Abstract
Mobilization of mechanical ventilation patients has broadened to include patients requiring prolonged mechanical ventilation (PMV). A previous systematic review outlined methodological flaws in the literature. The purpose of this integrative review is to evaluate existing publications to determine if mobilization interventions in PMV patients improve physical function, weaning rates, pulmonary mechanics, and hospital outcomes. An electronicsearch covering 2005–2016, included five bibliographic databases: CINHAL, PubMed, PEDro, EMBASE, and Web of Science. Key terms: PMV, mobilization, therapy, and rehabilitation. Eight research studies were identified; 3 RCT’s, 3 medical records reviews, 1 prospective cohort, and 1 undefined prospective interventional. Improvements in functional status, shorter duration of mechanical ventilation and hospitalization, decreased mortality, and superior 1-year survival rates in mobilized PMV patients were reported. Persistent methodological limitations impair the ability to determine if these outcomes were the result of improvements in pulmonary mechanics, overall functional status, or a combination of both.
Keywords: Prolonged mechanical ventilation, Mobilization, Review
Introduction
Scientific and technologic advances in medicine have resulted in the ability of the medical community to prolong life. One consequence of life-extending advancements in technology is the increasing numbers of patients requiring prolonged mechanical ventilation (PMV). Prolonged mechanical ventilation has been defined by the National Association for Medical Direction of Respiratory Care (NAMDRC) as the need for 21 days, or more, of consecutive mechanical ventilation for six or more hours a day.1 United States reports indicate that the number of PMV patients increased 5.2% between 2006 and 2008 and estimate that by 2020, approximately 625,298 patients will require PMV in the U.S. alone.2 However, the burgeoning PMV population is not isolated to the United States and is a trend noted globally. A Canadian national survey of intensive care units reported that patients who require PMV occupied 11% of the 2710 available ventilator-capable beds in Canada at the time of the study.3 From 1997 to 2007, researchers reporting on national trends in Taiwan reported 50,481 PMV patients.4 The conclusion from these statistics that the PMV population will continue to expand is incontrovertible.
The negative consequences of bed rest associated immobilization, previously felt to be necessary for patients undergoing mechanical ventilation, are widely recognized. Known complications can include: neuromuscular dysfunction5 and mechanical unloading,6 joint contracture,7 thromboembolism,8,9 atelectasis,9 insulin resistance,10 and pressure ulcers.11 Recognition of skeletal muscles weakness and the various other sequela that can result from immobility has resulted in a concentrated effort to study mobilization interventions aimed at improving functional status in the mechanical ventilation population. Mobilization in critical care is defined as “an interdisciplinary, goal-directed therapy used to facilitate movement and improve outcomes that involved energy expenditure”.12 Mobilization strategies employed by researchers are heterogenous in nature. A 2013 meta-analysis reported on a wide range of mobilization activities including: arm exercises, bed to chair transfers, standing training, ambulation, trunk control training at edge of bed, and ergometry training.13 Multiple researchers report reductions in ICU and hospital length of stay,14,15 improvements in strength and functional status,16,17 and reduction in the duration of mechanical ventilation weaning15 as a result of early critical care mobilization interventions such as sitting and walking with assistance. Importantly, completed feasibility studies have found mobilization of respiratory failure patients safe.18,19
Researchers in the aforementioned studies focused on short-term mechanical ventilation weaning (less than 21 consecutive days) and mobilization. However, researchers have applied many of these same constructs to the PMV population. Inspiratory muscle training20–23 and skeletal muscle training programs,24 both with and without electrical stimulation,25 have all been studied in the PMV population with varying degrees of success. While many of the studies and techniques aimed at the PMV population have potential as early interventions, a previously published literature review identified that most are burdened by substantial limitations that included small sample sizes, methodological limitations with a lack of control groups, and instrumentation reliability and validity concerns.26 Therefore, the purpose of this integrative review is to evaluate the strength of existing publications to determine if active mobilization interventions in PMV patients improves physical function, ventilator weaning rates, pulmonary mechanics, and clinical hospital outcomes such as length of stay and mortality. Additional goals are to evaluate for methodological improvement in the PMV mobility literature, as well as identify potential gaps in the research.
Methods
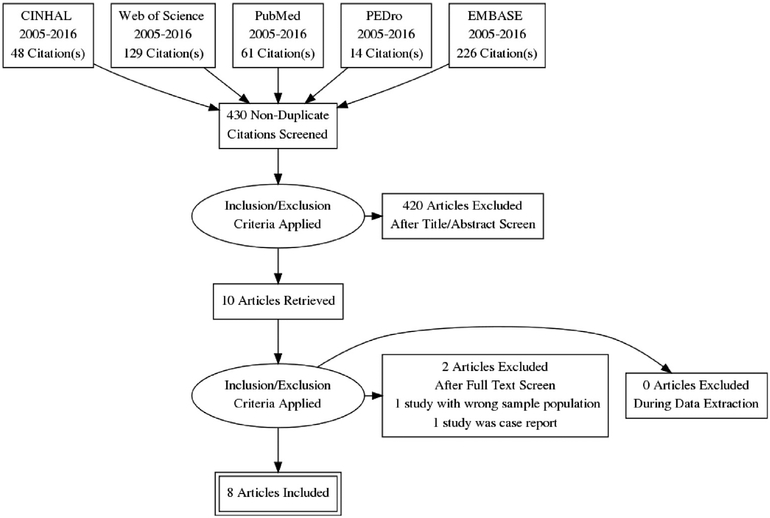
Focus on PMV and active mobilization interventions published after the literature review of the same topic published by Choi, Tasota, and Hoffman in 2008,26 guided the literature search. Choi, Tasota, and Hoffman26 included in their review research studies published from January 1990 to July of 2007. To ensure that quality research studies were not overlooked as the result of potential publication lag, a two-year overlap was allowed in our integrative review of the literature. Thus, this review of the literature covered an 11-year period, January 2005 to December 2016. Researchers completed an electronic search that included five bibliographic databases: CINHAL, PubMed, PEDro, EMBASE, and Web of Science. Initial key terms used included: “prolonged mechanical ventilation” and “mobilization”. This search resulted in 2 papers, both case study, for review.27,28 Therefore, key terms were broadened to include “therapy”, and “rehabilitation” which were used in various BOOLEAN combinations with search restrictions included a limitation to adults, human research, and inclusive dates of January 2005 to December 2016. This database search resulted in 478 potentially relevant studies for review (Fig. 1). After completion of each database search, records of interest were exported to Ref-Works web-based bibliographic management system, where duplicates were removed. Removal of duplicate records resulted in 430 potentially relevant studies, which were screened for inclusion via title and abstract review using predetermined inclusion and exclusion criteria. Title and abstract screen resulted in the removal of 420 additional records, resulting in ten articles retrieved for full article review.
Fig. 1.
Search strategies for identification of included studies.
Eligibility criteria include primary source material in the 11-year time frame referenced above which includes randomized control studies, controlled clinical trials, cohort studies, and observational studies, patients on PMV, active mobilization therapy, adult research, human research, and studies published in English. Exclusion criteria included abstracts, secondary and tertiary source material including review articles, meta-analysis, practice guidelines/protocols, unpublished research including dissertations, case study reports, pediatric research, research that relied on respiratory muscle training exclusively as the intervention (non-skeletal mobilization therapy), and animal research. For the purposes of this review, skeletal mobility intervention is defined as an activity in which the primary purpose is to prevent or reverse the complications of immobility, produces movement of the body, and requires energy expenditure on the part of the patient.12
The methodological framework suggested by Whittemore and Knafl29 was used for this integrative review as it allows for the inclusion of experimental, quasi-experimental, and non-experimental methodologies in a review of the evidence to more fully understand the phenomenon of mobilization activities in the PMV population.
A structured matrix form was used to facilitate data abstraction.30 Sackett’s levels of evidence scores were independently assigned to each extracted article at the completion of abstraction by the primary author and co-author (EC). Sackett’s levels of evidence scores were then compared for congruence. Sackett’s level of evidence scale measures overall scientific rigor of a study on a 1–5 ordinal scale.31 Scores of 1, 2, and 3 are further subdivided into categories a and b. Research studies of the highest scientific merit and quality are rated a 1a, these are systematic reviews of randomized controlled trials. At the opposite end of the spectrum, research studies relying on expert opinion are rated with the lowest score possible, a 5. Sackett’s level of evidence scale was used to evaluate methodological quality as it can be applied to a full range of experimental and non-experimental research designs.
Results
Using the search methods described above, 10 studies were selected for full article review. During full article review, 2 research studies did not meet inclusion criteria and were excluded. This integrated review has a final sample of eight research studies that met all inclusion and exclusion criteria (Fig. 1). Additional manual ancestral searching of the reference lists of the included studies identified in the electronic search was completed, which identified no additional studies for consideration. Each research study was evaluated in chronological order using a structured abstraction matrix.30 Extracted data include authors and corresponding country of origin, publication year, methodology/study design including sample size, sample characteristics, dependent variables with associated measurement instruments, independent variables, major findings, and limitations (Table 1).
Table 1.
Summary of included studies.
| Authors/Yr/Country | Methodology study design sample size | Sample characteristics | Dependent Variable(s)/Measure | Independent Variable(s) | Major findings/Outcomes | Limitations | Sackett’s/PEDro score |
|---|---|---|---|---|---|---|---|
| Martin et al, 2005 United States | Retrospective Medical record review N = 49 | Average age of sample was 58.5 ± 7 years Patients were ventilated for ≥ 14 consecutive days, and at least two failed weaning attempts prior to start of study Patients were intubated for 18.1 ± 7.7 days (range 9–29 days) before Tracheostomy Average admission APACHE II score was 20 | Pulmonary mechanics: - RSBI - NIFF Peripheral muscle strength: - 5-Point muscle strength score Function status: - FIM Weaning status: - Tolerance of 48 h of unassisted breathing |
Whole body rehabilitation program: - Conducted by physical therapists - Progressed from one session per day to two sessions per day once able to consistently tolerate > 45 min in one session |
Time to wean from mechanical ventilation, measured from the initial day of admission, was 16.0 ± 9 days 15 (30%) of patients weaned in ≤7 days Patients who weaned ≤7 days had higher upper limb motor strength scores than patients who took ≥7 days to wean, difference was not statistically significant All patients had significant improvement in FIM scores (admit score 1, discharge score 3) All patients were bed-bound at the time of admission; ambulation went from 0 to 52 ± 18 feet for the group (p = .005). A total of 40 patients (81%) were able to ambulate at the time of discharge Improvement in respiratory muscle strength evidenced by significant increase in maximal NIF (admission 24, discharge 35) A significant correlation was found between upper limb motor strength at the time of admission and time to wean (R = 0.72, R2 = 0.54, p < .001) On admission, the RSBI was <105 in 40% of patients. On the day of successful weaning, only 63% of patients had an RSBI <105 Three variables were found to be statistically significant in terms of predicting weaning time: upper motor strength (−7.1, SE 1.19, t −5.9, p = .001), exposure to neuromuscular blocking (4.6, SE 2.14, t 2.2, p = .03), and systemic steroids (4.8, SE 1.76, t 2.7, p = .0092) |
Retrospective analysis of data collected prospectively without a control group Sample is from a single facility Reliability and validity of 5-point muscle strength score not discussed Strength was not objectively quantified Did not perform routine electromyograms to rule out the presence of critical illness polyneuropathy |
4 |
| Chiang et al, 2006 Taiwan | Single-blinded, non-randomized prospective, repeated measures design. N = 32 (n = 17 treatment group |
Average age of control group was 79 (72.5 −82.8), average age of treatment group was 75 (63.0–80.3) All patients received mechanical ventilation via a tracheostomy tube All patient had received mechanical ventilation for > 14 days prior to start of study Strength of the 3 tested muscle groups were the same at baseline in both groups. |
Pulmonary mechanics: - PImax - PEmax - TLC Limb muscle strength: - Upper and lower extremity muscle strength via hand-held dynamometer Weaning status: - Time in hours off ventilator Functional status: - BI - FIM - 2-min walk test (only if off ventilator) |
Physical training program - Bedside upper extremity strengthening exercises including ROM exercises and use of weights - Bedside lower extremity strengthening exercises including ROM exercises and straight leg raises - Initially exercises were performed in supine position, progressed to seated position as tolerated - Progressed to side-to-side turning in bed, transfers from bed to chair, standing, and eventually, ambulation |
Limb strength increased in the treatment group (p = .001) at the 3rd and 6th weeks compared with baseline. After 3 and 6 weeks of physical training, strength of all 3 muscle groups was greater in the treatment group than in the control group (p = .05) Limb strength in control group deteriorated at both 3rd and 6th weeks of the study period compared with baseline (p < .05) PI max was greater in the treatment over control group at 6 weeks (p < .05); PEmax was greater in the treatment group over the control group at both 3 and 6 weeks (p < .05). After 6 weeks of physical training, BI and FIM scores were greater in the treatment group than in the control group (BI scores control group 0, treatment group 35 and total FIM score control group 26, treatment group 49; p < .05) All subjects at the time of enrollment into the study were unable to walk. After 6 weeks of physical training, 53% of the subjects in the treatment group regained their ambulation ability After 6 weeks of intervention, the average distance walked during the 2-min walk test was 42.9 ± 12.7 m (n = 9) in treatment group All subjects in control group remained bedridden and none could ambulate at the end of the 6-week study period Ventilator-free hours improved in the treatment group (p < .01) to an average of 8.9 h and increased to 4.8 h in the control group, however this change was not statistically significant (p = .10). There were no between group differences. At the end of the 6-weeks study period, 8 patients (47%) in the treatment group and 3 patients (20%) in the control group could tolerate unassisted breathing for at least 12 h |
Wide variety of patients with different diagnoses and etiologies Lack of randomization, patients were assigned in an alternating fashion to the treatment group of the control group, possible source of systematic bias Sample is from a single facility Small sample size |
2a/4 |
| Yang et al, 2010 | Prospective controlled clinical trial N = 126 (n = 55 treatment group and n = 71 control group) | Average age of sample was 68.9 ± 16.1 years Patients were ventilated for 20.4 ± 6.6 days before entering study Only 71(56%) of the sample had a tracheostomy at the start of the study Average admission APACHE II score was 16.4 ±6.5 Baseline difference in APACHE II severity of illness scores (χ2 = 2.23, p = .028), indicating that the severity of illness scores were lower in the therapy group than the non-therapy group (15.0 vs 17.5). Baseline differences also noted in BI score (t = 3.00, p = .004), indicating that mobility was higher in the therapy group than the non-therapy group (0.6 vs 0.2) |
Pulmonary mechanics: - RSBI Functional status: - BI |
Physical therapy - One session per day - Included abdominal breathing exercises, respiratory muscle weight training, passive and active joint exercises, upper and lower limb exercises, progressive mobility training. Patients averaged 14.8 ± 7.9 days of therapy |
RSBI before therapy was 75.7 ± 37.9, and after therapy was 80.0 ± 48.5, this was not significant (t = 0.540, p = .57), indicating that therapy did not have a significant impact on RSBI No significant difference in weaning rates between therapy group (32 ± 58.2) and control group (29 ± 40.9) at completion of study (t = 3.73, p = .054) There was a significant increase in BI in the therapy group increased from 0.8 ± 1.4, to 1.9 ± 2.5 at completion of therapy program (t = 0.004, p < .005). Therapy was positively associated with improved mobility Passive range of motion was most frequently provided therapy intervention (n = 37, 23.7%), only 18 patients (11.5%) progressed to progressive mobility |
No discussion of how patients were assigned to intervention or control group Sample is from a single facility Heterogenous sample population Most of exercises in the therapy group involved only passive range of motion of upper extremities, this could possibly explain lack of significant result |
2b |
| Chen, S. et al, 2011 Taiwan | Randomized control trial, prospective, repeated measures N= 34 (n = 18 treatment group and n = 16 control group) |
Average age of control group was 79 (72.5 −82.8), average age of treatment group was 75 (63.0–80.3) All patient had received mechanical ventilation for > 14 days prior to start of study, requiring mechanical ventilation for at least 6 h/day and had been attempting a spontaneous breathing trial each day All patients were tracheostomized prior to start of study |
Functional status: - FIM - BI 1-year survival rate Weaning status not defined |
Physical training program - Followed by independent exercise continuation - UE and LE strengthening - Active chair transfer and maintenance of sitting position for at least 20 min/day - Standing-ambulating with assist devices as needed |
In the rehab group FIM scores improved: Total FIM scores baseline 34 (30.3 −38.3); 6 weeks 49 (45–66.3); after independent therapy 78 (62 −126) Motor domain baseline 4.5 (13 −18.3); after independent therapy 47 (34–91) Cognitive domain baseline 19.5 (16.5–20.3); after independent therapy 33 (28–35) FIM scores remained unchanged for the control group The eating, comprehension, expression, and social interaction subscales reached the 7-point complete independence level at 6 months in the rehabilitation group, but not in the control group The 1-year survival rate for the rehabilitation group was 70%, which was significantly higher than the 25% for the control group (p = .0151) Five patients in the therapy group were discharged from the hospital during the one-year study, while only one was discharged in the control group No significant differences between groups for the percentage of survivors freed from mechanical ventilation after training or at the three follow-up times |
Sample size was relatively small Sample is from a single facility At the 3rd follow-up, there were only four patients in the Ctrl group and 11 patients in the Rehab group. The uneven sample size may have had an impact on the statistical analysis of the final data collection time point Entry criteria required that patients had undergone mechanical ventilation for at least 14 days. However, no restriction was placed on the duration of mechanical ventilation, which varied considerably in the study Lacked compliance records from caregivers for independent exercise training in the treatment group |
2a/5 |
| Clini et al, 2011 Italy | Prospective cohort study N = 77 | Average age of sample was 75 ± 7 years Male predominated sample (n = 46 60%) Average admission APACHE II score was 11.5 ± 4.4 Mean short term ICU hospitalization prior to admission to rehabilitation center was 24 days with SD ± 3 days Mean onset of acute respiratory failure was 24 days (range 18–29 days) Mean tracheostomy day was day 16 (range 12–21 days) No patients at the time of admission could tolerate a 2-h spontaneous breathing trial Mean length of hospitalization in the rehabilitation center was 51 days (range 12–115 days) |
Functional status: - BADL - FIM - 6-Point Kendall muscle testing scale Pulmonary mechanics: - PImax - PEmax Weaning status: Successfully weaned when could tolerate spontaneous respiration for at least 7 consecutive days |
Physical training program - Started 48 h after admission - Began with active movement of the limbs - Progressed to active muscular intervention consisting of trunk control, maintenance of body posture, and both upper and lower limb activities to facilitate transfer from bed to chair and standing up - As soon as possible patient begin to use a wheeled walker - Supported or unsupported limb training was incremented daily |
The mean ± SD Δ BADL was 2.5 ± 2.0 points for the entire cohort Statistically significant difference in survival and weaning rate between the groups: The group with the lowest Δ BADL had the worst clinical outcome 64 of the 77 patients improved in at least one BADL activity. Patients with less improvement or no change were less likely to be weaned and to live All the patients in the ΔBADL >2 category survived, and over 80% of them (51% of total) successfully weaned There were 67 respiratory-ICU survivors, and at respiratory-ICU discharge 23 patients went home, 15 were transferred to a medical ward, and 29 were transferred to a rehabilitation unit |
A single-center observational study Small sample size No control group |
4 |
| Chen, Y-H. et al, 2012 Taiwan | Randomized, single-blinded, prospective, repeated measures design. N = 27 (n = 12 treatment group and n = 15 control group) |
Average age of treatment group was 64.9 ± 21.3 and average age of control group was 66.5 ± 18.7 Significant difference in pulmonary mechanics between groups prior to intervention larger Vt in control group (230 ± 95.6 vs 143.6 ± 79.4) |
Pulmonary mechanics: - Vt - MV - PImax - RSBI - f, breaths/min Functional status: - BI - FIM Hospitalization outcomes: - LOS - Ventilator weaning rate: (Weaning from ventilation considered successful if subject free from ventilation continuously for > 5 days) - # of Total ventilator days Mortality rate: |
Exercise training program - Cardio/pulm endurance training with cycle ergometer - Muscle strength training with resisted arm activities with weights and weighted sand bag on abdomen for respiratory muscle training - Stretching exercises of cervical, upper limb, and upper chest |
After exercise intervention, treatment group had significant improvement in Vt(192.5 ± 75.0), while the control group was essentially unchanged (257.6 ± 125.1) Mid-study RSBI was decreased, when compared to pre-study measurements in the treatment group (110.6 ± 31.5 vs 162.2 ± 70.1), while essentially unchanged in the control group (123.1 ± 56.2vs 136.2 ± 48.8) In the treatment group, both FIM (28.1 ± 14.2 vs 44.6 ± 10.0, P = .005) and BI(4.3± 9.5 vs 19.3 ± 18.6, P = .004) scores improved after the exercise intervention. These were statistically significant for FIM, but not BI. Subjects in the training group had a higher weaning rate (75% vs 53.3%) and a lower mortality rate (0% vs 20%) during their hospitalization Patient in the training group had fewer days of mechanical ventilation than those in the control group, although not statistically significant. (32.7 ± 23.4d vs 54.6 ± 46.2d. p = .15) The mean Los for subject in the training group (36 d)was shorter than that for the control group(57 d) |
Small sample size Sample is from a single facility Baseline differences in some pulmonary mechanics measures between groups Did not account for the severity of illness, length of previous ICU stay, or etiologies for requiring PMV |
lb/6 |
| Patmanetal, 2012 Australia | Retrospective Medical record review N = 190 | Mean age was 52 ± 18 years Male predominated sample (n = 126 66%) Average APACHE II score was 20 ± 8 Median quartile ICU LOS 14 (10) days Median quartile acute care LOS 42 (38) days |
Functional Milestones: - Sit - Stand - Ambulate |
On discharge from acute care, 89 (47%; 95% CI, 40%−54%) were ambulating independently, of whom 54 (61%) did not require a gait aid 183 patients (96%) sat out of bed during their acute care stay. Of these. 124 (65%) achieved this milestone during the ICU admission Median time between admission to the ICU and when the patient first sat out of bed was 13 (8) days 163 patients (86%) stood during acute care stay. Of these. 42 (22%) achieved this during the ICU admission Median time between admission to the ICU and when the patient first stood was 19 (19) days 155 patients (82%) walked during their acute care stay. Of these. 15 (8%) achieved this during the ICU admission The median time between admission to the ICU and when the patient first walked was 23 (21) days Compared with those who stood within 30 days of admission to ICU. a delay in standing of between 30 and 60 days increased the odds of not being able to ambulate independently at the time of discharge 5-fold (95% CI. 2–11) A delay in standing of greater than 60 days increased the odds of not being able to ambulate independently at the time of acute care discharge 28-fold (95% CI, 6–122) |
Limited by retrospective data collection Sample is from a single facility No intervention Dependent variables measured at the nominal level resulting in potential loss of statistical power |
4 | |
| Hill etal, 2013 Australia | Retrospective Descriptive N = 181 | Mean age was 52 ± 19 years 22 patients (12%) did not survive the 12-month period after discharge from the acute care facility |
Mortality: - Death in the 12 month period after discharge from hospital Morbidity: - Hospital admission rate after discharge from hospital Functional Milestones: - Sit - Stand - Ambulate |
Those who died were older (50 [32] vs 69 [16] years, p = .001). had higher APACHE II scores (19 [10] vs 24 [9]. p = .008), and a longer hospital length of stay (40 [38] vs 64 [61] days, p = .005) In the 12-month period prior to the study. 66 (36%) patients had at least 1 admission and 11 (6%) had been admitted to an ICU and received MV. 2 (18%) had been mechanically ventilated for 7 days or longer. Median number of admissions was 2, median length of stay was 11 days In the 12-month period after discharge from the hospital, 148 (82%) patients had at least 1 admission and 17 (9%) had been admitted to an ICU and received MV, 7 (41%) had been mechanically ventilated for 7 days or longer. Median number of admissions was 2 and the median length of stay was 40 days With patients grouped according to functional status (independent ambulation vs dependent ambulation) there was no difference in the proportion of people who survived vs deceased over the 12-month period (χ2 = 0.023, P =.880) No difference when patients first sat out of bed (13 [8] days vs 12 [6] days, P = .282) or stood (19 [19] days vs 18 [23] days, P = .663) between those who survived and those who had deceased A longer time lapse between admission to the ICU and when the patient first stood was associated with greater health care use in the 12 months after the index admission When compared with those who could ambulate independently at the time of discharge, those who could not ambulate had 81% more admissions in the subsequent 12-month period |
Retrospective data collection methods Sample is from a single facility May lack statistical power for some of the analyses |
4 |
APACHE II + Acute Physiology and Chronic Health Evaluation; PImax = Maximum inspiratory pressure; PEmax = Maximum expiratory pressure; Vt = Tidal Volume; RSBI = Rapid Shallow Breathing Index; MV = Minute Volume; f, breaths/min = respiratory rate; TLC = Total Lung Capacity; NIFF = negative inspiratory force; FIM = Function Independence Measurement; BI = Barthel Index; BADL = Basic Activities of Daily Living; LOS = length of stay.
Study characteristics
Methodological designs were limited to two main types: randomized control trials and non-experimental, retrospective medical record review studies (Table 2). Clini E., et al was the one exception, using a prospective cohort design.32 Yang et al did not describe methodological procedures fully; the study was described as being prospective with an intervention and control group.36 Wide ranges of sample sizes in the included studies are noted (Table 2). Reported samples from the randomized control trials included in this synthesis were smaller (n = 27–34) than the other studies (n = 32–190). Researchers also reported a broad range of ages in the sample populations of the included studies (Table 2). The randomized control trials had the oldest patients.34,35 Importantly, advanced age did not limit functional outcome improvements or improvements in cognitive function. Significant improvements in Functional Independence Measurement Scale scores after completion of study specific therapy sessions were reported in those studies with older patients.34,35 Finally, when reported, there was a range of Acute Physiologic Assessment and Chronic Health Evaluation II score (APACHE II) baseline scores, from 11.5 to 21 (Table 2). APACHE II scores are a highly reliable and valid measure of severity of disease; an integer score from 0 to 71 is computed with higher scores representing increasing disease severity and higher rates of in-hospital mortality.41 Increasing disease severity did not negatively impact functional improvements in the sampled articles reviewed. Significant improvements in physical function at the completion of the respective studies were reported in those studies who enrolled patients with the highest average APACHE II scores.38,39 Overall, however, the average APACHE II scores reported by the authors of these eight studies were relatively low (Table 2), a possible reflection of the fact that PMV patients have survived their acute illness and have entered into a period of physiologic stability during their prolonged hospitalization.
Table 2.
Characteristics of included studies.
| Study design | Number |
|---|---|
| Randomized control trials | 3 |
| Retrospective medical records review | 3 |
| Prospective cohort | 1 |
| Prospective, unknown design | 1 |
| Sample characteristics | |
| Mean Sackett’s score | 2.875 (1b–4) |
| Mean PEDro score | 5 (4–6) |
| Mean sample size | 90 ± 63 subjects (27–190) |
| Age range | 52–79 years |
| APACHE II baseline score range | 11.5–21 |
APACHE II - Acute Physiologic Assessment and Chronic Health Evaluation II score.
Several studies struggled with lack of group equivalence despite reporting random assignment of study subjects. Chen, Y-H., et al reported nonequivalent groups with regards to pulmonary mechanics at the onset of the study.33 Additional group nonequivalence exists in regards to heterogeneity in the method of mechanical ventilation between the studies, as not all patients underwent tracheostomy before enrollment in the sampled studies. Yang et al36 reported that 56% of patients had been tracheostomized prior to the start of the research study, while all patients in the Martin et al39 and Chiang et al35 had been tracheostomized before enrollment. The presence of a tracheostomy is known to reduce the work of breathing and improves ventilator synchrony, particularly in patients who require PMV.40 The inclusion of a clinically heterogenous mechanically ventilated population, such as in the Yang et al36 study, increases the amount variance present in a study and impacts the ability of the researcher to measure a true intervention effect. Additionally, the lack of group equivalence is a threat to internal validity, a noted limitation in the design of the Yang et al36 study, and a potential explanation for the reported lack of improvement in functional outcomes in this study. Additionally, a wide variety of co-morbid conditions and underlying etiologies of respiratory failure is present in many of the selected research studies.32,33,35–37 Researchers did not indicate if there was an attempt to control for these variations in co-morbid conditions and disease severity, thus raising concerns regarding internal validity.
Sackett’s and PEDro’s levels of evidence
None of the studies extracted for this review scored the highest level of evidence score of 1a as this review was limited to primary source material and excluded systematic reviews. One study included in this review received a score of 1b, the next highest score possible.33 Three extracted articles received a score of 2b.34–36 Half of the extracted articles received a score of 4.32,37–39 Relatively low levels of evidence are present in the final sample of studies. Sackett’s level of evidence scores was used to evaluate methodological quality as only three of the extracted articles are RCT’s and therefore, have assigned PEDro score.33–35 The average PEDro score for the three RCT’s was 5, thus providing additional support for methodological rigor limitations.
Measured outcomes
Functional status
Researchers in all eight studies attempted to measure functional status, making it the most frequently measured outcome variable (Table 1). The Functional Independence Measurement Scale was used in five of the eight studies.32–35,39 The next most frequently utilized measure of functional status was the Barthel Index, used in four of the eight studies.33–36 The randomized control trials used both the Functional Independence Measurement Scale and the Barthel Index.
Ventilator-weaning rates and pulmonary mechanics
The second most frequently studied outcome variables were ventilator-weaning rates and pulmonary mechanics, both measured in six of the eight studies.32–36,39 There is significant heterogeneity in the measures used to assess these outcomes. Criteria used to define liberation from mechanical ventilation was not defined in four of the eight studies.34,36–38 The operational definition of ventilator liberation varied by study. Ventilator liberation was defined as 48 h of unassisted breathing,39 the number of hours free from mechanical ventilation,35 greater than five days of unassisted breathing,33 and at least seven days free from mechanical ventilation.32 In this sample of eight studies, rapid shallow breathing index, maximal inspiratory pressure, and maximal expiratory pressure are the most frequently used measures of pulmonary mechanics (Table 1).
Hospital outcomes
Six of the eight studies attempted to measure hospital outcomes (Table 1). As with ventilator liberation status, hospital outcomes were not operationalized uniformly across studies, making comparison difficult. Hospital discharge rates32,34 and ventilator liberation rates32,33,36,39 were the most commonly used indicators of hospital outcomes. Less commonly, hospital length of stay33 and 1-year survival rates37 were surrogate indicators of hospital outcomes.
Physical mobility training programs
Researchers in the prospective studies used a physical mobility-training program as the independent variable. While the use of a training program was consistent, there was significant variation in the delivery and implementation of the training interventions across studies (Table 3). For example, three of the eight researchers used a five-day per week therapy program.34,35,39 In contrast, Chen Y., et al used a therapy program that varied between four to six days per week.33 There was additional variation in the duration of the program, ranging from 10 sessions33 to 6 weeks34,35 to throughout the entire hospitalization.37,38 Length of individual therapy sessions also varied from a low of 15-min sessions33 to a maximum of 60-min sessions.39 However, 30-min sessions were the most frequently noted therapy session length across all articles describing therapy length.32,36,39 Also noted was a disparity in continuation of mechanical ventilation during therapy intervention35 versus therapy interventions conducted independently of ventilation weaning trials.32 The greatest incongruity identified was in the specific type of exercises employed, with no universally accepted approach. Two of the published articles discussed focus on truncal control and maintenance of posture via the use of resistance bands and low weights as an initial step in the therapy programs.32,39 Upper and lower limb exercises were the most common therapy activities noted, referenced in six of the extracted articles. The only consistent intervention across all studies was the use of weighted resistance of up to 600 gm of weight (Table 3). Less common than weight training is stationary cycle ergometry training, referenced by three researcher’s.32,33,39 Progressive mobility and functional activity were the goals of all therapy programs. However, the specifics of progression from sitting to standing to ambulation is only addressed by three authors.34–36 Respiratory muscle training was included in five of the published articles, but again there was significant variation in the approach. Methods varied from the use of a weighted sandbag placed on the abdomen,33,36 to the utilization of a threshold device,39 to nondescript “diaphragmatic breathing control”.34,35 The lack of homogeneity in therapy intervention was a finding also noted in previous literature reviews by Choi et al.26
Table 3.
Physical mobility program of interventional studies.
| Author year | Number of sessions per week | Length of sessions | Duration of therapy | Use of weights | Mechanical ventilation continued during training |
|---|---|---|---|---|---|
| Martin et al., 2005 | 5 | 30–45 min | Not defined | Yes | Yes |
| Chiang, et al, 2006 | 5 | Not defined | 6 weeks | Yes | Yes |
| Yang, et al., 2010 | 5 | 30 min | 7 – 21 days | Yes | Not defined |
| Chen, S. et al., 2011 | 5 | 20 | 6 weeks | Yes | Yes |
| Clini, et al., 2011 | 6 | 15 sessions minimum | Yes | Conducted independent from weaning trials | |
| Chen, Y-H. et al., 2012 | 4–6 | 30–40 min | 10 sessions | Yes | Not defined |
Effectiveness of physical mobility training programs
Functional status
All researchers reported improvements in functional status at the completion of prospective studies except Yang et al (Table 1). However, Yang, et al not only included a mixed mechanically ventilated population but also reported that the majority of intervention exercises were limited to passive range of motion (23.7%).36 This is contrary to a clinically homogenous tracheostomized population and reliance of active mobilization techniques in the other interventional studies included in this integrated review.32,34,35,39 These noted limitations may explain the lack of improvement reported in the Yang et al study. Researchers in the non-experimental, retrospective studies all reported improvements in patient’s functional status and respiratory muscle status.37–39
Weaning rate
There is marked heterogeneity in the reviewed ventilator-weaning rates, a likely reflection of the variety of clinical indicators used to measure successful ventilator liberation. Of the five researchers who reported on weaning rates, three indicated improvements in ventilator liberation in mobilized patients.32,33,35 Yang et al reported no differences in weaning rates or improvements in pulmonary mechanics, but again this study has noted internal validity limitations.36 Chen et al also reported no differences in weaning rates between groups but did report shorter hospitalizations and a significantly better one-year survival rate (70%) in mobilized patients.34 However, in contrast to these results, the retrospective research by Hill et al reported no one-year survival benefit.38
Pulmonary mechanics
Pulmonary mechanics, as measured by rapid shallow breathing index (RSBI), maximal inspiratory pressure (PI max), and maximal expiratory pressure (PEmax), were used by several researchers as a surrogate indictor of improvements in weaning status.32,35,36,39 Despite consistently reported and significant improvements in PI max and PEmax after physical mobility training programs, this did not always translate into statistical differences in weaning rates or duration.35 As such, RSBI has also been used to monitor improvements in pulmonary mechanics and weaning progression with inconsistent improvements. Martin et al reported statistically significant improvements (p = <0.001) in RSBI at the time of discharge, with admission RSBI’s of 104 and discharge RSBI’s of 80.39 In contrast, Yang, et al36 reported no statistically significant improvement in RSBI between groups (treatment group 88.7 ± 53.9, control group 80.7 ± 33.8, p = .42).
Hospital outcome
Patients who participated in physical mobility programs had shorter hospital lengths of stay,33 lower mortality rates,32,33 and improved one-year survival rates.34 However, this finding was not consistent across all studies examining hospital outcomes with Hill et al reporting no difference when patients first sat out of bed or stood between those patients who survived and those who died.37 Authors attempting to measure hospital outcomes relied on a medley of indicators, varying from hospital length of stay, discharge disposition, and one-year survival rates.32–34,37
Discussion
Overview of findings
This integrative review of eight studies evaluates the strength of existing publications to determine if active mobilization interventions in PMV patients improve physical function, ventilator weaning rates, pulmonary mechanics, and clinical hospital outcomes. All but one researcher reported improvements in functional status and shorter duration of mechanical ventilation.36 However, persistent methodological limitations in the available body of literature impair the ability to determine if improvements in ventilator liberation or mortality were the result of improvements in pulmonary mechanics, or improvements in overall functional status, or perhaps a combination of both.
Physical mobility program
This review of the literature has revealed that there has been little progress made toward the identification of specific mobilization interventions, and lack of homogeneity in defining the mobility intervention, a persistent finding also noted in the previously published literature review.26 Central to the development of beneficial mobility interventions is the availability of reliable and valid instruments to isolate effects of mobilization in the PMV population.26 The Functional Independence Measure (FIM) and the Barthel Index (BI) are not only the most frequently utilized measures to assess functional status in the articles included in this integrative review they are also widely used clinically to monitor outcomes of rehabilitative care world-wide.43,44 However, there have been questions raised about the validity of these measures in the PMV population.45 Recent attention has focused on the importance of using standardized mobility outcomes and the development of new instruments to measure functional outcomes in this challenging population. Researchers created The Function Status Score for the ICU (FSS-ICU) to address the need for critical care population-specific instruments. At this time, however, reliability and validity data are limited.46 The limited availability of mobilization research in the PMV population limits the ability to critically evaluate the reliability and validity of mobility measure. Additional psychometric testing of functional status instruments is needed to be able to identify specific mobility interventions that result in the greatest improvements in functional status and discharge outcomes amongst the PMV population.
Weaning rates
There is a noted lack of consistency in the operationalization of ventilator weaning rates in this review, thus limiting the ability of clinicians to make broader conclusions. This heterogeneity is not only problematic for inter-study comparisons of weaning status, but is also perplexing when considering the NAMDRC published a consensus statement on management of patients requiring prolonged mechanical ventilation in 2005. In this consensus statement, the authors clearly define successful ventilator weaning in the PMV population as complete liberation for mechanical ventilation, or the need for nocturnal non-invasive ventilation only, for seven consecutive days.1 All of the articles included in this review were published in 2005 or later, making the consensus definition accessible for use in ongoing research. Yet, only one researcher utilized the NAMDRC consensus definition.32 Of note, only one study included in this integrated review was conducted in the United States, which is also noted to be the oldest study included in this review.39 This is an indication that the most recent research in mobilization of the PMV population is being conducted internationally.39 It is possible that researchers outside the United States had not reached an international consensus regarding U.S. guideline statements or that the studies were ongoing prior to the definitions being published, thus limiting the application of the NAMDRC definitions. Additionally, the seven-day ventilator-free criterion may prove problematic in modern hospital practices where decreasing lengths of stay are the norm, and researchers may not be able to ensure that their study subjects will remain hospitalized for seven consecutive days post ventilator liberation.
The heterogeneity of weaning rates measures, coupled with the lack of established reliability and validity of pulmonary mechanics in the PMV population, leaves clinicians with a bedside management dilemma of what measure to rely upon as an indicator of success and progression. Recent research of Taiwanese PMV patients has indicated that tidal volume (p = .001) was the best predictor of liberation from prolonged ventilator weaning, while PI max and PEmax were not significant in predicting ventilator liberation (p = <0.055 and p = <0.06).46 RSBI has been noted in the literature to be a poor predictor of successful ventilator weaning in the PMV population.47 Consistent with this finding, Martin et al reports that only 63% of PMV patients in their study had RSBI’s of less than the established criterion indicator of success, 105, at the time of successful ventilator weaning.39 Despite the frequent use of RSBI’s in the PMV literature, inconsistent results raise a question of the utility of rapid shallow breathing index as an indication of improvement in pulmonary mechanics in the PMV population. As noted by Verceles et al,47 additional psychometric testing is needed to analyze the prognostic reliability and validity of RSBI trends in this population. The relationship between improvements in pulmonary mechanics and differences in ventilator-free hours remains unclear at this time.
Hospital outcomes
The multiparameter representation of hospital outcome is perhaps a reflection of the ethical and moral quandary regarding what exactly constitutes a positive outcome in this complex population with documented one-year mortality rates upwards of 52%.48 Health outcomes of the PMV population are a research area where qualitative approaches could be highly beneficial. Interviews and narrative from patients and family members may help to conceptualize the health outcomes of this specific population and more fully define their quality of life following termination of ventilator liberation attempts.
Gaps in the research and future directions
This integrative review of the literature has revealed that methodological and measurement concerns persist in the literature regarding the effectiveness of mobilization therapies on physical function, ventilator weaning rates, hospital outcomes in PMV patients. The non-experimental studies were limited by the retrospective nature of the designs, thus lessening the strength of the conclusions from the results.38,39,42 Randomized control trials remain very limited in number, with only three randomized control trials identified in the literature review.33–35 The randomized control trials suffered from multiple threats to internal validity including small sample sizes, lack of control over multiple co-morbidities, lack of group equivalence, and reliance on outcome measures without proven reliability and validity. These limitations raise concern over the statistical power necessary to detect between group change. Analysis of the Sackett’s levels of evidence and available PEDro scores also confirms the presence of methodological limitations in the published literature. Future multisite, randomized control trials with robust sample sizes, and homogenous sample populations are encouraged.
There is reliance on measures of pulmonary mechanics such as RSBI, PI max, and PEmax in the literature despite known limitations in this population. Future reliability and validity testing of measures of pulmonary mechanics, or the development of new novel measures in the PMV population is crucial. Overall, little progress has been made towards the definition of a successful outcome in this challenging population. Consistent use of the NAMDRC definitions of PMV and successful ventilator liberation will normalize sample populations reducing threats to internal validity and help to improve research quality. Qualitative research to conceptualize and define meaningful health outcomes amongst the PMV population is encouraged.
Finally, the lack of U.S. based research should be addressed. Only one study conducted in the United States, which is also noted to be the oldest study included in this review, was located for this review indicating that most recent research in this area is being conducted internationally.39 The reasons for the lack of U.S. based research are not immediately clear. It is possible that the paucity of U.S. based research is the indirect result of the fragmentation of PMV across a largely private, single-specialty hospital system combined with a complicated multipayer system.49 By comparison, Taiwan (the country that half of the research studies included in this integrative review originated from) has a national health insurance system50 and in July of 2000, implemented an integrated prospective payment program to encourage integrated care of mechanically ventilated patients.51 The level of organization that results from a national health insurance program and integrative payment programs for respiratory failure and PMV patients results in facilitated access to streamlined data, unlike what is available to researchers in the United States. Regardless of the reasons for the paltry representation of U.S. based research, external validity concerns remain regarding the generalizability of data from an international population to a U.S. population secondary to cultural variations in health care delivery. Despite that challenges of the fragmented, largely private hospital systems that prevail in the U.S. research is needed to guide best care practices of this growing population.
Limitations
This integrative review was limited by electronic database review of published literature and did not include unpublished research including dissertations or gray literature. Additionally, the author reviewed only studies published in English. With the known heavy representation of international research, it is possible that high-quality research exists that is published in a language other than English, and therefore does not appear in this review. The small number of identified studies for this review is an additional limitation. The majority of published reports identified in the literature search were case reports of limited methodological quality. Every attempt was made to conduct a comprehensive search, including the assistance of an experienced health-sciences librarian.
The reliance of The Functional Independence Measure, Barthel Index, and RSBI in the sampled studies is a considerable limitation. Use of instruments with known reliability and validity concerns affects the strength of the conclusions that can be drawn from the results. The broader application of study results to clinical application is negatively impacted until methodological advancements in instrumentation can be accomplished.
Conclusion
Based on the evaluation of these eight articles, mobilization may provide an outcome benefit to PMV patients. Certainly, the researchers in these eight studies did not report adverse outcomes, suggesting that mobilization activities are relatively safe interventions for PMV patients. However, methodological rigor is lacking and further research is needed to identify reliable and valid measures in this difficult patient population. The overall effectiveness of mobilization activities for the PMV population is difficult to establish at this time secondary to persistent methodological limitations in the existing literature.
Acknowledgments
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Abbreviations:
- PMV
Prolonged mechanical ventilation
- NAMDRC
National Association for Medical Direction of Respiratory Care
- FIM
Function Independence Measure
- BI
Barthel index
- FSS-ICU
The Function Status Score for the ICU
- BADL
Basic Activities of Daily Living
- LOS
Length of stay
References
- 1.MacIntyre NR, Epstein SK, Carson S, Scheinhorn D, Christopher K, Muldoon S. Management of patients requiring prolonged mechanical ventilation: report of a NAMDRC consensus conference. Chest. 2005;128(6):3937–3954. [DOI] [PubMed] [Google Scholar]
- 2.Zilberberg MD, de Wit M, Shorr AF. Accuracy of previous estimates for adult prolonged acute mechanical ventilation volume in 2020: update using 20002008 data*. Crit Care Med. 2012;40(1):18–20. [DOI] [PubMed] [Google Scholar]
- 3.Rose L, Fowler R,A, Fan E, et al. Prolonged mechanical ventilation in Canadian intensive care units: a national survey. J Crit Care. 2015;30(1):25–31. 10.1016/j.jcrc.2014.07.023. [DOI] [PubMed] [Google Scholar]
- 4.Hung M, Lu H, Chen L, et al. Life expectancies and incidence rates of patients under prolonged mechanical ventilation: a population-based study during 1998 to 2007 in taiwan. Crit Care. 2011;15(2). R107–R107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Jonghe B, Sharshar T, Lefaucheur J, et al. Caring for the critically ill patient. paresis acquired in the intensive care unit: a prospective multicenter study. JAMA. 2002;288(22):2859–2867. [DOI] [PubMed] [Google Scholar]
- 6.Chambers MA, Moylan JS, Reid MB. Physical inactivity and muscle weakness in the critically ill. Crit Care Med. 2009;37(10):S337–46. 10.1097/CCM.0b013e3181b6e974. [DOI] [PubMed] [Google Scholar]
- 7.Clavet H, Hébert PC, Fergusson D, Doucette S, Trudel G. Joint contracture following prolonged stay in the intensive care unit. CMAJ. 2008;178(6):691–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Geerts WH, Pineo GF, Heit JA, et al. Prevention of venous thromboembolism: the seventh ACCP conference on antithrombotic and thrombolytic therapy. Chest. 2004;126(3):338S–400S. [DOI] [PubMed] [Google Scholar]
- 9.Brower RG. Consequences of bed rest. Crit Care Med. 2009;37(10):S422–S428. 10.1097/CCM.0b013e3181b6e30a. [DOI] [PubMed] [Google Scholar]
- 10.McCowen KC, Malhotra A, Bistrian BR. Stress-induced hyperglycemia. Crit Care Clin. 2001;17(1):107–124. 10.1016/S0749-0704(05)70154-8. [DOI] [PubMed] [Google Scholar]
- 11.Cox J Predictors of pressure ulcers in adult critical care patients. Am J Crit Care. 2011;20(5):364–375. 10.4037/ajcc2011934. [DOI] [PubMed] [Google Scholar]
- 12.Amidei C Mobilisation in critical care: a concept analysis. Intensive Crit Care Nurs. 2012;28(2):73–81. 10.1016/j.iccn.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 13.Li Z, Peng X, Zhu B, Zhang Y, Xi X. Active mobilization for mechanically ventilated patients: a systematic review. Arch Phys Med Rehabil. 2013;94(3): 551–561. 10.1016/j.apmr.2012.10.023. [DOI] [PubMed] [Google Scholar]
- 14.Morris PE, Goad A, Thompson C, et al. Early intensive care unit mobility therapy in the treatment of acute respiratory failure. Crit Care Med. 2008;36(8):2238–2243. [DOI] [PubMed] [Google Scholar]
- 15.Malkoc M, Karadibak D, Yildirim Y. The effect of physiotherapy on ventilatory dependency and the length of stay in an intensive care unit. Int J Rehabil Res. 2009;32(1):85–88. 10.1097/MRR.0b013e3282fc0fce. [DOI] [PubMed] [Google Scholar]
- 16.Schweickert WD, Pohlman MC, Pohlman AS, et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet. 2009;373(9678):1874–1882. 10.1016/S0140-6736(09)60658-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brahmbhatt N, Murugan R, Milbrandt EB. Early mobilization improves functional outcomes in critically ill patients. Crit Care. 2010;14(5):321 10.1186/cc9262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bailey PP, Miller R, Clemmer TP. Culture of early mobility in mechanically ventilated patients. Crit Care Med. 2009;37(10):S429–S435. 10.1097/CCM.0b013e3181b6e227. [DOI] [PubMed] [Google Scholar]
- 19.Bourdin G, Barbier J, Burle J, et al. The feasibility of early physical activity in intensive care unit patients: a prospective observational one-center study. Respir Care. 2010;55(4):400–407. [PubMed] [Google Scholar]
- 20.Aldrich TK, Karpel JP, Uhrlass RM, Sparapani MA, Eramo D, Ferranti R. Weaning from mechanical ventilation: adjunctive use of inspiratory muscle resistive training. Crit Care Med. 1989;17(2):143–147. [PubMed] [Google Scholar]
- 21.Martin AD, Davenport PD, Franceschi AC, Harman E. Use of inspiratory muscle strength training to facilitate ventilator weaning: a series of 10 consecutive patients. Chest. 2002;122(1):192–196. doi: S0012-3692(16)46298-7 [pii]. [DOI] [PubMed] [Google Scholar]
- 22.Martin AD, Smith BK, Davenport PD, et al. Inspiratory muscle strength training improves weaning outcome in failure to wean patients: a randomized trial. Crit Care. 2011;15(2). R84–R84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sprague SS, Hopkins PD. Use of inspiratory strength training to wean six patients who were ventilator-dependent. Phys Ther. 2003;83(2):171–181. [PubMed] [Google Scholar]
- 24.Nava S Rehabilitation of patients admitted to a respiratory intensive care unit. Arch Phys Med Rehabil. 1998;79(7):849–854. doi: S0003-9993(98)90369-0 [pii]. [DOI] [PubMed] [Google Scholar]
- 25.Zanotti E, Felicetti G, Maini M, Fracchia C. Peripheral muscle strength training in bed-bound patients with COPD receiving mechanical ventilation: effect of electrical stimulation. Chest. 2003;124(1):292–296. doi: S0012-3692(15)36023-2 [pii]. [DOI] [PubMed] [Google Scholar]
- 26.Choi J, Tasota FJ, Hoffman LA. Mobility interventions to improve outcomes in patients undergoing prolonged mechanical ventilation: a review of the literature. Biol Res Nurs. 2008;10(1):21–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perme CS, Southard RE, Joyce DL, Noon GP, Loebe M. Early mobilization of LVAD recipients: who require prolonged mechanical ventilation. Tex Heart Inst J. 2006;33(2):130–133. [PMC free article] [PubMed] [Google Scholar]
- 28.Kendig TD, Nonaillada J, Ramaker J, Samuels F. Management of a patient with lung metastases requiring prolonged mechanical ventilation (PMV): rehabilitation and recovery of function. Rehabil Oncol. 2008;26(2): 3–14. [Google Scholar]
- 29.Whittemore R, Knafl K. The integrative review: updated methodology. J Adv Nurs. 2005;52(5):546–553. 10.1111/j.1365-2648.2005.03621.x. [DOI] [PubMed] [Google Scholar]
- 30.Gerrard J, ed. Health Sciences Literature Review Made Easy: The Matrix Method. 4th ed. Sudbury, MA: Jones and Bartlett Learning.; 2011. [Google Scholar]
- 31.Sackett DL. Evidence-based Medicine: How to Practice and Teach EBM. 2nd ed. New York; Edinburgh: Churchill Livingstone; 2000. [Google Scholar]
- 32.Clini EM, Crisafulli E, Degli Antoni F, et al. Functional recovery following physical training in tracheotomized and chronically ventilated patients. Respir Care. 2011;56(3):306–313. 10.4187/respcare.00956. [DOI] [PubMed] [Google Scholar]
- 33.Chen Y, Lin H, Hsiao H, et al. Effects of exercise training on pulmonary mechanics and functional status in patients with prolonged mechanical ventilation. Respir Care. 2012;57(5):727–734. 10.4187/respcare.01341. [DOI] [PubMed] [Google Scholar]
- 34.Chen S, Su CL, Wu YT, et al. Physical training is beneficial to functional status and survival in patients with prolonged mechanical ventilation. J Formos Med Assoc. 2011;110(9):572–579. 10.1016/j.jfma.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 35.Chiang L, Wang L, Wu C, Wu H, Wu Y. Effects of physical training on functional status in patients with prolonged mechanical ventilation. Phys Ther. 2006;86(9):1271–1281. 10.2522/ptj.20050036. [DOI] [PubMed] [Google Scholar]
- 36.Yang PH, Wang CS, Wang YC, et al. Outcome of physical therapy intervention on ventilator weaning and functional status. Kaohsiung J Med Sci. 2010;26(7): 366–372. 10.1016/S1607-551X(10)70060-7. [DOI] [PubMed] [Google Scholar]
- 37.Hill K, Dennis D,M, Patman S,M. Relationships between mortality, morbidity, and physical function in adults who survived a period of prolonged mechanical ventilation. J Crit Care. 2013;28(4):427–432. 10.1016/j.jcrc.2013.02.012. [DOI] [PubMed] [Google Scholar]
- 38.Hill K, Dennis DM, Patman SM. Relationships between mortality, morbidity, and physical function in adults who survived a period of prolonged mechanical ventilation. J Crit Care. 2013;28(4):427–432. Accessed 1 October 2015. [DOI] [PubMed] [Google Scholar]
- 39.Martin UJ, Hincapie L, Nimchuk M, Gaughan J, Criner GJ. Impact of whole-body rehabilitation in patients receiving chronic mechanical ventilation. Crit Care Med. 2005;33(10):2259–2265. [DOI] [PubMed] [Google Scholar]
- 40.Diehl JL, El Atrous S, Touchard D, Lemaire F, Brochard L. Changes in the work of breathing induced by tracheotomy in ventilator-dependent patients. Am Journal Respiratory Critical Care Medicine. 1999;159(2):383. [DOI] [PubMed] [Google Scholar]
- 41.Fau KW, Fau DE, Fau WD, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Medicine JID - 0355501. 1022. [PubMed] [Google Scholar]
- 42.Fau MF, Barthel DW. Functional evaluation: the Barthel index. Md state Medical Journal JID - 2985229R. 1201. [PubMed] [Google Scholar]
- 43.Granger CV, Hamilton BB, Keith RA, Zielezny M, Sherwin FS. Advances in functional assessment for medical rehabilitation. Top Geriatric Rehabilitation. 1986;1(3). [Google Scholar]
- 44.Thrush A, Rozek M, Dekerlegand J,L. The clinical utility of the functional status score for the intensive care unit (FSS-ICU) at a long-term acute care hospital: a prospective cohort study [corrected] [published erratum appears in PHYS THER 2013; 92(3):1484]. Phys Ther. 2012;92(12):1536–1545. 10.2522/ptj.20110412. [DOI] [PubMed] [Google Scholar]
- 45.Zanni JM, Korupolu R, Fan E, et al. Rehabilitation therapy and outcomes in acute respiratory failure: an observational pilot project. J Crit Care. 2010;25(2). 10.1016/j.crc.2009.10.010.254-2629p. [DOI] [PubMed] [Google Scholar]
- 46.Yao-Ling L, Wei C, Wei-Erh C, Shuo-Chueh C, Chuen-Ming S. Measurements of weaning index as a predictor of weaning outcome in long-term ventilator dependent patients in Taiwan. In: American Thoracic Society International Conference Abstract. Available at: http://www.atsjournals.org/doi/abs/10.1164/ajrccm-conference.2010.181.1_MeetingAbstracts.A3038; 2010. Accessed January 9 2017. [Google Scholar]
- 47.Verceles AC, Diaz-Abad M, Geiger-Brown J, Scharf SM. Testing the prognostic value of the rapid shallow breathing index in predicting successful weaning in patients requiring prolonged mechanical ventilation. Heart Lung. 2012;41(6): 546–552. 10.1016/j.hrtlng.2012.06.003.7p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kahn JM, Benson NM, Appleby D, Carson SS, Iwashyna TJ. Long-term acute care hospital utilization after critical illness. JAMA. 2010;303(22):2253–2259. 10.1001/jama.2010.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.The Medicare Payment Advisory Committee. Report to the Congress: Long-term Care Hospital Services. Available at: http://www.medpac.gov/docs/default-source/reports/chapter-10-long-term-care-hospital-services-march-2016-report-.pdf?sfvrsn=0; 2016. Accessed January 11 2017. [Google Scholar]
- 50.Wu T, Majeed A, Kuo KN. An overview of the healthcare system in taiwan. Lond J Prim Care. 2010;3(2):115–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu CJ, Chu CC, Chen W, et al. Impact of taiwan’s integrated prospective payment program on prolonged mechanical ventilation: a 6-year nationwide study. Respir Care. 2013;58(4):676–682. 10.4187/respcare.01242. [DOI] [PubMed] [Google Scholar]