Abstract
Objective
Ascites becomes refractory to diuretics in cirrhotic patients, who then require repeated large-volume paracentesis or cell-free and concentrated ascites reinfusion therapy (CART). The objective of this study was to confirm the safety and efficacy of CART, evaluate the actual situations with respect to the prescription of diuretics and determine the role of diuretics after the introduction of CART.
Patients and Methods
We recruited 34 cirrhotic patients who received CART with concomitant diuretics using furosemide (76.2%), spironolactone (48.5%), thiazide (4.0%) and tolvaptan (53.5%) from a post-marketing surveillance of CART.
Results
CART improved the tested clinical indices, i.e., body weight, abdominal circumference, performance status, dietary intake, total protein and albumin. The intervals of CART sessions were significantly prolonged in patients who received tolvaptan (mean, 22.5 days) compared to those not receiving tolvaptan (mean, 10.8 days) (p<0.001). The drop-out rate was significantly decreased in patients receiving tolvaptan compared to those not receiving tolvaptan when drop-out was defined as paracentesis (p<0.05).
Conclusion
We confirmed that CART is an effective treatment for refractory ascites occurring in cirrhotic patients. The administration of tolvaptan in combination with CART leads to a significantly reduced rate of ascites accumulation.
Keywords: liver cirrhosis, refractory ascites, cell-free and concentrated ascites reinfusion therapy, tolvaptan
Introduction
Ascites is the pathologic accumulation of fluid in the peritoneal cavity and is a cardinal sign of disease progression (1,2). Although sodium restriction and diuretics remain the cornerstones of management for ascites, many patients require additional therapy when they become refractory to this treatment. Patients who repeatedly receive large-volume paracentesis, which involves frequent hospital visits, experience a poor quality of life (QOL) (3,4). In order to manage refractory ascites, some cirrhotic patients have selected transjugular intrahepatic portosystemic shunt or peritoneovenous shunt to improve their QOL (5,6). Over the past several decades, new interventions and methodologies have been introduced, such as peritoneal-urinary drainage and cell-free and concentrated ascites reinfusion therapy (CART) (7,8).
The reinfusion of concentrated ascites was developed as a secondary modality to large-volume paracentesis in Japan. CART has been proven to be as safe and effective a treatment as large-volume paracentesis with albumin infusion (8). One of the primary benefits of CART is the reduced use of albumin. At present, the CART procedure may be more expensive than albumin infusion, depending on the amount of protein re-infused. However, albumin transfusion may cause infection and entails problems associated with the overuse of blood derivatives. In addition, insurance does not fully cover the cost of albumin. The manufacturer conducted post-marketing surveillance for CART from 2014 to 2015 and reported on the clinical data obtained from 147 patients undergoing a total of 356 sessions (9). Among the patients in this study, cancer was the most common primary disease (85.9%), followed by cirrhosis (11.4%).
The effect of the V2 receptor antagonist tolvaptan has been explored in combination with low-dose diuretics (furosemide ?40 mg/day and spironolactone ?25 mg/day; or furosemide ?20 mg/day and spironolactone ?50 mg/day) in cirrhotic patients with ascites (10,11). In Japan, tolvaptan was approved for administration to hepatic cirrhosis patients with fluid retention in 2013. Recently, the combination of CART with diuretics was thought to be a useful treatment in patients with refractory ascites, but it has not been validated. We therefore grappled with the research question, “Can diuretics, including tolvaptan, extend the intervals between CART sessions of ascites patients?”
In the present study, we selected cirrhotic patients with ascites from among post-marketing surveillance data. We then evaluated the prescription of diuretics after the introduction of CART and the intervals of CART sessions with respect to each diuretic administration. In addition, we reevaluated the safety and efficacy of CART in cirrhotic patients with refractory ascites.
Materials and Methods
Study design and patients
The post-marketing surveillance by the manufacturer was conducted in accordance with the Good Post-marketing Surveillance Practice, an ordinance of the Ministry of Health, Labour and Welfare. This study conforms to the provisions of the Declaration of Helsinki.
Twenty-two centers in Japan offering CART sessions were selected. All of the patients who underwent CART at each participating center from January 2014 to January 2015 were registered consecutively (9). Thirty-four of the 147 patients presented with liver cirrhosis and were included in this study. Cirrhosis was diagnosed based on liver function test values, diagnostic imaging with ultrasonography and computed tomography (CT), and the evaluation of esophageal varices on upper endoscopy. The diagnosis of hepatocellular carcinoma (HCC) was established via dynamic radiological findings using CT, magnetic resonance imaging (MRI) or angiography and clinical data. The HCC stage was determined as previously reported in studies for staging of HCC conducted by the Liver Cancer Study Group of Japan (12).
Survey items
The characteristics of patients in this study included the age, sex, the presence/absence of complications [i.e., chronic renal failure (estimate glomerular filtration rate (eGFR)<30 mL/min/m2)], overt hepatic encephalopathy (present or past), esophageal varices and HCC, a history of ascites therapy (i.e., large-volume paracentesis), concomitant use of anticancer drugs and diuretics and the number of CART sessions during the surveillance period.
The following patient characteristics were examined: body weight, abdominal circumference, Eastern Cooperative Oncology Group (ECOG) performance status (PS), dietary intake and blood test results (13). Blood tests included a complete blood count (white blood cells, red blood cells, hemoglobin, hematocrit [Hct], and platelets), serum levels of total protein, albumin, total bilirubin, urea nitrogen, eGFR and creatinine. All adverse events that had a possible association with CART procedures or concomitant drugs were documented by the physicians caring for the patients.
CART procedure
The standard CART procedure is comprised of three steps: i) drainage of the ascites into a designated bag by abdominal paracentesis; ii) removal of malignant cells and bacteria by filtration and removal of excess fluid and electrolytes by concentration; and iii) reinfusion of the filtered and concentrated ascites (14). The amount of ascites collected was dependent on the parameters set forth by the individual centers. We investigated the appearance of the original ascites and amount and composition of the original and processed ascites. The amounts of total protein and albumin in ascites were estimated by multiplying the volume of ascites by the total protein concentration and albumin concentration, respectively.
It was possible that the circulating blood volume might change due to an increase in colloidal osmotic pressure during the re-infusion process. Based on the assumption that the volume of the erythrocytes remained unaltered by the CART procedures, we corrected the clinical indices by the following equation, taking into account the change in the Hct value (9):
Adjusted post-CART value=Post-CART value×(pre-CART Hct value)/(post-CART Hct value).
Statistical analyses
All data were expressed as the mean±standard deviation (SD) or proportion (%). Continuous data were analyzed by Student's t-test or a one-way analysis of variance, while categorical data were analyzed by the χ2 test or Fisher's exact test. A paired t-test was used for comparing values before and after CART in the same patient. Relationships between variables were determined using the Spearman's correlation coefficient. The cumulative drop-out rates were estimated with the Kaplan-Meier method and compared between groups by the log-rank test. A logistic regression analysis was used for the multivariate analysis. Missing data were excluded from all analyses. P values less than 0.05 were considered statistically significant. Statistical analyses were performed using the JMP software program, ver. 12.0 (SAS Institute, Cary, USA).
Results
Patient characteristics
The characteristics of study patients are shown in Table 1. We recruited 34 cirrhotic patients (27 men and 7 women). Seventeen of the 34 patients had HCC in addition to cirrhosis. The etiology of ascites was categorized based on transudative serum-ascites albumin gradient values (15,16). Complications were found in 35.3% of cases. The number of CART sessions per patient during the surveillance period was 3.0±2.9 in cirrhotic patients. Concomitant medications taken during CART were anticancer drugs (3 doses, 3%), furosemide (77 doses, 76.2%), spironolactone (49 doses, 48.5%), thiazide (4 doses, 4%) and tolvaptan (54 doses, 53.5%).
Table 1.
Patient Characteristics before CART Procedures.
| Items | n=34, total 101 sessions | |
|---|---|---|
| Age, years | 68.7±10.6 (54-93) | |
| Male/Female | 27/7 | |
| Complications | ||
| HCC | 17 (50.0%) | |
| chronic renal failure | 8 (23.5%) | |
| hepatic encephalopathy | 3 (8.8%) | |
| esophageal varices | 7 (20.6%) | |
| Details of CART procedures the number of sessions | 3.0±2.9 (1-13) | |
| Body weight (kg) | 66.1±14.0 (47-104) | |
| Abdominal circumferences (cm) | 99.0±8.8 (76-120) | |
| Previous history of large volume paracentesis | 13 (12.9%) | |
| Concomitant drugs | ||
| anticancer drugs | 3 (3.0%) | |
| furosemide | 77 (76.2%) | |
| spironolactone | 49 (48.5%) | |
| thiazide | 4 (4.0%) | |
| tolvaptan | 54 (53.5%) | |
| Serum biochemistry | ||
| albumin (g/dL) | 2.4±0.5 (1.4-3.5) | |
| total bilirubin (mg/dL) | 2.1±2.9 (0.3-22.1) | |
| eGFR (mL/min/1.73?m2) | 51.9±21.7 (9.7-119.9) |
CART: cell-free and concentrated ascites reinfusion therapy, HCC: hepatocellular carcinoma, eGFR: estimate glomerular filtration rate
Efficacy of CART procedures
Albumin infusion between pre- and post-CART was done in 6 (for a total of 20 sessions) of the 34 patients (total 101 sessions). We analyzed the changes in the CART procedure due to albumin infusion (for a total 20 sessions) while excluding 6 patients.
The characteristics of the ascites before and after the CART procedures are shown in Table 2. The volume of processed ascites was 413±160 g after CART, giving a concentration ratio of 11.4 in terms of volume. The levels of total protein and albumin were 10.0±4.1 g/dL and 4.6±2.2 g/dL, and the calculated amounts of total protein and albumin were 40.6±22.1 g and 18.4±9.7 g, respectively. The clinical changes observed after CART compared to the patient status before CART are also shown in Table 2. Significant positive effects were observed in the body weight, abdominal circumference, dietary intake, ECOG PS and total protein and albumin levels. The Hct value after CART was significantly lower than that before CART, suggesting an increase in the circulating plasma volume, which potentiated a dilution effect. There was a significant elevation in the albumin level after CART (pre-CART, 2.3±0.5 g/dL vs. post-CART, 2.4±0.5 g/dL, p<0.01; post-CART corrected Hct, 2.5±0.5 g/dL, p<0.01). The degree of elevation in albumin is generally small due to a dilution effect. However, the results, when corrected for the changes in Hct, revealed a clear increase in the albumin level after CART, suggesting that CART replenished these factors. We observed no significant difference in other blood values, including white blood cells, red blood cells, hemoglobin, platelets, total bilirubin, urea nitrogen and creatinine (data not shown).
Table 2.
Changes of Clinical Indices between Pre- and Post-CART.
| Pre-CART | Post-CART | p value | ||||
|---|---|---|---|---|---|---|
| Amount and composition of ascites | Original ascites | Processed ascites | ||||
| ascitic fluid (g) | 4,727.4±2,206.7 | 412.9±160.0 | <0.001 | |||
| total protein concentration (g/dL) | 1.2±0.7 | 10.0±4.1 | <0.001 | |||
| albumin concentration (g/dL) | 0.6±0.4 | 4.6±2.2 | <0.001 | |||
| total protein amount (g) | 50.8±26.7 | 40.6±22.1 | <0.001 | |||
| albumin amount (g) | 23.5±12.3 | 18.4±9.7 | <0.001 | |||
| Patients characteristics | ||||||
| body weight (kg) | 75.0±15.7 | 70.3±15.0 | <0.001 | |||
| abdominal circumferences (cm) | 101.9±9.7 | 94.9±9.3 | <0.001 | |||
| dietary intake (%) | 54.4±28.1 | 63.2±28.9 | <0.001 | |||
| ECOG PS (%) | 2.4±1.0 | 2.2±1.1 | <0.01 | |||
| Serum biochemistry corrected Hct changes | ||||||
| total protein (g/dL) | 6.3±0.9 | 6.8±1.0 | <0.001 | |||
| albumin (g/dL) | 2.3±0.5 | 2.5±0.5 | <0.001 | |||
| Serum biochemistry | ||||||
| total protein (g/dL) | 6.3±0.9 | 6.5±0.9 | <0.05 | |||
| albumin (g/dL) | 2.3±0.5 | 2.4±0.5 | <0.01 |
CART: cell-free and concentrated ascites reinfusion therapy, ECOG PS: Eastern Cooperative Oncology Group performance status, Hct: hematocrit
Role of diuretics
No patients were transferred to another hospital or died during the surveillance period. We found that two patients (three sessions) received paracentesis from one CART session to the next. We compared the interval of CART sessions between patients with and without tolvaptan, excluding 3 paracentesis sessions (3 sessions) and 14 sessions using an albumin preparation.
The characteristics of the study patients with or without tolvaptan are shown in Table 3. The historical incidence rate of furosemide, spironolactone and thiazide use was significantly higher in cirrhotic patients who received tolvaptan than in those not receiving tolvaptan. The patients with coexisting HCC were only seen in the non-tolvaptan group. In addition, the serum albumin levels were significantly lower in the non-tolvaptan group than in the tolvaptan group. The intervals of CART sessions were significantly longer in patients who received tolvaptan (mean, 22.5 days) than in those not receiving tolvaptan (mean, 10.8 days) (p<0.001, Fig. 1).
Table 3.
Patient Characteristics and before CART Procedures in Patients with and without Tolvaptan.
| without tolvaptan (39 sessions) |
with tolvaptan (22 sessions) |
p value | ||||
|---|---|---|---|---|---|---|
| Age, years | 68.4±10.5 (56-93) | 63.2±5.8 (56-73) | 0.310 | |||
| Male/Female | 8/2 | 5/1 | 1.000 | |||
| Complications | ||||||
| HCC | 7 (70%) | 0 | 0.011 | |||
| (I/II/III/IV) | (0/0/1/6) | |||||
| chronic renal failure | 1 (10%) | 0 (0.0%) | 1.000 | |||
| hepatic encephalopathy | 1 (10%) | 1 (16.7%) | 1.000 | |||
| esophageal varices | 0 (0%) | 2 (33.3%) | 0.126 | |||
| Body weight (kg) | 63.8±7.1 (53-77) | 76.8±21.3 (50-101) | <0.01 | |||
| Abdominal circumferences (cm) | 98.8±7.5 (76-107) | 103.3±10.9 (90-120) | 0.179 | |||
| Previous history of large volume paracentesis | 3 (7.7%) | 0 (0.0%) | 0.547 | |||
| Concomitant drugs | ||||||
| anticancer drugs | 1 (2.6%) | 1 (4.5%) | 1.000 | |||
| furosemide | 18 (46.2%) | 22 (100%) | <0.001 | |||
| spironolactone | 9 (23.5%) | 21 (95.5%) | <0.001 | |||
| thiazide | 0 (0%) | 3 (13.6%) | 0.043 | |||
| Serum biochemistry | ||||||
| albumin (g/dL) | 2.0±0.4 | 2.7±0.4 | <0.001 | |||
| total bilirubin (mg/dL) | 2.0±0.5 | 2.0±1.1 | 0.912 | |||
| eGFR (mL/min/1.73 m2) | 56.1±23.8 | 55.8±13.4 | 0.958 | |||
| Amount and composition of ascites | ||||||
| ascitic fluid (g) | 3,825.3±1,429.7 | 5,458.9±2,296.7 | <0.01 | |||
| processed ascites (g) | 362.4±156.8 | 369.1±125.7 | 0.867 | |||
| total protein amount (g) | 54.2±25.1 | 48.1±22.8 | 0.435 | |||
| albumin amount (g) | 21.1±11.4 | 27.6±9.5 | 0.073 |
CART: cell-free and concentrated ascites reinfusion therapy, HCC: hepatocellular carcinoma, eGFR: estimate glomerular filtration rate
Figure 1.
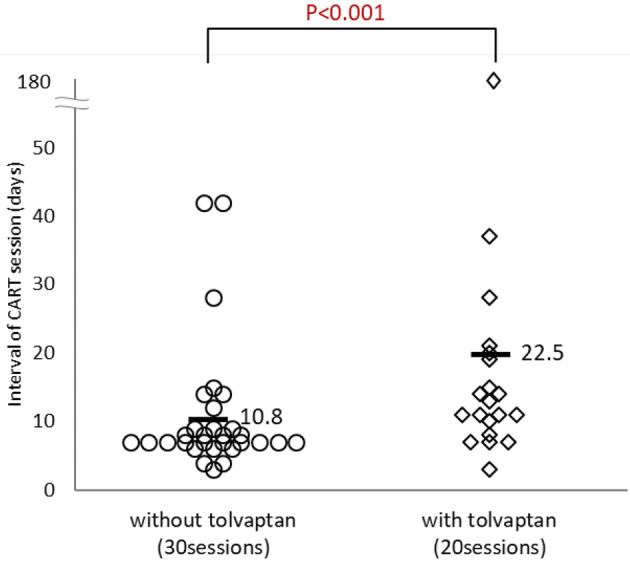
The intervals of CART sessions (days) in cirrhotic patients with or without tolvaptan, excluding patients who received paracentesis and albumin preparation. CART: cell-free and concentrated ascites reinfusion therapy
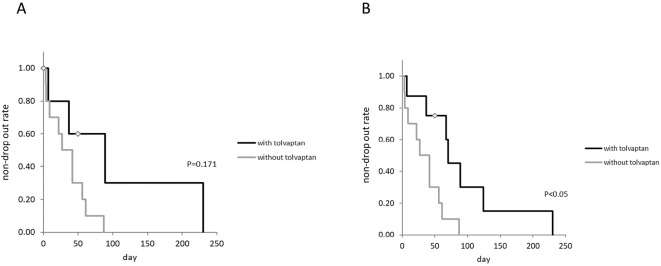
The drop-out rate tended to be lower in the patients receiving tolvaptan than in those not receiving tolvaptan when drop-out was defined as receiving paracentesis or using albumin (Fig. 2A). The drop-out rate was significantly lower in patients receiving tolvaptan than in those not receiving tolvaptan when drop-out was defined as receiving paracentesis (p<0.05, Fig. 2B). We also calculated the rate of ascites accumulation (volume of ascites collected divided by the CART interval). The accumulation rates of ascites were 492.3 mL and 536.9 mL with and without tolvaptan, respectively, showing no statistical significance.
Figure 2.
A: Drop-out tended to be lower in patients who received tolvaptan than in those not receiving tolvaptan when drop-out was defined as receiving paracentesis or using albumin. B: Drop-out was significantly lower in patients receiving tolvaptan than in those not receiving tolvaptan when drop-out was defined as receiving paracentesis.
With respect to differences between the two groups, such as in albumin levels, we further investigated whether or not the baseline albumin levels and changes in albumin were related to the intervals of CART sessions. There were no relationships between the albumin levels and intervals of CART sessions (r=0.269, ns; albumin, 1.4-2.2 g/dL vs. ?2.3 g/dL, p=0.715) nor between the changes in albumin levels and intervals of CART sessions (r=0.199, ns; changes in albumin, -0.5-0.1 g/dL vs. ?0.2 g/dL, p=0.591). In addition, we did not detect any association between coexisting HCC and the intervals of CART sessions (p=0.836) in this study.
Although a logistic regression analysis was performed to investigate the association between intervals of CART sessions and albumin levels, changes in albumin levels, presence of tolvaptan prescription and presence of HCC, none of these factors were found to be associated with CART intervals. There was also no marked difference in the intervals of CART sessions with respect to other diuretics, such as furosemide, thiazide or spironolactone.
Next, we evaluated the clinical changes between pre- and post-CART in patients with cirrhosis with or without tolvaptan treatment. The body weight was significantly higher after CART in patients with tolvaptan treatment than in those without tolvaptan treatment, although there were no marked difference between these patient groups concerning other factors, such as changes in abdominal circumference, dietary intake and EOCG PS (Table 4).
Table 4.
Changes of Clinical Indices between Pre- and Post-CART in Patients with or without Tolvaptan.
| without tolvaptan (39 sessions ) |
p value (pre vs. post) |
with tolvaptan (22 sessions) |
p value (pre vs. post) |
p value (without vs. with tolvaptan) |
||
|---|---|---|---|---|---|---|
| Patients characteristics | ||||||
| body weight (kg) | pre-CART | 63.8±7.1 | 76.8±21.3 | |||
| post-CART | 60.5±7.3 | <0.001 | 71.7±20.3 | <0.001 | ||
| changes in body weight (kg) | -3.4±1.7 | -5.1±1.7 | <0.01 | |||
| abdominal circumferences (cm) | pre-CART | 98.8±7.5 | 103.3±10.9 | |||
| post-CART | 91.1±6.7 | <0.001 | 94.4±12.1 | <0.001 | ||
| changes in abdominal circumferences (cm) | -7.7±3.4 | -8.9±4.0 | 0.373 | |||
| changes in dietary intake (%) | 6.6±12.4 | 6.3±25.3 | 0.967 | |||
| changes in ECOG PS (%) | -0.2±0.4 | -0.1±0.3 | 0.631 | |||
| Serum biochemistry corrected Hct changes | ||||||
| total protein (g/dL) | pre-CART | 6.0±0.9 | 6.6±0.8 | |||
| post-CART | 6.6±1.2 | <0.001 | 7.0±0.8 | <0.05 | ||
| changes in total protein (g/dL) | 0.7±0.7 | 0.3±0.5 | 0.105 | |||
| albumin (g/dL) | pre-CART | 2.0±0.4 | 2.7±0.4 | |||
| post-CART | 2.2±0.5 | <0.01 | 2.9±0.4 | <0.05 | ||
| changes in albumin (g/dL) | 0.2±0.3 | 0.2±0.3 | 0.672 | |||
| Serum biochemistry | ||||||
| total protein (g/dL) | pre-CART | 6.0±0.9 | 6.6±0.8 | |||
| post-CART | 6.3±1.1 | <0.001 | 6.5±0.7 | 0.359 | ||
| changes in total protein (g/dL) | 0.4±0.5 | -0.1±0.6 | <0.01 | |||
| albumin (g/dL) | pre-CART | 2.0±0.4 | 2.7±0.4 | |||
| post-CART | 2.2±0.5 | <0.01 | 2.7±0.4 | 1.000 | ||
| changes in albumin (g/dL) | 0.2±0.3 | 0.0±0.3 | 0.053 | |||
CART: cell-free and concentrated ascites reinfusion therapy, ECOG PS: Eastern Cooperative Oncology Group performance status, Hct: hematocrit
Frequencies of adverse events
Adverse events related to the drainage of ascites occurred in 4% of the recorded sessions (4/101). Adverse events included hypotension (3 cases), dyspnea (1 case) during/after drainage of the ascites and hyperammonemia after reinfusion (1 case). Adverse events related to the reinfusion process occurred in 13.9% of sessions (14/101). The majority of adverse events were a fever and chills, and none were considered severe, with patients fully recovering from the events. Although the common adverse events related to tolvaptan treatment are thirst and pollakiuria, severe case of these symptoms were not reported. Neither increased bilirubin or sodium, acute renal failure nor hepatic encephalopathy were recorded.
Discussion
First, we reevaluated the safety and efficacy of CART in a post-marketing surveillance setting in patients with cirrhosis. CART improved tested clinical indices, i.e., body weight, abdominal circumference, dietary intake and ECOG PS, presumably due to the favorable protein replenishment effect. The study by the Kansai CART Study Group showed no marked changes in total serum albumin values (published in Japanese). However, the results, when corrected for the changes in Hct, revealed a clear increase in both total protein and albumin levels after CART, likely leading to a reduction in the required doses of albumin preparations to be administered. This study also showed an improvement in the PS. The alleviation of symptoms and the recovery of activities in daily life in cases of malignancy-related ascites were reported after CART in a previous study, and this may be associated with an improvement in the PS (17). Generally, a fever is a significant problem in the clinical setting, but CART does not lead to a febrile state (18). Indeed, only mild and transient elevation of body temperature was observed in this study, which is in lockstep agreement with previous findings in patients with malignant ascites (9).
Both the European Association for the Study of the Liver and Japanese Society of Gastroenterology guidelines indicate that the first-line treatment for patients with refractory ascites is large-volume paracentesis coupled with the administration of intravenous albumin (2,19). CART was developed in Japan as a surrogate for large-volume paracentesis (2). We confirmed in the present study that many patients received concomitant diuretics during CART, i.e., furosemide (76.2%), spironolactone (48.5%), thiazide (4.0%) and tolvaptan (53.5%). This study also revealed that most patients underwent multiple CART sessions. We found that cirrhotic patients administered tolvaptan had long intervals between CART sessions. The Japanese government approved tolvaptan for the treatment of hepatic edema in 2013. This diuretic is used in combination therapy to treat fluid retention in cirrhosis when existing diuretics, such as spironolactone and furosemide, fail to achieve a sufficient therapeutic effect (10,11). Based on the results of this study, the combination of tolvaptan with CART may be useful for reducing the frequency of CART in patients with refractory ascites. Therapeutic large-volume paracentesis or CART instantaneously relieves symptoms; however, despite experienced personnel on staff carrying out large-volume paracentesis followed by colloid volume expansion, circulatory dysfunction can still be induced (20,21). This circulatory dysfunction may also facilitate the development of complications associated with paracentesis, such as hepatorenal syndrome, hyponatremia, hepatic encephalopathy and bacterial peritonitis (1). Therefore, patients who develop refractory ascites should not be exposed to multiple bouts of therapeutic large-volume paracentesis.
The present study had several limitations. There were differences between patients with and without tolvaptan, such as in the pre-CART albumin levels, frequency of HCC, amount of collected ascites, change in body weight and concomitant drugs. In addition, the results included multiple data from the same patients. We lacked information on the initiation of diuretics, including tolvaptan. This was an observational study, so the causal relationship of each factor with the outcome cannot be inferred. Prospective interventional studies in a large sample size will be required in the future.
The administration of tolvaptan in combination with CART leads to a significant reduction in the accumulation rate of ascites. This approach can be considered for patients who are not eligible for liver transplantation and who cannot tolerate repeated CART.
Author's disclosure of potential Conflicts of Interest (COI).
Motoh Iwasa: Honoraria, Otsuka Pharmaceutical. Ayako Isoai: Employment, Asahi Kasei Medical. Ryosuke Kobayashi: Employment, Asahi Kasei Medical. Naoko Torii: Employment, Asahi Kasei Medical. Noriko Soneda: Employment, Asahi Kasei Medical.
References
- 1. Moore KP, Wong F, Gines P, et al. . The management of ascites in cirrhosis: report on the consensus conference of the International Ascites Club. Hepatology 38: 258-266, 2003. [DOI] [PubMed] [Google Scholar]
- 2. Fukui H, Saito H, Ueno Y, et al. . Evidence-based clinical practice guidelines for liver cirrhosis 2015. J Gastroenterol 51: 629-650, 2016. [DOI] [PubMed] [Google Scholar]
- 3. Grabau CM, Crago SF, Hoff LK, et al. . Performance standards for therapeutic abdominal paracentesis. Hepatology 40: 484-488, 2004. [DOI] [PubMed] [Google Scholar]
- 4. Salerno F, Merli M, Riggio O, et al. . Randomized controlled study of TIPS versus paracentesis plus albumin in cirrhosis with severe ascites. Hepatology 40: 629-635, 2004. [DOI] [PubMed] [Google Scholar]
- 5. Gulberg V, Liss I, Bilzer M, Waggershauser T, Reiser M, Gerbes AL. Improved quality of life in patients with refractory or recidivant ascites after insertion of transjugular intrahepatic portosystemic shunts. Digestion 66: 127-130, 2002. [DOI] [PubMed] [Google Scholar]
- 6. Gines P, Arroyo V, Vargas V, et al. . Paracentesis with intravenous infusion of albumin as compared with peritoneovenous shunting in cirrhosis with refractory ascites. N Engl J Med 325: 829-835, 1991. [DOI] [PubMed] [Google Scholar]
- 7. Sola E, Sanchez-Cabus S, Rodriguez E, et al. . Effects of alfapump? system on kidney and circulatory function in patients with cirrhosis and refractory ascites. Liver Transpl 23: 583-593, 2017. [DOI] [PubMed] [Google Scholar]
- 8. Kozaki K, IInuma M, Takagi T, et al. . Cell-free and concentrated ascites reinfusion therapy for decompensated liver cirrhosis. Ther Apher Dial 20: 376-382, 2016. [DOI] [PubMed] [Google Scholar]
- 9. Hanafusa N, Isoai A, Ishihara T, et al. . Safety and efficacy of cell-free and concentrated ascites reinfusion therapy (CART) in refractory ascites: Post-marketing surveillance results. PLoS One 12: e0177303, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Okita K, Sakaida I, Okada M, et al. . A multicenter, open-label, dose-ranging study to exploratively evaluate the efficacy, safety, and dose-response of tolvaptan in patients with decompensated liver cirrhosis. J Gastroenterol 45: 979-987, 2010. [DOI] [PubMed] [Google Scholar]
- 11. Sakaida I, Kawazoe S, Kajimura K, ASCITES-DOUBLEBLIND Study Group, et al. . Tolvaptan for improvement of hepatic edema: a phase 3, multicenter, randomized, double-blind, placebo-controlled trial. Hepatol Res 44: 73-82, 2014. [DOI] [PubMed] [Google Scholar]
- 12. The Liver Cancer Study Group of Japan.. The General Rules for the Clinical and Pathological Study of Primary Liver Cancer. 6th ed. Kanehara, Tokyo, 2015: 26. [Google Scholar]
- 13. Oken MM, Creech RH, Tormey DC, et al. . Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 5: 649-655, 1982. [PubMed] [Google Scholar]
- 14. Ito T, Hanafusa N, Fukui M, et al. . Single center experience of cell-free and concentrated ascites reinfusion therapy in malignancy related ascites. Ther Apher Dial 18: 87-92, 2014. [DOI] [PubMed] [Google Scholar]
- 15. Runyon BA, Montano AA, Akriviadis EA, Antillon MR, Irving MA, McHutchison JG. The serum-ascites albumin gradient is superior to the exudate-transudate concept in the differential diagnosis of ascites. Ann Intern Med 117: 215-220, 1992. [DOI] [PubMed] [Google Scholar]
- 16. Light RW. Clinical practice. Pleural effusion. N Engl J Med 346: 1971-1977, 2002. [DOI] [PubMed] [Google Scholar]
- 17. Ito T, Hanafusa N, Iwase S, et al. . Effects of cell-free and concentrated ascites reinfusion therapy (CART) on symptom relief of malignancy-related ascites. Int J Clin Oncol 20: 623-628, 2015. [DOI] [PubMed] [Google Scholar]
- 18. Zaak D, Paquet KJ, Kuhn R. Prospective study comparing human albumin vs. reinfusion of ultrafiltrate-ascitic fluid after total paracentesis in cirrhotic patients with tense ascites. Z Gastroenterol 39: 5-10, 2001. [DOI] [PubMed] [Google Scholar]
- 19. European Association for the Study of the Liver EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. J Hepatol 53: 397-417, 2010. [DOI] [PubMed] [Google Scholar]
- 20. Salerno F, Merli M, Riggio O, et al. . Randomized controlled study of TIPS versus paracentesis plus albumin in cirrhosis with severe ascites. Hepatology 40: 629-635, 2004. [DOI] [PubMed] [Google Scholar]
- 21. Garcia-Compean D, Blanc P, Larrey D, et al. . Treatment of cirrhotic tense ascites with Dextran-40 versus albumin associated with large-volume paracentesis: a randomized controlled trial. Ann Hepatol 1: 29-35, 2002. [PubMed] [Google Scholar]