Abstract
Knowing how readily the skin produces melanin is invaluable in reducing photochemical and phototherapy overtreatment in dermatology and also in reducing the risk of actinic skin damage and skin cancer from excessive radiant light exposure. The commonly used Fitzpatrick skin type (FST) classification scale is often used to subjectively assess ultraviolet light sensitivity and susceptibility to sunburn following significant sunlight exposure. However, the FST scale falls short in the assessment of nonwhite skin types. Alternatively, commercially available melanin sensor devices, called melanometers, can be used to objectively quantify useful skin parameters such as the epidermal melanin concentration (EMC). This study reviews commercially available melanometers and their use in quantifying epidermal melanin concentration (EMC) and the individual maximum safe radiant exposure (IMSRE) for an individual in clinical, workplace and community settings.
Keywords: Melanin, Melanometers, Epidermis
Introduction
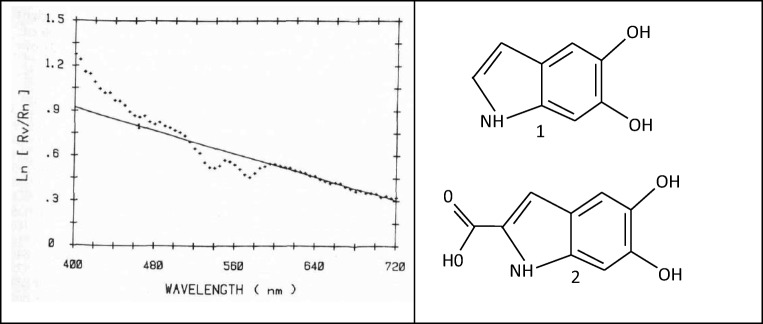
The structure and chemical composition of melanin has yet to be fully characterised. What is known, however, is summarised below. Melanin represents a small group of distinct bio-macromolecules that each has different properties and functional characteristics. In the skin, melanin is composed of a heterogenous mixture of macromolecule polymers that exist either as a brown/black coloured pigment called eumelanin or a yellow-reddish coloured pigment called pheomelanin (Watt et al. 2009). Eumelanin is also called “dark melanin,” and its epidermal concentration is a strong predictor of low skin cancer risk (Nisma et al. 2017). Melanocytes, the melanin-producing cells in the skin, are predominantly found in the stratum basale layer of the epidermis. These cells use tyrosine amino acids as an initial substrate to synthesis eumelanin and pheomelanin in melanosome organelles (Ito 2003). Within melanocytes, eumelanin is stored in eumelanosome organelles whilst pheomelanin is stored in pheomelanosome organelles (Ito 2003; Nakagawa and Imokawa 1996). These organelles are then transferred via dendritic cellular processes and internalised into cells called keratinocytes, which then distribute throughout the epidermis (Nisma et al. 2017). Importantly, not only is there difference in the shape, size and organisation structure between eumelanosomes and pheomelanosomes, but there are also marked variations in the structure between eumelanosome and pheomelanosome across different species (Cheun 2004). It is the broad absorption spectra, shown in Fig. 1a, and the distribution of these two distinct melanosomes within keratinocytes that give the epidermis its significant photoprotective benefits (Kollias and Baqer 1987). This photoprotective activity is limited by the superficial sloughing of keratinocytes rather than its natural half-life of several weeks (Nisma et al. 2017).
Fig. 1.
a A typical absorption spectrum, or apparent absorbance, of human melanin in vivo versus wavelength in nanometers. This was accomplished by calculating the logarithm (base e) of the ratio of the remitted intensity from vitiliginous to normal skin, at adjacent sites of the same volunteer. The straight line represents the best fit through the experimental points in the range 620–720 nm. The correlation coefficient for the straight line is 0.982 (Kollias and Baqer 1987). Reprinted from Journal of Investigative Dermatology, 89, Nikiforos Kollias, Ali H. Baqer, Absorption Mechanisms of Human Melanin in the Visible, 400–720 nm, 384–388, Copyright 1987, with permission from Elsevier. b Chemical structure of the eumelanin monomers 5,6-dihydroxyindole (DHI) (1) and 5,6-dihydroxyindole-2-carboxylic acid (DHICA) (2) (Watt et al. 2009)
During melanogenesis within melanocytes, eumelanin is polymerised from 5,6-dihydroxyindole (DHI) and 5,6-dihydroxyindole-2-carboxylic acid (DHICA) oligomer units, as shown in Fig. 1b (Ito 2003). Additionally, some indole rings are split to give pyrrole rings following reduced or oxidised species of DHI and DHICA. In comparison, pheomelanin is polymerised from benzothiazine and benzothiazole oligomer units (Ito 2003). The contribution of melanin to skin colour is attributable to the concentration and ratio of eumelanin and pheomelanin as shown in Table 1. This ratio to a certain extent depends on the action of dopachrome tautomerase, an enzyme that produces eumelanin from its immediate precursor (Ito 2003). The colour of eumelanin and pheomelanin is dependent upon their constituent oligomers within organelles, the redox reactions, isomeric configurations of these constituent oligomers and the intra- and intermolecular interactions of eumelanin and pheomelanin (Cheun 2004; Ito 2003). Skin type, classically defined according to the Fitzpatrick scale, is determined by the combined effects of melanin, haemoglobin and other pigments such as bilirubin and carotene (Karsten and Smit 2012). Importantly, there are numerous speculations regarding the secondary and tertiary structures of eumelanin and pheomelanin and how these contribute to their complex functions, such as melanin’s broad absorption spectra characteristics (Ito 2003; Watt et al. 2009).
Table 1.
Comparison of melanosome volume fractions found in different skin types when compared with dark skin types using the Fitzpatrick skin type classification (Karsten and Smit 2012). An estimate of the corresponding melanin concentration was made using liquid skin-like phantoms that represent skin types I–VI (Bashkatov et al. 2000; Karsten and Smit 2012)
| Skin type | Estimated Fitzpatrick skin type | Melanosome volume fraction (percentage as compared with dark skin type) | Melanin concentration (mg/mL) |
|---|---|---|---|
| Very fair | I–II | 0.0255 (8.4%) | 2.346 |
| Medium | IV–V | 0.155 (50.8%) | 14.26 |
| Dark | VI | 0.305 (100%) | 28.06 |
Cells and their DNA are protected against radiation-induced damage by melanin due to its ability to absorb ultraviolet light via properties that include broadband monotonic absorbance, extremely low radiative quantum yield and condensed phase electrical and photoconductivity (Meredith and Sarna 2006). The presence of a third form of melanin, neuromelanin, that is predominantly found in the substantia nigra of the brain, further suggests its importance in neurophysiology and disorders such as Parkinson’s disease. Additionally, given the functional versatility of melanin reported in biology, it is unsurprising that there has been much interest in melanin synthesis and its various potential applications in industry and medicine (Watt et al. 2009).
Discussion of melanometers
Optimal outcomes can be achieved during phototherapy when parameters such as laser pulse duration, wavelength, spot size and radiant exposure (RE) are well defined and customised for each patient (Asawanonda et al. 2000; Selvaag et al. 2000; Verkruysse et al. 2009). Of these parameters, RE lacks well established guidelines for phototherapy (Verkruysse et al. 2007) and so is often described in terms of the minimal erythema dose (MED), erythema index (EI) and individual maximum safe radiant exposure (IMSRE) (Asawanonda et al. 2000; Dolotov et al. 2004). In addition, the above parameters have significant interindividual and intraindividual variability that in part arise from variations in epidermal melanin concentrations (EMC) (Verkruysse et al. 2009), different skin pathologies and environmental factors (Dolotov et al. 2004). Such variability in patient responses poses a therapeutic challenge to the dermatologist who must predict both the IMSRE for each patient and attempt to quantify changes in patient EMC during and after topical agents or phototherapy (Bhargava et al. 2016; Sundaram 2014). For example, the severity of the depigmentation disorder, vitiligo, can be clinically evaluated according to the “vitiligo disease activity score” (VIDA) (Bhatnagar et al. 2007). This scoring system evaluates ongoing disease activity and severity based on the appearance of new vitiligo lesions or enlargement of preexisting lesions during a period ranging from less than 6 weeks to 1 year (Bhatnagar et al. 2007; Njoo et al. 1999).
Attempts at standardising EMC measurements have resulted in the creation of a “pigment index” (PI) or “melanin index” (MI) that describes the proportion of melanin to the average concentration of total pigments in the analysed sample skin site (Dolotov et al. 2004). Complicating the matter further, because no standardised guidelines exist in literature, it is technically challenging to calculate the IMSRE using MI values (Verkruysse et al. 2009). Classically, the unmodified FST scale has been used to subjectively classify patient skin types based on colour, tanning ability and propensity to burn following exposure to ultraviolet radiation (UVR). There are also biophysical correlations of the scale with EMC, stratum corneum hydration, wrinkle depth and dermal thickness (Tawakkul and Kerscher 2017). However, the unmodified FST scale has been reported to be unreliable in patients of coloured skin types (Kailas et al. 2017) and does not directly consider the EMC (Eilers et al. 2013). Furthermore, clinically using the subjective scale before and during phototherapy to carefully titrate dosing is intuitively less effective and efficient compared with the use of objective devices that accurately quantify the EMC, IMSRE (Verkruysse et al. 2009), PI, MI and erythema index (Dolotov et al. 2004) in real time (Ash et al. 2015).
To quantify therapeutic responses to phototherapy, commercially available devices could potentially use a variety of techniques that include spectrophotometry (such as infrared remittance spectroscopy) (Verkruysse et al. 2009; Wright et al. 2016), spectrofluorometry (that relies on skin autofluorescence) (Khairallah et al. 2018), diffuse reflectance spectroscopy (Wright et al. 2016), pulsed photothermal radiometry (that requires assessment 24 h after the initial measurement) (Verkruysse et al. 2007) and chromametry (J.-W. Shin et al. 2011). Table 2 lists the different devices and the techniques employed to quantify the melanin concentration of the skin.
Table 2.
Devices available to calculate epidermal melanin concentration (EMC) in skin. Tristimulus reflectance colorimetry quantifies the amount of light reflected through three broad wavelength filters and describes the colour using of three coordinates (XYZ) that are typically converted into other colour systems such as RGB and CIELAB (Van Der Wal et al. 2013)
| Device | Technique | Development and commercial availability | References |
|---|---|---|---|
| DRS probe (R200-7-UV-VIS, Ocean Optics, 830 Douglas Avenue, Dunedin, FL) |
Diffuse reflectance spectroscopy utilising a white light source, optical fibre and spectrometer, and measures the light diffusely reflected by the skin. Values for melanin and erythema are given in mol L−1 |
Commercially available probe | (Wright et al. 2016) |
| CM-2600d (Konica Minolta, Japan) | Remittance spectroscopy at 390 nm at a 15-nm bandwidth with an 8-mm aperture | Commercially available handheld spectrometer | (Verkruysse et al. 2009) |
| iPulse (CyDen Ltd., Swansea, UK) | Remittance spectroscopy at 460 nm | Preproduction handheld prototype consisting of three main components: an optical head, a control and data processing unit, and the power supply unit | (Ash et al. 2015) |
| DSM II and DSMIII ColorMeters (Cortex Technology ApS, 9560 Hadsund, Denmark) | Narrowband tristimulus reflectance spectrophotometry (470, 510 and 660 nm) with a 4 mm aperture. Can directly measure erythema, EMC and CIELAB values | Commercially available handheld colorimeters | (Ash et al. 2015; Van Der Wal et al. 2013) |
| Custom-built photothermal detector device with a PVI-5 probe (Boston Electronics, Boston) and Gentlelase Alexandrite laser (Gentlelase, Candela, Wayland, MA) | Photothermal measurements using an uncooled photovoltaic infrared photodetector PVI-5 probe (spectral bandwidth 2.5 to 5 μm, HgCdZnTe) that was aligned with the hand-piece of a Gentlelase Alexandrite laser (755 nm, 3-ms pulse duration) | Novel custom-built handheld device with a probe that was calibrated prior to each measurement with a commercial blackbody (BB701, Omega) | (Verkruysse et al. 2009) |
| Mexameter (MX18 probe) (Courage-Khazaka, Germany) | Narrowband tristimulus reflectance spectrophotometry (568 nm, 660 nm and 870 nm). Calculates erythema and melanin index values using an arbitrary scale ranging from 1 to 1000, with higher erythema and melanin index readings denoting more erythematous and darker skin or scar. Has a 5-mm aperture | Commercially available as a standalone device or as a MX18 probe that is connected to the company’s phototherapy device | (Bhargava et al. 2016; Matias et al. 2015; Van Der Wal et al. 2013) |
| Colorimeter (CL 400 probe) (Courage-Khazaka, Germany) | Tristimulus reflectance colorimetry using wavelength emissions ranging from 440 to 670 nm. The device interprets reflected light from white light-emitting diodes (LED) expressed in CIELAB system (represented as LAB1, LAB2 and LAB3, respectively) and individual typology angle (ITA) index values | Commercially available | (Matias et al. 2015; Van Der Wal et al. 2013) |
| CR200b and CR-400 chromameters (Minolta, Osaka, Japan) | Tristimulus reflectance spectrophotometry (450, 560 and 600 nm). The Minolta chromameter allows skin colour to be evaluated as a set of three parameters L, A and B in the colour space of CIELAB-system. | Commercially available | (Dolotov et al. 2004) |
| Custom EMM-01 device with three principle components: the optical head, the control/data processing unit and the power supply unit | Tristimulus reflectance colorimetry (560, 650 and 710 nm) | Novel and portable erythema/melanin meter device | (Dolotov et al. 2004) |
| Dermacatch photodetector | Visible-spectrum reflectance colorimeter (full visible light spectrum emission) that measures globally reflected light to compute erythema as well as melanin values (with arbitrary units). The measured area covers a disk of 5.5 mm in diameter | Commercially available | (Baquié and Kasraee 2014) |
| TLS850 Translucency Meter (Dia-Stron, Hampshire, UK) | Reflectance spectrophotometry that can be used to calculate the melanin index using the formula: melanin index = log10(905 nm/632 nm) × 1000 and the erythema index. The manufacturer reports the device’s fibre optic faceplate is insensitive to surface optical effects and only interprets internally scattered light. Measurements can be carried out using single colour modes of red, green or blue from the emission source | Commercially available reflectance spectrophotometer | (Ha et al. 2003; Kuchel et al. 2002) |
The turbidity and optical density of the skin observed during optical analysis is a reflection of its structurally different layers that are composed of the strongly absorbing epidermis with its melanin, the haemoglobin-rich papillary dermis and the diffusely reflecting reticular dermis (Dolotov et al. 2004). In addition, the optical absorption coefficients of the main chromophores in the skin have been well documented (Ash et al. 2015; Edwards and Duntley 1939). Studies have shown that melanin strongly absorbs in the 300–1000-nm spectrum, but mostly so in the near-UV wavelength spectrum (Kollias et al. 1991; Verkruysse et al. 2009). One of the technical difficulties encountered with devices that emit in the near-ultraviolet (UV) spectrum is that their accuracy is significantly affected by absorption of emitted light by haemoglobin (Hb) and oxygenated haemoglobin (HbO2) (Ash et al. 2015; Dolotov et al. 2004; Verkruysse et al. 2009). Absorption of Hb and HbO2 peaks in the ranges of 405–430 nm and 540–580 nm (Dolotov et al. 2004; Verkruysse et al. 2009), but HbO2 also has absorbance greater than 620 nm. The degree of interference by Hb and HbO2 also depends on the local dermal blood flow, smoking and tissue damage (Ash et al. 2015), which is why some studies advocate compression of the skin during measurements (Verkruysse et al. 2009).
To further minimise the interference, techniques have been proposed that emit near-infrared wavelengths (Michel et al. 2013; Verkruysse et al. 2007). One such technique involves narrow-band tristimulus reflectance spectrophotometry that calculates erythema and melanin index values based on differences in light absorption of red and green by haemoglobin and melanocytes, respectively (Van Der Wal et al. 2013). By analysing reflected light after emitting green and red spectrum light, this technique calculates two skin parameters. The first is the cutaneous haemoglobin content, the erythema value, with arbitrary units (Baquié and Kasraee 2014). The second parameter involves the analysis of reflected light from wavelengths in red and near-infrared to calculate skin melanin content, the melanin value, also with arbitrary units (Baquié and Kasraee 2014; Verkruysse et al. 2009). Such calculations typically rely on the linear relationship between EMC and the skin’s optical density in the red spectral range of 620–720 nm (Kollias and Baqer 1986). Published emission wavelengths of tristimulus reflectance colorimetry devices include 560 nm, 650 nm and 710 nm for the EMM-01 erythema/melanin meter (Dolotov et al. 2004), 470, 510 and 660 nm for the DSM II ColorMeter (Cortex Technology ApS, 9560 Hadsund, Denmark) (Ash et al. 2015).
More recently, simpler and encouraging studies use single remittance spectroscopy at single wavelengths such as 390 nm (Verkruysse et al. 2009) and 460 nm (Ash et al. 2015) to accurately predict EMC and IMSRE. Remittance spectroscopy typically quantifies EMC using the MI scale using calculations that rely upon the inverse relationship between EMC and remittance (Dawson et al. 1980; Matts et al. 2007; Stamatas et al. 2004; Verkruysse et al. 2009). Furthermore, the reported accuracy of using this single-wavelength technique is based on the assumption that light with wavelengths of 390 nm is highly scattered within the epidermis in a manner dependent upon the concentration of melanin (Verkruysse et al. 2009). This scattering results in shallow penetration that was found to be only mildly affected by both local dermal blood flow and whether the epidermal sample site was thick or thin. In addition, the remittance at this wavelength is minimally affected by the degree of local tissue oxygenation, since the wavelength represents an isosbestic point for Hb and HbO2 (Verkruysse et al. 2009). Improvement in the accuracy of the 390-nm remittance technique was postulated to result from narrowing the spectra bandwidth (below 15 nm) and the use of compression (diascopy with a UV-transparent windows) during measurements to minimise variations in tissue oxygenation saturation. Other significant advantages of using 390 nm over other wavelengths are its insensitivity to high dermal chromophore concentrations, such as tattoos and vascular lesions and the potential for smaller probe sizes that can reach skin regions such as the ears that are inaccessible to larger probes.
Another novel device includes the use of photothermal measurements as an independent estimate for EMC and IMSRE (Verkruysse et al. 2007). This technique involved the analysis of transient radiometric temperature increase, ΔT(t), after pulse duration, t, of the skin after irradiation with an Alexandrite laser (755 nm, 3-ms pulse duration, 6 J/cm2). ΔT(t) signals with 1-ms temporal resolution up to 1 s after slightly elliptical skin spots of the 18-mm diameter skin were irradiated with a laser at 6 J/cm2. Subsequently, an infrared (IR) detector measured the radiometric temperature increase in a slightly smaller area. At short times t less than 50 ms, ΔT(t) was found to be proportional to the volumetric melanin concentration, EMC. However, the authors of this study reported remittance at a single wavelength of 390 nm provided better estimates of EMC and IMSRE than the above photothermal device due to shallower penetration depths, dermal blood volume fraction and independence of blood oxygen saturation.
Conclusion
Similar to other optical methods, prototype devices need to be calibrated and validated with histochemical methods to express values that reflect absolute EMC values (Matts et al. 2007). There appears to still be plenty of room for new cost-effective portable devices employing other wavelengths or analytical techniques to investigate various clinically relevant skin parameters in dermatology and other fields (Liu and Zerubia 2015). For example, photoacoustic microscopy at 532 nm has demonstrated potential for the in vivo diagnosis of melanoma (Zhang et al. 2010). Lastly, the growing accuracy and versatility of melanin meters will allow their use on patient demographics with skin types that lie outside that typical of Caucasian (Kaidbey et al. 1979; Piérard et al. 2014; Thompson et al. 2018) and Asian (J. W. Shin et al. 2014; Treesirichod et al. 2014) populations (Wright et al. 2016).
Compliance with ethical standards
Conflict of interest
Vangelis George Kanellis declares that he has no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by the author.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Asawanonda P, Anderson RR, Chang Y, Taylor CR. 308-nm excimer laser for the treatment of psoriasis: a dose-response study. JAMA Dermatol. 2000;136(5):619–624. doi: 10.1001/archderm.136.5.619. [DOI] [PubMed] [Google Scholar]
- Ash C, Town G, Bjerring P, Webster S. Evaluation of a novel skin tone meter and the correlation between Fitzpatrick skin type and skin color. Photonics Lasers Med. 2015;4:177–186. doi: 10.1515/plm-2013-0056. [DOI] [Google Scholar]
- Bashkatov AN, Genina EA, Kochubey VI, Stolnitz MM, Bashkatova TA, Novikova OV, Peshkova AY, Tuchin VV (2000). Optical properties of melanin in the skin and skinlike phantoms. Proc SPIE 4162:219–226. 10.1117/12.405946
- Baquié M, Kasraee B. Discrimination between cutaneous pigmentation and erythema: comparison of the skin colorimeters Dermacatch and Mexameter. Skin Res Technol. 2014;20(2):218–227. doi: 10.1111/srt.12109. [DOI] [PubMed] [Google Scholar]
- Bhargava P, Prakash C, Tiwari S, Lakhani R. Correlating melanin index to repigmentation potential: a novel prognostic tool in vitiligo. Pigment Int. 2016;3(2):72–76. doi: 10.4103/2349-5847.196296. [DOI] [Google Scholar]
- Bhatnagar A, Kanwar A, Parsad D, De D. Psoralen and ultraviolet A and narrow-band ultraviolet B in inducing stability in vitiligo, assessed by vitiligo disease activity score: an open prospective comparative study. J Eur Acad Dermatol Venereol. 2007;21(10):1381–1385. doi: 10.1111/j.1468-3083.2007.02283.x. [DOI] [PubMed] [Google Scholar]
- Cheun WL. The chemical structure of melanin. Pigment Cell Res. 2004;17(4):422–423. doi: 10.1111/j.1600-0749.2004.00165_1.x. [DOI] [PubMed] [Google Scholar]
- Dawson JB, Barker DJ, Ellis DJ, Cotterill JA, Grassam E, Fisher GW, Feather JW. A theoretical and experimental study of light absorption and scattering by in vivo skin. Phys Med Biol. 1980;25(4):695–709. doi: 10.1088/0031-9155/25/4/008. [DOI] [PubMed] [Google Scholar]
- Dolotov LE, Sinichkin YP, Tuchin VV, Utz SR, Altshuler GB, Yaroslavsky IV. Design and evaluation of a novel portable erythema-melanin-meter. Lasers Surg Med. 2004;34(2):127–135. doi: 10.1002/lsm.10233. [DOI] [PubMed] [Google Scholar]
- Edwards EA, Duntley SQ. The pigments and color of living human skin. Am J Anat. 1939;65(1):1–33. doi: 10.1002/aja.1000650102. [DOI] [Google Scholar]
- Eilers S, Bach DQ, Gaber R, Blatt H, Guevara Y, Nitsche K, Kundu RV, Robinson JK. Accuracy of self-report in assessing Fitzpatrick skin phototypes I through VI. JAMA Dermatol. 2013;149(11):1289–1294. doi: 10.1001/jamadermatol.2013.6101. [DOI] [PubMed] [Google Scholar]
- Ha T, Javedan H, Waterston K, Naysmith L, Rees JL. The relationship between constitutive pigmentation and sensitivity to ultraviolet radiation induced erythema is dose–dependent. Pigment Cell Res. 2003;16(5):477–479. doi: 10.1034/j.1600-0749.2003.00076.x. [DOI] [PubMed] [Google Scholar]
- Ito S. A chemist’s view of melanogenesis. Pigment Cell Res. 2003;16(3):230–236. doi: 10.1034/j.1600-0749.2003.00037.x. [DOI] [PubMed] [Google Scholar]
- Kaidbey KH, Agin PP, Sayre RM, Kligman AM. Photoprotection by melanin--a comparison of black and Caucasian skin. J Am Acad Dermatol. 1979;1(3):249–260. doi: 10.1016/S0190-9622(79)70018-1. [DOI] [PubMed] [Google Scholar]
- Kailas A, Solomon J, Mostow E, Rigel D, Kittles R, Taylor S, et al. Use of a modified Fitzpatrick scale in the understanding of skin cancer risk among people of color. J Am Acad Dermatol. 2017;76(6):AB276–AB276. doi: 10.1016/j.jaad.2017.04.1073. [DOI] [Google Scholar]
- Karsten AE, Smit JE. Modeling and verification of melanin concentration on human skin type. Photochem Photobiol. 2012;88(2):469–474. doi: 10.1111/j.1751-1097.2011.01044.x. [DOI] [PubMed] [Google Scholar]
- Khairallah G, Amouroux M, Plénat F, Rakotomanga P, Soussen C, Marchal F, Delconte A, Chen H & Blondel W. (2018) Spatially resolved spectroscopy for guiding margin delineation during human skin carcinomas resection: first clinical results on diffuse reflectance and autofluorescence spectra and in vivo skin optical properties, Proc. SPIE, 10685, Biophotonics: photonic solutions for better health care VI, 106851J. 10.1117/12.2309516
- Kollias N, Baqer A. On The assessment of melanin in human skin in vivo. Photochem Photobiol. 1986;43(1):49–54. doi: 10.1111/j.1751-1097.1986.tb05590.x. [DOI] [PubMed] [Google Scholar]
- Kollias N, Baqer AH. Absorption mechanisms of human melanin in the visible, 400-720 nm. J Investig Dermatol. 1987;89(4):384–388. doi: 10.1111/1523-1747.ep12471764. [DOI] [PubMed] [Google Scholar]
- Kollias N, Sayre RM, Zeise L, Chedekel MR. Photoprotection by melanin. J Photochem Photobiol B Biol. 1991;9(2):135–160. doi: 10.1016/1011-1344(91)80147-A. [DOI] [PubMed] [Google Scholar]
- Kuchel JM, Barnetson RSC, Halliday GM. Ultraviolet A augments solar-simulated ultraviolet radiation-induced local suppression of recall responses in humans. J Investig Dermatol. 2002;118(6):1032–1037. doi: 10.1046/j.1523-1747.2002.01773.x. [DOI] [PubMed] [Google Scholar]
- Liu Z, Zerubia J. Skin image illumination modeling and chromophore identification for melanoma diagnosis. Phys Med Biol. 2015;60(9):3415–3431. doi: 10.1088/0031-9155/60/9/3415. [DOI] [PubMed] [Google Scholar]
- Matias AR, Ferreira M, Costa P, Neto P. Skin colour, skin redness and melanin biometric measurements: comparison study between Antera 3D, Mexameter and colorimeter. Skin Res Technol. 2015;21(3):346–362. doi: 10.1111/srt.12199. [DOI] [PubMed] [Google Scholar]
- Matts P, Dykes PJ, Marks R. The distribution of melanin in skin determined in vivo. Br J Dermatol. 2007;156(4):620–628. doi: 10.1111/j.1365-2133.2006.07706.x. [DOI] [PubMed] [Google Scholar]
- Meredith P, Sarna T. The physical and chemical properties of eumelanin. Pigment Cell Res. 2006;19(6):572–594. doi: 10.1111/j.1600-0749.2006.00345.x. [DOI] [PubMed] [Google Scholar]
- Michel APM, Liakat S, Bors K, Gmachl CF. In vivo measurement of mid-infrared light scattering from human skin. Biomed Opt Express. 2013;4(4):520. doi: 10.1364/BOE.4.000520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa H, Imokawa G. Characterization of melanogenesis in normal human epidermal melanocytes by chemical and ultrastructural analysis. Pigment Cell Res. 1996;9(4):175–178. doi: 10.1111/j.1600-0749.1996.tb00106.x. [DOI] [PubMed] [Google Scholar]
- Nisma M, Yanke L, Ryo M, Hwan GC, Allison SD, Jinhua W, Suita Y, Weng QY, Allouche J, Kemeny LV, Hermann, Andrea L, Roider EM, Gray NS, Fisher DE. A UV-independent topical small-molecule approach for melanin production in human skin. Cell Rep. 2017;19(11):2177–2184. doi: 10.1016/j.celrep.2017.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Njoo MD, Das PK, Bos JD, Westerhof W. Association of the Köbner phenomenon with disease activity and therapeutic responsiveness in vitiligo vulgaris. Arch Dermatol. 1999;135(4):407–413. doi: 10.1001/archderm.135.4.407. [DOI] [PubMed] [Google Scholar]
- Piérard GE, Hermanns-Lê T, Piérard SL, Dewalque L, Charlier C, Piérard-Franchimont C, Delvenne P. In vivo skin fluorescence imaging in young Caucasian adults with early malignant melanomas. Clin Cosmet Investig Dermatol. 2014;7:225–230. doi: 10.2147/CCID.S66929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvaag E, Caspersen L, Bech-Thomsen N, Olivarius FDF, Wulf HC. Optimized UVB treatment of psoriasis: a controlled, left-right comparison trial. J Eur Acad Dermatol Venereol. 2000;14(1):19–21. doi: 10.1046/j.1468-3083.2000.00012.x. [DOI] [PubMed] [Google Scholar]
- Shin JW, Lee DH, Choi SY, Na JI, Park KC, Youn SW, Huh CH. Objective and non-invasive evaluation of photorejuvenation effect with intense pulsed light treatment in Asian skin. J Eur Acad Dermatol Venereol. 2011;25(5):516–522. doi: 10.1111/j.1468-3083.2010.03815.x. [DOI] [PubMed] [Google Scholar]
- Shin JW, Yoon SW, Jeong JB, Park KC. Different responses of the melanin index to ultraviolet irradiation in relation to skin color and body site. Photodermatol Photoimmunol Photomed. 2014;30(6):308–315. doi: 10.1111/phpp.12133. [DOI] [PubMed] [Google Scholar]
- Stamatas GN, Zmudzka BZ, Kollias N, Beer JZ. Non-invasive measurements of skin pigmentation in situ. Pigment Cell Res. 2004;17(6):618–626. doi: 10.1111/j.1600-0749.2004.00204.x. [DOI] [PubMed] [Google Scholar]
- Sundaram H. Prospective pilot evaluation of a novel unipolar radiofrequency device with dermal rolling mechanism for rejuvenation of Fitzpatrick skin phototypes III to V. J Am Acad Dermatol. 2014;70(5):AB200. doi: 10.1016/j.jaad.2014.01.829. [DOI] [Google Scholar]
- Tawakkul S, Kerscher M. Comparative evaluation of the skin physiology of Fitzpatrick skin phototypes I through IV by biophysical measuring methods. J Am Acad Dermatol. 2017;76(6):AB77–AB77. doi: 10.1016/j.jaad.2017.04.316. [DOI] [Google Scholar]
- Thompson MJW, Jones G, Aitken DA. Constitutive melanin density is associated with higher 25-hydroxyvitamin D and potentially total body BMD in older Caucasian adults via increased sun tolerance and exposure. Osteoporos Int. 2018;29(8):1887–1895. doi: 10.1007/s00198-018-4568-8. [DOI] [PubMed] [Google Scholar]
- Treesirichod A, Chansakulporn S, Wattanapan P. Correlation between skin color evaluation by skin color scale chart and narrowband reflectance spectrophotometer. Indian J Dermatol. 2014;59(4):339. doi: 10.4103/0019-5154.135476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Wal M, Bloemen M, Verhaegen P, Tuinebreijer W, De Vet H, Van Zuijlen P, Middelkoop E. Objective color measurements: clinimetric performance of three devices on normal skin and scar tissue. J Burn Care Res. 2013;34(3):e187–e194. doi: 10.1097/BCR.0b013e318264bf7d. [DOI] [PubMed] [Google Scholar]
- Verkruysse W, Jia W, Franco W, Milner TE, Nelson JS. Infrared measurement of human skin temperature to predict the individual maximum safe radiant exposure (IMSRE) Lasers Surg Med. 2007;39(10):757–766. doi: 10.1002/lsm.20581. [DOI] [PubMed] [Google Scholar]
- Verkruysse W, Svaasand LO, Franco W, Nelson JS. Remittance at a single wavelength of 390 nm to quantify epidermal melanin concentration. J Biomed Opt. 2009;14(1):014005. doi: 10.1117/1.3065542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt AAR, Bothma JP, Meredith P. The supramolecular structure of melanin. Soft Matter. 2009;5(19):3754–3760. doi: 10.1039/B902507C. [DOI] [Google Scholar]
- Wright CY, Karsten AE, Wilkes M, Singh A, Plessis J, Albers PN, Karsten PA. Diffuse reflectance spectroscopy versus Mexameter® MX18 measurements of melanin and erythema in an African population. Photochem Photobiol. 2016;92(4):632–636. doi: 10.1111/php.12607. [DOI] [PubMed] [Google Scholar]
- Zhang C, Maslov K, Wang LV. Subwavelength-resolution label-free photoacoustic microscopy of optical absorption in vivo. Opt Lett. 2010;35(19):3195. doi: 10.1364/OL.35.003195. [DOI] [PMC free article] [PubMed] [Google Scholar]