Abstract
Dental forensics for the resolution of unnatural death remains an underdeveloped field. Accordingly, an experimental study was conducted with six to seven months old Wistar rats that were drowned in order to identify key postmortem features and pattern of dental decomposition. The visual, structural and elemental changes were assessed periodically. Based on mode of death, they were designated as SB (euthanized and soil buried), FWD (fresh water drowned) and SWD (sea water drowned). Postmortem features as well as the structural and elemental patterns of decomposition of teeth were analyzed with Field Emission Scanning Electron Microscopy (FE-SEM) and Energy Dispersive Spectroscopy (EDAX) periodically for two months. The periodic observation of elemental changes in the teeth of SB, FWD and SWD rats allowed us to derive an equation using linear regression analysis to relate the degree of dental decomposition with the time since death. The difference in pattern of surface deterioration was also observed. The present findings could provide a better knowledge in resolving unnatural deaths and supporting evidence for legal prosecution.
KEYWORDS : Unnatural death, Forensics, teeth, Scanning electron microscopy, EDAX, Time since death
Introduction
Drowning is one of the most common forms of asphyxial death. It is broadly classified as typical and atypical based on the extent of fluid accumulation within lung’s air passages. (1) In drowning, it is always difficult to confirm the cause and time since death because of decomposition and because the bodies are frequently mutilated by aquatic animals. (1) Also, putrefaction inevitably leads to gas formation in soft tissues, which adds further uncertainties in the determination of time of death. (2) The teeth, by contrast, are comparatively preserved due to their hard and calciferous properties. The manifestation of drowning occurs throughout the body with pink tooth being one of the most prominent features. The reason behind this appearance is not clear, but such a phenotype is predominant in asphyxial death. (3, 19)
Thomas Bell (1829) was the first one to note the pink tooth. (4) The Christie murder in 1953 was one of the landmark cases where pink tooth became an important Forensic biomarker since. The exhumed body expressed pink coloration of both tooth and skin. The accused confessed that the victim was strangulated and thus was subjected to carbon monoxide poisoning. The evidence of pink tooth reconfirmed the
strangulation. (4) Beeley and Harvey (1973), Whittaker et.al (1976), Clark and Law (1984), Brondum and Simonsen (1987) and Van Wyk (1987) did major work on pink tooth. (5, 6)
In animal models, the appearance of pink tooth was demonstrated in cat, golden hamsters, female dog and rats. (5, 6, 20) These studies were only qualitative in nature wherein the appearance of pink tooth under various conditions and modes of death were highlighted.
In the present work, we made an attempt to find out postmortem changes apart from pink tooth appearance in rats that were subjected to fresh and sea water drowning (FWD and SWD rat) experimentally. The SB rat was euthanized and buried to simulate natural death and decomposition was used as a reference for comparisons to FWD and SWD rats. The pathophysiology of death in fresh and sea water drowning were different (1) which was the reason for the present work with FWD and SWD rats. The visual, elemental, and structural features exhibited in rat teeth (SB, FWD and SWD) were studied over a period of two months.
Currently, there are two methods that allow to determine the time of death: Rate method and concurrence method, with the former used in the present work. (7)
MATERIALS AND METHODS:
Study design
This study was approved by the Institutional Animal Ethics Committee (IAEC) of Saveetha Medical College and the approval number is SU/CLAR/RD/027/2017. The animals in the lab were maintained according to the guidelines of CPCSEA (Committee for the Purpose of Control and Supervision of Experiments on Animals, India). Three Wistar rats of six to seven months old were used in the experiment and based on their mode of death induced they were designated as SB (euthanized and soil buried), FWD (fresh water drowned) and SWD (sea water drowned). The dental formula of Wistar rat is I 1/1 C 0/0 M 3/3 and hence a rat contains 16 teeth. (8) Overall, 48 teeth (including SB, FWD and SWD rats) were observed to record the postmortem changes in this study. This includes 12 incisors and 36 molars. Fresh water was collected from a Lake, sea water from Bay of Bengal and soil collected from the college campus was categorized as red soil. The other materials utilized for research were isoflurane (Raman and Weil, Mumbai), surgical blade no 22, BP handle no 4, toothed forceps, desiccator, Mackintosh sheet, diamond disc, micro motor and 10% formalin (SB chems, Tamilnadu).
Procedures
SB rat were subjected to overdose of anaesthesia and euthanized, while FWD and SWD rats were subjected to fresh and sea water drowning respectively after mild anaesthesia in the laboratory at an optimum room temperature of 26ºC. Video recordings of both fresh and sea water drowning of rats were collected (data not shown). Immediately upon death confirmation, SB rat was buried in soil while FWD and SWD rats were kept in a tub containing fresh and sea water respectively for 24 hours. After 24 hours, the maxilla and mandible of rats were resected, buried in soil in the case of SB rat, immersed in fresh and sea water filled containers for FWD and SWD rats correspondingly. The containers were kept open and maintained at an optimum temperature ranging from 25 to 28 ºC. To prevent complications from contagious disease such as bubonic or pneumonic plague, the maxilla and mandible of the rats were resected from dead animals and monitored for two months. The FWD and SWD rat lungs were removed by dissection and preserved in 10% formalin for histopathological examination. Visual observation was done to record the changes taking place in the skeleton (maxilla and mandible) and teeth of the rats. Both fresh and sea water utilized for experimental drowning were analyzed by ICP-MS inductively coupled plasma mass spectrometry to understand their chemical composition. (9) The ICP-MS equipment used for water analysis was the Agilent 7700X instrument (model#-G3281A) with an octopole based collision/reaction cell. After every ten days, one incisor tooth from each rat was taken and subjected to FE-SEM with EDAX to monitor the changes happening elementally. The model of the instrument used for FE-SEM with EDAX was CARL ZEISS SUPRA 55 FE-SEM which has resolution capacity of 0·8nm (magnification ranging from 100X to 1,000kx). Before analyzing the sample in FE-SEM with EDAX, sample preparation was done that includes sectioning (cross section) of the tooth with diamond disc. The standard size of the sample for observing in FE-SEM with EDAX is less than 1×1cm, so specifically from the cervical to apex region was sectioned excluding apical end. This sectioned part was observed under FE-SEM at three different but fixed points for consistency. The cross sectioned rat teeth were subjected to gold coating with sputtering instrument and analyzed by FE-SEM with EDAX. (10)
In this study, only the circumpulpal dentin and pulpal region of the incisor tooth were examined by FESEM with EDAX and elemental quantification determined. In total, analysis with FE-SEM with EDAX was done four times with each session having an interval of ten days. Hematoxylin and eosin staining were performed for lung tissue sections. Light microscope was used to observe the slides and optical images were captured.
Statistical analysis
The elemental changes of the tooth for two months were observed periodically by EDAX. Standard two-tailed unequal variance T-test was used for statistical analysis of the results. The elemental changes, taking place in the dentine and pulpal region of four incisor teeth over time were assessed by linear regression analysis using Sigmaplot 13 (Systat software, USA)
RESULTS
The elemental quantification of fresh and sea water were given in the Appendix Table 1. On observation of the drowned rats, copious white froth with bloody tinge was noted on the nasal and oral cavity of SWD rat alone. However these signs were not seen in FWD rat. The nasal and oral cavity images of SB, FWD and SWD rats are given in Fig 1 - A, B, C. The isolated maxilla and mandible of the three rats were observed regularly to study the decomposition changes. On checking the mobility of tooth with probes, it was found that the mobility of tooth started earlier in SWD rat compared to others. Within a time period of 72 hrs after drowning, most of the teeth of SWD rat got mobilized. In SB and FWD rats, the teeth were firm in the socket. However, the maxilla and mandible of all three rats showed observable color differences. In particular, the maxilla and mandible of SB, FWD and SWD rats appeared black, white and pale white in color respectively. The images of the maxilla and mandible of the three rats after 72 hrs post death are shown in Fig 2 - A-C.
Fig. 1.
Images showing the oral and nasal cavity of dead rats after euthanasia or SB (A), fresh water drowning or FWD (B) and sea water drowning or SWD (C). All rats shown were subjected to anaesthesia before death. The copious froth is evident in the oral and nasal regions of SWD rat alone.
Fig. 2.
The appearance of maxilla and mandible regions of SB(A), FWD (B) and SWD (C) rats 72 hrs after death
The onset of pink tooth was evident 96 hrs after death in both FWD and SWD rats, while in SB rat there was no such signs. The pink tooth evident in FWD and SWD rats are displayed in Fig 3 (A, B).
Fig. 3.
Evidence of pink tooth in the incisors of FWD (A) and SWD (B) rats, 96 hrs after death.
The intensity of color and extent of pink tooth in FWD and SWD rats were compared and found to be quite phenotypically different. Within 2 to 3 days, the pink tooth slowly faded off in the FWD rat while in SWD rat it was present until 14 days postmortem. After 14 days, the pinkish coloration slowly started to fade from the SWD samples as well. Mainly, pink tooth was evident in the mandibular incisor teeth of the FWD rat while in SWD rat it was found in all the four incisors which included both the maxillary and the mandibular archs. The remaining maxillary and mandibular molars and maxillary incisors tooth of FWD rat and all molars of SWD rat didn’t manifest pink tooth. In SB rat, none of the tooth expressed pink tooth phenomena. The quantification of pink teeth among three rats are given in the table 1.
Table 1. the evidence of pink tooth among three rats were given below.
| Evidence of pink teeth among three groups | |
|---|---|
| Number of rats | 3 |
| Number of teeth(overall) | 48 |
| Number of pink teeth evident in SB rat | 0 |
| Number of pink teeth evident in FWD rat | 2 |
| Number of pink teeth evident in SWD rat | 4 |
| Number of pink teeth evident in anterior region of FWD rat (incisors) | 2 |
| Number of pink teeth evident in posterior region of FWD rat (molars) | 0 |
| Number of pink teeth evident in anterior region Of SWD rat (incisors) | 4 |
| Number of pink teeth evident in posterior region Of SWD rat (molars) | 0 |
SB-euthanized and soil buried rat: FWD- fresh water drowned rat: SWD sea water drowned rat.
Collapse or atelectatic changes of the alveoli is common in both fresh and sea water drowning but is more prominent in the latter.
Alveoli appeared empty and without the lining pneumocytes in FWD and SWD rat lungs. Capillaries were dilated and more congested in SWD compared to FWD (Appendix Fig. 1 - A, B). Edema which is more pronounced grossly can also be detected microscopically in both drowning interventions, but are more predominant in SWD cases. Inflammatory infiltrates composed of macrophages and neutrophils are seen as a reaction to the pulmonary edema due to hemodynamic disturbances (Appendix Fig 1C). Endothelial lining is injured and denuded in SWD, but not in the FWD (Appendix Fig 1D, E).
Through EDAX data, the elements such as oxygen, calcium and phosphorous were consistently detected in the dentine region of the incisor teeth (SB, FWD and SWD rats) in all the four sessions. When observing the dentine from SB rat, potassium and aluminium were found in addition to oxygen, calcium and phosphorous. However in the dentine of FWD rat incisors, sodium and magnesium were found other than the three elements of oxygen, calcium and phosphorous. In the dentine of SWD rat incisors, the sodium, magnesium and chlorine are evident in addition to the three common elements. Other elements such as zinc, carbon and Sulphur were detected in the dentine of incisors from SB rat. Copper was evident in the fourth session analysis of dentine for both SB and FWD rat incisors. A linear regression analysis was used for analyzing all the four sessions EDAX data (Fig.4 A-D).
Fig 4.
A. The oxygen element weight % in the dentine and pulpal region of the incisor teeth of Wistar rats after a time period of asphyxial (drowning) death with reference control. Continuous line (SB rat (n=4 incisors) – simulating natural death and buried in soil); medium line (FWD rat (n=4 incisors) - fresh water drowned); dash dot (SWD rat (n=4 incisors) - Sea water drowned). B. The calcium element weight % in the dentine and pulpal region of the incisor teeth of Wistar rats after a time period of asphyxial (drowning) death with reference control. Continuous line (SB rat (n=4 incisors) - simulating natural death and buried in soil); medium line (FWD rat (n=4 incisors) – fresh water drowned); dash dot (SWD rat (n=4 incisors) - Sea water drowned). C. The phosphorus element weight % in the dentine and pulpal region of incisor teeth of Wistar rats after a time period of asphyxial (drowning) death with reference control. Continuous line (SB rat (n=4 incisors) - simulating natural death and buried in soil); medium line (FWD rat (n=4 incisors) - fresh water drowned); Dash dot (SWD rat (n=4 incisors) – Sea water drowned). D. The chlorine element weight % in the dentine and pulpal region of incisor teeth of Wistar rats after a time period of asphyxial (drowning) death with reference control. Continuous line (SB rat (n=4 incisors) - simulating natural death and buried in soil); medium line (FWD rat (n=4 incisors) - fresh water drowned); dash dot (SWD rat (n=4 incisors) - Sea water drowned).
While analyzing the oxygen element content of four sessions of the dentine region in SB, FWD and SWD rats, the r2 value was significant in the dentine region of SB rat and the probability of prediction was 87%. The oxygen element percentage shows a positive correlation with days in the dentine region of SB rat incisor tooth. An equation was derived for SB rat(y=33·84+0·6629x) to estimate the time since death approximately with the help of percentage of oxygen element present in dentine region. For FWD and SWD rats, the r2 value of oxygen analysis was not significant both in dentine and pulpal region. For the calcium EDAX data analysis of dentine and pulpal region, the r2 value was significant in the dentine region of SB and FWD rats. The percentage of calcium negatively correlated with days in the dentine region of SB and FWD rats. Based on that, an equation was derived for estimating time since death for SB rat (y=56·10 -0·89x) and FWD rat (y= 46·95-0·599x) approximately with the help of calcium composition in the dentine region. The probabilities of estimating time since death with the above equations are 88% and 96% respectively. For SWD rat, the r2 value obtained for calcium percentage was not significant both in the dentine and pulpal region. While analyzing the phosphorous EDAX data, the r2 value was significant for FWD and SWD rats in the dentine region. The percentage of the phosphorous element in the dentine region of FWD and SWD rats were negatively correlated. Based on the above data, the derived equation for FWD and SWD rats was(y=22·10-0·257x) and (y=18·27-0·062x) respectively. The probability of prediction of time since death with the above equations derived from phosphorous percentages in the dentin are 94% and 72% correspondingly. While observing the dentine region of SWD rat incisor tooth for four sessions, the level of chlorine consistently increased on every succeeding session and hence positively correlated. By assessing the quantity of chlorine in the dentine region for the four sessions, an equation was derived for SWD rat (y=0·443+0·0255x) with probability of prediction as 98%.The rest of the elements are not consistently evident in the all four sessions of analysis, so they are not statistically assessed. Table 2 shows the equation derived from the elements. Correlation of various elements (%) with days post death and the differential detection of the elemental percentages in the dentine and pulpal region in SB, FWD and SWD rats were given in Appendix Table 2 and Appendix Table 3.
Table 2. Derived Equations for estimating time since death from elemental degradation with the help of linear regression.
| Element | Region | Equations | ||
|---|---|---|---|---|
| SB | FWD | SWD | ||
| Oxygen | Dentine | y=33·84+0·6629x | - | - |
| Pulpar | - | - | - | |
| Calcium | Dentine | y= 56·10-0·89x | y=46·95-0·599x | - |
| Pulpar | - | - | - | |
| Phosphorus | Dentine | - | y= 22·10-0·257x | y= 18·27-0·062x |
| Pulpar | - | - | ||
| Chlorine | Dentine | - | - | y = 0·443+0·0255x |
| Pulpar | - | - | - | |
The FE-SEM images of dentine and pulpal region of incisor teeth of SB, FWD and SWD rats were studied and interpreted results are given below.
1. Dentine region
The results in surface morphology were not consistent, with select areas of the sample in each of the groups suffering from severe degradation even by day 10, while some areas appear relatively intact after day 50. Thus, morphology of such regions cannot be reliably used for sample differentiation and dating.
11. Pulpal region
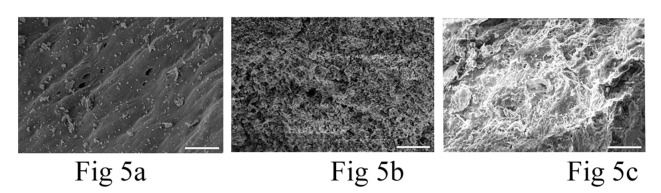
The damage to the pulpal surface of teeth in SB, FWD and SWD rats are more mild and much more differentiated compared to the dentine region. In particular, the pulpal surface of SB rat teeth (Fig 5A) exhibited minimal damage even after 50 days. On the contrary, the damage to the pulpal surface is progressively worse for FWD and SWD rat’s incisor teeth. FWD rat incisor teeth shows deep pores in the pulpal surface (Fig 5B) but the surface roughness of the latter is highest among the three (SB, FWD and SWD rats) and is most pronounced at day 50 without noticeable pores (Fig 5C). Calcification of the pulpal surface seems to be diminished going from SB, FWD and SWD rats teeth, although the difference has not been determined to be significant.
Fig 5.
FE-SEM image of incisor tooth of SB (A), FWD (B) and SWD(C) rats captured on 50th day after death showing pulpal surface manifests the variations in surface structural decomposition features.
DISCUSSION
The occurrence of pink tooth in asphyxial death is a constant feature in forensic medicine but literature on this topic are not sufficient in scientific databases. (11) Consequently, studies carried out on pink tooth (a key manifestation of asphyxia death) in humans and laboratory animals are very scarce. Any investigations were restricted to qualitative studies. Hence, the present study has been focused on quantitatively assesing some unexplored features to the pink tooth phenomenon from drowning cases. The features include visual, elemental and structural changes observed over a period of 2 months after death in these animals.
After death due to diagenetic changes, the enamel and dentine allow entry of water which leads to mineral deposition in teeth that consequently changes the color, composition and surface texture of the teeth. (12) Quantitative elemental analysis of fresh and sea water was carried out in this study to establish the effect of elements on rats teeth of FWD and SWD.
Atelactic changes in alveoli, capillaries dilatations, oedema and macrophage infiltration proves the ante mortem drowning in the Wistar rats. (13) The oedema, the capillary dilatation and congestion are predominant in sea water drowning indicating higher level of destruction of lungs than in the fresh water. (14)
At 96 hours after death, the entire maxilla and mandible regions of SB rat appeared black indicating loss of vascularity. This shows the vascular tissue decomposition rate is faster in soil buried as compared to the maxilla and mandible kept in fresh and sea water. The cause for early decomposition of vascular tissues could be the effect of microorganisms in soil that enters through the vascular bundles and cellular region consequently leading to decomposition. This rapid decomposition of vascularity in the skeletal part would have caused the early necrosis in the skeletal and dental part of SB rat as compared to FWD and SWD rats. (15) Interestingly, the teeth of SB rat were rigidly fixed and could not be extracted easily from the maxilla and mandible of SB rat at 96 hours which indicates the outer surface of the skeletal structures (maxilla and mandible) are intact. The comparatively mobility of teeth, 72 hours after death in SWD rat indicates that decomposition in bone tend to follow the peripheral region rather than the vascular bundles as reported for inhumed cases. The early mobility of teeth in SWD rat could be possibly due to faster rate of decomposition around the neck of the tooth. (15)
The time taken for pink tooth occurrence in fresh and sea water drowning were around 96 hrs. The reason for time delay in the occurrence of pink teeth appearance in humans was explained as follows: The diameter of erythrocytes (7·5μm) are larger than the diameter of dentinal tubules (3μm). Thus, there should be the resistance to entry of erythrocytes by the dentinal tubules for several hours after death. (6) When autolysis of erythrocytes is accomplished in teeth, it leads to seepage of blood components into dentinal tubules which results in pink tooth. Obviously, a similar kind of mechanism would have occurred in rat teeth which delayed the pink tooth. The pink tooth in FWD rat faded off at an earlier time period as compared to the SWD rat, an observation which can be explained by the FE-SEM results. The pores are deep in the pulpal surface of FWD rat teeth due to deterioration. These deep pores encouraged easy seepage of erythrocytes which consequently led to earlier fading of pink tooth in FWD rat. FE-SEM image of the pulpal surface of SWD rat incisor tooth show that the pores are not very evident which may be the reason for late disappearance of pink tooth. The extent and color variation of pink tooth visualized in fresh and sea water drowning may be due to the variations in mineral content, carbon dioxide and salinity level. The intense color of pink tooth in SWD rat could be attributed to the increased salinity of sea water, (5) which is reflected in the present work (Fig 3b).
There are more likely, species variations in the appearance and distribution of pink tooth. For instance, pink tooth was evident only in anterior teeth in FWD and SWD rats while in posterior region, it was not observable. The distribution of pink tooth given by Campobasso et al (2006) in ship wreckage case shows that pink tooth was evident in both anterior and posterior region indicating the difference in occurrence between humans and animals. (16) The reason for predominance of pink tooth in anterior region as compared to posteriors in rats could be due to the difference in the root morphology and dimensions which plays the role in pooling of blood in the cervical region of the tooth. The anterior tooth of rats have single root and tooth are tubular in structure which may allow pink tooth occurrence predominantly in anterior than posterior teeth.
The elements present in the Wistar rat dentin are Ca, P, F, Mg, Na and Zn. Based on the different areas of dentin like mantle and circumpulpal, the percentage of the element varies. (17)
In SB rat, the level of oxygen increased in the dentine region of incisor tooth for every succeeding analysis whereas the calcium levels were found to be decreased. The work of Pipenbrick et.al (1987) on bones explains about the calcium carbonate formation in the Volkmann’s canals of carcasses. Their findings revealed that calcium from hydroxy apatite crystals reacted with carbonate in soil to form calcium carbonate which leads to further decomposition of bone. (18) The present EDAX data of incisor teeth from four sessions of SB rat shows similar kind of results such as calcium decrease and oxygen increase on every succeeding sessions which correlates with Pipenbrick’s findings of decomposition mechanism in the inhumed bone. The FE-SEM interpretation also reconfirms the EDAX data showing maximum calcium decreases in the dentine region of the incisor tooth of SB rat compared to others. While analyzing phosphorous content in the dentine of SB rat, it appeared the levels kept fluctuating during the sessions assessed. In contrast, Pipenbrick et al (1987) mentioned that phosphorous was reduced very prominently in inhumed as compared to the original bone. (18) Therefore, a long term observation of inhumed teeth might give results comparable with Pipenbrick’s findings. The degree of pulpal elemental changes in SB rat is not substantial to provide any clues for the study. The elements such as oxygen, calcium and phosphorous consistently decreased in the dentine region of FWD rat teeth with succeeding sessions. In the dentine region of SWD rat teeth, oxygen and chlorine increased while calcium and phosphorus decreased. The cause for such elemental changes in the dentine region of FWD and SWD rat’s incisor teeth needs further exploration.
CONCLUSION
The subtle differences in pink tooth features observed between fresh and sea water drowning allows one to discriminate between the two and is focus of the present study. Even though pink tooth occurrence is believed to be due to internal mechanism like increased intrapulpal pressure, the extent and duration of pink tooth as well as color intensity could be influenced by the external environment. The tracking of elemental and structural changes during decomposition of teeth over time helps to determine the approximate time since death. Although the present study is limited by the small sample size of studied animals, the results were consolidated with reasonable number of tooth samples from each group for both morphological evaluation and element analysis. More robust and discrete conclusions on elemental and structural alterations of teeth are likely to be generated, if the number of rats is increased in a future study. Undoubtedly, this work can create a base for many future works on asphyxial deaths and post-mortem pink tooth evaluation.
Acknowledgement
We thank Dr N.D. Jayakumar, Dean of Saveetha Dental College for the motivation and encouragement. We also thank Dr. E. Sukumar, Dean-Research, Saveetha Institute of Medical and Technical Sciences for critically reading the manuscript. We thank scientists K. Viswanathan and Suman of sathyabama University for providing their technical assistance in FE-SEM with EDAX and ICP-MS respectively. We also extend thanks to Dr G. Barathi, Assistant Professor, Department of Pathology, Sri Ramachandra Medical College and Research Institute for supporting histopathology work. We thank Madhan Kumar, Lab Attendant, Department of Research and Development, Saveetha Institute of Medical and Technical Sciences for lab assistance during experiments. We extend sincere thanks to Dr Edwin Chang, Department of Radiology, Stanford University school of Medicine, CA, USA for doing English language editing. This study didn’t receive any funds and we declare no other potential conflicts of interest with respect to the authorship and/or publication of this article.
SUPPLEMENTAL APPENDIX.
Appendix.
Figure 1: Dilatation of the alveoli and the capillaries in fresh water drowning 40X(A), Dilatation and edema of the alveoli and more congested capillaries in sea water drowning 40X (B), Inflammation composed of macrophages and neutrophils in sea water drowning 400X (C), Endothelial injury and denudation of the lining cells in the sea water drowning 100X (D), Higher power view of the denuded endothelium in sea water drowning 200X (E), Bacterial colonies as evident of sea water drowning as against fresh water 400X (F).
Appendix. Table 1: Elemental analysis of fresh and sea water by ICP-MS.
| Elements | Concentration | |
|---|---|---|
| Fresh water | Sea water | |
| Na23 | 50mg/dl | 6450mg/dl |
| Mg24 | 84mg/dl | 849mg/dl |
| S34 | 643mg/dl | 1587mg/l |
| Cl35 | 2·210mg/ | 79·665mg/l |
| K39 | 2·412mg/l | 2·412mg/l |
| Ca43 | 0·16421mg/l | 2·206mg/l |
| Fe56 | 0·00621mg/l | 0·003704mg/l |
| Zn66 | 0·007718mg/l | 0·004831mg/l |
Appendix. Table 2: Correlation of various elements (%) with days post death in the dentine and pulpal region in SB, FWD and SWD rats.
| Element | Region | SB rat | FWD rat | SWD rat |
|---|---|---|---|---|
| Oxygen | Dentine | r2=0·7544 | r2=0·00924 | r2=0·094 |
| β0=33·64 | β0=45·27 | β0=45·22 | ||
| β1=0·6229 | β1= -0·026 | β1=0·096 | ||
| Pulpal | r2=0·018 | r2=0·425 | r2=0·0072 | |
| β0=65·16 | β0=85·25 | β0=53·93 | ||
| β1= -0·213 | β1= -0·69 | β1=0·029 | ||
| Calcium | Dentine | r2=0·792 | r2=0·929 | r2=0·339 |
| β0=56·10 | β0=46·95 | β0=35·44 | ||
| β1= -0·89 | β1= -0·599 | β1= -0·151 | ||
| Pulpal | r2=0·299 | r2=0·021 | r2=0·011 | |
| β0=0·459 | β0=28·25 | β0=25·94 | ||
| β1=0·371 | β1= -0·081 | β1=0·046 | ||
| Phosphorous | Dentine | r2=0·0035 | r2=0·891 | r2=0·521 |
| β0=11·54 | β0=22·10 | β0=18·27 | ||
| β1=0·012 | β1= -0·257 | β1= -0·062 | ||
| Pulpal | r2=1 | r2=1 | r2=0·165 | |
| β0= - | β0= - | β0=13·88 | ||
| β1= - | β1= - | β1=7·21 | ||
| Chlorine | Dentine | r2= - | r2= - | r2=0·973 |
| β0= - | β0= - | β0=0·443 | ||
| β1= - | β1= - | β1=0·0255 | ||
| Pulpal | r2= - | r2= - | r2= 0.100 | |
| β0= - | β0= - | β0=1.733 | ||
| β1= - | β1= - | β1= -0.011 |
The statistical result of the elements in the dentine and pulpal region of SB, FWD and SWD rats were given above. *the bolded figures indicate the significant values. Y (element %) = β0 + β1 x(days)
Appendix. Table 3: The differential detection of the elemental percentages among the SB, FWD and SWD rats.
| Elements | Dentine region | Pulpar region | ||||
|---|---|---|---|---|---|---|
| SB | FWD | SWD | SB | FWD | SWD | |
| Oxygen | +++ | - | - | - | + | - |
| Calcium | +++ | +++ | + | - | - | - |
| Phosporous | - | +++ | ++ | - | - | - |
| Chlorine | - | - | +++ | - | - | - |
The + for elemental percentage was given based on the r value. r>0·3= +, r>0·5=++ and r>0·7= +++.
Footnotes
The authors declare that they have no conflict of interest.
References
- 1.Parikh CK .Textbook of Medical Jurisprudence and toxicology. 6thEd. New Delhi.CBS publishers and distributors; 2008.
- 2.Roberts ISD, Benamore RE, Benbow EW, Lee SH, Harris JN, Jackson A, et al. Post-mortem imaging as an alternative to autopsy in the diagnosis of adult deaths: a validation study. Lancet. 2012;379:136–42. 10.1016/S0140-6736(11)61483-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clark DH, Law M. Postmortem pink teeth. Med Sci Law. 1984;24(2):130–4. 10.1177/002580248402400214 [DOI] [PubMed] [Google Scholar]
- 4.Beeley JA, Harvey W. Pink teeth appearing as a postmortem phenomenon. J Forensic Sci Soc. 1973;13:297–305. 10.1016/S0015-7368(73)70829-X [DOI] [PubMed] [Google Scholar]
- 5.Borrman H, Chesne AD, Brinkmann B. Medico-legal aspects of postmortem pink tooth. Int J Legal Med. 1994;106:225–31. 10.1007/BF01225410 [DOI] [PubMed] [Google Scholar]
- 6.Dye TJ, Lucy D, Pollard AM. The Occurrence and implications of post-mortem ‘pink teeth’ in forensic and archaeological cases. Int J Osteoarchaeol. 1995;5:339–48. 10.1002/oa.1390050404 [DOI] [Google Scholar]
- 7.Senn RD, Stimson PG. Forensic Dentistry.2nded.Boca Raton: Taylor and Francis Group; 2010. [Google Scholar]
- 8.Prasanth T, Saraswathi TR. Histopathological and radiographic evaluation of rat molar teeth after traumatic injury a pilot study. JOMFP. 2012;16(3):313–7. 10.4103/0973-029X.102473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aimoto M, Kato T, Kiso E, Tsutsumi N, Miki O. Analysis of Trace iron (Fe) in sea water by using inductively coupled plasma mass spectrometry with solid phase chelate extraction technique. Nippon Steel technical report. 2011; 100: 85-91.
- 10.Olea-Mejia O, Contreras-Bulnes R, Zamudio-Ortega CM, Morales-Luckie RA, Olea-Cardoso O. Lopez- Castanares R. Scanning electron microscopy and energy dispersive spectroscopy microanalysis applied to human dental specimens under laser irradiation for caries prevention. Microscopy: advances in scientific research and education. In: Mendez-vilas A (ed). Badajoz: Formatex; 2014.p70-77. [Google Scholar]
- 11.Soriano EP, De Carvalho MVD, Santos FBD, De Mendoza CC, De Araujo MDSD, Campello RIC. The post-mortem pink teeth phenomenon: A case report. Med Oral Patol Oral Cir Bucal. 2009; 14(7): E 337-339. [PubMed]
- 12.Manoilescu I, Ion A, Ioan BG .Post-Mortem changes in teeth – Forensic issues. International Journal of Medical dentistry.2015; 5(4): 249-252.
- 13.McEwen BJ, Gerdin J. Veterinary forensic pathology: Drowning and bodies recovered from water. Vet Pathol. 2016;53:1049–56. 10.1177/0300985815625757 [DOI] [PubMed] [Google Scholar]
- 14.Jin F, Li C. Seawater-drowning-induced acute lung injury: from molecular mechanisms to potential treatments [review]. Exp Ther Med. 2017;13:2591–8. 10.3892/etm.2017.4302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bell LS, Boyde A, Jones SJ. Diagenetic alteration to teeth in situ illustrated by Backscattered electron Imaging. Scanning. 1991;13(2):173–83. 10.1002/sca.4950130204 [DOI] [Google Scholar]
- 16.Campobasso CP, Di Vella G, De Donno A, Santoro V, Favia G, Introna F. Pink teeth in a series of bodies recovered from a single shipwreck. Am J Forensic Med Pathol. 2006;27(4):313–6. 10.1097/01.paf.0000233544.58567.81 [DOI] [PubMed] [Google Scholar]
- 17.Tjäderhane L, Hietala EL, Larmas M. Mineral element analysis of carious and sound rat dentin by electron probe micro analyzer combined with Back-scattered electron image. J Dent Res. 1995;74(11):1770–4. 10.1177/00220345950740110901 [DOI] [PubMed] [Google Scholar]
- 18.Piepenbrink H, Schutkowski H. Decomposition of skeletal remains in desert dry soil A roentgenological study. Hum Evol. 1987;2(6):481–91. 10.1007/BF02437423 [DOI] [Google Scholar]
- 19.de Almeida CA, Daruge E, Daruge E, Jr, el-Guindy M. Comparative study of experimentally induced and post-mortem pink teeth. J Forensic Odontostomatol. 1996;14(2):25–7. [PubMed] [Google Scholar]
- 20.Van Wyk CW. Pink teeth of the dead: 1. A clinical and histological description. J Forensic Odontostomatol. 1987;5(2):41–50. [PubMed] [Google Scholar]