Abstract
A 69-year-old Japanese woman was admitted to our hospital with progressive muscle weakness and dysphagia. She was taking pitavastatin for dyslipidemia. Her serum creatine kinase was 6,300 U/L. Pitavastatin was stopped, but her symptoms deteriorated, and cardiac congestion appeared. A muscle biopsy showed necrotizing myopathy (NM), and anti-signal recognition particle (SRP) antibody was positive. 18F-fluorodeoxyglucose-positron emission tomography showed an abnormal uptake, and magnetic resonance imaging showed abnormal gadolinium enhancement in the left ventricular wall. An endomyocardial biopsy revealed inflammatory cardiomyopathy. Steroid, tacrolimus, and intravenous immunoglobulins were effective against the symptoms. This is the first case of biopsy-proven secondary cardiomyopathy due to anti-SRP-positive NM.
Keywords: anti-signal recognition particle (SRP) antibody, necrotizing myopathy, cardiomyopathy, myocardial biopsy, positron-emission tomography (PET)
Introduction
Immune-mediated necrotizing myopathy (IMNM) presents with subacute proximal limb muscle weakness and a high serum creatine kinase (CK) level, and biopsied muscle from IMNM patients shows prominent fiber necrosis and regeneration with minimal or no inflammation (1,2). Specific autoantibodies associated with IMNM are anti-signal recognition particle (SRP) and anti-3-hydroxy-3-methylglutaryl-CoA reductase (HMGCR) (2). Anti-SRP antibody-positive necrotizing myopathy causes severe and rapidly progressive muscle weakness and disability (3), and its neurological outcomes are unsatisfactory, regardless of the combination of immunotherapies used (4,5).
Several studies have reported cases of IMNM involving cardiac symptoms, including electrocardiographic and echocardiographic abnormalities (2,6,7), but the usefulness of cardiac magnetic resonance imaging (CMRI), nuclear medicine imaging studies, and myocardial biopsies in IMNM has not been examined in detail.
We herein report the first case of anti-SRP IMNM with biopsy-proven secondary cardiomyopathy.
Case Report
A 69-year-old Japanese woman was referred to our hospital with progressive proximal limb muscle weakness. She had been well until she noticed muscle weakness of the lower extremities three months before her visit. She had a 4-year history of dyslipidemia, which had been treated with pitavastatin (1 mg/day).
A laboratory examination showed a markedly increased serum creatine kinase level (6,300 U/L; normal range 24-170). The pitavastatin was discontinued because statin-induced myopathy was suspected, but the patient subsequently experienced rapidly progressive weakness of the extremities and swallowing disturbance. She became bed-ridden within one month and was therefore admitted to our hospital. She had no family history of myopathy/cardiomyopathy.
On admission, a physical examination showed peripheral pitting edema and a sacral pressure ulcer. The patient's heart and lung sounds were normal. A neurological examination showed proximal limb muscle weakness [manual muscle testing (MMT) score: 2/5] and severe dysphagia. Her serum CK level was 4,193 U/L, and her creatine kinase-muscle/brain (CK-MB) level was 217 U/L (normal <12). C-reactive protein was <0.30 mg/dL. Her plasma brain natriuretic peptide (BNP) level was also elevated (97.6 pg/mL; normal <18.4) as well as human atrial natriuretic peptide (HANP, 54.3 pg/mL; normal <43.0) and N-terminal prohormone of brain natriuretic peptide (NT-proBNP, 391.4 pg/mL; normal <124.99). Her serum levels of angiotensin-converting enzyme and lysozyme were within the normal range. Tests for anti-nuclear antibodies, anti-aminoacyl-transfer ribonucleic acid synthetase (ARS) antibodies, and anti-mitochondrial M2 antibodies were negative. In addition, a test for serum anti-HMGCR antibody with an enzyme linked immunosorbent assay (ELISA) was negative (<1.0 IU/L), but a test for anti-SRP antibody with an ELISA produced a positive result (2.2 IU/L; normal <1.0).
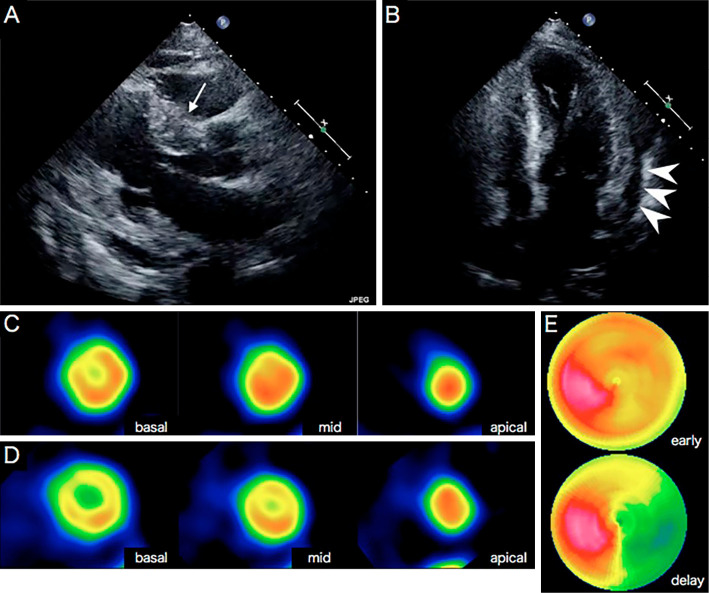
Chest X-ray revealed cardiomegaly (cardiothoracic ratio: 61%), pulmonary congestion, and pleural effusion. An electrocardiogram (ECG) showed a sinus rhythm and a heart rate of 74 beats per minute, but frequent ventricular and atrial extrasystoles were detected on a Holter ECG. Transthoracic echocardiography demonstrated a relatively well-preserved left ventricular (LV) ejection fraction (61%), but thinning and severe hypokinesis of the postero-infero-lateral wall and hypertrophy of the anteroseptal basal wall (18 mm) in the left ventricle, marked global pericardial effusion, and diastolic dysfunction were seen (Fig. 1A and B). The septal and lateral mitral annular early diastolic velocity (e') were 3.3 cm/s (normal ≥7) and 4.2 cm/s (normal ≥10) respectively, and the average ratio of the mitral E-wave velocity (E) to e' (E/e') was 12.6 (normal ≤14). The left atrial volume index (LAVI) was elevated at 51 mL/m2 (normal ≤34), and the tricuspid regurgitation (TR) velocity was 2.5 m/s (normal ≤2.8). These findings suggested diastolic left ventricular dysfunction (8).
Figure 1.
Transthoracic echocardiography and myocardial scintigraphy. Echocardiography (A, long axis; B, four-chamber view) showed hypertrophy of the anteroseptal basal wall (18 mm, white arrow), thinning of the posterior wall (5 mm), and pericardial effusion (arrowhead). (C) 201Thallium(I) chloride (TlCl) and (D) 123I-15-4-iodophenyl-3-(R, S)-methyl-pentadecanoic acid (BMIPP) scintigraphy showed a mildly decreased uptake in the anteroseptal basal area. (E) 123I-metaiodobenzylguanidine (MIBG) myocardial scintigraphy showed increased washout in the inferolateral area.
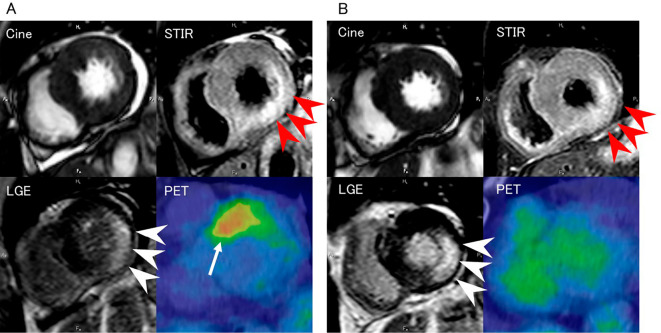
Cardiac 201thallium(I) chloride (TlCl) and 123I-15-4-iodophenyl-3-(R, S)-methyl-pentadecanoic acid (BMIPP) scintigraphy showed a mildly decreased uptake in the anteroseptal basal wall (Fig. 1C and D), and 123I-metaiodobenzylguanidine (MIBG) scintigraphy demonstrated increased washout in the inferolateral wall (Fig. 1E). CMRI showed pericardial effusion, hypokinesis of the basal inferolateral wall, a high-intensity area in the inferolateral wall on short inversion time inversion recovery (STIR) imaging, and late transmural (especially in the media) gadolinium enhancement of the inferolateral wall (Fig. 2A). 18F-fluorodeoxyglucose (FDG)-positron emission tomography (PET) showed an abnormal uptake in the anteroseptal wall of the left ventricle (Fig. 2A). Coronary angiography revealed that the coronary arteries were normal (data not shown). Needle electromyography showed a myopathic pattern of proximally predominant low-amplitude/short-duration motor unit potentials, preserved interference, and fibrillation potentials and positive sharp waves at rest. Muscle MRI-STIR imaging of the proximal upper and lower limbs revealed high-intensity areas suggestive of muscle edema and inflammation (data not shown).
Figure 2.
Cardiac magnetic resonance imaging (CMRI) [cine imaging, short inversion time inversion recovery (STIR) imaging, and late gadolinium enhancement (LGE) imaging] and 18F-fluorodeoxyglucose (FDG)-positron emission tomography (PET) performed on admission (A) and at one year after admission (B). On admission, cine imaging showed pericardial effusion, and STIR imaging detected a high-intensity region in the inferolateral wall (red arrowheads). LGE imaging revealed late transmural gadolinium enhancement of the inferolateral wall (white arrowheads). 18F-FDG-PET showed an abnormal uptake in the anteroseptal wall of the left ventricle (arrow). These findings had improved at one year after admission, but the late gadolinium enhancement persisted [cine, repetition time (TR) 2.44 ms, echo time (TE) 1.2 ms; STIR (T2-weighted), TR 2,400 ms, TE 60 ms; LGE, TR 4.03 ms, TE 1.2 ms].
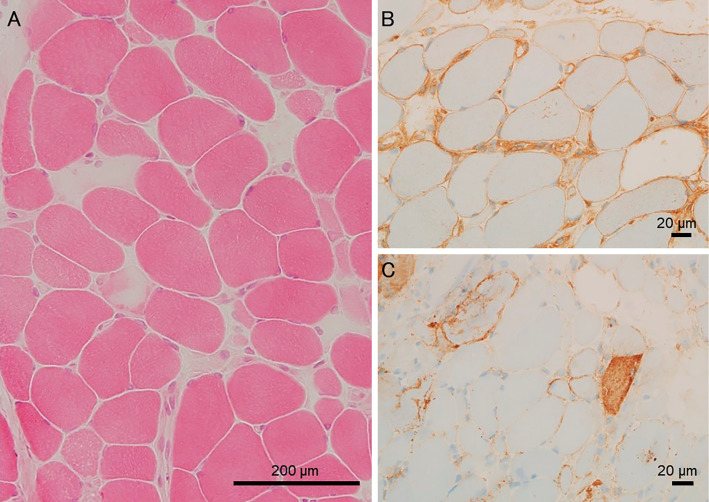
A muscle biopsy examination of the left biceps brachii performed on the fourth hospital day showed necrotic and regenerating fibers along with positive major histocompatibility complex (MHC) class I and membrane attack complex (MAC) immunostaining of myocytes (Fig. 3) with slight CD4- and CD8-positive mononuclear cell infiltration but few CD20-positive cells.
Figure 3.
Histopathological findings of the left biceps brachii muscle. Hematoxylin and Eosin staining showed moderate variations in fiber size and many necrotic fibers (A), positive major histocompatibility complex (MHC) class I (B), and membrane attack complex (MAC) (C) immunostaining.
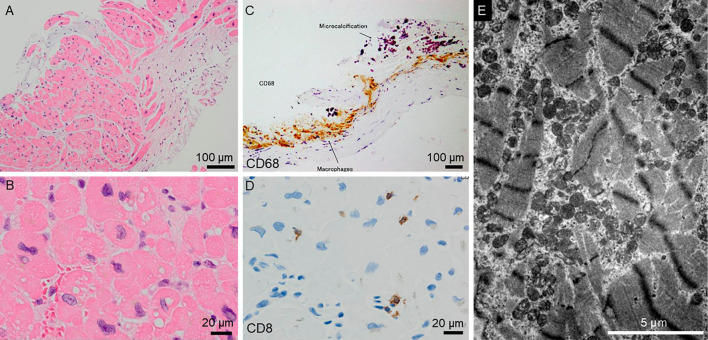
To differentiate secondary cardiomyopathy, such as sarcoidosis, a myocardial biopsy examination of the septal wall was conducted on the 13th hospital day. It showed interstitial fibrosis, binucleated myocytes, hypertrophic myocytes, and variations in the sizes of the myocyte nuclei along with infiltration of CD68-positive macrophages and CD8-positive lymphocytes, findings that were consistent with inflammatory cardiomyopathy (Fig. 4A-D). Electron microscopy revealed disarranged myofibrils and proliferating mitochondria in the myocytes (Fig. 4E).
Figure 4.
Histopathological findings of the septal wall obtained during the right ventricle biopsy examination. Hematoxylin and Eosin staining showed interstitial fibrosis (A), binucleated myocytes, hypertrophic myocytes, and variations in the sizes of the myocyte nuclei (B). Immunostaining showed the infiltration of CD68-positive macrophages (C) and CD8-positive lymphocytes (D). Electron microscopy revealed myofibril disarrangement and the proliferation of mitochondria in the myocytes (E).
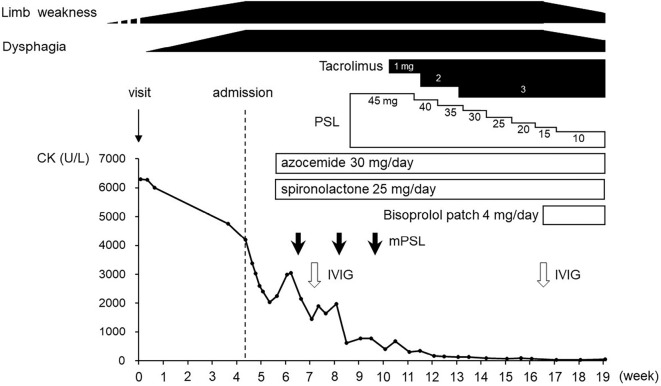
The patient was diagnosed with anti-SRP antibody-positive necrotizing myopathy combined with secondary cardiomyopathy and was treated with 3 rounds of steroid pulse therapy (intravenous administration of 1,000 mg methylprednisolone for 3 days) followed by 45 mg/day prednisolone (PSL; 1 mg/kg/day), tacrolimus (3 mg/day), and intravenous immunoglobulins (IVIG) [20 g/day (400 mg/kg) for 5 days]. Heart failure and pleural effusion were treated with azosemide (30 mg/day) and spironolactone (25 mg/day). A transdermal bisoprolol patch (4 mg/day) was added later because of frequent paroxysmal atrial and ventricular extrasystoles. Although the patient's serum CK and CK-MB levels gradually normalized, her muscle weakness did not improve. IVIG therapy was administered again on the 83rd hospital day. The patient's condition improved markedly after the second round of IVIG therapy, and she was able to walk independently with a cane. In addition, swallowing became possible. The PSL dose was tapered, and the patient was moved to a rehabilitation institution on the 106th hospital day (Fig. 5). Her symptoms gradually improved (deltoid MMT 4+ and iliopsoas MMT 3), and she left the institution at one year after the onset of the condition.
Figure 5.
Clinical course of this case. CK: creatine kinase, IVIG: intravenous immunoglobulin, mPSL: methylprednisolone, PSL: prednisolone
She visited our hospital again two weeks after she left the institution, and her serum anti-SRP antibody titer had decreased (1.3 IU/L after one year; <1.0 IU/L after two years). CMRI showed a marked reduction in pericardial effusion, and STIR imaging revealed that the area of high signal intensity in the posterior wall of the left ventricle had shrunk, as the LV mass had reduced. However, the area of late gadolinium enhancement in the interolateral wall of the left ventricle persisted. 18F-FDG-PET no longer showed an abnormal uptake (Fig. 2B). The patient's BNP, HANP, and NT-proBNP showed the delayed peaks but improved gradually. Follow-up echocardiography showed that the pericardial effusion had disappeared, but the thickness of the LV wall and hypokinesis were unchanged (data not shown). The values of septal e', lateral e', E/e', and LAVI after treatment remained unchanged although TR disappeared.
Discussion
We herein report the first myocardial biopsy-proven case of anti-SRP-positive necrotizing myopathy. Previous studies have described various cardiac symptoms in cases of IMNM including chest pain, palpitations, congestive heart failure, as well as electrocardiographic and echocardiographic changes and MRI changes (2,7,9,10). Cardiac fibrosis has also been reported, but no detailed pathological findings were provided (11).
In the present case, a myocardial biopsy revealed interstitial fibrosis and myofibril disarrangement but relatively mild mononuclear cell infiltration, which was similar to the changes in the skeletal muscle. A thorough investigation excluded other heart diseases, such as ischemia, hypertension, valvular disease, congenital heart disease, sarcoidosis, infections, amyloidosis, hypothyroidism, hemochromatosis, and beriberi. The detection of the anti-SRP antibody, the inflammatory myocardial changes, and the favorable response to immunotherapy suggested that the cardiac symptoms were caused by an immune-mediated condition, and the similarity of the pathological changes between the myocardium and the skeletal muscle suggested that these changes shared the same etiology. The mechanisms responsible for cardiac and skeletal muscle involvement in IMNM are yet to be elucidated, but T cell-mediated cytotoxic processes or antibody-mediated processes are plausible mechanisms. The pathological role of the anti-SRP antibody remains unclear, but B cell depletion therapy with rituximab was effective in patients with anti-SRP antibody-associated myopathy, and the substantial reduction in anti-SRP antibody levels after rituximab treatment suggested that B cells and anti-SRP antibodies might play a role in the pathogenesis of IMNM (4).
This case also provided detailed information about the utility of CMRI and FDG-PET for detecting and following up myocardial lesions in IMNM. Only one previous article reported the identification of cardiac involvement in IMNM using CMRI (9). In the current case, CMRI showed late gadolinium enhancement that might have been due to active inflammation or a myocardial injury, whereas in the chronic phase, such findings are indicative of residual focal fibrosis (12). Aside from the present study, there have been no reports on the utility of cardiac FDG-PET for evaluating IMNM. One plausible explanation for the increased FDG-PET uptake is inflammatory changes (13), but disturbed aerobic metabolism and/or increased anaerobic glycolysis due to mitochondrial abnormalities in the myocytes are also possible explanations (14).
The treatment of IMNM often requires combination therapy involving steroids, immunosuppressants, immunoglobulins, and/or plasmapheresis (2), and the anti-SRP antibody titer has been reported to decrease after treatment (5), as seen in the present case. It is likely that the anti-SRP antibody titer is associated with the patient's clinical state in IMNM, although the pathological mechanisms underlying the effects of autoantibodies in inflammatory myopathies remain unclear (15). Our patient's cardiac symptoms also seemed to improve after immunotherapy, although diuretics and bisoprolol were also administered.
Several limitations associated with the present study warrant mention. First, the myocardial biopsy samples were collected from the septal wall, so we were unable to obtain pathological information about the LV free wall or the whole left ventricle. In this case, the imaging examinations showed patchy abnormalities in the left ventricle, and the lesion exhibited different findings on FDG-PET and CMRI, but the reason for such discrepancies is unclear. One possible explanation is that these differences might reflect temporary pathological changes in the lesion, but we were unable to determine whether the changes detected on PET or CMRI occurred first. Second, we did not perform a second myocardial biopsy in this case because of the invasiveness, so the pathological changes after treatment could not be assessed.
In conclusion, we encountered a case of anti-SRP antibody-positive necrotizing myopathy combined with secondary cardiomyopathy, which was the first myocardial biopsy-proven case of anti-SRP antibody-positive necrotizing myopathy. Multimodal imaging and a myocardial biopsy revealed morphological, functional, and microstructural changes in the myocardium, and this information will facilitate our understanding of the pathophysiology of this disease.
The authors state that they have no Conflict of Interest (COI).
Financial Support
This study was supported in part by an Intramural Research Grant (29-4) for Neurological and Psychiatric Disorders from National Center of Neurology and Psychiatry (NCNP).
Shiori Takeguchi-Kikuchi and Taiki Hayasaka contributed equally to this work.
References
- 1. Stenzel W, Goebel HH, Aronica E. Review: immune-mediated necrotizing myopathies - a heterogeneous group of diseases with specific myopathological features. Neuropathol Appl Neurobiol 38: 632-646, 2012. [DOI] [PubMed] [Google Scholar]
- 2. Kassardjian CD, Lennon VA, Alfugham NB, et al. . Clinical features and treatment outcomes of necrotizing autoimmune myopathy. JAMA Neurol 72: 996-1003, 2015. [DOI] [PubMed] [Google Scholar]
- 3. Miller T, Al-Lozi MT, Lopate G, et al. . Myopathy with antibodyes to the signal recognition particle: clinical and pathological features. J Neurol Neurosurg Psychiatry 73: 420-428, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Valiyil R, Casciola-Rosen L, Hong G, et al. . Rituximab therapy for myopathy associated with anti-signal recognition particle antibodyes: A case series. Arthritis Care Res 62: 1328-1334, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Suzuki S, Nishikawa A, Kuwana M, et al. . Inflammatory myopathy with anti-signal recognition particle antibodies: case series of 100 patients. Orphanet J Rare Dis 10: 61, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kao AH, Lacomis D, Lucas M, Fertig N, Oddis CV. Anti-signal recognition particle autoantibody in patients with and patients without idiopathic inflammatory myopathy. Arthritis Rheum 50: 209-215, 2004. [DOI] [PubMed] [Google Scholar]
- 7. Hengstman GJ, ter Laak HJ, Vree Egberts WT, et al. . Anti-signal recognition particle autoantibodies: marker of a necrotising myopathy. Ann Rheum Dis 65: 1635-1638, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nagueh SF, Smiseth OA, Appleton CP, et al. . Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 29: 277-314, 2016. [DOI] [PubMed] [Google Scholar]
- 9. Love LA, Leff RL, Fraser DD, et al. . A new approach to the classification of idiopathic inflammatory myopathy: myositis-specific autoantibodies define useful homogeneous patient groups. Medicine (Baltimore) 70: 360-374, 1991. [DOI] [PubMed] [Google Scholar]
- 10. Thiébaut M, Terrier B, Menacer S, et al. . Antisignal recognition particle antibodies-related cardiomyopathy. Circulation 127: e434-e436, 2013. [DOI] [PubMed] [Google Scholar]
- 11. Targoff IN, Johnson AE, Miller FW. Antibody to signal recognition particle in polymyositis. Arthritis Rheum 33: 1361-1370, 1990. [DOI] [PubMed] [Google Scholar]
- 12. Lagan J, Schmitt M, Miller CA. Clinical applications of multi-parametric CMR in myocarditis and systemic inflammatory diseases. Int J Cardiovasc Imaging 34: 35-54, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ferda J, Hromádka M, Baxa J. Imaging of the myocardium using 18F-FDG-PET/MRI. Eur J Radiol 85: 1900-1908, 2016. [DOI] [PubMed] [Google Scholar]
- 14. Croteau E, Renaud JM, Richard MA, Ruddy TD, Bénard F, deKemp RA. PET metabolic biomarkers for cancer. Biomark Cancer 8 (Suppl): 61-69, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Suzuki S, Uruha A, Suzuki N, Nishino I. Integrated diagnosis project for inflammatory myopathies: an association between autoantibodies and muscle pathology. Autoimmun Rev 16: 693-700, 2017. [DOI] [PubMed] [Google Scholar]