Abstract
During the malignant phase of hypertension in patients with primary aldosteronism complicated with severe renal failure, the plasma renin activity may markedly increase with a false negative screening result for primary aldosteronism, thus potentially leading to a missed diagnosis of primary aldosteronism. We herein report the case of 37-year-old man who presented with accelerated-malignant hypertension complicated with severe renal insufficiency. The plasma renin activity was markedly increased in the malignant phase of hypertension, which were atypical results for primary aldosteronism. However, a plain abdominal computed tomography scan revealed a left adrenal nodule, which was diagnosed as aldosterone-producing adenoma by adrenal venous sampling.
Keywords: primary aldosteronism, accelerated-malignant hypertension, plasma renin activity, plasma aldosterone concentration
Introduction
Primary aldosteronism (PA) is the most common cause of secondary hypertension accounting for 5 to 15% of all cases of hypertension (1). PA is characterized by autonomously increased aldosterone secretion and a consequently suppressed plasma renin activity (PRA). Therefore, the simultaneous measurement of the plasma aldosterone concentration (PAC) and PRA, usually resulting in high levels of PAC and low levels of PRA in patients with PA, is recommended as a screening for PA (2-4). However, PRA in patients with PA could be increased under the conditions in which renin release from the kidney is overstimulated by glomerular ischemia, potentially leading to an incorrect diagnosis of PA. We herein report a case of aldosterone-producing adenoma (APA) with markedly increased renin activity caused by accelerated-malignant hypertension complicated with severe renal insufficiency.
Case Report
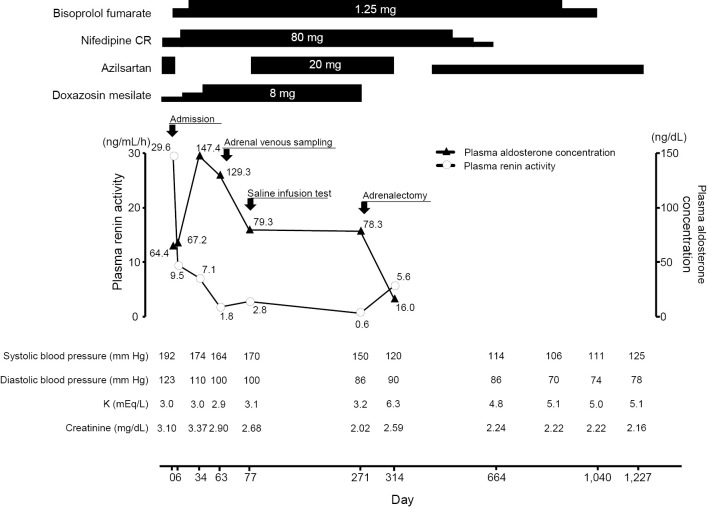
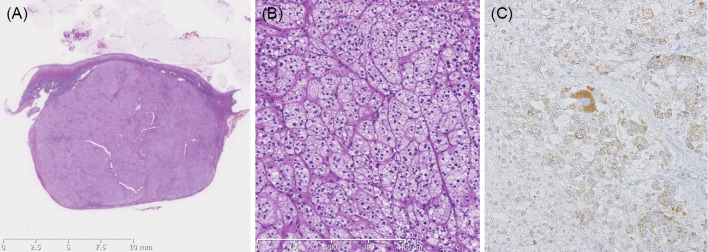
A 37-year-old man with headache and fatigue that had started 1 month earlier was referred to Hiroshima University Hospital because of severe hypertension with a systolic blood pressure of more than 200 mm Hg, even after the initiation of antihypertensive therapy with a calcium-channel blocker and α-blocker, and renal dysfunction. On admission, his blood pressure was 192/122 mm Hg, his pulse was 68 beats per minute, and his body mass index was 19.3 kg/m2. Abdominal bruit was inaudible. The laboratory data revealed renal failure with an elevated serum creatinine level of 3.1 mg/dL (estimated glomerular filtration rate of 20 mL/min/1.73 m2) and hypokalemia with a reduced serum potassium level of 3.1 mEq/L. He had mild proteinuria and hematuria. An electrocardiogram (ECG) and an echocardiogram showed left ventricular (LV) hypertrophy with an increased LV size (end diastolic diameter of 59 mm) and impaired LV systolic function (ejection fraction of 48%). A fundus examination showed hypertensive retinopathy with the Keith-Wagener's grade III, suggesting accelerated-malignant hypertension. Endocrinological tests revealed markedly high PRA of 29.6 ng/mL/h and high PAC of 64.4 ng/dL with PAC/PRA ratio of 2.1, which were atypical results for PA (Fig. 1). Magnetic resonance angiography showed no renal artery stenosis. A plain abdominal computed tomography scan revealed an 18-mm left adrenal nodule (Fig. 2). Although PRA remained high after the malignant phase of hypertension improved (9.5 ng/mL/h at 6 days after admission and 7.1 ng/mL/h at 34 days after admission) (Fig. 1), the possibility of PA was not excluded because of the presence of severe hypertension, onset at a young age, the presence of hypokalemia despite the existence of renal failure, and a left adrenal nodule. We confirmed autonomous aldosterone secretion with high PAC level of 97.0 ng/dL and a low PRA level of 1.8 ng/mL/h after 2-liter saline infusion (Table 1). A definitive diagnosis of an APA in the left adrenal gland was made by adrenal venous sampling (Table 2). Before the operation, PRA further decreased to 0.6 ng/mL/h with PAC/PRA ratio of 130.5, which were typical results for PA (Fig. 1). At 8 months after the first admission, he underwent laparoscopic left adrenalectomy. The histological findings were consistent with adrenocortical adenoma (Fig. 3A and B). The adenoma cells were focally positive for CYP11B2 on immunohistochemistry (Fig. 3C). At 2 months after the left adrenalectomy, PRA was 5.6 ng/mL/h and PAC was 16.0 ng/dL (Fig. 1). After the adrenalectomy, the number of antihypertensive agents required for blood pressure control was reduced. At 3 years after the operation, the blood pressure was well controlled by antihypertensive monotherapy. ECG was normalized and no further impairment of the renal function was observed after the operation (Fig. 1).
Figure 1.
Clinical course.
Figure 2.
An axial section (A) and a coronal section (B) of a plain abdominal computed tomography scan. Arrows indicate the left adrenal tumor.
Table 1.
Saline Infusion Test.
| Before saline infusion | After saline infusion | |||
|---|---|---|---|---|
| PAC (ng/dL) | 79.3 | 97.0 | ||
| PRA (ng/mL/h) | 2.8 | 1.8 | ||
| Blood pressure (mm Hg) | 178/100 | 173/100 | ||
| Pulse rate (bpm) | 48 | 47 |
PAC: plasma aldosterone concentration, PRA: plasma renin activity
Table 2.
Adrenal Venous Sampling.
| Before ACTH stimulation | After ACTH stimulation | |||||||
|---|---|---|---|---|---|---|---|---|
| PAC (ng/dL)/Cortisol (µg/dL) | Ratio | PAC (ng/dL)/Cortisol (µg/dL) | Ratio | |||||
| Left adrenal vein | 2,571.3/21.5 | 119.6 | 32,902.6/931.6 | 35.32 | ||||
| Right adrenal vein | 136.9/13.9 | 9.85 | 4,356/820 | 5.31 | ||||
| Inferior vena cava | 78.3/12.7 | 6.17 | 152/19.1 | 7.96 | ||||
| Lateralized ratio | 119.6/9.86 | 12.14 | 35.32/5.31 | 6.65 | ||||
| Contralateral ratio | 9.86/6.17 | 1.6 | 5.31/7.96 | 0.67 | ||||
PAC: plasma aldosterone concentration, PRA: plasma renin activity
Figure 3.
Histological examination of the left adrenal tumor (size, 19×19×12 mm) (A) consistent with an adrenocortical adenoma (B). Immunohistochemistry for CYP11B2 (C).
Discussion
Measuring the PAC and PRA is recommended as a screening for secondary hypertension. PA, the most common cause of secondary hypertension, is characterized by autonomously increased aldosterone production and consequently suppressed PRA, leading to high PAC and low PRA in patients with PA (4,5). However, the present case with APA exhibited a markedly increased PRA without suppression, which may be attributed to accelerated-malignant hypertension and severe renal failure (6,7). Renal injury induced by accelerated-malignant hypertension might cause glomerular ischemia in concert with reduced renal perfusion due to hypertensive nephrosclerosis, resulting in an overstimulation of renin release. PRA was gradually decreased after control of the acute presentation and markedly decreased to 0.6 ng/mL/h after 8 months (Fig. 1). A serial assessment of PAC and PRA is needed to detect PA in patients with accelerated-malignant hypertension complicated with renal insufficiency.
Accelerated-malignant hypertension is rare in patients with PA (8). Although we do not know the precise pathology underlying the occurrence of accelerated-malignant hypertension in the present case, excess dietary salt intake might have been associated with the occurrence of accelerated-malignant hypertension in the present case. The patient liked salty food and often ate instant noodles for many years. He was aware of his elevated blood pressure 3 years before admission. However, his dietary habits remained unchanged and his high blood pressure was left untreated, which might have caused the accelerated-malignant hypertension.
The renal function was severely impaired in the present case. Previous studies in which a renal biopsy was performed during adrenalectomy in patients with APA showed arteriolosclerosis of intrarenal vessels with hyalinized glomeruli and interstitial fibrosis, findings that are consistent with hypertensive vascular damage (7,9). Although clinical evidence supporting a direct association between aldosterone and renal damage is limited, experimental studies have indicated that aldosterone per se causes renal injury (10). Therefore, the impairment of renal function in the present case might have been caused by severe systemic hypertension, highly excreted aldosterone from the left APA, and accelerated-malignant hypertension.
A previous study has shown that creatinine clearance correlates inversely with PRA in patients with PA, indicating that PRA tends to be higher in PA patients with renal failure (9). There have been several reports on PA cases with renal failure (7,8,11-18). To our knowledge, however, only 6 PA cases with a clinical course similar to that of the present case (i.e., PA with renal failure, accelerated-malignant hypertension, increased PRA, and false-negative result of PAC/PRA ratio) have been reported (11-16). PRA is markedly increased in PA patients with renal failure complicated with accelerated-malignant hypertension, resulting in a false-negative screening result for PA with a low PAC/PRA ratio (11-16), whereas PRA is not suppressed, but it is also not markedly increased in PA patients with renal failure who are not complicated with accelerated-malignant hypertension (7).
We performed a saline infusion test as a confirmatory test for PA in the present case. A captopril challenging test or a furosemide upright test was not an appropriate confirmatory test in the present case because of the already elevated PRA at baseline. Although there was a possibility that further blood pressure elevation or pulmonary congestion was caused by the saline infusion test in the present case with high blood pressure and renal failure, we had no choice but to select the saline infusion test for confirmation of autonomous aldosterone secretion. The blood pressure level at baseline (178/100 mm Hg) and that after the saline infusion (173/100 mm Hg) were not different and no symptoms were observed during the test (Table 1).
There was a possibility that the use of a contrast medium during adrenal venous sampling or adrenalectomy performed under general anesthesia led to further impairment of the renal function in the present case. However, previous studies have shown that the rates of cardiovascular morbidity and mortality are higher in patients with PA than in patients with essential hypertension and that adrenalectomy decreases the long-term all-cause mortality in patients with APA (19-21). The clinical potential benefits of adrenalectomy, such as an improvement of cardiovascular morbidity and mortality and a reduction in the number of antihypertensive agents were likely to outweigh the risk of a further impairment of the renal function in this young patient with target organ damage, including renal failure, left ventricular hypertrophy, and hypertensive retinopathy. Therefore, adrenal venous sampling and adrenalectomy were performed in the present case. We used only 4 mL of contrast medium during the adrenal venous sampling and contrast-induced nephropathy did not occur. In addition, no further impairment of the renal function was observed after the adrenalectomy. Moreover, after the adrenalectomy, the patient's blood pressure gradually decreased and was well controlled by antihypertensive monotherapy and ECG was normalized. Therefore, adrenalectomy is thought to have been clinically beneficial for the present case.
Informed consent was obtained from all the patients included in the study.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Young WF. Primary aldosteronism: renaissance of a syndrome. Clin Endocrinol (Oxf) 66: 607-618, 2007. [DOI] [PubMed] [Google Scholar]
- 2. Nishikawa T, Omura M, Satoh F, et al. Guidelines for the diagnosis and treatment of primary aldosteronism-the Japan Endocrine Society 2009. Endocr J 58: 711-721, 2011. [DOI] [PubMed] [Google Scholar]
- 3. Shimamoto K, Ando K, Fujita T, et al. The Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2014). Hypertens Res 37: 253-390, 2014. [DOI] [PubMed] [Google Scholar]
- 4. Funder JW, Carey RM, Mantero F, et al. The management of primary aldosteronism: case detection, diagnosis, and treatment: an endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 101: 1889-1916, 2016. [DOI] [PubMed] [Google Scholar]
- 5. Monticone S, Viola A, Tizzani D, et al. Primary aldosteronism: who should be screened? Horm Metab Res 44: 163-169, 2012. [DOI] [PubMed] [Google Scholar]
- 6. Kawazoe N, Eto T, Abe I, et al. Pathophysiology in malignant hypertension: with special reference to the renin-angiotensin system. Clin Cardiol 10: 513-518, 1987. [DOI] [PubMed] [Google Scholar]
- 7. Oelkers W, Diederich S, Bahr V. Primary hyperaldosteronism without suppressed renin due to secondary hypertensive kidney damage. J Clin Endocrinol Metab 85: 3266-3270, 2000. [DOI] [PubMed] [Google Scholar]
- 8. Zarifis J, Lip GY, Leatherdale B, Beevers G. Malignant hypertension in association with primary aldosteronism. Blood Press 5: 250-254, 1996. [DOI] [PubMed] [Google Scholar]
- 9. Catena C, Colussi G, Nadalini E, et al. Relationships of plasma renin levels with renal function in patients with primary aldosteronism. Clin J Am Soc Nephrol 2: 722-731, 2007. [DOI] [PubMed] [Google Scholar]
- 10. Hollenberg NK. Aldosterone in the development and progression of renal injury. Kidney Int 66: 1-9, 2004. [DOI] [PubMed] [Google Scholar]
- 11. Baglin A, Weiss Y, Safar M, Milliez P. Primary hyperaldosteronism with malignant hypertension. Nouv Presse Med 2: 295-297, 1973(in French). [PubMed] [Google Scholar]
- 12. Iyori S, Saruta T, Kondo K, Ozawa Y, Hata J. Renin and juxtaglomerular apparatus in a patient with primary aldosteronism complicated by chronic renal failure. South Med J 69: 951-952, 1976. [DOI] [PubMed] [Google Scholar]
- 13. Iwaoka T, Umeda T, Sato T, Katsuragi S, Takeuchi T. High plasma renin activities in primary aldosteronism with malignant hypertension. A case report. Jpn Heart J 21: 423-428, 1980. [DOI] [PubMed] [Google Scholar]
- 14. Ideishi M, Kishikawa K, Kinoshita A, et al. High-renin malignant hypertension secondary to an aldosterone-producing adenoma. Nephron 54: 259-263, 1990. [DOI] [PubMed] [Google Scholar]
- 15. Oka K, Hayashi K, Nakazato T, Suzawa T, Fujiwara K, Saruta T. Malignant hypertension in a patient with primary aldosteronism with elevated active renin concentration. Intern Med 36: 700-704, 1997. [DOI] [PubMed] [Google Scholar]
- 16. Suzuki H, Asano K, Eiro M, et al. Recovery from renal failure in malignant hypertension associated with primary aldosteronism: effect of an ACE inhibitor. QJM 95: 128-130, 2002. [DOI] [PubMed] [Google Scholar]
- 17. Prejbisz A, Klisiewicz A, Januszewicz A, et al. 22-year-old patient with malignant hypertension associated with primary aldosteronism. J Hum Hypertens 27: 138-140, 2013. [DOI] [PubMed] [Google Scholar]
- 18. Ito H, Sasaoka A, Takao T, et al. Aldosterone-producing adrenocortical adenoma complicated by chronic renal failure. Case report and review of the literature. Am J Nephrol 18: 541-546, 1998. [DOI] [PubMed] [Google Scholar]
- 19. Milliez P, Girerd X, Plouin PF, Blacher J, Safar ME, Mourad JJ. Evidence for an increased rate of cardiovascular events in patients with primary aldosteronism. J Am Coll Cardiol 45: 1243-1248, 2005. [DOI] [PubMed] [Google Scholar]
- 20. Reincke M, Fischer E, Gerum S, et al. Observational study mortality in treated primary aldosteronism: the German Conn's registry. Hypertension 60: 618-624, 2012. [DOI] [PubMed] [Google Scholar]
- 21. Wu VC, Wang SM, Chang CH, et al. Long term outcome of Aldosteronism after target treatments. Sci Rep 6: 32103, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]