Abstract
Objective
Cryptococcal meningoencephalitis (CM) causes significant morbidity and mortality in human immunodeficiency virus (HIV)-negative and HIV-positive populations. White matter lesions (WMLs) have been reported in both populations of CM patients; however, the mechanisms underlying WML formation remain unknown. We herein report the relationship between the intrathecal immune response and the development of WMLs in HIV-negative patients with CM.
Methods
Eleven consecutive HIV-negative patients with CM who presented at one of three emergency hospitals in Japan from April 2001 to March 2018 were enrolled. For all patients, we retrospectively assessed the relationships between clinical and laboratory information and the presence of WMLs.
Results
At presentation, 6 patients had WMLs on magnetic resonance imaging (MRI). The cerebrospinal fluid immunoglobulin G (CSF IgG) index was significantly higher in the patients with WMLs than in those without WMLs (mean, 1.34 vs. 0.70, p=0.017). The time from the symptom onset to initial neuroimaging was also significantly longer in the patients with WMLs than in those without WMLs (median, 31.5 vs. 7.0 days; p=0.008). The clinical outcome was comparable among the patients with and without WMLs.
Conclusion
In HIV-negative patients with CM, a persistent, aberrant immune response to Cryptococcus, such as intrathecal IgG synthesis, may induce WML formation.
Keywords: cryptococcal meningitis, leukoencephalopathy, immune reconstitution inflammatory syndrome, autoimmune diseases
Introduction
Cryptococcal meningoencephalitis (CM) is a life-threatening fungal infection of the central nervous system (CNS). Although CM is common in human immunodeficiency virus (HIV)-positive patients, HIV-negative CM patients have also been increasingly frequently encountered because of the widespread use of immunologic drugs against cancers and autoimmune disorders and the prevalence of diabetes mellitus, chronic liver and renal diseases especially in developed countries (1-3).
In HIV-negative patients with CM, brain magnetic resonance imaging (MRI) often reveals dilated Virchow-Robin spaces, hydrocephalus, intracerebral nodules and pseudocysts, cortical and lacunar infarcts and gadolinium-enhanced leptomeningeal lesions (4-6). In addition, white matter lesions (WMLs) have been reported in a few patients (7,8); however, little is known about the pathogenic mechanisms of the lesions. In such cases, WMLs were found prior to the initiation of antifungal treatment and resolved almost completely in response to corticosteroids therapy. The pathologic study of an autopsied patient revealed perivascular lymphocytic inflammation in the cerebral white matter, which suggested aberrant immune reaction as a possible etiology of WMLs (8).
We hypothesized that aberrant immune responses triggered by Cryptococcus infection in the CNS was associated with the development of WMLs in HIV-negative CM patients. In the present study, we used the cerebrospinal fluid immunoglobulin G (CSF IgG) index, an indicator of intrathecal IgG production, as a surrogate marker of the immune-mediated response in the CNS to investigate the relationships between clinical profiles and the development of WMLs.
Materials and Methods
Study cohort
This was a retrospective study conducted in Japan at Tokyo Medical and Dental University, Yokosuka Kyosai Hospital and Tsuchiura Kyodo General Hospital between April 2001 and March 2018. CM was defined as symptomatic meningoencephalitis with the presence of Cryptococcus neoformans in one or more CSF cultures or positive CSF India ink staining or cryptococcal antigen findings. We enrolled 11 HIV-negative CM patients who had not begun antifungal treatment for CM. All enrolled patients received complete medical and neurological examinations at the time of hospital admission.
Each participating hospital received either human research approval to enroll patients without individual patient consent under the Common Rule or a waiver of authorization and exemption from subsequent review by the ethics committees of Tokyo Medical and Dental University, Yokosuka Kyosai Hospital, and Tsuchiura Kyodo General Hospital. The study was conducted in compliance with the Declaration of Helsinki.
Clinical variables
As part of the participating hospitals' standard clinical practice, patient characteristics, including the age, gender, medical history, clinical manifestations, laboratory data, the time from the symptom onset to initial MRI and neuroimaging findings, were collected. The white blood cell count, glucose content and total protein content in the CSF were determined. The CSF IgG index was calculated using the following formula: (CSF IgG×serum albumin)/(serum IgG×CSF albumin). A CSF IgG index above 0.7 was indicative of intrathecal IgG synthesis (9). The blood-CSF barrier integrity was assessed by calculating the ratio of the albumin concentration in CSF to that in serum (Qalb; normal value, <11), which increases with decreasing blood-CSF barrier integrity (10,11). Exploratory outcomes included the in-hospital mortality, discharge disposition (home vs. nursing home) and modified Rankin Scale score [from 0 (no neurologic deficit) to 6 (death)] at discharge (12).
Radiological investigations
All of the patients underwent MRI upon admission before receiving antifungal treatment. MRI findings were examined by one of two neurologists (T.O. and Y.KT.) who were blinded to clinical events. For the purpose of this study, the neurologists retrospectively reviewed all available MR images collected in HIV-negative patients with CM. For each enrolled patient, MRI sequences were obtained with different ≥1.5-tesla scanners and included at least an axial T1-weighted (T1WI) sequence and a T2-weighted (T2WI) or fluid-attenuated inversion recovery (FLAIR) sequence at a slice thickness of 5 mm. WMLs were identified as hyperintensity lesions on T2WI or FLAIR sequences; however, cases with acute ischemic stroke and white matter leukoaraioses, whose putative causes are aging or small-vessel disease (13), were excluded. Pseudocysts and cryptococcoma are typically found in the basal ganglia, thalamus and midbrain with surrounding edema that shows hyperintensity on T2WI or FLAIR. Pseudocysts are round or oval with hypointensity on both T1WI and FLAIR and hyperintensity on T2WI, and cryptococcoma are masses that are isointense at the walls with some hypointense areas in the core on T1WI and hypointensity at the walls with some hyperintense areas in the core on T2WI (14). Cases with these MRI findings were also excluded.
Statistical analyses
Data were reported as the mean and standard deviation when distributions were confirmed to follow a normal distribution; the median and interquartile range (IQR) were reported otherwise. Categorical variables within two groups were compared using Fisher's exact test, and continuous variables within two groups were compared using the Mann-Whitney U test for non-parametric data. P values <0.05 were considered statistically significant. All statistical analyses were conducted using EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria).
Results
Patient characteristics
Eleven HIV-negative patients with CM (men:women, 9:2; median age, 75 years; IQR, 63-81) were enrolled (Supplementary material 1). Eight of the patients had underlying conditions, the most common being glucocorticoid or immunosuppressant use (n=4), diabetes mellitus (n=3), colorectal or nasopharyngeal cancer (n=3) and interstitial lung disease (n=3). The first-noticed neurological symptom was, in order of frequency, abnormal mental status (72.7%), headache (63.6%), a fever (63.6%) and seizure (9.1%). Although the treatment regimen and treatment duration varied among patients, all patients received amphotericin B or liposomal amphotericin B with or without flucytosine as primary therapy, and 63.6% of patients received fluconazole as maintenance therapy.
Radiological findings
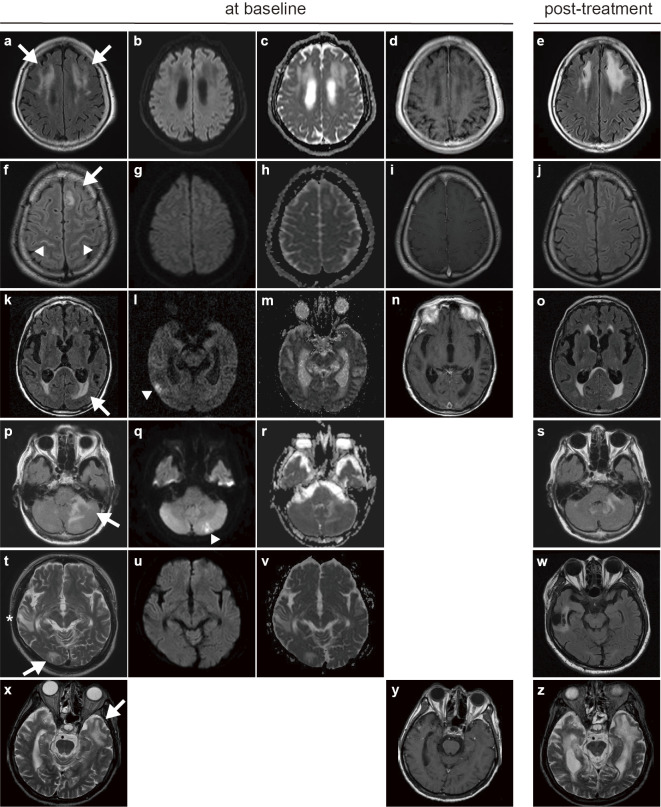
The median duration from the symptom onset to initial MRI was 17.0 days (IQR, 7-32). Six (54.5%) patients had WMLs identified on baseline MRI. Representative MR images showing WMLs are presented in Figure a, f, k, p, t and x. Post-gadolinium MRI sequences were collected in four of the patients presenting with WMLs, and the WMLs demonstrated no enhancement (Figure d, i, n, y). There were no high intensities on diffusion-weighted imaging (Figure b, g, l, q, u), although two patients presenting with WMLs developed additional high intensities indicating ischemic strokes as a complication of CM (Figure l, q, arrowhead). The apparent diffusion coefficient values of the WMLs were increased (Figure c, h, m, r, v). Two patients who presented with WMLs showed resolution of the WMLs on MRI (Figure j, w), and one patient with WMLs showed partial improvement in the WMLs (Figure s); however, the remaining three patients showed either worsening of the WMLs or they remained unchanged (Figure e, o, z).
Figure.
Representative magnetic resonance (MR) images of white matter lesions (WMLs) in HIV-negative patients with cryptococcal meningoencephalitis (CM). Case 1, 80-year-old woman (a-e); case 2, 33-year-old man (f-j); case 3, 79-year-old man (k-o); case 4, 89-year-old woman (p-s); case 5, 63-year-old man (t-w); case 6, 81-year-old man (x-z). (a, f, k, p, t, x) Axial T2-weighted or fluid-attenuated inversion recovery images showing WMLs (arrows), leptomeningeal high intensities (f, arrowhead) and a previous brain contusion (t, *). (b, g, l, q, u) Axial diffusion-weighted images showing no high intensities at the location of the WMLs, suggesting infarction as a complication of CM (l, q, arrowhead). (c, h, m, r, v) Axial apparent diffusion coefficient maps showing increased apparent diffusion coefficient values in WMLs. (d, i, n, y) Axial T1-weighted images with contrast infusion displaying no enhancement in WMLs. (e, j, o, s, w, z) Post-antifungal treatment follow-up MR images showing progression (e, z), unchanging (o), partially improvement (s), and improvement of WMLs (j, w) since the initial imaging.
A longer time from symptom onset to initial neuroimaging is associated with the development of WMLs
Patients were divided into two groups according to whether or not they presented with WMLs. We then investigated whether or not the development of WMLs was associated with various clinical features (Table 1). Patients who presented with WMLs had a significantly longer median time from the symptom onset to initial neuroimaging (31.5 vs. 7.0 days, p=0.008). Based on the results, we found follow-up MRI in 3 out of 5 patients presenting without WMLs at baseline, and WMLs were not observed on follow-up MRI performed after a median of 41 days from the onset of symptoms (IQR 34-46). There were no significant differences in the underlying condition or initial symptom between the two groups. The in-hospital mortality rates were 16.7% for CM patients presenting with WMLs and 20.0% for those not presenting with WMLs (Supplementary material 1).
Table 1.
Clinical Data of 11 Human Immunodeficiency Virus-negative Patients with Cryptococcal Meningitis (CM) Presenting with or without White Matter Lesions (WMLs).
| Clinical characterization | All participants (n=11) |
CM with WMLs (n=6) |
CM without WMLs (n=5) |
p value* | ||||
|---|---|---|---|---|---|---|---|---|
| Patient characteristics | ||||||||
| Age, y, median (range) | 75 (29-89) | 80 (33-89) | 75 (29-82) | 0.410b | ||||
| Male sex, n (%) | 10 (90.9%) | 5 (83.3%) | 5 (100%) | >0.999a | ||||
| Medical history, n (%) | ||||||||
| Use of glucocorticoids/ immunosuppressants | 4 (36.3%) | 2 (33.3%) | 2 (40.0%) | >0.999a | ||||
| Diabetes | 3 (27.3%) | 1 (16.7%) | 2 (40.0%) | 0.546a | ||||
| Cancer | 3 (27.3%) | 1 (16.7%) | 2 (40.0%) | 0.546a | ||||
| Interstitial lung disease | 3 (27.3%) | 0 (0%) | 3 (60.0%) | 0.061a | ||||
| Rheumatoid arthritis | 1 (9.1%) | 1 (16.7%) | 0 (0%) | >0.999a | ||||
| Autoimmune uveitis | 1 (9.1%) | 1 (16.7%) | 0 (0%) | >0.999a | ||||
| Brain contusion | 1 (9.1%) | 1 (16.7%) | 0 (0%) | >0.999a | ||||
| First-noticed neurological symptom, n (%) | ||||||||
| Abnormal mental status | 8 (72.7%) | 4 (66.7%) | 4 (80.0%) | >0.999a | ||||
| Headache | 7 (63.6%) | 3 (50.0%) | 4 (80.0%) | 0.546a | ||||
| Fever | 7 (63.6%) | 4 (66.7%) | 3 (60.0%) | >0.999a | ||||
| Seizure | 1 (9.1%) | 0 (0%) | 1 (20.0%) | 0.455a | ||||
| Time from symptom onset to initial MRI, days, median (IQR) | 17.0 (24.5) | 31.5 (13.0) | 7.0 (2.0) | 0.008b | ||||
| mRS score at discharge (mean±standard deviation) | 2.5±2.6 | 3.5±2.4 | 1.4±2.6 | 0.259b |
*p values were determined by using Fisher’s exact testa for categorical variables and the Mann-Whitney U testb for continuous variables. mRS: modified Rankin Scale
A higher CSF IgG index is associated with the development of WMLs
Next, we assessed the relationship between the presence of WMLs and various laboratory findings (Table 2). Patients presenting with WMLs had a significantly higher CSF IgG index at baseline than those without WMLs (1.34 vs. 0.70, p=0.017); however, the CSF IgG index in patients presenting with WMLs was not correlated with the WML size according to Spearman's rank correlation coefficient (ρ=0.60, p=0.242). In addition, in patients not presenting with WMLs, the CSF IgG index measured at about the same time as follow-up MRI was not higher than that at baseline (p=0.75) and remained significantly lower than that in patients presenting with WMLs at baseline (0.71 vs. 1.34, p=0.048). Notably, there was no significant difference in the Qalb between the 2 groups (202 vs. 200, p<0.999), suggesting that both groups had comparable blood-CSF barrier dysfunction. There were no other significant differences in laboratory findings between the two groups.
Table 2.
Laboratory Data of 11 Human Immunodeficiency Virus-negative Patients with Cryptococcal Meningitis (CM) Presenting with or without White Matter Lesions (WMLs).
| Parameter | CM with WMLs (n=6) |
CM without WMLs (n=5) |
p value | |||
|---|---|---|---|---|---|---|
| C-reactive protein (mg/dL) | 0.82±1.02 | 1.52±0.92 | 0.177 | |||
| Serum IgG (mg/dL) | 1,068±389 | 1,212±460 | 0.792 | |||
| CSF white blood cell count (cells/3 µL) | 174±152 | 518±587 | 0.537 | |||
| CSF protein (g/dL) | 208±72 | 159±40 | 0.329 | |||
| CSF glucose (mg/dL) | 31.8±10.6 | 51.2±24.4 | 0.082 | |||
| CSF IgG index | 1.34±0.61 | 0.70±0.23 | 0.017 | |||
| Qalb | 202±128 | 200±110 | >0.999 |
Data are presented as mean±standard deviation.
p values were determined by using the Mann-Whitney U test for continuous variables.
CSF: cerebrospinal fluid, Qalb: quotient of the albumin concentration in CSF to that in serum
Discussion
In this study of 11 consecutive HIV-negative patients with CM, we found that WMLs observed on MRI before the initiation of antifungal treatment were associated with a higher CSF IgG index at hospital admission and longer time from symptom onset to initial MRI. Previous case reports of HIV-negative CM patients with WMLs suggested the involvement of immunological responses triggered by Cryptococcus infection as a possible pathomechanism of the WMLs (7,8). Our present study further supports the hypothesis that sustained, aberrant immune responses in the CNS affect the development of WMLs.
A biopsy report of CM patients with WMLs showed perivascular lymphocyte collections and Cryptococcus engulfed within a small collection of macrophages (7). The WMLs and clinical conditions of the patient responded evidently to corticosteroids therapy. The effect of adjunctive corticosteroid therapy on brain inflammatory lesions was also reported in non-HIV patients with CM, even after microbiological clearance has been documented by negative CSF cultures (7,15). Although the CSF IgG index was not determined in those previous reports, the reported pathological findings and dramatic response of WMLs to glucocorticoids support the involvement of the immune response to Cryptococcus infection in the WML formation. Furthermore, C. neoformans is known to be a major pathogen capable of eliciting a lymphocyte response as a mitogen (16). In experimental cryptococcosis, antibody-mediated immunity can be harmful to the host, depending on the degree of its activation relative to the inoculum of C. neoformans (17). These findings suggest the possibility that immune activation by Cryptococcus infection produces an inappropriate immune response or inflammatory imbalance, resulting in the development of WMLs.
Previous reports examining neuroradiological findings in HIV-negative patients with CM refer little to WMLs (4-6); however, a recent study using diffusion tensor imaging has revealed marked white matter structural damage in HIV-negative patients with CM, which was associated with an increased CSF cryptococcal antigen titer (18). The prevalence of WMLs in HIV-negative patients with CM was more frequent than that previously reported despite the small number of patients included in our study. The WMLs found in the present study predominantly involved juxtacortical white matter without gadolinium enhancement or mass effect and showed hypointensity on T1-weighted imaging, isointensity on diffusion-weighted imaging (DWI) and an increase in the apparent diffusion coefficient (ADC). ADCs have been shown to be increased as a consequence of vasogenic edema or perivascular infiltration in acute inflammatory demyelinating lesions, and their subsequent increase in some lesions may represent the destruction of the matrix, demyelination and axonal loss (19,20). The MR appearance on DWI of meningoencephalitis is also related to pathologic changes that occur following infectious involvement (21,22). In the acute phase of meningoencephalitis, perivascular infiltration or congestion is likely the pathological cause of cytotoxic edema, which leads to restricted diffusion. In the subacute phase, the ADC starts to increase with the gradual development of prominent inflammatory edematous changes. This phase is also accompanied by vasogenic and interstitial edema, which contribute to the lesion becoming visible on T2-weighted MRI (21). Indeed, our present study showed that the time from the symptom onset to initial neuroimaging was associated with the development of WMLs, and CM patients with WMLs in previous reports also tended to have a long duration (>20 days) from the symptom onset to the diagnosis (7,8). In these situations, a persistent immune response, which is originally necessary for the clearance of Cryptococcus from the CNS, may cause secondary inflammation sufficient to elicit WML formation (23).
Several limitations associated with the present study warrant mention. First, this was a retrospective study that included three emergency hospitals with a relatively small sample size. Second, other potential risk factors, such as inflammatory indicators and immunological factors, including the CSF cryptococcal antigen titer or oligoclonal band, were not available for an analysis because of incomplete records. Third, the majority of patients enrolled in this study were men; one potential reason for this is that men more often present with CM-related lesions than women (4). Additional prospective studies with larger sample sizes and more complete records are required to confirm and extend our findings.
Conclusion
We found that WMLs in HIV-negative patients with CM were associated with a higher CSF IgG index and longer time from the symptom onset to initial neuroimaging. These findings suggest that a persistent, aberrant immune response in the CNS may be a crucial part of the pathogenesis of WMLs in patients with CM. Although more information on pathologic and immunologic alternations in the CNS caused by Cryptococcus infection is crucial for understanding the pathogenesis of WMLs, the possible contribution of persistent, aberrant immune responses in WMLs may be a clue suggesting better therapeutic strategies for HIV-negative CM patients, such as antifungal therapy with immunosuppressants like corticosteroids. Furthermore, when treating patients with a longer duration of disease and with WMLs on pretreatment MRI, corticosteroids may be useful for resolving WMLs and help prevent irreversible CNS damage and neurologic sequelae.
This retrospective study was approved by the ethics committees of Tokyo Medical and Dental University, Yokosuka Kyosai hospital, and Tsuchiura Kyodo General Hospital.
The authors state that they have no Conflict of Interest (COI).
Supplementary Materials
Clinical characteristics of 11 human immunodeficiency virus-negative patients with cryptococcal meningoencephalitis (CM) presenting with or without white matter lesions (WMLs).
Case 7, 29-year-old man (a, f); case 8, 62-year-old man (b); case 9, 75-year-old man (c); case 10, 75-year-old man (d, g); case 11, 82-year-old-man (e, h). (a-e) Initial axial fluid attenuated inversion recovery images. (f-h) Follow-up axial fluid-attenuated inversion recovery images.
References
- 1. Williamson PR, Jarvis JN, Panackal AA, et al. Cryptococcal meningitis: epidemiology, immunology, diagnosis and therapy. Nat Rev Neurol 13: 13-24, 2017. [DOI] [PubMed] [Google Scholar]
- 2. Pyrgos V, Seitz AE, Steiner CA, Prevots DR, Williamson PR. Epidemiology of cryptococcal meningitis in the US: 1997-2009. PLoS One 8: e56269, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhu LP, Wu JQ, Xu B, Ou XT, Zhang QQ, Weng XH. Cryptococcal meningitis in non-HIV-infected patients in a Chinese tertiary care hospital, 1997-2007. Med Mycol 48: 570-579, 2010. [DOI] [PubMed] [Google Scholar]
- 4. Zhong Y, Zhou Z, Fang X, Peng F, Zhang W. Magnetic resonance imaging study of cryptococcal neuroradiological lesions in HIV-negative cryptococcal meningitis. Eur J Clin Microbiol Infect Dis 36: 1367-1372, 2017. [DOI] [PubMed] [Google Scholar]
- 5. Tan ZR, Long XY, Li GL, Zhou JX, Long L. Spectrum of neuroimaging findings in cryptococcal meningitis in immunocompetent patients in China - A series of 18 cases. J Neurol Sci 368: 132-137, 2016. [DOI] [PubMed] [Google Scholar]
- 6. Sarkis RA, Mays M, Isada C, Ahmed M. MRI findings in cryptococcal meningitis of the non-HIV population. Neurologist 19: 40-45, 2015. [DOI] [PubMed] [Google Scholar]
- 7. Wilcox RA, Thyagarajan D, Kempster P. Two cases of Cryptococcus meningitis presenting as leukoencephalopathy prior to amphotericin therapy. Eur J Neurol 14: 350-352, 2007. [DOI] [PubMed] [Google Scholar]
- 8. Kuwahara H, Tsuchiya K, Kobayashi Z, Inaba A, Akiyama H, Mizusawa H. Cryptococcal meningitis accompanying lymphocytic inflammation predominantly in cerebral deep white matter: a possible manifestation of immune reconstitution inflammatory syndrome. Neuropathology 34: 45-48, 2014. [DOI] [PubMed] [Google Scholar]
- 9. Link H, Tibbling G. Principles of albumin and IgG analyses in neurological disorders. III. Evaluation of IgG synthesis within the central nervous system in multiple sclerosis. Scand J Clin Lab Invest 37: 397-401, 1977. [DOI] [PubMed] [Google Scholar]
- 10. Hegen H, Auer M, Zeileis A, Deisenhammer F. Upper reference limits for cerebrospinal fluid total protein and albumin quotient based on a large cohort of control patients: implications for increased clinical specificity. Clin Chem Lab Med 54: 285-292, 2016. [DOI] [PubMed] [Google Scholar]
- 11. Andersson M, Alvarez-Cermeno J, Bernardi G, et al. Cerebrospinal fluid in the diagnosis of multiple sclerosis: a consensus report. J Neurol, Neurosurg Psychiatr 57: 897-902, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rankin J. Cerebral vascular accidents in patients over the age of 60. II. Prognosis. Scott Med J 2: 200-215, 1957. [DOI] [PubMed] [Google Scholar]
- 13. Wardlaw JM, Smith EE, Biessels GJ, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol 12: 822-838, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chen S, Chen X, Zhang Z, et al. MRI findings of cerebral cryptococcosis in immunocompetent patients. J Med Imaging Radiat Oncol 55: 52-57, 2011. [DOI] [PubMed] [Google Scholar]
- 15. Panackal AA, Wuest SC, Lin YC, et al. Paradoxical immune responses in non-HIV cryptococcal meningitis. PLoS Pathog 11: e1004884, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mody CH, Wood CJ, Syme RM, Spurrell JC. The cell wall and membrane of Cryptococcus neoformans possess a mitogen for human T lymphocytes. Infect Immun 67: 936-941, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Taborda CP, Casadevall A. Immunoglobulin M efficacy against Cryptococcus neoformans: mechanism, dose dependence, and prozone-like effects in passive protection experiments. J Immunol 166: 2100-2107, 2001. [DOI] [PubMed] [Google Scholar]
- 18. Lu CH, Chen HL, Chang WN, et al. Assessing the chronic neuropsychologic sequelae of human immunodeficiency virus-negative cryptococcal meningitis by using diffusion tensor imaging. AJNR Am J Neuroradiol 32: 1333-1339, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gass A, Niendorf T, Hirsch JG. Acute and chronic changes of the apparent diffusion coefficient in neurological disorders--biophysical mechanisms and possible underlying histopathology. J Neurol Sci 186(Suppl 1): S15-S23, 2001. [DOI] [PubMed] [Google Scholar]
- 20. Castriota-Scanderbeg A, Sabatini U, Fasano F, et al. Diffusion of water in large demyelinating lesions: a follow-up study. Neuroradiology 44: 764-767, 2002. [DOI] [PubMed] [Google Scholar]
- 21. Kiroglu Y, Karabulut N, Alkan A. The role of diffusion-weighted echo planar MRI in central nervous system infections regarding etiopathogeneses. Diagn Interv Radiol 16: 257-262, 2010. [DOI] [PubMed] [Google Scholar]
- 22. Tsuchiya K, Katase S, Yoshino A, Hachiya J. Diffusion-weighted MR imaging of encephalitis. AJR Am J Roentgenol 173: 1097-1099, 1999. [DOI] [PubMed] [Google Scholar]
- 23. Johnson TP, Nath A. Neurological syndromes driven by postinfectious processes or unrecognized persistent infections. Curr Opin Neurol 31: 318-324, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Clinical characteristics of 11 human immunodeficiency virus-negative patients with cryptococcal meningoencephalitis (CM) presenting with or without white matter lesions (WMLs).
Case 7, 29-year-old man (a, f); case 8, 62-year-old man (b); case 9, 75-year-old man (c); case 10, 75-year-old man (d, g); case 11, 82-year-old-man (e, h). (a-e) Initial axial fluid attenuated inversion recovery images. (f-h) Follow-up axial fluid-attenuated inversion recovery images.