological evidence. Spine 2015;40:1057–71. [DOI] [PubMed] [Google Scholar]
- [61].Sions JM, Coyle PC, Velasco TO, Elliott JM, Hicks G. Multifidi muscle characteristics and physical function among older adults with and without chronic low back pain. Arch Phys Med Rehabil 2017;98:51–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Kader DF, Wardlaw D, Smith F. Correlation between the MRI changes in the lumbar multifidus muscles and leg pain. Clin Radiol 2000;55:145–9. [DOI] [PubMed] [Google Scholar]
- [63].Fortin M, Dobrescu O, Courtemanche M, et al. Association between paraspinal muscle morphology, clinical symptoms and functional status in patients with degenerative cervical myelopathy. Spine 2017;42:232–9. [DOI] [PubMed] [Google Scholar]
- [64].Gerber C, Schneeberger AG, Hoppeler H, Meyer DC. Correlation of atrophy and fatty infiltration on strength and integrity of rotator cuff repairs: a study in thirteen patients. J Shoulder Elbow Surg 2007;16:691–6. [DOI] [PubMed] [Google Scholar]
- [65].Gladstone JN, Bishop JY, Lo IK, Flatow EL. Fatty infiltration and atrophy of the rotator cuff do not improve after rotator cuff repair and correlate with poor functional outcome. Am J Sports Med 2007;35:719–28. [DOI] [PubMed] [Google Scholar]
- [66].Nozaki T, Tasaki A, Horiuchi S, et al. Predicting retear after repair of full-thickness rotator cuff tear: two-point Dixon MR imaging quantification of fatty muscle degeneration—initial experience with 1-year follow-up. Radiology 2016;280:500–9. [DOI] [PubMed] [Google Scholar]
- [67].Nozaki T, Tasaki A, Horiuchi S, et al. Quantification of fatty degeneration within the supraspinatus muscle by using a 2-point Dixon method on 3-T MRI. Am J Roentgenol 2015;205:116–22. [DOI] [PubMed] [Google Scholar]
- [68].Nardo L, Karampinos DC, Lansdown DA, et al. Quantitative assessment of fat infiltration in the rotator cuff muscles using water-fat MRI. J Magn Reson Imaging 2014;39:1178–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Kumar D, Karampinos DC, MacLeod TD, et al. Quadriceps intramuscular fat fraction rather than muscle size is associated with knee osteoarthritis. Osteoarthritis Cartilage 2014;22:226–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Davison MJ, Maly MR, Adachi JD, Noseworthy MD, Beattie K. Relationships between fatty infiltration in the thigh and calf in women with knee osteoarthritis. Aging Clin Exp Res 2017;29:291–9. [DOI] [PubMed] [Google Scholar]
- [71].Smith AC, Parrish TB, Hoggarth MA, et al. Potential associations between chronic whiplash and incomplete spinal cord injury. Spinal Cord Ser Cases 2015;15024:doi: 10.1038/scsandc.2015.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Gorgey AS, Dudley GA. Skeletal muscle atrophy and increased intramuscular fat after incomplete spinal cord injury. Spinal Cord 2007;45:304–9. [DOI] [PubMed] [Google Scholar]
- [73].Crawford RJ, Volken T, Valentin S, Melloh M, Elliott J. Rate of lumbar paravertebral muscle fat infiltration versus spinal degeneration in asymptomatic populations: an age-aggregated cross-sectional simulation study. Scoliosis Spinal Disord 2016;11:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Crawford RJ, Filli L, Elliott JM, et al. Age- and level-dependence of fatty infiltration in lumbar paravertebral muscles of healthy volunteers. Am J Neuroradiol 2015;37:742–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Lee SH, Park SW, Kim YB, Nam TK, Lee Y. The fatty degeneration of lumbar paraspinal muscles on computed tomography scan according to age and disc level. Spine J 2017;17:81–7. [DOI] [PubMed] [Google Scholar]
- [76].Pearce J A critical appraisal of the chronic whiplash syndrome. J Neurol Neurosurg Psychiatry 1999;66:273–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Elliott JM, Noteboom JT, Flynn TW, Sterling M. Characterization of acute and chronic whiplash-associated disorders. J Orthop Sports Phys Ther 2009;39:312–23. [DOI] [PubMed] [Google Scholar]
- [78].Sterling M, McLean SA, Sullivan MJ, Elliott JM, Buitenhuis J, Kamper S. Potential processes involved in the initiation and maintenance of whiplash-associated disorders: discussion paper 3. Spine 2011;36(25 Suppl):S322–9. [DOI] [PubMed] [Google Scholar]
- [79].Myran R, Zwart JA, Kvistad KA, et al. Clinical characteristics, pain and disability in relation to alar ligament MRI findings. Spine 2011;36:E862–7. [DOI] [PubMed] [Google Scholar]
- [80].Siegmund GP, Winkelstein BA, Ivancic PC, Svensson MY, Vasavada A. The anatomy and biomechanics of acute and chronic whiplash injury. Traffic Inj Prev 2009;10:101–12. [DOI] [PubMed] [Google Scholar]
- [81].Pettersson K, Hildingsson C, Toolanen G, et al. Disc pathology after whiplash injury: a prospective magnetic resonance imaging and clinical investigation. Spine 1997;22:283–8. [DOI] [PubMed] [Google Scholar]
- [82].Pettersson K, Hildingsson C, Toolanen G, Fagerlund M, Bjornebrink J. MRI and neurology in acute whiplash trauma. No correlation in prospective examination of 39 cases. Acta Orthop Scand 1994;65:525–8. [DOI] [PubMed] [Google Scholar]
- [83].Ronnen HR, de Korte PJ, Brink PR, van der Bijl HJ, Tonino AJ, Franke C. Acute whiplash injury: is there a role for MR imaging? A prospective study of 100 patients. Radiology 1996;201:93–6. [DOI] [PubMed] [Google Scholar]
- [84].Malleson A. Whiplash and other useful illnesses. Montreal: McGill-Queen’s University Press; 2005. [Google Scholar]
- [85].Abbott R, Pedler A, Sterling M, et al. The geography of fatty infiltrates within the cervical multifidus and semispinalis cervicis in individuals with chronic whiplash-associated disorders. J Orthop Sports Phys Ther 2015;45:281–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Matsumoto M, Ichihara D, Okada E, et al. Modic changes of the cervical spine in patients with whiplash injury: a prospective 11-year follow-up study. Injury 2013;44:819–24. [DOI] [PubMed] [Google Scholar]
- [87].Matsumoto M, Okada E, Ichihara D, et al. Modic changes in the cervical spine: prospective 10-year follow-up study in asymptomatic subjects. J Bone Joint Surg Br 2012;94:678–83. [DOI] [PubMed] [Google Scholar]
- [88].Fuchs B, Weishaupt D, Zanetti M, Hodler J, Gerber C. Fatty degeneration of the muscles of the rotator cuff: assessment by computed tomography versus magnetic resonance imaging. J Shoulder Elbow Surg 1999;8:599–605. [DOI] [PubMed] [Google Scholar]
- [89].Goutallier D, Postel JM, Bernageau J, Lavau L, Voisin MC. Fatty muscle degeneration in cuff ruptures. Pre- and postoperative evaluation by CT scan. Clin Orthop Relat Res 1994;304:78–83. [PubMed] [Google Scholar]
- [90].Oh JH, Kim SH, Choi JA, Kim Y, Oh CH. Reliability of the grading system for fatty degeneration of rotator cuff muscles. Clin Orthop Relat Res 2010;468:1558–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Elliott JM, Kerry R, Flynn T, Parrish T. Content not quantity is a better measure of muscle degeneration in whiplash. Manual Ther 2013;18:578–82. [DOI] [PubMed] [Google Scholar]
- [92].Crawford RJ, Cornwall J, Abbott R, Elliott J. Manually defining regions of interest when quantifying paravertebral muscles fatty infiltration from axial magnetic resonance imaging: a proposed method for the lumbar spine with anatomical cross-reference. BMC Musculoskelet Disord 2017;18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Elliott JM, Owen M, Bishop MD, et al. Measuring pain for patients seeking physical therapy: can functional magnetic resonance imaging (fMRI) help? Phys Ther J 2017;97:145–55. [DOI] [PubMed] [Google Scholar]
- [94].Elliott JM, Dewald JP, Hornby TG, Walton DM, Parrish TB. Mechanisms underlying chronic whiplash: contributions from an incomplete spinal cord injury? Pain Med 2014;15:1938–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Elliott JM, Dayanidhi S, Hazle C, et al. Advancements in imaging technology: do they (or will they) equate to advancements in our knowledge of recovery in whiplash? J Ortho Sports Phys Ther 2016;46:861–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Haase A, Frahm J, Hänicke W, Matthaei D. 1H NMR chemical shift selective (CHESS) imaging. Phys Med Biol 1985;30:341–4. [DOI] [PubMed] [Google Scholar]
- [97].Elliott JM, Walton DM, Rademaker A, Parrish T. Quantification of cervical spine muscle fat: a comparison between T1-weighted and multi-echo gradient echo imaging using a variable projection algorithm (VARPRO). BMC Med Imaging 2013;13:30. doi: 10.1186/1471-2342-13-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Bydder GM, Steiner RE, Blumgart L. MR imaging of the liver using short TI inversion recovery. J Comput Assist Tomogr 1985;9:1084–9. [DOI] [PubMed] [Google Scholar]
- [99].Dixon W Simple proton spectroscopic imaging. Radiology 1984;153:189–94. [DOI] [PubMed] [Google Scholar]
- [100].Elliott JM, Flynn TW, Press J, Al-Najjar A, Nguyen BC, Noteboom JT. The pearls and pitfalls of magnetic resonance imaging for the spine. J Orthop Sports Phys Ther 2011;14:848–60. [DOI] [PubMed] [Google Scholar]
- [101].Wang J, Mao W, Qiu M, Smith MB, Constable RT. Factors influencing flip angle mapping in MRI: RF pulse shape, slice-select gradients, off-resonance excitation, and B0 inhomogeneities. Magn Reson Med 2006;56:463–8. [DOI] [PubMed] [Google Scholar]
- [102].Reeder SB, Wen Z, Yu H, et al. Multicoil Dixon chemical species separation with an iterative least squares estimation method. Magn Reson Med 2004;51:35–45. [DOI] [PubMed] [Google Scholar]
- [103].Reeder SB, Pineda AR, Wen Z, et al. Iterative decomposition of water and fat with echo asymmetry and least-squares estimation (IDEAL): application with fast spin-echo imaging. Magn Reson Med 2005;54:636–44. [DOI] [PubMed] [Google Scholar]
- [104].Valentin S, Licka T, Essigbeck A, Elliott J. In vivo magnetic resonance imaging features of spinal muscles in the ovine model. J Orthop Trans 2016;6:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Smith AC, Parrish TB, Abbott R, et al. Muscle-fat magnetic resonance imaging: 1.5 Tesla and 3.0 Tesla versus histology. Muscle Nerve 2014;50:170–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Hernando D, Kellman P, Haldar JP, Liang ZP. Estimation of water/fat images, B0 field map and T2* map using VARPRO. In Proceedings of the 16thAnnual Meeting of ISMRM, Toronto, Canada 1517, 2008. [Google Scholar]
- [107].Ni Mhuiris Á, Volken T, Elliott JM, Hoggarth MA, Samartzis D, Crawford R. Reliability of quantifying the spatial distribution of fatty infiltration in lumbar paravertebral muscles using a new segmentation method for T1-weighted MRI. BMC Musculoskelet Disord 2016;17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Dufton JA, Bruni SG, Kopec JA, Cassidy JD, Quon J. Delayed recovery in patients with whiplash-associated disorders. Injury 2012;43:1141–7. [DOI] [PubMed] [Google Scholar]
- [109].Ferrari R, Russell AS, Carroll LJ, Cassidy JD. A re-examination of the whiplash associated disorders (WAD) as a systemic illness. Ann Rheum Dis 2005;64:1337–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Bagley LJ, McGowan JC, Grossman RI, et al. Magnetization transfer imaging of traumatic brain injury. J Magn Reson Imaging 2000;11:1–8. [DOI] [PubMed] [Google Scholar]
- [111].McGowan JC, Yang JH, Plotkin RC, et al. Magnetization transfer imaging in the detection of injury associated with mild head trauma. AJNR Am J Neuroradiol 2000;21:875–80. [PMC free article] [PubMed] [Google Scholar]
- [112].Sinclair CD, Morrow JM, Miranda MA, et al. Skeletal muscle MRI magnetisation transfer ratio reflects clinical severity in peripheral neuropathies. J Neurol Neurosurg Psychiatry 2012;83:29–32. [DOI] [PubMed] [Google Scholar]
- [113].Filippi M, Rovaris M. Magnetisation transfer imaging in multiple sclerosis. J Neurovirol 2000;6(Suppl. 2):S115–20. [PubMed] [Google Scholar]
- [114].Hanyu H, Asano T, Iwamoto T, Takasaki M, Shindo H, Abe K. Magnetization transfer measurements of the hippocampus in patients with Alzheimer’s disease, vascular dementia, and other types of dementia. AJNR Am J Neuroradiol 2000;21:1235–42. [PMC free article] [PubMed] [Google Scholar]
- [115].Hanyu H, Asano T, Sakurai H, Takasaki M, Shindo H, Abe K. Magnetization transfer measurements of the hippocampus in the early diagnosis of Alzheimer’s disease. J Neurol Sci 2001;188:79–84. [DOI] [PubMed] [Google Scholar]
- [116].Hanyu H, Shimizu S, Tanaka Y, Kanetaka H, Iwamoto T, Abe K. Differences in magnetization transfer ratios of the hippocampus between dementia with Lewy bodies and Alzheimer’s disease. Neurosci Lett 2005;380:166–9. [DOI] [PubMed] [Google Scholar]
- [117].Kabani NJ, Sled JG, Chertkow H. Magnetization transfer ratio in mild cognitive impairment and dementia of Alzheimer’s type. Neuroimage 2002;15:604–10. [DOI] [PubMed] [Google Scholar]
- [118].Kabani NJ, Sled JG, Shuper A, Chertkow H. Regional magnetization transfer ratio changes in mild cognitive impairment. Magn Reson Med 2002;47:143–8. [DOI] [PubMed] [Google Scholar]
- [119].Eckert T, Sailer M, Kaufmann J, et al. Differentiation of idiopathic Parkinson’s disease, multiple system atrophy, progressive supranuclear palsy, and healthy controls using magnetization transfer imaging. Neuroimage 2004;21:229–35. [DOI] [PubMed] [Google Scholar]
- [120].Tambasco N, Pelliccioli GP, Chiarini P, et al. Magnetization transfer changes of grey and white matter in Parkinson’s disease. Neuroradiology 2003;45:224–30. [DOI] [PubMed] [Google Scholar]
- [121].Wolff SD, Balaban RS. Magnetization transfer contrast (MTC) and tissue water proton relaxation in vivo. Magn Reson Med 1989;10:135–44. [DOI] [PubMed] [Google Scholar]
- [122].Wolff SD, Eng J, Balaban RS. Magnetization transfer contrast: method for improving contrast in gradient-recalled-echo images. Radiology 1991;179:133–7. [DOI] [PubMed] [Google Scholar]
- [123].Cohen-Adad J, El Mendili MM, Lehericy S, et al. Demyelination and degeneration in the injured human spinal cord detected with diffusion and magnetization transfer MRI. Neuroimage 2011;55:1024–33. [DOI] [PubMed] [Google Scholar]
- [124].Elkin BS, Elliott JM, Siegmund G. Whiplash injury or concussion? A possible biomechanical explanation for concussion symptoms in some individuals following a rear-end collision. J Ortho Sports Phys Ther 2016;46:874–85. [DOI] [PubMed] [Google Scholar]
- [125].De Leener B, Levy S, Dupont SM, et al. SCT: spinal cord toolbox, an open-source software for processing spinal cord MRI data. Neuroimage 2017;145(pt A):24–43. [DOI] [PubMed] [Google Scholar]
- [126].Fonov VS, Le Troter A, Taso M, et al. Framework for integrated MRI average of the spinal cord white and gray matter: the MNI-Poly-AMU template. Neuroimage 2014;102(pt 2):817–27. [DOI] [PubMed] [Google Scholar]
- [127].Levy S, Benhamou M, Naaman C, Rainville P, Callot V, Cohen-Adad J. White matter atlas of the human spinal cord with estimation of partial volume effect. Neuroimage 2015;119:262–71. [DOI] [PubMed] [Google Scholar]
- [128].Martin AR, De Leener B, Cohen-Adad J, et al. 163 Microstructural MRI quantifies tract-specific injury and correlates with global disability and focal neurological deficits in degenerative cervical myelopathy. Neurosurgery 2016;63(Suppl. 1):165. [Google Scholar]
- [129].Jensen MC, Brantzawadzki MN, Obuchowski N, Modic MT, Malkasian D, Ross JS. Magnetic-resonance-imaging of the lumbar spine in people without back pain. New Engl J Med 1994;331:69–73. [DOI] [PubMed] [Google Scholar]
- [130].Li SY, Suyou LT, Chen J, et al. Comparison of Modic changes in the lumbar and cervical spine, in 3167 patients with and without spinal pain. PLoS ONE 2014;9. [DOI] [PMC free article] [PubMed] [Google Scholar]