Abstract
Purpose
Various immune cells, including eosinophils and neutrophils, are known to contribute to the development of chronic rhinosinusitis with nasal polyps (CRSwNP). However, the current understanding of the role of neutrophils in the development of CRSwNP still remains unclear. Therefore, we investigated risk factors for refractoriness of CRSwNP in an Asian population.
Methods
Protein levels of 17 neutrophil-related mediators in nasal polyps (NPs) were determined by multiplex immunoassay, and exploratory factor analysis using principal component analysis was performed. Immunofluorescence analysis was conducted to detect human neutrophil elastase (HNE) or myeloperoxidase (MPO)-positive cells. Tissue eosinophilic nasal polyp (ENP) and tissue neutrophilia (Neuhigh) were defined as greater than 70 eosinophils and 20 HNE-positive cells, otherwise was classified into non-eosinophilic nasal polyp (NENP) and absence of tissue neutrophilia (Neulow).
Results
In terms of disease control status, NENP-Neulow patients showed the higher rate of disease control than NENP-Neuhigh and ENP-Neuhigh patients. Linear by linear association demonstrated the trend in refractoriness from NENP-Neulow to NENP-Neuhigh or ENP-Neulow to ENP-Neuhigh. When multiple logistic regression was performed, tissue neutrophilia (hazard ratio, 4.38; 95% confidence interval, 1.76-10.85) was found as the strongest risk factor for CRSwNP refractoriness. Additionally, exploratory factor analysis revealed that interleukin (IL)-18, interferon-γ, IL-1Ra, tumor necrosis factor-α, oncostatin M, and MPO were associated with good disease control status, whereas IL-36α and IL-1α were associated with refractory disease control status. In subgroup analysis, HNE-positive cells and IL-36α were significantly upregulated in the refractory group (P = 0.0132 and P = 0.0395, respectively), whereas MPO and IL-18 showed higher expression in the controlled group (P = 0.0002 and P = 0.0009, respectively). Moreover, immunofluorescence analysis revealed that IL-36R+HNE+-double positive cells were significantly increased in the refractory group compared to the control group. We also found that the ratio of HNE-positive cells to α1 anti-trypsin was increased in the refractory group.
Conclusions
Tissue neutrophilia had an influence on treatment outcomes in the Asian CRSwNP patients. HNE-positive cells and IL-36α may be biomarkers for predicting refractoriness in Asians with CRSwNP. Additionally, imbalances in HNE and α1 anti-trypsin may be associated with pathophysiology of neutrophilic chronic rhinosinusitis.
Keywords: Neutrophils, leukocyte elastase, interleukin 36, risk factors, rhinitis, sinusitis, nasal polyps, Asians
INTRODUCTION
Chronic rhinosinusitis (CRS) is one of the most prevalent chronic diseases and is characterized by inflammation of nasal mucosa and paranasal sinuses that can persist for 12 weeks or longer.1 Its estimated prevalence in Canada, the United States, Europe, and South Korea is 5%, 12%, 11%, and 7%, respectively,2,3,4,5 and it is commonly diagnosed in the areas of primary care, allergy, and otolaryngology. In addition, CRS poses considerable direct and indirect healthcare costs to healthcare systems and society as a whole.6,7,8 CRS is a highly heterogeneous disease and is currently defined as either accompanied by nasal polyps (CRSwNP) or without nasal polyps (CRSsNP).
To date, numerous evidence from Western countries suggests that CRSsNP is characterized by a predominant type 1 inflammatory response, whereas CRSwNP is driven by type 2 inflammation and increased eosinophil infiltration.9,10,11 However, unlike Western countries, non-eosinophilic CRSwNP is more common in Asian countries.12,13,14 Additionally, several studies have reported that nasal polyps (NPs) tissues, including those of the eosinophilic type, show significantly increased levels of neutrophils.9,13,15 These findings suggest that in addition to eosinophils, various kinds of inflammatory cells that infiltrate NP tissues may play a pathological role in the development of CRSwNP.
Recently, one study revealed that increased neutrophils in CRSwNP reduced response to oral corticosteroid therapy.16 Another study also showed that the interleukin (IL)-36γ/IL-36R pathway may contribute to the development of neutrophilic inflammation and corticosteroid resistance in CRS.17 Moreover, an eosinophilic CRSwNP study suggested that neutrophils play a pathogenic role in nasal polypogenesis by disrupting epithelial barriers via the production of oncostatin M (OSM).5 However, the clinical importance of neutrophils is not yet completely understood in CRSwNP. Therefore, we investigated neutrophils-related biomarkers contributing to the refractoriness of CRSwNP in an Asian population.
MATERIALS AND METHODS
Patients and tissue samples
NP tissues were obtained from patients with CRSwNP during routine functional endoscopic sinus surgery. All subjects provided written informed consent for study participation, and the study was approved by the Institutional Review Board of the Seoul National University Hospital, Seoul Metropolitan Government-Seoul National University Boramae Medical Center (No. 10-2018-80). The diagnosis of CRS was based on personal history, physical examination, nasal endoscopy, and sinus computed tomography (CT) findings according to a 2012 European position paper on rhinosinusitis and nasal polyps (EPOS) guidelines.1 Exclusion criteria were as follows: 1) younger than 18 years of age, 2) prior treatment with antibiotics, systemic or topical corticosteroids, or other immune-modulating drugs for 4 weeks before surgery, and 3) unilateral rhinosinusitis, antrochoanal polyp, allergic fungal sinusitis, cystic fibrosis, or immotile ciliary disease. Each sample was divided into 2 parts; one was fixed in 10% formaldehyde and embedded in paraffin for histological analysis, and the other was submersed in 1 mL phosphate-buffered saline (PBS) supplemented with 0.05% Tween-20 (Sigma-Aldrich, St Louis, MO, USA) and 1% PIC (Sigma-Aldrich) per 0.1 g of tissue. The latter tissue was homogenized with a mechanical homogenizer at 1,000 rpm for 5 minutes on ice. After homogenization, the suspensions were centrifuged at 3,000 rpm for 10 minutes at 4°C, and the supernatants were separated and stored at −80°C for further analysis of cytokines and other inflammatory mediators. The atopic status of study subjects was evaluated using the ImmunoCAP® assay (Phadia, Uppsala, Sweden) to detect immunoglobulin E (IgE) antibodies against 6 mixtures of common aeroallergens (house dust mites; molds; trees; weed and grass pollen; and animal dander). Subjects were considered atopic if the allergen-specific IgE level was greater than 0.35 IU/mL to any one or more of the allergens. The diagnosis of asthma was based on medical history and lung function analysis, including challenge tests by an allergist. Lund-Mackay CT scores and global osteitis scoring scale were calculated with the preoperative CT scan.18 Odor threshold was evaluated using a butanol threshold test (BTT), performed as previously described.19 According to National Institute on Alcohol Abuse and Alcoholism (NIAAA) standards, 14 g of alcohol was defined as one “standard drink”. One quarter bottle of soju (Korean distilled spirits), 350 mL beer, or 1 glass of wine contain the same amount of alcohol as one “standard drink”. Based on these guidelines, alcohol consumption of patients was calculated in grams. The disease control status (DCS) of individual patients was evaluated 1 year after surgery and was classified into controlled and refractory (partly controlled plus uncontrolled) groups according to the EPOS guidelines.1 In brief, CRS control status takes into account the presence and severity of the four major sinonasal symptoms, sleep disturbance and/or fatigue, nasal endoscopic evaluation and need for oral medication. “Controlled” was defined postoperatively when there were no symptoms and abnormal endoscopic findings without medication. “Refractory” was classified 1 year after surgery, when there were symptoms or abnormal findings on endoscope which needs systemic medication such as antibiotics and steroids. Prior study demonstrated that the cutoff value of 70 eosinophils/high power field (HPF) presented the most significant difference in NP recurrence. 20 Thus, we defined eosinophilic nasal polyp (ENP) as the presence of greater than 70 eosinophils/HPF, while the presence of fewer than 70 eosinophils/HPF was designated as non-eosinophilic nasal polyp (NENP).20 Additionally, another previous study for subclassification of CRS showed that patients with 20 neutrophils/HPF exhibited the minimum P values for NP recurrence.21 Thus, in this study, tissue neutrophilia (Neuhigh) was defined as greater than 20 human neutrophil elastase (HNE)-positive cells/HPF, while less than 20 HNE-positive cells/HPF was designated as the absence of tissue neutrophilia (Neulow).21
Immunohistochemistry
As previously described,22,23,24 immunohistochemical staining was performed using the polink-2 plus polymerized horseradish peroxidase (HRP) broad DAB-Detection System (Golden Bridge International Labs., Bothell, WA, USA). Briefly, sections were incubated in 3% hydrogen peroxidase to inhibit endogenous peroxidases. Heat-induced epitope retrieval was then performed by microwaving samples in 10 mmol/L citrate buffer (pH 6.0). The sections were incubated with a 1:500 dilution of mouse anti-human neutrophil elastase primary antibody (R&D Systems, Minneapolis, MN, USA) for 60 minutes at room temperature. Sections were then incubated with a broad antibody enhancer and polymer-HRP before staining with the DAB Detection System. Finally, slides were counterstained with hematoxylin. Positive cells were counted in the ten densest visual fields (400×), and the average value from 2 independent observers was determined.
Measurement of cytokines and total IgE in tissue homogenates
As previously described,14,25 protein concentrations of tissue extracts were determined using the Pierce 660 nm Protein Assay Kit (Thermo Scientific Inc., Rochester, NY, USA), and samples were thawed and vortexed at room temperature to ensure a well-mixed sample. Tissue homogenates were then assayed for periostin, HNE and α-1 anti-trypsin using commercially available ELISA kits (R&D Systems). Multiple cytokine analysis kits (tumor necrosis factor [TNF]-α, interleukin [IL]-1α, IL-1β, IL-1Ra, IL-5, IL-6, IL-17A, IL-18, IL-22, IL-23, IL-36α, OSM, chemokine [C-X-C motif] ligand [CXCL]-1, CXCL-8, myeloperoxidase [MPO], matrix metallopeptidase (MMP)-9, granulocyte-macrophage colony-stimulating factor (GM-CSF), interferon (IFN)-γ, and IL-5) were obtained from R&D systems (catalog No. LMSAHM), and data were collected using a Luminex 100 reader (Luminex, Austin, TX, USA). Data analysis was performed using MasterPlex QT version 2.0 (MiraiBio, Alameda, CA, USA), and total IgE was measured using the Human IgE ELISA kit (K3231066: KOMA, Seoul, Korea). All assays were run in duplicate according to the manufacturer protocol. All protein levels in the tissue homogenate were normalized to the concentration of total protein. The sensitivity of the ELISA assays is presented in Supplementary Table S1.
Immunofluorescence analysis
To verify the relationship between markers expressed on HNE or MPO-positive cells and IL36R, NP tissues were plated. Immunofluorescence was evaluated using primary antibodies directed against HNE (1:500, MAB91671; R&D Systems), MPO (1:300, Abcam, Cambridge, UK), and IL36R (1:150, PA5-38013; Invitrogen, Carlsbad, CA, USA). After 24 hours incubation with primary antibodies, tissues were incubated for 1 h with an Alexa Fluor® 488-conjugated anti-rabbit IgG (1:1,000, ab150077; Abcam) or Cy3-conjugated anti-mouse IgG (1:500, ab97035; Abcam) secondary antibody. The nucleus was stained with 4′,6-Diamidino-2-phenylindole dihydrochloride (1:1,000, Sigma) for 2 min, and tissues were mounted with aqueous-based medium. Fluorescent images were obtained using a fluorescence microscope (CELENA® S; Logos Biosystems, Annandale, VA, USA). HNE, MPO, and IL-36R-positive cells were counted and analyzed in three randomly selected fields, with non-specific signals excluded.
Statistical analysis
Statistical analyses were performed using IBM SPSS 21 (SPSS, Inc., Chicago, IL, USA), R version 3.4.2 software (R Foundation for Statistical Computing, Vienna, Austria), and GraphPad Prism software 7.0 (GraphPad Software Inc, La Jolla, CA, USA). A χ2 test and 2-tailed Mann-Whitney U test were used for unpaired comparisons. Bonferroni correction was applied to multiple comparison analyses. Linear by linear associations were also performed to evaluate the trend in proportion of refractoriness of each subtype. Multiple variate analyses were performed to identify the risk factor for disease refractoriness. The parameters statistically significant in univariate analysis were involved in binary logistic regression analysis for multivariate analysis, after adjusting for confounding factors. The significance level was set at α = 0.05. Exploratory factor analysis using principal component analysis (PCA) was used to describe patterns of inflammatory mediators. Variables with loadings greater than 0.3 were selected.
RESULTS
Characterization and treatment outcomes in CRSwNP patients based on tissue eosinophilia and neutrophilia
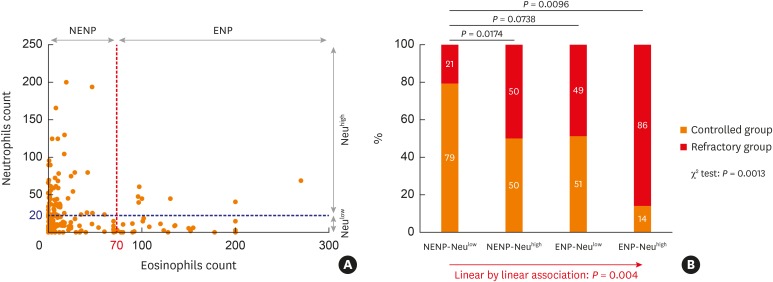
Subjects were divided into 4 groups based on tissue eosinophilia and neutrophilia. ENP and NENP accounted for 40 (25%) and 120 (75%) subjects, respectively. Out of the patients with ENP, 7 (4.4%) had tissue neutrophilia (ENP-Neuhigh) and 33 (20.6%) did not have tissue neutrophilia (ENP-Neulow). For patients with NENP, 69 (43.1%) had tissue neutrophilia (NENP-Neuhigh) and 51 (31.9%) did not have tissue neutrophilia (NENP-Neulow). In addition, we observed that tissue neutrophilia was inversely correlated with tissue eosinophilia in NP (r = −0.2932, P = 0.0001; Fig. 1A).
Fig. 1. Treatment outcomes according to tissue eosinophilia and neutrophilia in CRSwNP. (A) Distribution of NP tissues according to the tissue eosinophilia and tissue neutrophilia (B) Comparison of disease control status according to the endotype of NP tissues based on tissue eosinophilia and neutrophilia.
NP, nasal polyp; ENP, eosinophilic nasal polyp; NENP, non-eosinophilic nasal polyp; Neuhigh, tissue neutrophilia; Neulow, without tissue neutrophilia.
Patients with ENP-Neuhigh had an increased incidence of asthma and a higher likelihood of smoking, in addition to an increased amount of smoking (packs/year) and alcohol intake (g/month), compared with ENP-Neulow patients. However, there were no significant differences in smoking and alcohol intake between NENP-Neulow and NENP-Neuhigh patients, except the proportion of asthma was lower in NENP-Neuhigh patients (Table 1).
Table 1. Demographics of enrolled patients.
| No. of subjects (n = 160) | Eosinophilic CRSwNP (n = 40) | Non-eosinophilic CRSwNP (n = 120) | ||||
|---|---|---|---|---|---|---|
| With neutrophilia (n = 7) | Without neutrophilia (n = 33) | P value | With neutrophilia (n = 69) | Without neutrophilia (n = 51) | P value | |
| Sex (female) | 1 | 13 | 0.386 | 10 | 10 | 0.655 |
| Age (yr) | 53 (36–56) | 48 (38–54) | 0.941 | 53 (42–60) | 55 (44–60) | 0.687 |
| Atopic (number) | 4 | 12 | 0.425 | 29 | 25 | 0.719 |
| Asthma (number) | 4 | 3 | 0.012* | 3 | 9 | 0.029* |
| Symptom duration (mon) | 27 (4–60) | 42 (14–180) | 0.244 | 43 (12–120) | 60 (12–120) | 0.600 |
| Lund-Mackay score | 18 (12–23) | 17 (13–19) | 0.727 | 16 (11–20) | 16 (12–20) | 0.554 |
| Global-osteitis score | 20 (8–29) | 15 (6–20) | 0.336 | 13 (8–21) | 13 (4–22) | 0.257 |
| BTT | 3 (0–8) | 0 (0–7) | 0.632 | 3 (0–6) | 3 (0–8) | 0.563 |
| Tissue eosinophil (cells/HPF) | 100.0 (97.2–165.3) | 91.0 (71.0–135.3) | 0.132 | 6.2 (1.0–12.1) | 8.0 (1.9–21.7) | 0.175 |
| Tissue neutrophil (cells/HPF) | 45.0 (43.0–54.5) | 4.5 (1.0–10.3) | < 0.001* | 39.0 (25.0–67.0) | 9.0 (3.3–13.8) | < 0.001* |
| Current smoking (number) | 6 | 5 | < 0.001* | 26 | 17 | 0.707 |
| Smoking history (pack/year) | 7.5 (4.5–43.0) | 0 (0–25.0) | < 0.001* | 0 (0–18.4) | 0 (0–10.0) | 0.207 |
| Alcohol intake (g/mon) | 336 (195–448) | 20 (0–160) | 0.023* | 56 (0–560) | 0 (0–392) | 0.424 |
| BMI (kg/m2) | 25.2 (23.4–29.0) | 23.3 (21.6–24.6) | 0.138 | 24.2 (22.1–26.5) | 24.2 (22.3–26.1) | 0.691 |
Value is presented as median with interquartile range.
CRSwNP, chronic rhinosinusitis with nasal polyps; BTT, butanol threshold test; HPF, high power field; BMI, body mass index.
*Means P value < 0.05.
NENP-Neulow patients showed the higher rate of disease control (35/44, 79%) than NENP-Neuhigh (25/50, 50%) and ENP-Neuhigh (1/7, 14%) patients (Fig. 1B). Linear by linear association was performed to show the trend in refractoriness from NENP-Neulow to NENP-Neuhigh or ENP-Neulow to ENP-Neuhigh. These results imply that tissue eosinophilia may be correlated with a worse disease outcome, which is consistent with previous studies,26,27,28,29 and tissue neutrophilia was also associated with a worse disease outcome in CRSwNP.
Assessment of risk factors for disease refractoriness in CRSwNP
To further identify risk factors for disease refractoriness in Asians with CRSwNP, we performed binary multivariate analysis (Table 2). We found that tissue neutrophilia was the strongest risk factor for CRSwNP refractoriness (hazard ratio [HR], 4.38, 95% confidence interval [CI], 1.76-10.85). The Lund-Mackay score (HR, 1.14; 95% CI, 1.04–1.23), age (HR, 0.96; 95% CI, 0.93–0.99), and tissue eosinophilic count (HR, 1.01; 95% CI, 1.002–1.01) were also risk factors for CRSwNP refractoriness.
Table 2. Analysis of risk factors for refractoriness.
| No. of subjects (n = 130) | Controlled group (n = 76) | Refractory group (n = 54) | Multi-variate P value (hazard ratio) |
|---|---|---|---|
| Sex (female) | 16 (21.1) | 16 (29.6) | |
| Age (yr) | 54 (48–61) | 44 (34–58) | 0.015 (0.96) |
| Atopic (number) | 33 (43.4) | 25 (49.0) | |
| Asthma (number) | 7 (9.2) | 10 (18.5) | |
| Symptom duration (mon) | 30 (6–120) | 60 (18–120) | |
| Lund-Mackay score | 14 (11–18) | 19 (13–22) | 0.004 (1.14) |
| Global-osteitis score | 10 (5–17) | 20 (9–24) | |
| Butanol threshold test | 4 (0–8) | 2 (0–7) | |
| Tissue eosinophil count (cells/HPF) | 10.0 (2.3–40.7) | 25.5 (4.1–92.0) | 0.014 (1.01) |
| Tissue eosinophilia (≥ 70 cells/HPF) | 16 (21.1) | 20 (37.0) | |
| Tissue neutrophil count (cells/HPF) | 11.9 (6.0–23.5) | 24.0 (8.9–59.86) | |
| Tissue neutrophilia (≥ 20 cells/HPF) | 26 (34.2) | 31 (57.4) | 0.001 (4.38) |
| Current smoking (number) | 22 (28.9) | 18 (33.3) | |
| Smoking history (pack/year) | 0 (0–10) | 0 (0–5) | |
| Alcohol intake (g/mon) | 56 (0–488) | 10 (0–280) | |
| BMI (kg/m2) | 24.6 (22.5–26.4) | 23.6 (21.5–25.2) |
Value is presented as number (%) or median with interquartile range. Bold text indicates statistical significance (P < 0.05).
BTT, butanol threshold test; HPF, high power field; BMI, body mass index
Neutrophil-associated biomarkers are related to disease control status
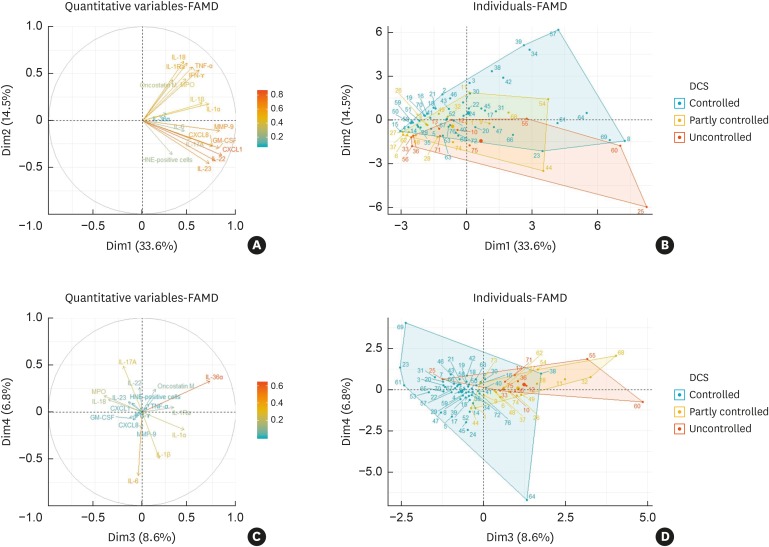
Next, to determine the influence of neutrophils-related biomarkers on disease refractoriness, we performed exploratory factor analysis using PCA, with a correlation matrix of 17 neutrophils-associated mediators. The PCA retained 6 components, accounting for 74.7% of all variance in the data (Supplementary Table S2). The first, second, third, and fourth components accounted for 33.6%, 14.5%, 8.6% and 6.8% of the variance, respectively (Fig. 2). We found that IL-18, IFN-γ, IL-1Ra, TNF-α, OSM and MPO were associated with good disease control status (Fig. 2A and B), whereas IL-36α and IL-1α were associated with refractory disease control (Fig. 2C and D). Therefore, we speculated that neutrophils-associated mediators have distinct phenotypes related to disease control status in CRSwNP.
Fig. 2. Biplots for neutrophils-associated markers according to disease control status.
Arrows are displayed for neutrophils-associated markers against the first 4 principal components. The dots are displayed for individual patients, and they are divided into three colors based on their disease control status; the refractory group consists of the partly controlled and uncontrolled patients. (A) Neutrophils-associated markers against the first 2 components. (B) Individual patients categorized by their disease control status against the first 2 components. (C) Neutrophils-associated markers against the third and fourth 2 components. (D) Individual patients categorized by their disease control status against the third and fourth 2 components.
FAMD, factor analysis of mixed data; IL, interleukin; TNF, tumor necrosis factor; IFN, interferon; MPO, myeloperoxidase; MMP, matrix metallopeptidase; CXCL, chemokine (C-X-C motif) ligand; GM-CSF, granulocyte-macrophage colony-stimulating factor; OSM, oncostatin M; DCS, disease control status.
To confirm this, we compared various neutrophil-associated inflammatory mediators in CRSwNP, according to disease control status (Table 3). Among those, we found that HNE-positive cells, HNE, and IL-36α expression were significantly up-regulated in the refractory group (P = 0.0132, P < 0.0001, and P = 0.0395, respectively), whereas the expression of MPO and IL-18 was higher in the controlled group (P = 0.0002 and P = 0.0009, respectively). Additionally, there was no difference in the expression of type 2 inflammatory markers such as IL-5 and total IgE between the controlled (IL-5: 0.1 [0–7.22] and total IgE: 20.71 [7.77–44.45]) and refractory (IL-5: 0.1 [0–2.99] and total IgE: 21.01 [7.99–36.74]) groups.
Table 3. Comparison of various neutrophils-associated inflammatory mediators in CRSwNP according to disease control status.
| No. of subjects (n = 76) | Disease control status | |
|---|---|---|
| Controlled group (n = 48) | Refractory group (n = 28) | |
| HNE-positive cells | 9.75 (5.4–21) | 23.5 (16.75–45.75)* |
| HNE (ng/mg) | 4.35 (4.00–5.64) | 76.04 (50.84–86.12)* |
| MPO (ng/mg) | 63.74 (34.38–89.36)* | 34.24 (19.91–48.33) |
| CXCL1 (ng/mg) | 0.75 (0.38–1.29) | 0.77 (0.44–1.65) |
| CXCL8 (pg/mg) | 177 (76–296) | 159 (71–391) |
| GM-CSF (pg/mg) | 0.24 (0.19–0.60) | 0.21 (0.16–0.39) |
| IFN-γ (pg/mg) | 6.01 (1.04–8.14) | 3.31 (0.52–6.91) |
| IL-1α (pg/mg) | 2.58 (1.55–3.74) | 2.40 (1.10–3.54) |
| IL-1β (pg/mg) | 0.30 (0.23–2.74) | 0.84 (0.28–2.43) |
| IL-1Ra (ng/mg) | 4.38 (2.14–13.14) | 3.49 (2.24–10.62) |
| IL-6 (pg/mg) | 5.64 (2.05–17.51) | 6.84 (2.61–17.34) |
| IL-17A (pg/mg) | 0.55 (0.46–0.78) | 0.64 (0.49–1.81) |
| IL-18 (pg/mg) | 27.40 (19.93–42.13)* | 18.45 (12.49–24.09) |
| IL-22 (pg/mg) | 0.78 (0.68–1.03) | 0.86 (0.63–3.53) |
| IL-23 (pg/mg) | 11.61 (9.76–20.22) | 12.13 (8.44–18.21) |
| IL-36α (pg/mg) | 9.36 (0.49–94) | 44.84 (2.77–132.73)* |
| TNF-α (pg/mg) | 0.18 (0–0.66) | 0.23 (0–0.89) |
| MMP-9 (ng/mg) | 12.46 (6.80–20.21) | 17.20 (10.88–28.23) |
| OSM (pg/mg) | 288 (238–373) | 246 (162–316) |
Value is presented as median with IQR.
CRSwNP, chronic rhinosinusitis with nasal polyps; HNE, human neutrophil elastase, MPO, myeloperoxidase; CXCL, chemokine (C-X-C motif) ligand; GM-CSF, granulocyte-macrophage colony-stimulating factor; IFN, interferon; IL, interleukin; TNF, tumor necrosis factor; MMP, matrix metallopeptidase; OSM, oncostatin M.
*Means P value < 0.05.
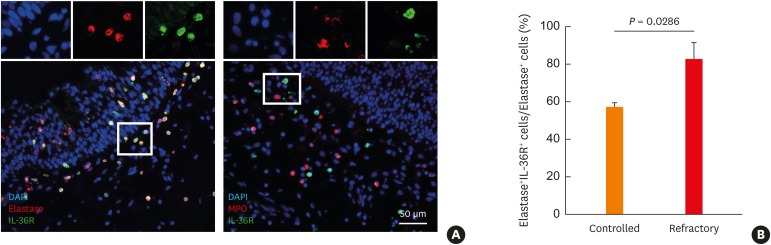
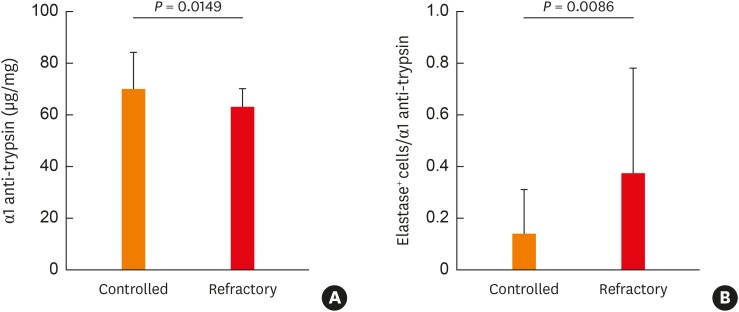
Interestingly, our findings imply that expression of a neutrophil marker such as HNE or MPO in Asian NPs may have a different clinical outcome. Thus, to identify a possible association between expression of HNE or MPO-positive cells in Asian NP tissues and disease control status, we performed immunofluorescence analysis. Among HNE-positive cells, IL36R+HNE+ double-positive cells were significantly increased in the refractory group compared to the controlled group, whereas IL-36R+MPO+ positive cells were infrequently observed in NP tissues (Fig. 3). Additionally, we found that the concentration of α1 anti-trypsin was lower and the ratio of HNE-positive cells to α1 anti-trypsin was higher in the refractory group than in the controlled group (Fig. 4). The α-1 anti-trypsin is known to be one of the potent inhibitors of neutrophil elastase.30,31 Therefore, we suggest that an imbalance of HNE-positive neutrophils to α-1 anti-trypsin might have an influence on refractoriness in Asian CRSwNP patients.
Fig. 3. Phenotypes of neutrophils in the refractory NPs. (A) Representative images of HNE-positive cells, MPO-positive cells, and IL-36R-positive cells (B) Ratio of double positive (HNE and IL-36R) cells/HNE-positive cells. Cell nuclei were counterstained with DAPI (blue).
NP, nasal polyp; HNE, human neutrophil elastase; MPO, myeloperoxidase; IL, interleukin; DAPI, 4′,6-Diamidino-2-phenylindole dihydrochloride.
Fig. 4. Imbalance of neutrophil elastase and α1 anti-trypsin in the refractory nasal polyps. (A) Concentration of α1 anti-trypsin between the controlled and the refractory groups. (B) The ratio of human elastase-positive cells/α1 anti-trypsin between the controlled and the refractory groups.
DISCUSSION
Limited studies have reported the role of neutrophils in nasal polypogenesis.13,14,15,16,17 In the present study, we classified CRSwNP patients into 4 groups according to the presence of tissue eosinophilia and tissue neutrophilia. In accordance with the findings of previous studies,32 tissue eosinophilia was related to a worse disease outcome, regardless of whether tissue neutrophilia was present. Moreover, we also observed that HNE-positive neutrophils were associated with poor treatment outcomes in Asians with CRSwNP. Specifically, the HR of refractoriness on CRSwNP in Asians was the highest for tissue neutrophilia (HR, 4.38; 95% CI, 1.76–10.85). Although some studies supported that patients with the neutrophilic CRSwNP have less response to treatment with corticosteroids,16,33 unlike eosinophils, tissue neutrophilia has not yet been established as a cellular biomarker in NP tissues. Meanwhile, several studies have chosen diverse markers for neutrophils, such as MPO, elastase, and morphologic findings.13,14,15,17 This diversity in markers may be the reason why neutrophil phenotype was not previously found to be significantly associated with treatment outcome. However, we did find an association in this study.
Previously, one Western cluster study of inflammatory endotypes reported that a high amount of neutrophils-related markers was detected in both IL-5 negative and IL-5 high clusters.34 This finding suggests that some patients have increased neutrophilic inflammation along with eosinophilic inflammation. Another cluster study in China showed that clusters characterized by predominant neutrophilic inflammation with elevated levels of IL-8 were also associated with poor disease outcomes.35 That study also revealed that CRS patients with high numbers of neutrophils had increased expression of granulocyte-colony stimulating factor, which stimulates survival and function of neutrophils, and IgG1, which promotes the local recruitment of neutrophils via complement. Moreover, one study of Asian CRSsNP patients demonstrated that neutrophils-related markers were associated with disease severity, although the immune response was mixed (types 2 and 3).25 These prior findings indicate that neutrophilic inflammation plays an important role in developing nasal polypogenesis in some patient groups.
Neutrophils are important cells in the first line of defense. However, there is emerging evidence that neutrophils are involved not only in the killing of extracellular pathogens, but also in contributing to immune responses through cross-talk with other immune cells, such as lymphocytes, dendritic cells, and natural killer cells.36,37 Interestingly, PCA revealed that a distinct pattern of neutrophil-related mediators is associated with disease control status; namely, IL-18, IFN-γ, IL-1Ra, TNF-α, OSM, and MPO were correlated with better treatment outcome. A previous study demonstrated that higher levels of IFN-γ at the first surgery are associated with a lower recurrence risk of CRSwNP, which is in agreement with our findings.38 Additionally, subgroup analysis demonstrated that MPO was upregulated in the controlled group, but HNE-positive cells and HNE levels were upregulated in the refractory group. Moreover, double positive cells (IL36R+HNE+) were observed more frequently in the refractory group than in the controlled group. MPO expression is usually consistent with HNE expression in neutrophils. In the present study, our data also showed that MPO expression was positively correlated with HNE expression (r = 0.2572, P = 0.0249), though it was weak. However, we found that there may be a different phenotype of neutrophils according to disease severity. HNE may be a phenotypic marker of neutrophils in refractory neutrophilic CRS rather than MPO. HNE and IL-36/IL-36R axis was known to be associated with each other and IL-36 promoted the mRNA expression of IL-17A, MMP-9, CXCL1, CXCL2, and CXCL8, but not MPO in NP cell culture.17 These findings were consistent with our results showing two different groups of neutrophil markers (Fig. 2A).
Generally, neutrophil activation by cytokines or chemokines leads to a rapid granule translocation to the cell surface, followed by extracellular secretion. Primary neutrophil granules are known as azurophilic granules and contain MPO in addition to neutrophil serine proteases.39 Specifically, neutrophil serine proteases consist of proteinase-3, cathepsin-G, and neutrophil elastase; these highly active proteolytic enzymes are formed during the promyelocytic phase of neutrophil maturation and are mainly stored in azurophilic granules.40 Inhibitors of these proteins are necessary to prevent excessive damage and can be found in the plasma. In particular, one of these proteins, α-1 anti-trypsin, inhibits neutrophil elastase,30,41 which is known for its role in chronic obstructive pulmonary disease (COPD).31 Generally, deficiency of α-1 anti-trypsin in the lung leads to an imbalance between elastase and anti-elastase activity, resulting in progressive, irreversible destruction of lung tissue. Ultimately, this imbalance contributes to the development of COPD with early-onset emphysema.42 In this study, we found that α-1 anti-trypsin expression was higher in the controlled group than in the refractory group, and that the ratio of HNE-positive cells to α1 anti-trypsin was increased in the refractory group. Consistent with our results, HNE plays a role in airway remodeling in COPD and is a major predictor of disease severity, particularly of the emphysematous component.31,43,44 Therefore, these findings suggest that HNE-positive, rather than MPO-positive neutrophils, might be more strongly associated with refractoriness in Asians with CRSwNP.
However, our study has some limitations. This study has enrolled a relatively small number of patients with eosinophilic CRSwNP due to the regional difference in CRS endotype. Thus, this makes it difficult to investigate the role of type 2 inflammatory markers for CRS refractoriness. Moreover, a very small portion of ENP-Neuhigh (4.4%) did not enable us to elucidate the roles of neutrophils in eosinophilic inflammation. Meanwhile, one prior study based on the Asian population demonstrated that Th17/Th1-associated mediators may play a role in revision surgery cases of CRSwNP patient.45 Another study also proposed a 2-track treatment strategy (Th2-targeted and Th1/Th17-targeted treatment) according to the CRS subclassification.46 It means that neutrophilic inflammation might be more important in refractoriness of CRSwNP patients. Therefore, we need further studies with a large scale prospective design to validate our findings.
In conclusion, this study suggests that tissue neutrophilia has an influence on treatment outcome for CRSwNP in the Asian population. Specifically, HNE-positive neutrophils may be a cellular marker in refractory CRSwNP. Additionally, the imbalance of HNE and α-1 antitrypsin may contribute to disease refractoriness in Asian CRSwNP patients.
ACKNOWLEDGMENTS
This research was supported by a grant from the Seoul National University Hospital Research Fund (No. 03-2019-0360 to Dae Woo Kim) and a clinical research grant-in-aid from the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and Future Planning (NRF-2018R1D1A3B07040862 to Dong-Kyu Kim and NRF-2019R1A2C2087170 to Dae Woo Kim).
Footnotes
Disclosure: There are no financial or other issues that might lead to conflict of interest.
SUPPLEMENTARY MATERIALS
The lower detection limits of ELISA assays according to the mediators
Coordinates of principal component analysis for the first 6 orthogonally rotated principal components: neutrophil-related mediators
References
- 1.Fokkens WJ, Lund VJ, Mullol J, Bachert C, Alobid I, Baroody F, et al. EPOS 2012: European position paper on rhinosinusitis and nasal polyps 2012. A summary for otorhinolaryngologists. Rhinology. 2012;50:1–12. doi: 10.4193/Rhino12.000. [DOI] [PubMed] [Google Scholar]
- 2.Chen Y, Dales R, Lin M. The epidemiology of chronic rhinosinusitis in Canadians. Laryngoscope. 2003;113:1199–1205. doi: 10.1097/00005537-200307000-00016. [DOI] [PubMed] [Google Scholar]
- 3.Blackwell DL, Lucas JW, Clarke TC. Summary health statistics for U.S. adults: national health interview survey, 2012. Vital Health Stat 10. 2014:1–161. [PubMed] [Google Scholar]
- 4.Hastan D, Fokkens WJ, Bachert C, Newson RB, Bislimovska J, Bockelbrink A, et al. Chronic rhinosinusitis in Europe--an underestimated disease. A GA2LEN study. Allergy. 2011;66:1216–1223. doi: 10.1111/j.1398-9995.2011.02646.x. [DOI] [PubMed] [Google Scholar]
- 5.Kim YS, Kim NH, Seong SY, Kim KR, Lee GB, Kim KS. Prevalence and risk factors of chronic rhinosinusitis in Korea. Am J Rhinol Allergy. 2011;25:117–121. doi: 10.2500/ajra.2011.25.3630. [DOI] [PubMed] [Google Scholar]
- 6.Caulley L, Thavorn K, Rudmik L, Cameron C, Kilty SJ. Direct costs of adult chronic rhinosinusitis by using 4 methods of estimation: Results of the US Medical Expenditure Panel Survey. J Allergy Clin Immunol. 2015;136:1517–1522. doi: 10.1016/j.jaci.2015.08.037. [DOI] [PubMed] [Google Scholar]
- 7.Halawi AM, Smith SS, Chandra RK. Chronic rhinosinusitis: epidemiology and cost. Allergy Asthma Proc. 2013;34:328–334. doi: 10.2500/aap.2013.34.3675. [DOI] [PubMed] [Google Scholar]
- 8.Smith KA, Orlandi RR, Rudmik L. Cost of adult chronic rhinosinusitis: a systematic review. Laryngoscope. 2015;125:1547–1556. doi: 10.1002/lary.25180. [DOI] [PubMed] [Google Scholar]
- 9.Van Zele T, Claeys S, Gevaert P, Van Maele G, Holtappels G, Van Cauwenberge P, et al. Differentiation of chronic sinus diseases by measurement of inflammatory mediators. Allergy. 2006;61:1280–1289. doi: 10.1111/j.1398-9995.2006.01225.x. [DOI] [PubMed] [Google Scholar]
- 10.Polzehl D, Moeller P, Riechelmann H, Perner S. Distinct features of chronic rhinosinusitis with and without nasal polyps. Allergy. 2006;61:1275–1279. doi: 10.1111/j.1398-9995.2006.01132.x. [DOI] [PubMed] [Google Scholar]
- 11.Van Bruaene N, Pérez-Novo CA, Basinski TM, Van Zele T, Holtappels G, De Ruyck N, et al. T-cell regulation in chronic paranasal sinus disease. J Allergy Clin Immunol. 2008;121:1435–1441. 1441.e1–1433. doi: 10.1016/j.jaci.2008.02.018. [DOI] [PubMed] [Google Scholar]
- 12.Cao PP, Li HB, Wang BF, Wang SB, You XJ, Cui YH, et al. Distinct immunopathologic characteristics of various types of chronic rhinosinusitis in adult Chinese. J Allergy Clin Immunol. 2009;124:478–484. 484.e1–472. doi: 10.1016/j.jaci.2009.05.017. [DOI] [PubMed] [Google Scholar]
- 13.Shi LL, Xiong P, Zhang L, Cao PP, Liao B, Lu X, et al. Features of airway remodeling in different types of Chinese chronic rhinosinusitis are associated with inflammation patterns. Allergy. 2013;68:101–109. doi: 10.1111/all.12064. [DOI] [PubMed] [Google Scholar]
- 14.Kim DK, Eun KM, Kim MK, Cho D, Han SA, Han SY, et al. Comparison between signature cytokines of nasal tissues in subtypes of chronic rhinosinusitis. Allergy Asthma Immunol Res. 2019;11:201–211. doi: 10.4168/aair.2019.11.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pothoven KL, Norton JE, Suh LA, Carter RG, Harris KE, Biyasheva A, et al. Neutrophils are a major source of the epithelial barrier disrupting cytokine oncostatin M in patients with mucosal airways disease. J Allergy Clin Immunol. 2017;139:1966–1978.e9. doi: 10.1016/j.jaci.2016.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wen W, Liu W, Zhang L, Bai J, Fan Y, Xia W, et al. Increased neutrophilia in nasal polyps reduces the response to oral corticosteroid therapy. J Allergy Clin Immunol. 2012;129:1522–1528.e5. doi: 10.1016/j.jaci.2012.01.079. [DOI] [PubMed] [Google Scholar]
- 17.Wang H, Li ZY, Jiang WX, Liao B, Zhai GT, Wang N, et al. The activation and function of IL-36γ in neutrophilic inflammation in chronic rhinosinusitis. J Allergy Clin Immunol. 2018;141:1646–1658. doi: 10.1016/j.jaci.2017.12.972. [DOI] [PubMed] [Google Scholar]
- 18.Georgalas C, Videler W, Freling N, Fokkens W. Global Osteitis Scoring Scale and chronic rhinosinusitis: a marker of revision surgery. Clin Otolaryngol. 2010;35:455–461. doi: 10.1111/j.1749-4486.2010.02218.x. [DOI] [PubMed] [Google Scholar]
- 19.Kim DW, Kim JY, Jeon SY. The status of the olfactory cleft may predict postoperative olfactory function in chronic rhinosinusitis with nasal polyposis. Am J Rhinol Allergy. 2011;25:e90–e94. doi: 10.2500/ajra.2011.25.3617. [DOI] [PubMed] [Google Scholar]
- 20.Tokunaga T, Sakashita M, Haruna T, Asaka D, Takeno S, Ikeda H, et al. Novel scoring system and algorithm for classifying chronic rhinosinusitis: the JESREC Study. Allergy. 2015;70:995–1003. doi: 10.1111/all.12644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ikeda K, Shiozawa A, Ono N, Kusunoki T, Hirotsu M, Homma H, et al. Subclassification of chronic rhinosinusitis with nasal polyp based on eosinophil and neutrophil. Laryngoscope. 2013;123:E1–9. doi: 10.1002/lary.24154. [DOI] [PubMed] [Google Scholar]
- 22.Kim DK, Jin HR, Eun KM, Mo JH, Cho SH, Oh S, et al. The role of interleukin-33 in chronic rhinosinusitis. Thorax. 2017;72:635–645. doi: 10.1136/thoraxjnl-2016-208772. [DOI] [PubMed] [Google Scholar]
- 23.Kim DK, Jin HR, Eun KM, Mutusamy S, Cho SH, Oh S, et al. Non-eosinophilic nasal polyps shows increased epithelial proliferation and localized disease pattern in the early stage. PLoS One. 2015;10:e0139945. doi: 10.1371/journal.pone.0139945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim DW, Kulka M, Jo A, Eun KM, Arizmendi N, Tancowny BP, et al. Cross-talk between human mast cells and epithelial cells by IgE-mediated periostin production in eosinophilic nasal polyps. J Allergy Clin Immunol. 2017;139:1692–1695.e6. doi: 10.1016/j.jaci.2016.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim DW, Eun KM, Roh EY, Shin S, Kim DK. Chronic rhinosinusitis without nasal polyps in Asian patients shows mixed inflammatory patterns and neutrophil-related disease severity. Mediators Inflamm. 2019;2019:7138643. doi: 10.1155/2019/7138643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van Crombruggen K, Zhang N, Gevaert P, Tomassen P, Bachert C. Pathogenesis of chronic rhinosinusitis: inflammation. J Allergy Clin Immunol. 2011;128:728–732. doi: 10.1016/j.jaci.2011.07.049. [DOI] [PubMed] [Google Scholar]
- 27.Gevaert E, Zhang N, Krysko O, Lan F, Holtappels G, De Ruyck N, et al. Extracellular eosinophilic traps in association with Staphylococcus aureus at the site of epithelial barrier defects in patients with severe airway inflammation. J Allergy Clin Immunol. 2017;139:1849–1860.e6. doi: 10.1016/j.jaci.2017.01.019. [DOI] [PubMed] [Google Scholar]
- 28.Schleimer RP. Immunopathogenesis of chronic rhinosinusitis and nasal polyposis. Annu Rev Pathol. 2017;12:331–357. doi: 10.1146/annurev-pathol-052016-100401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stevens WW, Ocampo CJ, Berdnikovs S, Sakashita M, Mahdavinia M, Suh L, et al. Cytokines in chronic rhinosinusitis. Role in eosinophilia and aspirin-exacerbated respiratory disease. Am J Respir Crit Care Med. 2015;192:682–694. doi: 10.1164/rccm.201412-2278OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Janciauskiene S, Wrenger S, Immenschuh S, Olejnicka B, Greulich T, Welte T, et al. The multifaceted effects of alpha1-antitrypsin on neutrophil functions. Front Pharmacol. 2018;9:341. doi: 10.3389/fphar.2018.00341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meijer M, Rijkers GT, van Overveld FJ. Neutrophils and emerging targets for treatment in chronic obstructive pulmonary disease. Expert Rev Clin Immunol. 2013;9:1055–1068. doi: 10.1586/1744666X.2013.851347. [DOI] [PubMed] [Google Scholar]
- 32.Lou H, Meng Y, Piao Y, Wang C, Zhang L, Bachert C. Predictive significance of tissue eosinophilia for nasal polyp recurrence in the Chinese population. Am J Rhinol Allergy. 2015;29:350–356. doi: 10.2500/ajra.2015.29.4231. [DOI] [PubMed] [Google Scholar]
- 33.Cao Y, Chen F, Sun Y, Hong H, Wen Y, Lai Y, et al. LL-37 promotes neutrophil extracellular trap formation in chronic rhinosinusitis with nasal polyps. Clin Exp Allergy. 2019;49:990–999. doi: 10.1111/cea.13408. [DOI] [PubMed] [Google Scholar]
- 34.Tomassen P, Vandeplas G, Van Zele T, Cardell LO, Arebro J, Olze H, et al. Inflammatory endotypes of chronic rhinosinusitis based on cluster analysis of biomarkers. J Allergy Clin Immunol. 2016;137:1449–1456.e4. doi: 10.1016/j.jaci.2015.12.1324. [DOI] [PubMed] [Google Scholar]
- 35.Liao B, Liu JX, Li ZY, Zhen Z, Cao PP, Yao Y, et al. Multidimensional endotypes of chronic rhinosinusitis and their association with treatment outcomes. Allergy. 2018;73:1459–1469. doi: 10.1111/all.13411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mayadas TN, Cullere X, Lowell CA. The multifaceted functions of neutrophils. Annu Rev Pathol. 2014;9:181–218. doi: 10.1146/annurev-pathol-020712-164023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Soehnlein O, Steffens S, Hidalgo A, Weber C. Neutrophils as protagonists and targets in chronic inflammation. Nat Rev Immunol. 2017;17:248–261. doi: 10.1038/nri.2017.10. [DOI] [PubMed] [Google Scholar]
- 38.Van Zele T, Holtappels G, Gevaert P, Bachert C. Differences in initial immunoprofiles between recurrent and nonrecurrent chronic rhinosinusitis with nasal polyps. Am J Rhinol Allergy. 2014;28:192–198. doi: 10.2500/ajra.2014.28.4033. [DOI] [PubMed] [Google Scholar]
- 39.Faurschou M, Borregaard N. Neutrophil granules and secretory vesicles in inflammation. Microbes Infect. 2003;5:1317–1327. doi: 10.1016/j.micinf.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 40.Campanelli D, Melchior M, Fu Y, Nakata M, Shuman H, Nathan C, et al. Cloning of cDNA for proteinase 3: a serine protease, antibiotic, and autoantigen from human neutrophils. J Exp Med. 1990;172:1709–1715. doi: 10.1084/jem.172.6.1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Witko-Sarsat V, Rieu P, Descamps-Latscha B, Lesavre P, Halbwachs-Mecarelli L. Neutrophils: molecules, functions and pathophysiological aspects. Lab Invest. 2000;80:617–653. doi: 10.1038/labinvest.3780067. [DOI] [PubMed] [Google Scholar]
- 42.Janciauskiene SM, Bals R, Koczulla R, Vogelmeier C, Köhnlein T, Welte T. The discovery of α1-antitrypsin and its role in health and disease. Respir Med. 2011;105:1129–1139. doi: 10.1016/j.rmed.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 43.Kim S, Nadel JA. Role of neutrophils in mucus hypersecretion in COPD and implications for therapy. Treat Respir Med. 2004;3:147–159. doi: 10.2165/00151829-200403030-00003. [DOI] [PubMed] [Google Scholar]
- 44.Vlahos R, Wark PA, Anderson GP, Bozinovski S. Glucocorticosteroids differentially regulate MMP-9 and neutrophil elastase in COPD. PLoS One. 2012;7:e33277. doi: 10.1371/journal.pone.0033277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ryu G, Kim DK, Dhong HJ, Eun KM, Lee KE, Kong IG, et al. Immunological characteristics in refractory chronic rhinosinusitis with nasal polyps undergoing revision surgeries. Allergy Asthma Immunol Res. 2019;11:664–676. doi: 10.4168/aair.2019.11.5.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim DK, Kang SI, Kong IG, Cho YH, Song SK, Hyun SJ, et al. Two-track medical treatment strategy according to the clinical scoring system for chronic rhinosinusitis. Allergy Asthma Immunol Res. 2018;10:490–502. doi: 10.4168/aair.2018.10.5.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The lower detection limits of ELISA assays according to the mediators
Coordinates of principal component analysis for the first 6 orthogonally rotated principal components: neutrophil-related mediators