Abstract
Background
The objective of this study was to evaluate the efficiency and safety of microwave thermal ablation in the treatment of skip metastases in extremity osteosarcomas. Osteosarcoma of extremities with skip metastases has a poor prognosis, and thus, microwave thermal ablation presents an attractive minimally invasive option in this patient group.
Methods
A retrospective review included a cohort of 76 patients with extremity osteosarcoma in one institute, of which five cases (6.6%) showed skip metastases. Skip lesions located in proximal femur and primary sites were distal femur in all five patients. The authors treated skip lesions using microwave thermal ablation after primary tumors were removed at wide margins. Procedural efficacy and safety were determined with postoperative MSTS score and follow-ups of 12–62 months (median 22 months).
Results
The ablation time was five to nine minutes (mean seven minutes). Taking advantage of Microwave-induced hyperthermia, wide resections of distal femur and endoprosthesis reconstructions were performed instead of total femoral resection and replacement in four patients, and above-knee amputation was performed instead of hip disarticulation in one patient. The postoperative hip functions were intact and the mean lower extremity MSTS score was 26. Three patients died at 12–22 months after definitive surgery because of pulmonary metastases, and two patients remained disease-free at 44 and 62 months after surgery, respectively. No local recurrence either at sites of primary tumors or skip lesions was found at time of the latest follow up.
Conclusion
Microwave thermal ablation is efficacious in treating skip metastases of osteosarcoma in extremities. The modality has promise for good local control of tumors, less invasive surgeries, and intact and satisfied lower extremity functions in these relatively poor prognosis patients.
Level of evidence
Therapeutic Level III.
Keywords: microwave ablation, skip metastases, osteosarcoma, extremity
Osteosarcoma tends to localize at metaphysis of long bone in children and adolescent extremities. Skip metastases (synchronous regional bone metastases) may present in high grade ostersarcoma1 With the development of diagnostic imaging especially for the progress of Magnetic Resonance Imaging, skip metastases in osteosarcoma of extremities could be clearly identified before definitive surgery. The incidence rate of skip metastases was reported to be 1.4–10% in the literature.2,5
Surgical treatment of skip metastases in extremity osteosarcoma remains a challenge. It has been widely accepted that the entire tumor-bearing bone should be removed as one compartment according to wide resection principle if skip metastasis occurred, no matter tumor sizes of these skip lesions, leading to more invasive surgeries, difficulties in reconstruction and relatively poor joint functions. Unfortunately, osteosarcoma patients with skip metastases had relatively poor prognosis.2,3 So a better short-term joint function should be concerned to improve life quality. But the existence of skip metastases may impede the surgical choice.
The authors treated skip metastases in extremity osteosarcoma with microwave ablation and achieved very good balance between surgical margins and functional outcomes.
Materials And Methods
Seventy-six patients of osteosarcoma in extremities were retrospectively analyzed from October 2012 through September 2018. Five cases (6.6%) were found to have skip metastases by preoperative plain radiographs, MR imaging and Tc-99m bone scan. Two cases were male, three female, aged eight to 18 years with a median age of 13 years. Primary sites were all located at distal femur and tumors skip metastasized to proximal femur alone synchronous intramedullary canal. The primary tumors and skip metastases were identified with histologically confirmed high grade osteosarcomas in all five patients. At least one course of preoperative chemotherapy was administered and the response of chemotherapy varied. MR imaging showed skip metastases still existed without cortex involvement after preoperative chemotherapy. Four wide resections of tumor in distal femur and prosthetic reconstructions were performed when limb-salvages were indicated. One mid-femur amputation was performed in a patient with major neurovascular bundle involved by massive tumor. Skip metastases were treated using microwave thermal ablation in all five patients (Table 1). Written informed consents of patients or parents (for patients under 18 years of age) were obtained before enrolling into the study. This study was approved by the local Ethics Committee of the Fourth Medical Center of PLA General Hospital and conformed to the principles outlined in the declaration of Helsinki.
Table 1.
Patient Data From This Skip Metastases Series
| Patient Number | Age (Years) | Gender | Primary Site | Skip Lesion Site | Response To Preop Chemo | Definitive Surgery | Local Recurrence | Follow-up (Months) |
|---|---|---|---|---|---|---|---|---|
| 1 | 9 | F | Distal femur | Proximal femur | Good | Wide resection | No | 62 |
| 2 | 8 | M | Distal femur | Proximal femur | Good | Wide resection | No | 22 (died) |
| 3 | 18 | F | Distal femur | Proximal femur | Poor | Wide resection | No | 14 (died) |
| 4 | 11 | M | Distal femur | Proximal femur | Poor | Amputation | No | 12 (died) |
| 5 | 13 | F | Distal femur | Proximal femur | Good | Wide resection | No | 44 |
Preoperative planning was made based on imaging findings after chemotherapy to determine the osteotomy planes. Primary tumors were resected at a wide margin 3 cm beyond abnormal uptake, as determined by preoperative studies. The proximal marrows were sampled and sent to pathology for frozen section analysis. The surgical margins were confirmed to be free of tumor.
After wide resection of primary tumors, a 9-gauge microwave ablation antenna (3.0 mm, 20 cm long, Yigao Inc. Nanjing) and a thermocouple were inserted to the remaining intramedullary canal of femur. Using fluoroscopic guidance, the emitter of antenna was placed into or near the skip lesions. According to the preoperative plan, the angle and depth of the antenna were adjusted to ablate skip lesions (Figure 1). The temperature inside the tumor should reach 80°C or higher with monitoring of thermocouple thermometer, and the surrounding soft tissues were protected with cold saline to ensure the temperature was lower than 40°C during the ablation. The ablation time of skip metastases was five to nine minutes, with an average of seven minutes depending on the size and the number of the skip lesions. Temperature at any part of the oval ablation zone exceeded 80°C and maintained for five minutes was considered as successful ablation. We also ablated all marrow tissues distal to skip lesions with interval of 2 cm gradually (the ablation time was shorter than 30 seconds each time) when the antenna was pulling out, particularly focused on the osteotomy site, even they were normal as appeared in preoperative imaging.
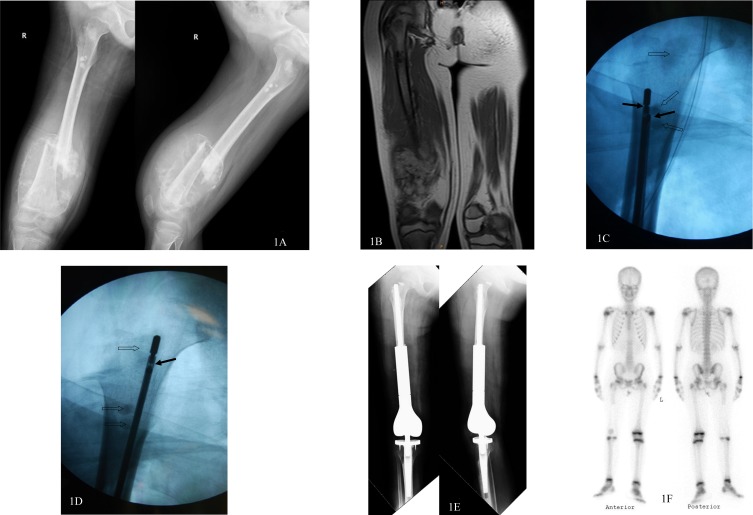
Figure 1.
A nine-year old girl with osteosarcoma in the right distal femur got a very good response for preoperative chemotherapy. Skip metastases in proximal femur and intertrochanteric area were clearly shown in plain radiographs (A) and MR imaging (B) after preoperative chemo. A microwave antenna and a thermocouple (solid arrows) were inserted into the remaining intramedullary canal of the femur and the ablation of skip metastases (hollow arrows) were performed with fluoroscopic guidance (C, D). After the ablation, the bone defect was reconstructed with distal femoral endoprosthesis and hip function was retained (E). No local recurrence of skip metastasis was found in Tc-99m bone scan 36 months after the operation (F).
The ablated tumor tissues in the intramedullary canal were removed with a thorough curettage after the ablation. The bony reconstruction with endoprosthesis, or myodesis and myoplasty were performed subsequently. Adjuvant chemotherapy was administered postoperatively.
Lower extremities functions were assessed with MSTS score one month after surgery. Local recurrences were assessed with MRI or Tc-99m bone scan every three months in the first year after surgery and every six months thereafter.
Results
Taking advantage of microwave-induced hyperthermia, wide resections of distal femur and endoprosthesis reconstructions were performed instead of total femoral resection and replacement in four patients, and above-knee amputation was performed instead of hip disarticulation in one patient (Figure 2). There was no wound complication and deep infection. The postoperative hip functions were intact in all patients allowing them to walk with crutches one week after surgery. The postoperative lower extremity functions were assessed one month after surgery, the mean MSTS score was 26. Five patients were followed up for 12 to 62 months, with a median of 22 months. Three patients (including patient with amputation) died at 12 to 22 months after definitive surgery because of pulmonary metastases, and two patients remained disease-free at 44 and 62 months after surgery, respectively. No local recurrence either at sites of primary tumors or skip lesions was found at time of the latest follow up. No long-term complication such as fracture and prosthetic infection occurred.
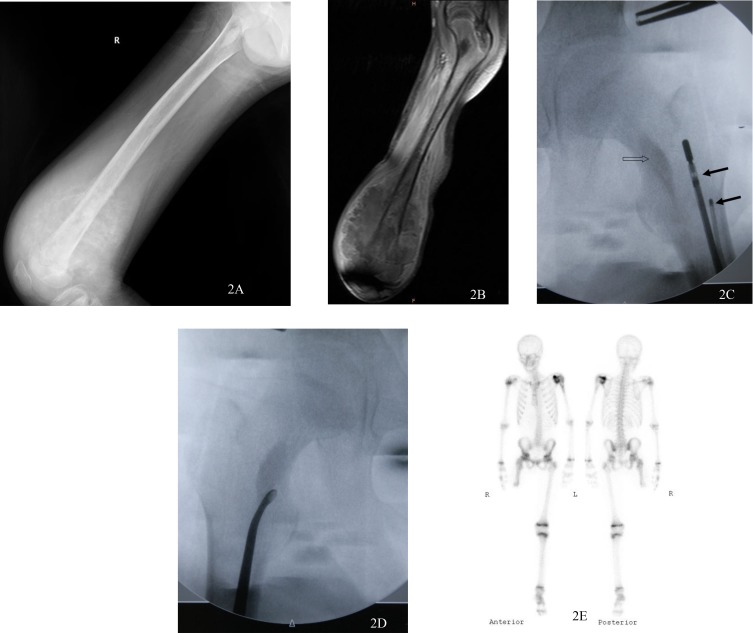
Figure 2.
An 11-year old boy with massive osteosarcoma in the right distal femur got a very poor response for preoperative chemotherapy. Skip metastasis in intertrochanteric area was found in plain radiograph (A) and MR imaging (B) after preoperative chemo. The major neurovascular bundle was surrounded by tumor, leading to a mid-femur above-knee amputation. With fluoroscopic guidance, a microwave antenna and a thermocouple (solid arrows) were inserted into the remaining intramedullary canal of the femur near the skip metastasis (hollow arrows) (C). Skip metastasis was removed with a thorough curettage (D) after the ablation. Myodesis and myoplasty were carried out and hip function was retained. No local recurrence of skip metastasis was found in Tc-99m bone scan 12 months after the operation (E).
Discussions
Skip metastases in patients with osteosarcoma usually indicates poor prognosis. In the early 1990s, Wuisman et al1 had reported that skip metastasis should be regarded as another criterion for considering an osteosarcoma to be Stage III. Kager et al2 analyzed the collected data of 1765 consecutive patients with newly diagnosed high-grade osteosarcoma and identified 24 patients (1.4%) with unequivocally proven skip metastases. All 24 patients were treated by an aggressive surgical approach coupled with polychemotherapy and 12 patients were alive. Sajadi et al3 reported a group of 155 patients of osteosarcoma, 10 (6.5%) patients with skip metastases were identified. All 10 patients eventually died. Ahmed4 reported 116 cases of osteosarcoma patients, of which 12 cases (10%) showed skip metastases. All skip lesions were accompanied with the distant metastases. The author believed that skip metastasis was equal to systemic metastases and the prognosis was poor. In this study, we reported a cohort of 76 patients of osteosarcoma, five patients (6.6%) with skip metastases were identified preoperatively. Three patients eventually died and two patients are alive free of tumor.
When considering the surgical management of skip metastases in extremity osteosarcoma, surgeons may encounter challenges. For instance, in the most common skip metastases of osteosarcoma which metastasize from distal femur to proximal femur along the intramedullary canal, total femur resection with endoprosthetic reconstruction should be performed in most cases6 in terms of wide margin principle, leading to a more invasive surgery and a poorer short-term hip function. The surgeons may pay more attention to short-term limb function because of the poor prognosis in this patient group. In addition, most patients of osteosarcoma with skip metastases are children and adolescent whose acetabulum is immature. It is likely to cause instability and even hip dislocation if total femur replacement is performed7 With regard to amputation, the function of above-knee prosthesis is better than that of hip disarticulation. But methods of salvaging the proximal femur are limited when the proximal femur is contaminated with skip metastases.
Microwave thermal ablation has been used in the treatment of hepatic malignancies for decades and is considered to be an effective and safe procedure8 This technology was extended to treatments of pulmonary9 renal10 breast11 and thyroid12 tumors recently. Microwave ablation has been wide accepted in the treatment of various benign and malignant musculoskeletal lesions as well13 especially in osteoid osteoma14,15 and palliation of metastatic bone lesions.16,17 Most of these ablations were performed percutaneously. Fan et al18–20 have introduced microwave thermal ablation into treatment of primary bone malignancies in definitive surgery for more than two decades. Microwave ablation has also been used in treatment of spinal metastases combined with minimally invasive open decompression21 The authors have used microwave thermal ablation in treatment of skip metastases in osteosarcoma of extremities and summarized it has the following advantages:
Microwave thermal ablation can satisfy the principle of wide resection of osteosarcoma. Earlier experimental studies have showed that the tumor cells will be ablated in five minutes in excess of 60°C.22 Because the intramedullary canal of long bone is a relatively closed and narrow compartment, it is capable of generating very high temperatures in skip lesions, often in excess of 100°C in the center of an oval ablation zone, achieving a “wide” surgical margin. The cortical bone is an ideal barrier to isolate thermal power in the confined canal, and the temperature of surrounding normal tissues is easy to be controlled below 40°C. Negative histological results of osteotomy plane based on preoperative MR imaging were identified in all five patients in this study. Even if osteotomy planes are histological positive, the marrow of stump can be ablate readily using microwave ablation to achieve satisfactory local control, as long as there is no extraosseous mass around.
The procedure of microwave thermal ablation is feasible and easy to operate, which could reduce surgical invasiveness and operation time. The proximal femur is salvaged without any wide detachment. The only thing needs to do is inserting the microwave ablation antenna into the intramedullary canal of remaining femur. Reconstruction after the ablation is relatively easy as well. The distal femur replacement takes shorter time compared with total femur replacement.
The postoperative hip function is intact and short-term limb function is good. The short-term function is very important in osteosarcoma patients with skip metastases because of poor prognosis.
There are some disadvantages of microwave thermal ablation when treating skip metastases in extremity osteosarcoma. Microwave thermal ablation of metastases in the proximal femur may cause damage to the cartilage of the femoral head when lesions are located in femoral neck, leading to secondary hip degeneration if a longer survival is anticipated. Other limitations of microwave system in treating tumors percutaneously, such as shaft heating, large diameter antenna and long ablation zone8 are not obstacles in treating intramedullary metastases in surgery.
The authors believe that microwave thermal ablation may meet the principle of oncologic resection, decrease the surgical invasion, and provide good short-term function in dealing with skip metastases in extremity osteosarcoma.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Wuisman P, Enneking WF. Prognosis for patients who have osteosarcoma with skip metastasis. J Bone Joint Surg Am. 1990;72(1):60–68. doi: 10.2106/00004623-199072010-00010 [DOI] [PubMed] [Google Scholar]
- 2.Kager L, Zoubek A, Kastner U, et al., Cooperative Osteosarcoma Study Group. Skip metastases in osteosarcoma: experience of the Cooperative Osteosarcoma Study Group. J Clin Oncol. 2006;24(10):1535–1541. doi: 10.1200/JCO.2005.04.2978 [DOI] [PubMed] [Google Scholar]
- 3.Sajadi KR, Heck RK, Neel MD, et al. The incidence and prognosis of osteosarcoma skip metastases. Clin Orthop Relat Res. 2004;426:92–96. doi: 10.1097/01.blo.0000141493.52166.69 [DOI] [PubMed] [Google Scholar]
- 4.Ahmed AR. Secondary bone lesions in the affected limb in osteosarcoma (skip lesions), its classification and prognosis. Arch Orthop Trauma Surg. 2011;131(10):1351–1355. doi: 10.1007/s00402-011-1304-7 [DOI] [PubMed] [Google Scholar]
- 5.Leavey PJ, Day MD, Booth T, Maale G. Skip metastasis in osteosarcoma. J Pediatr Hematol Oncol. 2003;25(10):806–808. doi: 10.1097/00043426-200310000-00013 [DOI] [PubMed] [Google Scholar]
- 6.Ruggieri P, Bosco G, Pala E, Errani C, Mercuri M. Local recurrence, survival and function after total femur resection and megaprosthetic reconstruction for bone sarcomas. Clin Orthop Relat Res. 2010;468(11):2860–2866. doi: 10.1007/s11999-010-1476-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sewell MD, Spiegelberg BG, Hanna SA, et al. Total femoral endoprosthetic replacement following excision of bone tumours. J Bone Joint Surg Br. 2009;91(11):1513–1520. doi: 10.1302/0301-620X.91B11.21996 [DOI] [PubMed] [Google Scholar]
- 8.Lubner MG, Brace CL, Ziemlewicz TJ, Hinshaw JL, Lee FT Jr. Microwave thermal ablation of hepatic malignancy. Semin Intervent Radiol. 2013;30(1):56–66. doi: 10.1055/s-0033-1333654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ridge CA, Solomon SB, Thornton RH. Thermal ablation of stage I non-small cell lung carcinoma. Semin Intervent Radiol. 2014;31(2):118–124. doi: 10.1055/s-0034-1373786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wagstaff P, Ingels A, Zondervan P, de la Rosette JJ, Laguna MP. Thermal ablation in renal cell carcinoma management: a comprehensive review. Curr Opin Urol. 2014;24(5):474–482. doi: 10.1097/MOU.0000000000000084 [DOI] [PubMed] [Google Scholar]
- 11.Fornage BD, Hwang RF. Current status of imaging-guided percutaneous ablation of breast cancer. AJR Am J Roentgenol. 2014;203(2):442–448. doi: 10.2214/AJR.13.11600 [DOI] [PubMed] [Google Scholar]
- 12.Korkusuz H, Happel C, Heck K, Ackermann H, Grünwald F. Percutaneous thermal microwave thermal ablation of thyroid nodules. Preparation, feasibility, efficiency. Nuklearmedizin. 2014;53(4):123–130. doi: 10.3413/Nukmed-0631-13-10 [DOI] [PubMed] [Google Scholar]
- 13.Foster RC, Stavas JM. Bone and soft tissue ablation. Semin Intervent Radiol. 2014;31(2):167–179. doi: 10.1055/s-00000068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kostrzewa M, Diezler P, Michaely H, et al. Microwave thermal ablation of osteoid osteomas using dynamic MR imaging for early treatment assessment: preliminary experience. J Vasc Interv Radiol. 2014;25(1):106–111. (Epub 2013 Nov 26). doi: 10.1016/j.jvir.2013.09.009 [DOI] [PubMed] [Google Scholar]
- 15.Rinzler ES, Shivaram GM, Shaw DW, Monroe EJ, Koo KSH. Microwave ablation of osteoid osteoma: initial experience and efficacy. Pediatr Radiol. 2019;49(4):566–570. doi: 10.1007/s00247-018-4327-1 [DOI] [PubMed] [Google Scholar]
- 16.Kastler A, Alnassan H, Aubry S, Kastler B. Microwave thermal ablation of spinal metastatic bone tumors. J Vasc Interv Radiol. 2014;25(9):1470–1475. (Epub 2014 Jul 4). doi: 10.1016/j.jvir.2014.06.007 [DOI] [PubMed] [Google Scholar]
- 17.Kurup AN, Callstrom MR. Expanding role of percutaneous ablative and consolidative treatments for musculoskeletal tumours. Clin Radiol. 2017;72(8):645–665. doi: 10.1016/j.crad.2017.02.019 [DOI] [PubMed] [Google Scholar]
- 18.Fan QY, Ma BA, Qlu XC, Li YL, Ye J, Zhou Y. Preliminary report on treatment of bone tumors with microwave-induced hyperthermia. Bioelectromagnetics. 1996;17(3):218–222. doi: [DOI] [PubMed] [Google Scholar]
- 19.Fan QY, Ma BA, Zhou Y, Zhang MH, Hao XB. Bone tumors of the extremities or pelvis treated by microwave-induced hyperthermia. Clin Orthop Relat Res. 2003;406:165–175. doi: 10.1097/00003086-200301000-00026 [DOI] [PubMed] [Google Scholar]
- 20.Fan QY, Zhou Y, Zhang M, et al. Microwave ablation of malignant extremity bone tumors. Springerplus. 2016;5(1):1373. doi: 10.1186/s40064-016-3005-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu B, Yuan Z, Wei CY. Combined microwave ablation and minimally invasive open decompression for the management of thoracic metastasis in breast cancer. Cancer Manag Res. 2018;10:1397–1401. doi: 10.2147/CMAR [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fan Q, Ma B, Guo A, et al. Surgical treatment of bone tumors in conjunction with microwave-induced hyperthermia and adjuvant immunotherapy. A preliminary report. Chin Med J (Engl). 1996;109(6):425–431. [PubMed] [Google Scholar]