Abstract
Improved understanding of the contribution of immune-inflammatory mechanisms in allergic diseases and asthma has encouraged development of biologicals and small molecules specifically targeting the innate and adaptive immune response. There are several critical points impacting the efficacy of this stratified approach, from the complexity of disease endotypes to the effectiveness in real-world settings. We discuss here how these barriers can be overcome to facilitate the development of implementation science for allergic diseases and asthma.
Keywords: Biological products; phenotype, precision medicine; hypersensitivity; asthma
INTRODUCTION
Biologicals and small molecules targeting specific inflammatory pathways have emerged as promising stratified approach for the treatment of severe allergic diseases. Regardless of the initial enthusiasm several drawbacks are yet to be overcome.
Allergic diseases pathogenesis involves a complex network of innate, adaptive immune and resident cells, epithelial barriers, cytokines, chemokines, growth factors, lipid and neuro-mediators, etc. These complex disease endotypes are continuously modulated by external and internal factors such as the exposome, epigenetic factors, microbiome, etc.1,2,3,4 The redundancy and plasticity of the pathogenetic network is difficult to be tackled through a very specific intervention targeting one cytokine or one receptor. Same holds true for selecting responders to a targeted intervention based on a few selected biomarkers.1,2,5 Last but not least, achieving selective immune modulation without altering the healthy immune response and with a long-lasting disease modifying effect is still not reached.
The effectiveness of the stratified approach in real life is hampered by many unknown factors such as the validity of the stringent selection criteria from randomized clinical trials for the general population or the accessibility and affordability of innovative diagnostic and therapeutic approaches. Multidirectional and multidisciplinary integration of basic, patient-oriented, and population-based research and implementation science are stringent unmet needs for facilitating the transition from the stratified to the precision medicine approach in allergic diseases and asthma.6
We discuss here several critical points for the use of biologicals in severe allergic disease, from disease mechanisms and recent discoveries to the real-world evidence of their effectiveness.
UNDERSTANDING BETTER THE MECHANISMS CURRENTLY TARGETED WITH BIOLOGICS IN ALLERGIC DISEASES
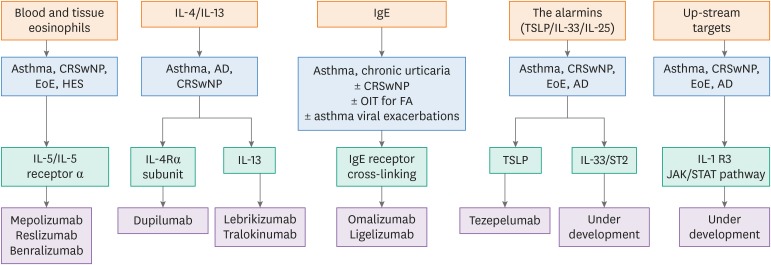
Mechanistic studies have improved our understanding of molecular and cellular components involved in allergic diseases and our ability to treat severe patients (Figure). Omalizumab directed against immunoglobulin (Ig) E has become an established add-on therapy for patients with uncontrolled allergic asthma and chronic spontaneous urticaria (CSU), while monoclonal antibodies (mAbs) against interleukin (IL)-5 (reslizumab, mepolizumab), IL-5 receptor α chain (IL-5Rα; benralizumab), and IL-4 receptor α chain (IL-4Rα; dupilumab) have been approved as add-on treatments for uncontrolled eosinophilic asthma. Dupilumab is also approved for atopic dermatitis (AD) and chronic rhinosinusitis with nasal polyps (CRSwNP). All these mAbs have complex pharmacokinetic profiles, dependent on their structure and administration route (intravenous or subcutaneous) and their efficacy is markedly influenced by the biology of their target antigen.
Figure. Targeted interventions in allergic diseases and asthma.
AD, atopic dermatitis; CRSwNP, chronic rhinosinusitis with nasal polyps; EoE, eosinophilic esophagitis; FA, food allergy; HES, hypereosinophilic syndrome; Ig, immunoglobulin; IL, interleukin; IL-4Rα, interleukin-4 receptor α chain; JAK, Janus-activated kinase; OIT, oral immunotherapy; STAT, signal transducer and activator of transcription; TSLP, thymic stromal lymphopoietin.
The eosinophils
Eosinophils are prominent pathogenic cells involved in asthma, AD, CRSwNP, eosinophilic esophagitis (EoE), and hypereosinophilic syndrome (HES) and are found in high numbers in local tissue and/or circulating blood of affected patients. In healthy individuals, eosinophils contribute to protective immune responses directed against parasites, viral, bacterial, and fungal pathogens, are crucial for the survival of long-lived plasma cells and are critical regulators of local immunity and remodeling/repair in both health and disease.7,8 Homeostatic eosinophils present in healthy individuals in various tissues are related to the control of glucose homeostasis, protection against obesity, regulation of mammary gland development, and preparation of the uterus for pregnancy.9 In the lung homeostatic eosinophils that have been shown to suppress Th2-driven allergic airway responses.10
Most human diseases accompanied by hypereosinophilia are associated with increased IL-5 production. The main sources of IL-5 are group 2 innate lymphoid cells (ILC2s), T helper (Th) 2 lymphocytes and in some cases transformed epithelial cells. IL-5 is a key modulator of the eosinophil's biology acting at many levels and during different time points, from proliferation, differentiation and maturation of IL-5Rα-expressing eosinophil-committed progenitors in the bone marrow, to their pheresis, recruitment and activation in the tissues and inhibition of their apoptosis.11,12 IL-5 was described as having a very narrow set of cellular targets in humans, mainly eosinophils, basophils and a subset of mast cells expressing the IL-5Rα. However, recent data showed that IL-5Rα capable of signal transduction is expressed by neutrophils from the bronchoalveolar lavage (BAL) fluid collected from children with treatment-refractory asthma and thus can play a role bridging atopic type 2 (T2) and innate anti-microbial immunity.13 Although IL-5 plays a central role in eosinophil biology, it is neither necessary nor sufficient for inducing fully an eosinophil mediated disease. IL-5 transgenic mice have marked eosinophilia in blood and certain tissues, without associated organ dysfunction.14 The reduction of bone-marrow eosinophils with benralizumab did not abolish eosinophilic infiltration in bronchial biopsies or eosinophil cationic protein levels in the sputum.15 Local mechanisms and/or of other cytokines promote eosinophils priming, recruitment, activation, and survival in the tissues. In humans, IL-5 is often co-expressed with other cytokines including IL-4 and IL-13 and associated in atopic individuals with increased IgE production.
Two types of antibodies have been developed to target IL-5: mepolizumab and reslizumab, directed against the cytokine itself, and benralizumab, directed against the IL-5Rα. Anti-IL-5 antibodies bind to IL-5 and interfere with occupation of the IL-5R, whereas anti-IL-5Rα antibodies bind to the membrane-expressed receptor, inhibit signaling and induce cell lysis via antibody-dependent cytotoxicity. Both types of antibodies have been shown to rapidly reduce eosinophil counts in peripheral blood and in tissues in humans. A recent study showed that benralizumab modulates blood proteins or genes associated with eosinophils or basophils, most prominent in eosinophil-high vs. eosinophil-low patients.15 However, only half of the patients respond to anti IL-5 interventions, there is dissociated effect (decrease in exacerbations and oral glucocorticoids [OCS] without any major impact on lung function, airway hyperreactivity [AHR], rescue medication use, or quality of life [QoL] in asthma; no improvement in symptoms or histological remission in EoE) and the effect is lost when the biological is interrupted.16,17,18 Even more, rebound eosinophilia after cessation of anti-IL-5 interventions and attenuation of the treatment response with repeated dosing had been reported.18,19 One of the key questions is whether residual eosinophils following IL-5-targeted therapy are an intrinsically nonresponsive subset or residual homeostatic eosinophils or there is under-dosing of the mAbs or an autoimmune process changes the endotype.20,21 Anti IL-5 antibodies decrease lung eosinophils by roughly 50%, without any major change in their functional phenotype.22,23,24,25 The IL-5Rα is shed after eosinophils migration into the tissue, thus its expression is lower compared to blood.26,27 In addition, anti-IL-5 antibodies prolong the half-life of serum IL-5 and might potentiate IL-5 activity in certain conditions, although the clinical significance is unknown.28 In humans, the effects of IL-5 are restricted to basophils and eosinophils. The expression of IL-5Rα on basophils is threefold lower compared to mature eosinophils and their differentiation is not dependent on IL-5 but rather IL-3. However, a complete depletion of basophils in peripheral blood was reported following benralizumab suggesting that even a low membrane expression of IL-5Rα can induce their apoptosis via antibody dependent cytotoxicity .29 ILC2s play a crucial role in eosinophils homeostasis. Whether ILC2 express IL-5Rα is controversial.30,31
The IL-4/IL-13 pathway
IL-4 and IL-13 are both pivotal cytokines involved in the pathogenesis of allergic diseases.32 Of particular interest is that by blocking the IL‐4Rα signaling downstream processes such as local IgE formation (with a particular importance in nasal polyps), as well as the expression of chemokines attracting inflammatory cells to the tissue, including eosinophils, are downregulated.33 IL-13 is overexpressed in the lesional skin and has a significant impact on skin biology, including the recruitment of inflammatory cells, the alteration of the skin microbiome, and the decrease in the epidermal barrier function. The IL-13-rich local milieu causes barrier dysfunction by downregulating the OVOL1-filaggrin axis and upregulating the periostin-IL-24 axis.34 Recent data show that dupilumab restores the barrier function and the skin microbiome.35,36
Despite sharing the IL-4Rα in their signaling cascades, IL-4 and IL-13 have different functions in atopic inflammation. IL-13 preferentially participates in the peripheral tissues because tissue-resident ILC2 produce IL-13 but not IL-4. In contrast, lymph node T follicular helper cells express IL-4 but not IL-13 to regulate B cell immunity.34,37,38
Development of antagonistic antibody against IL-4Rα subunit of IL-4/IL-13 receptors is a promising therapeutic strategy for T2-mediated allergic diseases such as asthma, AD and CRSwNP. Both affinity and epitope are critical factors for the efficacy of anti-IL-4Rα targeted interventions.39 Currently, besides dupilumab, which blocks the binding of both IL-13 and IL-4 to their receptors, a number of new pharmacologic entities have been designed to target both IL-4 and IL-13 and/or their receptors and/or receptor-associated signal transduction machinery such as Janus kinases. Biologics targeting IL-13, such as the anti-IL-4Rα antibody dupilumab and the anti-IL-13 antibody tralokinumab and lebrikizumab, successfully improve AD lesions and further highlight the importance of IL-13 in the pathogenesis of AD. Anti-IL13 antibodies were however less successful in asthma.
IL-4 is a pleiotropic anti-inflammatory cytokine that is known to play an important role in the in the modulation of the hepatic immune system. The α subunit of the IL-4 receptor has been reported to promote liver regeneration through hepatocyte proliferation and regulate both the progression and reversal of liver fibrosis.40,41 Additionally, IL-4 has also been implicated in the progression to cirrhosis in patients with hepatitis B virus (HBV). The decision to initiate anti IL-4 treatment should be made with careful consideration and in collaboration with a hepatologist. Additionally, prophylaxis with antivirals should be considered to prevent a catastrophic hepatitis flare, liver failure, or HBV reactivation in the setting of immunomodulation therapy with anti-IL-4.42
The IgE pathway
IgE has been convincingly linked to the pathophysiology of allergic asthma and other allergic conditions.43 In the Mechanisms of the Development of Allergy (MeDALL) study IgE sensitization was associated with the frequency, persistence, and severity of allergic symptoms.44 Besides promoting T2 inflammation, the activation of allergen-specific memory Th2 cells by antigen-presenting cells via IgE-facilitated allergen presentation contributes to disease chronicity.43,45 IgE/FcεR1 cross-linking inhibits virus-induced interferon-α responses of plasmacytoid dendritic cells (DCs) explaining the increased susceptibility to viral infections in allergic asthma and the effect of omalizumab in blunting viral exacerbations of asthma.46,47
The alarmins
The alarmins, thymic stromal lymphopoietin (TSLP), IL-25, and IL-33, upstream regulators of T2 inflammation are expressed at high levels in T2 asthma, AD, CRSwNP and EoE. The alarmins initiate allergic and non-allergic inflammation through activation of ILC2, which are a rich source of cytokines such as IL-5 and IL-13. There is widespread expression of alarmins and their receptors across many effector cells, and recent studies have emphasized alarmin regulation of cluster of differentiation (CD) 4 T lymphocytes, eosinophils and basophils, and their progenitors. Furthermore, a link between alarmins and lipid mediators is being uncovered. Alarmins can drive well defined inflammatory pathways through activation of DCs and polarizing T cells to produce T2 cytokines, as well as they can directly activate many other effector cells that play a central role in allergic and nonallergic inflammation.48,49,50,51
TSLP is a pleiotropic cytokine exerting its biological effects by binding to a high-affinity heteromeric complex composed of thymic stromal lymphopoietin receptor chain and IL-7Rα. TSLP is produced by activated lung and intestinal epithelial cells, keratinocytes, fibroblasts, DCs and mast cells. In human tissues there are two variants for TSLP: the main isoform expressed in steady state is the short form (sf) TSLP, which plays a homeostatic role, whereas the long form is upregulated in inflammatory conditions.52 mAbs used to neutralize TSLP should not interact or hamper the homeostatic effects of sfTSLP. Several cellular targets for TSLP have been identified, including immune (DCs, ILC2, T and B cells, natural killer T and regulatory T cells, eosinophils, neutrophils, basophils, monocytes, mast cells, and macrophages) and non-immune cells (platelets and sensory neurons). IL-33 is as potent eosinophil activator, similar to IL-3, IL-5 and eotaxin-1, thus, important to consider for modulating eosinophil function. Through ILC2/IL-5 IL-33 promotes eosinophilopoiesis.53,54,55 Epithelial-derived IL-33 uniquely induces type-2 cytokines in mast cells, which regulate the expression of epithelial IL33 in a feedforward loop.56
The broad pathophysiologic profile of TSLP has motivated therapeutic targeting of this cytokine. Tezepelumab is a first-in-class human mAb that binds to TSLP inhibiting its interaction with its receptor complex. Clinical trials with tezepelumab support a central role for TSLP in driving airway inflammation and asthma exacerbations,57 while ongoing trials blocking IL-33 and IL-25 will help to define their respective role in asthma and other allergic diseases.
Upstream targets
In diseases driven by multiple cytokines such as allergic diseases, a single antagonistic agent targeting up-stream multiple pathways is a therapeutic option with considerable translational benefit. Interleukin-1 R3 is the co-receptor in three signaling pathways that involve six cytokines of the IL-1 family (IL-1α, IL-1β, IL-33, IL-36α, IL-36β, and IL-36γ). In vivo (animal models) targeting IL-1R3 significantly attenuated heterogeneous cytokine-driven inflammation and disease severity.58
The Janus-activated kinase (JAK) family together with signal transducer and activator of transcription (STAT) signaling pathway has a key role in regulating the expression and function of many inflammatory cytokines.59,60 Several JAK inhibitors are already on the market with proven anti-inflammatory efficiency. In a dose escalating study, a JAK inhibitor, ASN002 significantly suppressed key AD inflammatory pathways, corresponding to clinical response.61 Unfortunately, the oral route is hampered by adverse events, thus topical administration is currently investigated. Topical inhibition of JAK in the lungs, without relevant systemic exposure, is sufficient to reduce lung inflammation and improve lung functions in a rat asthma model.62
SHORT UPDATE-CURRENT AND NOVEL APPROACHES
Asthma
Five mAbs are available for uncontrolled severe asthma targeting IgE (omalizumab), IL-4/IL-13 (dupilumab) and IL-5 (reslizumab, mepolizumab, and benralizumab). In the absence of endotype-predictive biomarkers, the choice largely depends on patient factors. Future studies should focus on cost-effectiveness of treatment, drug-drug comparisons, and long-term efficacy and safety. Recently evaluated in clinical trials are mAbs against TSLP, IL-33 and its receptor ST2, small molecule antagonists to the chemoattractant receptor-homologous molecule expressed on Th2 cells (CRTH2), the receptor for stem cell factor on mast cells, a DNA enzyme directed at GATA3 and CCJM112, an anti-IL17A. In addition, a number of antagonists directed against other potential targets are under consideration for future trials, including C-X-C chemokine receptor type 2/IL-8, IL-25, IL-6, tumor necrosis factor-like ligand 1A, CD6, and activated cell adhesion molecule. Clinical data from ongoing and future trials will be important in determining whether these new medications will offer benefits in place of or in addition to existing therapies for allergic diseases.
Of note, patients with severe eosinophilic asthma show a comparable clinical benefit when targeting the IL-4/IL/13 pathway with dupilumab, or when targeting the IgE pathway with omalizumab, while the number of eosinophils in circulation and in sputum merely changes.63,64 The two pathways seem somehow independent as benralizumab treatment decreased exacerbations and improved lung function for patients with severe, uncontrolled eosinophilic asthma regardless of serum IgE concentrations and atopy status.65 Furthermore, dupilumab reduced severe exacerbation rates, improved forced expiratory volume in 1 second (FEV1) and asthma control, and suppressed type 2 inflammatory biomarkers in uncontrolled, moderate-to-severe asthma patients with or without evidence of allergic asthma.66
Simultaneous control of severe asthma and its multi-morbidities is a topic of major interest, while prescribing a biological. Efficacy on both asthma and CRSwNP symptoms is reported for all 5 biologicals approved for asthma. Dupilumab significantly improved allergic rhinitis (AR)-associated l symptoms in patients with uncontrolled persistent asthma and comorbid perennial AR.67 Both randomized controlled and observational-type clinical studies have demonstrated the effectiveness and safety of omalizumab in patients with asthma and AR.68 A recent real-life study reported on the benefit of omalizumab for patients with asthma and food allergy (FA).69
Algorithms may facilitate the identification of responders and non-responders during treatment, thus supporting the decision to continue therapy or the stop of ineffective treatment. For omalizumab the Global Evaluation of Treatment Effectiveness (GETE) score was validated and is currently under use.70 For reslizumab a similar evaluation after 16 weeks of treatment based on exacerbations, FEV1, Asthma Control Questionnaire and Asthma QoL scores, can correctly predict a positive response at 52 weeks in 90% of cases with a sensitivity of 95.4%–95.5%. The algorithm had however a low specificity, thus it cannot reliably predict non-responders.71
Chronic rhinosinusitis with nasal polyps
CRSwNP is an inflammatory disease of the nasal and paranasal mucosa, which causes nasal obstruction, hyposmia, and rhinorrhea. Conventional therapy includes intranasal corticosteroids (INCS) and polypectomy, but INCS offer only modest benefits, and recurrence after surgery is common. Therefore, effective pharmacologic therapies for CRSwNP are being actively sought.
The mAbs under investigation, omalizumab, dupilumab, reslizumab, mepolizumab, benralizumab, and etokinumab target key players in the pathophysiology of CRSwNP.72,73,74,75,76 A recent systematic review evaluating omalizumab, reslizumab, mepolizumab, and dupilumab in CRSwNP showed all these biologicals effective in reducing total nasal endoscopic polyp score, opacification in computed tomography and T2 biomarkers, while improving quality of life measures, nasal airflow, and olfaction. Overall, the use of these agents was deemed safe and well-tolerated.77 Dupilumab has just completed phase III trials for CRSwNP with positive results (reduced disease severity, significantly improved HRQoL, and improved productivity) and was recently approved by Food and Drug Administration (FDA), while the other biologicals are currently in phase III trials for this indication. Other potential targets include TSLP, IL-25, IL-33, Siglec-8, and nuclear factor-κB.78
Atopic dermatitis
AD is one of the most common inflammatory skin diseases affecting children and adults characterized by pruritus, inflammatory erythematous skin lesions, and skin-barrier defect. The intense pruritus and rash can be debilitating, significantly impairing QoL. Current mainstay treatments with emollients, topical or systemic corticosteroids, calcineurin inhibitors, and immunosuppressants have limited efficacy and potentially serious side effects.
Recent advances and understanding of the pathogenesis of AD have resulted in new therapies that target specific pathways with increased efficacy and the potential for less systemic side effects. A systematic literature review of 41 studies showed that the strongest evidence currently exists for dupilumab and cyclosporine at improving clinical disease severity.79 New FDA-approved therapies for AD are crisaborole and duplimab. Dupliumab trials of up to 52 weeks demonstrated efficacy and a favorable safety profile in patients with moderate-to-severe AD inadequately controlled with topical medications.80,81,82 A favorable benefit-risk profile can be achieved all racial subgroups83 and a recent safety study showed that dupilumab does not require laboratory monitoring.84
Lebrikizumab, tralokinumab and tezepelumab showed promising results in phase II trials.85,86,87 In exploratory analyses, additional anti IL-13 mAbs benefits were observed in DPP-4- and periostin-high subgroups. The JAK-STAT inhibitors (baricitinib, upadacitinib, PF-04965842, ASN002, tofacitinib, ruxolitinib, and delgocitinib) have the most promising results of the emerging therapies. Other drugs with potential include the aryl hydrocarbon receptor modulating agent tapinarof and the IL-31Rα antagonist nemolizumab. A long-term prospective observational safety study is essential to fully characterize the safety profile of systemic immunomodulating therapies for patients with AD. The TREatment of ATopic eczema (TREAT) Registry Taskforce offers a large platform to conduct such research using national registries that collect the same data using a predefined core dataset. Adult and pediatric patients who start treatment with dupilumab or another systemic immunomodulating agent for their AD will be included. The primary endpoint is the incidence of malignancies (excluding non-melanoma skin cancer) compared between the treatment groups. Secondary endpoints include other serious adverse events and adverse events of special interest, such as eye disorders and eosinophilia.88
Chronic urticaria
CSU has a significant effect on patients' QoL. Current therapies include antihistamines, leukotriene receptor antagonists, and immunosuppressants. Omalizumab is the treatment of choice in patients with antihistamine-resistant CSU. Total IgE levels and their changes predict the response.89,90 The presence of antinuclear antibodies in CSU is a predictor of poor response to omalizumab, as this endotype seems driven by IgG antibodies.91 Of note, in responders, omalizumab reverts the transcriptional signatures associated with CSU lesion phenotype to non-lesional/healthy skin phenotype.92 Omalizumab should further be explored for the use in CSU, in children <12 years old with CSU, and at higher doses. The off-label use of dupilumab, reslizumab, mepolizumab, and benralizumab can be effective in CSU. Ligelizumab and UB-221, 2 novel anti-IgE mAbs are in clinical trials for CSU. Other promising drugs that are currently under development for CSU are a CRTH2 antagonist (AZD1981), a mAb to Siglec-8 (AK002), Bruton's tyrosine kinase inhibitors (Fenebrutinib and Lou064), topical Syk inhibitors (GSK2646264). and dupilumab. Promising targets of future therapies include the Mas-related G-protein coupled receptor X2, the histamine H4 receptor, C5a and its receptor, inhibitory mast cell receptors other than Siglec-8, IL-33/IL-25/TSLP, and stem cell factor.93
Food allergy
Omalizumab has been extensively used to improve the efficacy and safety of oral immunotherapy (OIT) for FA.94,95,96 Results suggest particular benefit in patients with high risk of fatal anaphylaxis. An alternative approach is to use omalizumab instead of OIT to prevent severe allergic reactions upon accidental exposure.
Eosinophilic esophagitis
EoE is a chronic, allergen driven, immune mediated disorder of the esophagus, characterized by symptoms of esophageal dysfunction and eosinophil-predominant inflammation. Persistent, uncontrolled esophageal inflammation, frequently relapsing after discontinuation of the treatment, is associated with esophageal remodeling and stricture formation. Current treatment options consist of dietary intervention, endoscopic dilatation, and pharmacotherapy. The pathogenesis of EoE involves the activation of IL-5 and IL-13 pathways and local IgE production. Mepolizumab, reslizumab, omalizumab, an anti-IL-13 mAb (QAX576 and RPC4046), vedolizumab (anti α4β7 integrin), and infliximab have been evaluated but more data are needed.97,98,99,100,101,102,103,104 Dupilumab recently received orphan drug status for the treatment of EoE from the Orphan Drug Designation program of the FDA. Of note, a recent network meta-analysis showed viscous budesonide as the most effective pharmacologic therapy for EoE, superior to mepolizumab. Several other promising therapeutic agents are Siglec 8, TLSP, and IL-15 blocking antibodies.105,106,107
Hypereosinophilic disorders
HES is a group of diseases defined by marked eosinophilia in blood or tissue and eosinophil-related clinical manifestations. Anti-IL-5 therapy has a glucocorticoid-sparing effect in glucocorticoid-sensitive HES. Response is more likely in subjects with idiopathic or overlap forms of HES.108,109,110,111
Mepolizumab was successful in treating eosinophilic granulomatosis with polyangiitis (EGPA) and is approved by FDA for this condition. However, only 50% of EGPA patients respond to mepolizumab and further exploration of the genotype or of the anti-neutrophil cytoplasmic antibodies positive and negative phenotypes is warranted to select responders.112,113 Phase II trials including reslizumab and benralizumab for EGPA are currently ongoing. Small phase 2 trials and case reports or document the efficacy and safety of anti-IL-5 interventions in allergic bronchopulmonary aspergillosis, Gleich syndrome, bullous pemphigoid, Drug Rash with Eosinophilia and Systemic Symptoms (DRESS) syndrome or cutaneous mastocytosis associated with hypereosinophilia.114,115,116,117
EFFICACY VERSUS EFFECTIVENESS – BIOLOGICALS IN REAL LIFE
While omalizumab, benralizumab, mepolizumab, reslizumab and dupilumab have proven highly effective in T2 severe asthma, some patients with severe T2 asthma, as well as most patients with severe non-T2 disease, have poorly controlled disease. A post hoc analysis (129 patients, 64% women) of the international Identification and Description of Severe Asthma Patients (IDEAL) study aimed to evaluate the proportion of patients with severe asthma eligible for treatment with omalizumab, mepolizumab and reslizumab. The majority were overweight and 85% had at least one medical comorbidity. Asthma was poorly controlled in 67% and 24% had maintenance OCS. In this population 40% of patients were eligible for omalizumab, 27% for mepolizumab, and 2% for reslizumab. These findings show that a considerable proportion of patients with severe asthma remain uncontrolled and are not eligible for any of the available biological treatments.118
Of note patient selection for biologicals in real life might not be optimal: many omalizumab users have low or very low adherence rates for ICSs and/or ICS-LABA in the 12 months before omalizumab initiation compared the matched cohort of nonusers.119 In addition, there might be a selection bias as patients prescribed mepolizumab had a different prevalence of certain comorbidities such as CRSwNP, higher disease burden, higher healthcare resource utilization and costs compared with patients prescribed omalizumab.120 Last but not least, use of biologicals remains uncommon, with prevalence peaking in 2006 at 3 in 1,000 individuals with asthma and there is inequity in access to biologicals, as higher likelihood of use was related with middle age, higher income, commercial insurance, and access to a specialist.121
A validated assessment tool is needed to adequately evaluate response to biologicals in real-world settings. The Real-life Effectiveness of Omalizumab Therapy (REALITY) study evaluated The Standardized Measure to Assess Response to Therapy (SMART) tool was designed to define response by physician's subjective assessment of asthma symptoms and control and objective assessment of 6 parameters (exacerbations, steroid bursts, emergency department visits, and hospitalizations; lung function; ACT score). True responders are defined as meeting both subjective and objective criteria.122
Uncontrolled asthma is associated with considerable clinical burden and costs to payers and patients. The cost-effectiveness of biologicals based on real-world treatment patterns is unknown. Based on real-world outcomes, omalizumab may be cost-effective for uncontrolled asthma from the US payer perspective.123 Including broader evidence on treatment discontinuation, caregiver burden, and OCS reduction from real-world studies and severe asthma registries may better reflect the effects and value of omalizumab for all healthcare stakeholders.
INDIRECT TREATMENT COMPARISONS BETWEEN BIOLOGICALS
As there are no head-to-head comparison between biologicals targeting the same phenotype and no good biomarkers exist for selecting responders to a selected intervention indirect treatment comparisons (ITC) and network metanalysis (NMA) tried to offer a solution for the choice of a particular biological. The ITC conducted by Busse et al.124 using licensed doses of the biologicals targeting the IL-5 pathway suggested that in patients with similar blood eosinophil counts mepolizumab was associated with significantly greater improvements in clinically significant exacerbations and asthma control compared with reslizumab or benralizumab.124 Casale et al.125 claimed that reslizumab is superior to benralizumab for Asthma Control Questionnaire score, Asthma Quality of Life Questionnaire score, FEV1, and clinical asthma exacerbations. Another indirect comparison suggested a better efficacy of benralizumab versus mepolizumab in reducing the OCS dose.126
A key requirement of ITC and NMAs is that included studies have sufficiently similar designs, treatment durations and patient baseline characteristics to justify cross-study comparisons. Baseline asthma severity, atopic status definition, lung function, eosinophil cut-offs or exacerbation history and asthma duration are all important modulators of treatment efficacy. These differ across trials because of different inclusion or exclusion criteria, thus the ITC may be erroneous or biased. Matching-adjusted indirect comparisons (MAICs) are population-adjusted ITC attempting to reduce bias in treatment comparisons by matching patient-level data from the clinical trials of one treatment to aggregate data reported for comparator trials.127 Treatment-effect-modifying variables that differ across studies are used to weight the patient-level data to reflect the characteristics of the comparator's patient population. Data similar to the aggregate of the comparator population are weighted more heavily when modelling study outcomes, similar to a propensity score, while data quite different from the comparator population will weigh less on the outcome. After matching the effective sample size (ESS) is considered.128 A small ESS means that the weighted population and non-weighted population have little overlap, which may result in unstable, invalid estimates. Thus, if the ESS is small the comparison cannot be done. In addition, unmeasured/unreported differences between trials cannot be matched, thus there is a degree of uncertainty. When baseline patient characteristics were matched across asthma trials, benralizumab and mepolizumab yielded similar efficacy in decreasing exacerbations and improving lung function.129 Benralizumab and reslizumab patient populations were too dissimilar to generate a sufficient ESS for a reliable estimate using MAIC.
CONCLUSION
Even if biologicals do not prove to be effective in all patients, studying their clinical impact and their associated immunologic markers will definitely help to better understand the endotypes of allergic diseases. Ultimately, what must be determined are the clinical effectiveness and duration of benefit under real-world conditions. Most of real-world patients are not included in phase 2 and 3 trials or eligible for currently available biologicals based on regulatory approved criteria. Comparisons on cost-effectiveness of biologicals compared with standard care, particularly in vulnerable populations at high risk for poor outcomes are urgently needed.
Footnotes
Disclosure: There are no financial or other issues that might lead to conflict of interest.
References
- 1.Agache I. Severe asthma phenotypes and endotypes. Semin Immunol. 2019;101301:101301. doi: 10.1016/j.smim.2019.101301. [DOI] [PubMed] [Google Scholar]
- 2.Agache I, Akdis CA. Precision medicine and phenotypes, endotypes, genotypes, regiotypes, and theratypes of allergic diseases. J Clin Invest. 2019;130:1493–1503. doi: 10.1172/JCI124611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agache I, Akdis CA. Endotypes of allergic diseases and asthma: an important step in building blocks for the future of precision medicine. Allergol Int. 2016;65:243–252. doi: 10.1016/j.alit.2016.04.011. [DOI] [PubMed] [Google Scholar]
- 4.Agache I, Miller R, Gern JE, Hellings PW, Jutel M, Muraro A, et al. Emerging concepts and challenges in implementing the exposome paradigm in allergic diseases and asthma: a Practall document. Allergy. 2019;74:449–463. doi: 10.1111/all.13690. [DOI] [PubMed] [Google Scholar]
- 5.Agache I, Rogozea L. Asthma biomarkers: do they bring precision medicine closer to the clinic? Allergy Asthma Immunol Res. 2017;9:466–476. doi: 10.4168/aair.2017.9.6.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Agache I, Annesi-Maesano I, Bonertz A, Branca F, Cant A, Fras Z, et al. Prioritizing research challenges and funding for allergy and asthma and the need for translational research-the European Strategic Forum on Allergic Diseases. Allergy. 2019;74:2064–2076. doi: 10.1111/all.13856. [DOI] [PubMed] [Google Scholar]
- 7.Ravin KA, Loy M. The eosinophil in infection. Clin Rev Allergy Immunol. 2016;50:214–227. doi: 10.1007/s12016-015-8525-4. [DOI] [PubMed] [Google Scholar]
- 8.Jacobsen EA, Helmers RA, Lee JJ, Lee NA. The expanding role(s) of eosinophils in health and disease. Blood. 2012;120:3882–3890. doi: 10.1182/blood-2012-06-330845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marichal T, Mesnil C, Bureau F. Homeostatic eosinophils: characteristics and functions. Front Med (Lausanne) 2017;4:101. doi: 10.3389/fmed.2017.00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mesnil C, Raulier S, Paulissen G, Xiao X, Birrell MA, Pirottin D, et al. Lung-resident eosinophils represent a distinct regulatory eosinophil subset. J Clin Invest. 2016;126:3279–3295. doi: 10.1172/JCI85664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takatsu K. Interleukin-5 and IL-5 receptor in health and diseases. Proc Jpn Acad, Ser B, Phys Biol Sci. 2011;87:463–485. doi: 10.2183/pjab.87.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Molfino NA, Gossage D, Kolbeck R, Parker JM, Geba GP. Molecular and clinical rationale for therapeutic targeting of interleukin-5 and its receptor. Clin Exp Allergy. 2012;42:712–737. doi: 10.1111/j.1365-2222.2011.03854.x. [DOI] [PubMed] [Google Scholar]
- 13.Gorski SA, Lawrence MG, Hinkelman A, Spano MM, Steinke JW, Borish L, et al. Expression of IL-5 receptor alpha by murine and human lung neutrophils. PLoS One. 2019;14:e0221113. doi: 10.1371/journal.pone.0221113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dent LA, Strath M, Mellor AL, Sanderson CJ. Eosinophilia in transgenic mice expressing interleukin 5. J Exp Med. 1990;172:1425–1431. doi: 10.1084/jem.172.5.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sridhar S, Liu H, Pham TH, Damera G, Newbold P. Modulation of blood inflammatory markers by benralizumab in patients with eosinophilic airway diseases. Respir Res. 2019;20:14. doi: 10.1186/s12931-018-0968-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Agache IO. From phenotypes to endotypes to asthma treatment. Curr Opin Allergy Clin Immunol. 2013;13:249–256. doi: 10.1097/ACI.0b013e32836093dd. [DOI] [PubMed] [Google Scholar]
- 17.Wang FP, Liu T, Lan Z, Li SY, Mao H. Efficacy and safety of anti-interleukin-5 therapy in patients with asthma: a systematic review and meta-analysis. PLoS One. 2016;11:e0166833. doi: 10.1371/journal.pone.0166833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haldar P, Brightling CE, Singapuri A, Hargadon B, Gupta S, Monteiro W, et al. Outcomes after cessation of mepolizumab therapy in severe eosinophilic asthma: a 12-month follow-up analysis. J Allergy Clin Immunol. 2014;133:921–923. doi: 10.1016/j.jaci.2013.11.026. [DOI] [PubMed] [Google Scholar]
- 19.Kim YJ, Prussin C, Martin B, Law MA, Haverty TP, Nutman TB, et al. Rebound eosinophilia after treatment of hypereosinophilic syndrome and eosinophilic gastroenteritis with monoclonal anti-IL-5 antibody SCH55700. J Allergy Clin Immunol. 2004;114:1449–1455. doi: 10.1016/j.jaci.2004.08.027. [DOI] [PubMed] [Google Scholar]
- 20.Mukherjee M, Aleman Paramo F, Kjarsgaard M, Salter B, Nair G, LaVigne N, et al. Weight-adjusted intravenous reslizumab in severe asthma with inadequate response to fixed-dose subcutaneous mepolizumab. Am J Respir Crit Care Med. 2018;197:38–46. doi: 10.1164/rccm.201707-1323OC. [DOI] [PubMed] [Google Scholar]
- 21.Mukherjee M, Lim HF, Thomas S, Miller D, Kjarsgaard M, Tan B, et al. Airway autoimmune responses in severe eosinophilic asthma following low-dose Mepolizumab therapy. Allergy Asthma Clin Immunol. 2017;13:2. doi: 10.1186/s13223-016-0174-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kelly EA, Esnault S, Liu LY, Evans MD, Johansson MW, Mathur S, et al. Mepolizumab attenuates airway eosinophil numbers, but not their functional phenotype in asthma. Am J Respir Crit Care Med. 2017;196:1385–1395. doi: 10.1164/rccm.201611-2234OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lim HF, Nair P. Efficacy and safety of reslizumab in patients with moderate to severe eosinophilic asthma. Expert Rev Respir Med. 2015;9:135–142. doi: 10.1586/17476348.2015.1000867. [DOI] [PubMed] [Google Scholar]
- 24.Sehmi R, Lim HF, Mukherjee M, Huang C, Radford K, Newbold P, et al. Benralizumab attenuates airway eosinophilia in prednisone-dependent asthma. J Allergy Clin Immunol. 2018;141:1529–1532.e8. doi: 10.1016/j.jaci.2018.01.008. [DOI] [PubMed] [Google Scholar]
- 25.Laviolette M, Gossage DL, Gauvreau G, Leigh R, Olivenstein R, Katial R, et al. Effects of benralizumab on airway eosinophils in asthmatic patients with sputum eosinophilia. J Allergy Clin Immunol. 2013;132:1086–1096.e5. doi: 10.1016/j.jaci.2013.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu LY, Sedgwick JB, Bates ME, Vrtis RF, Gern JE, Kita H, et al. Decreased expression of membrane IL-5 receptor alpha on human eosinophils: I. Loss of membrane IL-5 receptor alpha on airway eosinophils and increased soluble IL-5 receptor alpha in the airway after allergen challenge. J Immunol. 2002;169:6452–6458. doi: 10.4049/jimmunol.169.11.6452. [DOI] [PubMed] [Google Scholar]
- 27.Liu LY, Sedgwick JB, Bates ME, Vrtis RF, Gern JE, Kita H, et al. Decreased expression of membrane IL-5 receptor alpha on human eosinophils: II. IL-5 down-modulates its receptor via a proteinase-mediated process. J Immunol. 2002;169:6459–6466. doi: 10.4049/jimmunol.169.11.6459. [DOI] [PubMed] [Google Scholar]
- 28.Stein ML, Villanueva JM, Buckmeier BK, Yamada Y, Filipovich AH, Assa’ad AH, et al. Anti-IL-5 (mepolizumab) therapy reduces eosinophil activation ex vivo and increases IL-5 and IL-5 receptor levels. J Allergy Clin Immunol. 2008;121:1473–1483. 1483.e1–1474. doi: 10.1016/j.jaci.2008.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kolbeck R, Kozhich A, Koike M, Peng L, Andersson CK, Damschroder MM, et al. MEDI-563, a humanized anti-IL-5 receptor alpha mAb with enhanced antibody-dependent cell-mediated cytotoxicity function. J Allergy Clin Immunol. 2010;125:1344–1353.e2. doi: 10.1016/j.jaci.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 30.Wright AK, Weston C, Rana BM, Brightling CE, Cousins DJ. Human group 2 innate lymphoid cells do not express the IL-5 receptor. J Allergy Clin Immunol. 2017;140:1430–1433.e4. doi: 10.1016/j.jaci.2017.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith SG, Chen R, Kjarsgaard M, Huang C, Oliveria JP, O’Byrne PM, et al. Increased numbers of activated group 2 innate lymphoid cells in the airways of patients with severe asthma and persistent airway eosinophilia. J Allergy Clin Immunol. 2016;137:75–86.e8. doi: 10.1016/j.jaci.2015.05.037. [DOI] [PubMed] [Google Scholar]
- 32.Gandhi NA, Pirozzi G, Graham NM. Commonality of the IL-4/IL-13 pathway in atopic diseases. Expert Rev Clin Immunol. 2017;13:425–437. doi: 10.1080/1744666X.2017.1298443. [DOI] [PubMed] [Google Scholar]
- 33.Jonstam K, Swanson BN, Mannent LP, Cardell LO, Tian N, Wang Y, et al. Dupilumab reduces local type 2 pro-inflammatory biomarkers in chronic rhinosinusitis with nasal polyposis. Allergy. 2019;74:743–752. doi: 10.1111/all.13685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Furue K, Ito T, Tsuji G, Ulzii D, Vu YH, Kido-Nakahara M, et al. The IL-13-OVOL1-FLG axis in atopic dermatitis. Immunology. 2019 doi: 10.1111/imm.13120. Forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Callewaert C, Nakatsuji T, Knight R, Kosciolek T, Vrbanac A, Kotol P, et al. IL-4Rα blockade by dupilumab decreases Staphylococcus aureus colonization and increases microbial diversity in atopic dermatitis. J Invest Dermatol. 2019 doi: 10.1016/j.jid.2019.05.024. Forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guttman-Yassky E, Bissonnette R, Ungar B, Suárez-Fariñas M, Ardeleanu M, Esaki H, et al. Dupilumab progressively improves systemic and cutaneous abnormalities in patients with atopic dermatitis. J Allergy Clin Immunol. 2019;143:155–172. doi: 10.1016/j.jaci.2018.08.022. [DOI] [PubMed] [Google Scholar]
- 37.León B, Lund FE. Compartmentalization of dendritic cell and T-cell interactions in the lymph node: anatomy of T-cell fate decisions. Immunol Rev. 2019;289:84–100. doi: 10.1111/imr.12758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Prout MS, Kyle RL, Ronchese F, Le Gros G. IL-4 Is a key requirement for IL-4- and IL-4/IL-13-expressing CD4 Th2 subsets in lung and skin. Front Immunol. 2018;9:1211. doi: 10.3389/fimmu.2018.01211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim JE, Jung K, Kim JA, Kim SH, Park HS, Kim YS. Engineering of anti-human interleukin-4 receptor alpha antibodies with potent antagonistic activity. Sci Rep. 2019;9:7772. doi: 10.1038/s41598-019-44253-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goh YP, Henderson NC, Heredia JE, Red Eagle A, Odegaard JI, Lehwald N, et al. Eosinophils secrete IL-4 to facilitate liver regeneration. Proc Natl Acad Sci U S A. 2013;110:9914–9919. doi: 10.1073/pnas.1304046110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weng SY, Wang X, Vijayan S, Tang Y, Kim YO, Padberg K, et al. IL-4 Receptor alpha signaling through macrophages differentially regulates liver fibrosis progression and reversal. EBioMedicine. 2018;29:92–103. doi: 10.1016/j.ebiom.2018.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mastroianni CM, Lichtner M, Citton R, Del Borgo C, Rago A, Martini H, et al. Current trends in management of hepatitis B virus reactivation in the biologic therapy era. World J Gastroenterol. 2011;17:3881–3887. doi: 10.3748/wjg.v17.i34.3881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Palomares Ó, Sánchez-Ramón S, Dávila I, Prieto L, Pérez de Llano L, Lleonart M, et al. dIvergEnt: how IgE axis contributes to the continuum of allergic asthma and anti-IgE therapies. Int J Mol Sci. 2017;18:E1328. doi: 10.3390/ijms18061328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Anto JM, Bousquet J, Akdis M, Auffray C, Keil T, Momas I, et al. Mechanisms of the Development of Allergy (MeDALL): introducing novel concepts in allergy phenotypes. J Allergy Clin Immunol. 2017;139:388–399. doi: 10.1016/j.jaci.2016.12.940. [DOI] [PubMed] [Google Scholar]
- 45.Wilcock LK, Francis JN, Durham SR. IgE-facilitated antigen presentation: role in allergy and the influence of allergen immunotherapy. Immunol Allergy Clin North Am. 2006;26:333–347. doi: 10.1016/j.iac.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 46.Gill MA, Liu AH, Calatroni A, Krouse RZ, Shao B, Schiltz A, et al. Enhanced plasmacytoid dendritic cell antiviral responses after omalizumab. J Allergy Clin Immunol. 2018;141:1735–1743.e9. doi: 10.1016/j.jaci.2017.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Teach SJ, Gill MA, Togias A, Sorkness CA, Arbes SJ, Jr, Calatroni A, et al. Preseasonal treatment with either omalizumab or an inhaled corticosteroid boost to prevent fall asthma exacerbations. J Allergy Clin Immunol. 2015;136:1476–1485. doi: 10.1016/j.jaci.2015.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Martinez-Gonzalez I, Steer CA, Takei F. Lung ILC2s link innate and adaptive responses in allergic inflammation. Trends Immunol. 2015;36:189–195. doi: 10.1016/j.it.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 49.Roan F, Obata-Ninomiya K, Ziegler SF. Epithelial cell-derived cytokines: more than just signaling the alarm. J Clin Invest. 2019;129:1441–1451. doi: 10.1172/JCI124606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Morita H, Moro K, Koyasu S. Innate lymphoid cells in allergic and nonallergic inflammation. J Allergy Clin Immunol. 2016;138:1253–1264. doi: 10.1016/j.jaci.2016.09.011. [DOI] [PubMed] [Google Scholar]
- 51.Kim HJ, Lee SH, Jeong S, Hong SJ. Protease-activated receptors 2-antagonist suppresses asthma by inhibiting reactive oxygen species-thymic stromal lymphopoietin inflammation and epithelial tight junction degradation. Allergy Asthma Immunol Res. 2019;11:560–571. doi: 10.4168/aair.2019.11.4.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Varricchi G, Pecoraro A, Marone G, Criscuolo G, Spadaro G, Genovese A, et al. Thymic stromal lymphopoietin isoforms, inflammatory disorders, and cancer. Front Immunol. 2018;9:1595. doi: 10.3389/fimmu.2018.01595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Anderson EL, Kobayashi T, Iijima K, Bartemes KR, Chen CC, Kita H. IL-33 mediates reactive eosinophilopoiesis in response to airborne allergen exposure. Allergy. 2016;71:977–988. doi: 10.1111/all.12861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Johnston LK, Hsu CL, Krier-Burris RA, Chhiba KD, Chien KB, McKenzie A, et al. IL-33 precedes IL-5 in regulating eosinophil commitment and is required for eosinophil homeostasis. J Immunol. 2016;197:3445–3453. doi: 10.4049/jimmunol.1600611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Angulo EL, McKernan EM, Fichtinger PS, Mathur SK. Comparison of IL-33 and IL-5 family mediated activation of human eosinophils. PLoS One. 2019;14:e0217807. doi: 10.1371/journal.pone.0217807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Altman MC, Lai Y, Nolin JD, Long S, Chen CC, Piliponsky AM, et al. Airway epithelium-shifted mast cell infiltration regulates asthmatic inflammation via IL-33 signaling. J Clin Invest. 2019;129:4979–4991. doi: 10.1172/JCI126402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Corren J, Parnes JR, Wang L, Mo M, Roseti SL, Griffiths JM, et al. Tezepelumab in adults with uncontrolled asthma. N Engl J Med. 2017;377:936–946. doi: 10.1056/NEJMoa1704064. [DOI] [PubMed] [Google Scholar]
- 58.Højen JF, Kristensen ML, McKee AS, Wade MT, Azam T, Lunding LP, et al. IL-1R3 blockade broadly attenuates the functions of six members of the IL-1 family, revealing their contribution to models of disease. Nat Immunol. 2019;20:1138–1149. doi: 10.1038/s41590-019-0467-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zak M, Hanan EJ, Lupardus P, Brown DG, Robinson C, Siu M, et al. Discovery of a class of highly potent Janus kinase 1/2 (JAK1/2) inhibitors demonstrating effective cell-based blockade of IL-13 signaling. Bioorg Med Chem Lett. 2019;29:1522–1531. doi: 10.1016/j.bmcl.2019.04.008. [DOI] [PubMed] [Google Scholar]
- 60.Fragoulis GE, McInnes IB, Siebert S. JAK-inhibitors. New players in the field of immune-mediated diseases, beyond rheumatoid arthritis. Rheumatology (Oxford) 2019;58:i43–i54. doi: 10.1093/rheumatology/key276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pavel AB, Song T, Kim HJ, Del Duca E, Krueger JG, Dubin C, et al. Oral Janus kinase/SYK inhibition (ASN002) suppresses inflammation and improves epidermal barrier markers in patients with atopic dermatitis. J Allergy Clin Immunol. 2019;144:1011–1024. doi: 10.1016/j.jaci.2019.07.013. [DOI] [PubMed] [Google Scholar]
- 62.Calbet M, Ramis I, Calama E, Carreño C, Paris S, Maldonado M, et al. Novel inhaled pan-JAK inhibitor, LAS194046, reduces allergen-induced airway inflammation, late asthmatic response, and pSTAT activation in brown Norway rats. J Pharmacol Exp Ther. 2019;370:137–147. doi: 10.1124/jpet.119.256263. [DOI] [PubMed] [Google Scholar]
- 63.Wenzel S, Ford L, Pearlman D, Spector S, Sher L, Skobieranda F, et al. Dupilumab in persistent asthma with elevated eosinophil levels. N Engl J Med. 2013;368:2455–2466. doi: 10.1056/NEJMoa1304048. [DOI] [PubMed] [Google Scholar]
- 64.Katsaounou P, Buhl R, Brusselle G, Pfister P, Martínez R, Wahn U, et al. Omalizumab as alternative to chronic use of oral corticosteroids in severe asthma. Respir Med. 2019;150:51–62. doi: 10.1016/j.rmed.2019.02.003. [DOI] [PubMed] [Google Scholar]
- 65.Chipps BE, Newbold P, Hirsch I, Trudo F, Goldman M. Benralizumab efficacy by atopy status and serum immunoglobulin E for patients with severe, uncontrolled asthma. Ann Allergy Asthma Immunol. 2018;120:504–511.e4. doi: 10.1016/j.anai.2018.01.030. [DOI] [PubMed] [Google Scholar]
- 66.Corren J, Castro M, O'Riordan T, Hanania NA, Pavord ID, Quirce S, et al. Dupilumab efficacy in patients with uncontrolled, moderate-to-severe allergic asthma. J Allergy Clin Immunol Pract. 2019:Forthcoming. doi: 10.1016/j.jaip.2019.08.050. [DOI] [PubMed] [Google Scholar]
- 67.Weinstein SF, Katial R, Jayawardena S, Pirozzi G, Staudinger H, Eckert L, et al. Efficacy and safety of dupilumab in perennial allergic rhinitis and comorbid asthma. J Allergy Clin Immunol. 2018;142:171–177.e1. doi: 10.1016/j.jaci.2017.11.051. [DOI] [PubMed] [Google Scholar]
- 68.Vignola AM, Humbert M, Bousquet J, Boulet LP, Hedgecock S, Blogg M, et al. Efficacy and tolerability of anti-immunoglobulin E therapy with omalizumab in patients with concomitant allergic asthma and persistent allergic rhinitis: SOLAR. Allergy. 2004;59:709–717. doi: 10.1111/j.1398-9995.2004.00550.x. [DOI] [PubMed] [Google Scholar]
- 69.Fiocchi A, Artesani MC, Riccardi C, Mennini M, Pecora V, Fierro V, et al. Impact of omalizumab on food allergy in patients treated for asthma: a real-life study. J Allergy Clin Immunol Pract. 2019;7:1901–1909.e5. doi: 10.1016/j.jaip.2019.01.023. [DOI] [PubMed] [Google Scholar]
- 70.Bousquet J, Rao S, Manga V. Global evaluation of treatment effectiveness (GETE) is an accurate predictor of response to omalizumab in patients with severe allergic asthma: a pooled analysis. Eur Respir J. 2014;44:3483. [Google Scholar]
- 71.Bateman ED, Djukanović R, Castro M, Canvin J, Germinaro M, Noble R, et al. Predicting responders to reslizumab after 16 weeks of treatment using an algorithm derived from clinical studies of patients with severe eosinophilic asthma. Am J Respir Crit Care Med. 2019;199:489–495. doi: 10.1164/rccm.201708-1668OC. [DOI] [PubMed] [Google Scholar]
- 72.Bachert C, Hellings PW, Mullol J, Hamilos DL, Gevaert P, Naclerio RM, et al. Dupilumab improves health-related quality of life in patients with chronic rhinosinusitis with nasal polyposis. Allergy. 2019 doi: 10.1111/all.13984. Forthcoming. [DOI] [PubMed] [Google Scholar]
- 73.Bachert C, Sousa AR, Lund VJ, Scadding GK, Gevaert P, Nasser S, et al. Reduced need for surgery in severe nasal polyposis with mepolizumab: randomized trial. J Allergy Clin Immunol. 2017;140:1024–1031.e14. doi: 10.1016/j.jaci.2017.05.044. [DOI] [PubMed] [Google Scholar]
- 74.Bachert C, Mannent L, Naclerio RM, Mullol J, Ferguson BJ, Gevaert P, et al. Effect of subcutaneous dupilumab on nasal polyp burden in patients with chronic sinusitis and nasal polyposis: a randomized clinical trial. JAMA. 2016;315:469–479. doi: 10.1001/jama.2015.19330. [DOI] [PubMed] [Google Scholar]
- 75.Gevaert P, Calus L, Van Zele T, Blomme K, De Ruyck N, Bauters W, et al. Omalizumab is effective in allergic and nonallergic patients with nasal polyps and asthma. J Allergy Clin Immunol. 2013;131:110–116.e1. doi: 10.1016/j.jaci.2012.07.047. [DOI] [PubMed] [Google Scholar]
- 76.Kartush AG, Schumacher JK, Shah R, Patadia MO. Biologic agents for the treatment of chronic rhinosinusitis with nasal polyps. Am J Rhinol Allergy. 2019;33:203–211. doi: 10.1177/1945892418814768. [DOI] [PubMed] [Google Scholar]
- 77.Tsetsos N, Goudakos JK, Daskalakis D, Konstantinidis I, Markou K. Monoclonal antibodies for the treatment of chronic rhinosinusitis with nasal polyposis: a systematic review. Rhinology. 2018;56:11–21. doi: 10.4193/Rhino17.156. [DOI] [PubMed] [Google Scholar]
- 78.Jung HJ, Zhang YL, Kim DK, Rhee CS, Kim DY. The role of NF-κB in chronic rhinosinusitis with nasal polyps. Allergy Asthma Immunol Res. 2019;11:806–817. doi: 10.4168/aair.2019.11.6.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Seger EW, Wechter T, Strowd L, Feldman SR. Relative efficacy of systemic treatments for atopic dermatitis. J Am Acad Dermatol. 2019;80:411–416.e4. doi: 10.1016/j.jaad.2018.09.053. [DOI] [PubMed] [Google Scholar]
- 80.Thaçi D, L Simpson E, Deleuran M, Kataoka Y, Chen Z, Gadkari A, et al. Efficacy and safety of dupilumab monotherapy in adults with moderate-to-severe atopic dermatitis: a pooled analysis of two phase 3 randomized trials (LIBERTY AD SOLO 1 and LIBERTY AD SOLO 2) J Dermatol Sci. 2019;94:266–275. doi: 10.1016/j.jdermsci.2019.02.002. [DOI] [PubMed] [Google Scholar]
- 81.Simpson EL, Bieber T, Guttman-Yassky E, Beck LA, Blauvelt A, Cork MJ, et al. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med. 2016;375:2335–2348. doi: 10.1056/NEJMoa1610020. [DOI] [PubMed] [Google Scholar]
- 82.Blauvelt A, Rosmarin D, Bieber T, Simpson EL, Bagel J, Worm M, et al. Improvement of atopic dermatitis with dupilumab occurs equally well across different anatomical regions: data from phase III clinical trials. Br J Dermatol. 2019;181:196–197. doi: 10.1111/bjd.17703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Alexis AF, Rendon M, Silverberg JI, Pariser DM, Lockshin B, Griffiths CE, et al. Efficacy of dupilumab in different racial subgroups of adults with moderate-to-severe atopic dermatitis in three randomized, placebo-controlled phase 3 trials. J Drugs Dermatol. 2019;18:804–813. [PubMed] [Google Scholar]
- 84.Wollenberg A, Beck LA, Blauvelt A, Simpson EL, Chen Z, Chen Q, et al. Laboratory safety of dupilumab in moderate-to-severe atopic dermatitis: results from three phase III trials (LIBERTY AD SOLO 1, LIBERTY AD SOLO 2, LIBERTY AD CHRONOS) Br J Dermatol. 2019 doi: 10.1111/bjd.18434. Forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Simpson EL, Flohr C, Eichenfield LF, Bieber T, Sofen H, Taïeb A, et al. Efficacy and safety of lebrikizumab (an anti-IL-13 monoclonal antibody) in adults with moderate-to-severe atopic dermatitis inadequately controlled by topical corticosteroids: A randomized, placebo-controlled phase II trial (TREBLE) J Am Acad Dermatol. 2018;78:863–871.e11. doi: 10.1016/j.jaad.2018.01.017. [DOI] [PubMed] [Google Scholar]
- 86.Wollenberg A, Howell MD, Guttman-Yassky E, Silverberg JI, Kell C, Ranade K, et al. Treatment of atopic dermatitis with tralokinumab, an anti-IL-13 mAb. J Allergy Clin Immunol. 2019;143:135–141. doi: 10.1016/j.jaci.2018.05.029. [DOI] [PubMed] [Google Scholar]
- 87.Simpson EL, Parnes JR, She D, Crouch S, Rees W, Mo M, et al. Tezepelumab, an anti-thymic stromal lymphopoietin monoclonal antibody, in the treatment of moderate to severe atopic dermatitis: a randomized phase 2a clinical trial. J Am Acad Dermatol. 2019;80:1013–1021. doi: 10.1016/j.jaad.2018.11.059. [DOI] [PubMed] [Google Scholar]
- 88.Bosma AL, Spuls PI, Garcia-Doval I, Naldi L, Prieto-Merino D, Tesch F, et al. TREatment of ATopic eczema (TREAT) Registry Taskforce: protocol for a European safety study of dupilumab and other systemic therapies in patients with atopic eczema. Br J Dermatol. 2019 doi: 10.1111/bjd.18452. Forthcoming. [DOI] [PubMed] [Google Scholar]
- 89.Ertas R, Ozyurt K, Atasoy M, Hawro T, Maurer M. The clinical response to omalizumab in chronic spontaneous urticaria patients is linked to and predicted by IgE levels and their change. Allergy. 2018;73:705–712. doi: 10.1111/all.13345. [DOI] [PubMed] [Google Scholar]
- 90.Weller K, Ohanyan T, Hawro T, Ellrich A, Sussman G, Koplowitz J, et al. Total IgE levels are linked to the response of chronic spontaneous urticaria patients to omalizumab. Allergy. 2018;73:2406–2408. doi: 10.1111/all.13586. [DOI] [PubMed] [Google Scholar]
- 91.Ertaş R, Hawro T, Altrichter S, Özyurt K, Erol K, Ketenci Ertaş Ş, et al. Antinuclear antibodies are common and linked to poor response to omalizumab treatment in patients with CSU. Allergy. 2019 doi: 10.1111/all.14033. Forthcoming. [DOI] [PubMed] [Google Scholar]
- 92.Metz M, Torene R, Kaiser S, Beste MT, Staubach P, Bauer A, et al. Omalizumab normalizes the gene expression signature of lesional skin in patients with chronic spontaneous urticaria: A randomized, double-blind, placebo-controlled study. Allergy. 2019;74:141–151. doi: 10.1111/all.13547. [DOI] [PubMed] [Google Scholar]
- 93.Min TK, Saini SS. Emerging therapies in chronic spontaneous urticaria. Allergy Asthma Immunol Res. 2019;11:470–481. doi: 10.4168/aair.2019.11.4.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wood RA, Kim JS, Lindblad R, Nadeau K, Henning AK, Dawson P, et al. A randomized, double-blind, placebo-controlled study of omalizumab combined with oral immunotherapy for the treatment of cow's milk allergy. J Allergy Clin Immunol. 2016;137:1103–1110.e11. doi: 10.1016/j.jaci.2015.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.MacGinnitie AJ, Rachid R, Gragg H, Little SV, Lakin P, Cianferoni A, et al. Omalizumab facilitates rapid oral desensitization for peanut allergy. J Allergy Clin Immunol. 2017;139:873–881.e8. doi: 10.1016/j.jaci.2016.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yee CS, Albuhairi S, Noh E, El-Khoury K, Rezaei S, Abdel-Gadir A, et al. Long-term outcome of peanut oral immunotherapy facilitated initially by omalizumab. J Allergy Clin Immunol Pract. 2019;7:451–461.e7. doi: 10.1016/j.jaip.2018.09.015. [DOI] [PubMed] [Google Scholar]
- 97.Loizou D, Enav B, Komlodi-Pasztor E, Hider P, Kim-Chang J, Noonan L, et al. A pilot study of omalizumab in eosinophilic esophagitis. PLoS One. 2015;10:e0113483. doi: 10.1371/journal.pone.0113483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Markowitz JE, Jobe L, Miller M, Frost C, Laney Z, Eke R. Safety and efficacy of reslizumab for children and adolescents with eosinophilic esophagitis treated for 9 years. J Pediatr Gastroenterol Nutr. 2018;66:893–897. doi: 10.1097/MPG.0000000000001840. [DOI] [PubMed] [Google Scholar]
- 99.Otani IM, Anilkumar AA, Newbury RO, Bhagat M, Beppu LY, Dohil R, et al. Anti-IL-5 therapy reduces mast cell and IL-9 cell numbers in pediatric patients with eosinophilic esophagitis. J Allergy Clin Immunol. 2013;131:1576–1582. doi: 10.1016/j.jaci.2013.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rothenberg ME, Wen T, Greenberg A, Alpan O, Enav B, Hirano I, et al. Intravenous anti-IL-13 mAb QAX576 for the treatment of eosinophilic esophagitis. J Allergy Clin Immunol. 2015;135:500–507. doi: 10.1016/j.jaci.2014.07.049. [DOI] [PubMed] [Google Scholar]
- 101.Hirano I, Collins MH, Assouline-Dayan Y, Evans L, Gupta S, Schoepfer AM, et al. RPC4046, a monoclonal antibody against IL13, reduces histologic and endoscopic activity in patients with eosinophilic esophagitis. Gastroenterology. 2019;156:592–603.e10. doi: 10.1053/j.gastro.2018.10.051. [DOI] [PubMed] [Google Scholar]
- 102.Nhu QM, Chiao H, Moawad FJ, Bao F, Konijeti GG. The anti-α4β7 integrin therapeutic antibody for inflammatory bowel disease, vedolizumab, ameliorates eosinophilic esophagitis: a novel clinical observation. Am J Gastroenterol. 2018;113:1261–1263. doi: 10.1038/s41395-018-0145-1. [DOI] [PubMed] [Google Scholar]
- 103.Straumann A, Bussmann C, Conus S, Beglinger C, Simon HU. Anti-TNF-alpha (infliximab) therapy for severe adult eosinophilic esophagitis. J Allergy Clin Immunol. 2008;122:425–427. doi: 10.1016/j.jaci.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 104.Tomizawa Y, Melek J, Komaki Y, Kavitt RT, Sakuraba A. Efficacy of pharmacologic therapy for eosinophilic esophagitis: a systematic review and network meta-analysis. J Clin Gastroenterol. 2018;52:596–606. doi: 10.1097/MCG.0000000000000878. [DOI] [PubMed] [Google Scholar]
- 105.Youngblood BA, Brock EC, Leung J, Falahati R, Bochner BS, Rasmussen HS, et al. Siglec-8 antibody reduces eosinophils and mast cells in a transgenic mouse model of eosinophilic gastroenteritis. JCI Insight. 2019;4:126219. doi: 10.1172/jci.insight.126219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Noti M, Wojno ED, Kim BS, Siracusa MC, Giacomin PR, Nair MG, et al. Thymic stromal lymphopoietin-elicited basophil responses promote eosinophilic esophagitis. Nat Med. 2013;19:1005–1013. doi: 10.1038/nm.3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Vicari AP, Schoepfer AM, Meresse B, Goffin L, Léger O, Josserand S, et al. Discovery and characterization of a novel humanized anti-IL-15 antibody and its relevance for the treatment of refractory celiac disease and eosinophilic esophagitis. MAbs. 2017;9:927–944. doi: 10.1080/19420862.2017.1332553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Roufosse FE, Kahn JE, Gleich GJ, Schwartz LB, Singh AD, Rosenwasser LJ, et al. Long-term safety of mepolizumab for the treatment of hypereosinophilic syndromes. J Allergy Clin Immunol. 2013;131:461–647.e1-5. doi: 10.1016/j.jaci.2012.07.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kuang FL, Legrand F, Makiya M, Ware J, Wetzler L, Brown T, et al. Benralizumab for PDGFRA-negative hypereosinophilic syndrome. N Engl J Med. 2019;380:1336–1346. doi: 10.1056/NEJMoa1812185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kuang FL, Fay MP, Ware J, Wetzler L, Holland-Thomas N, Brown T, et al. Long-term clinical outcomes of high-dose mepolizumab treatment for hypereosinophilic syndrome. J Allergy Clin Immunol Pract. 2018;6:1518–1527.e5. doi: 10.1016/j.jaip.2018.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Roufosse F, de Lavareille A, Schandené L, Cogan E, Georgelas A, Wagner L, et al. Mepolizumab as a corticosteroid-sparing agent in lymphocytic variant hypereosinophilic syndrome. J Allergy Clin Immunol. 2010;126:828–835.e3. doi: 10.1016/j.jaci.2010.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wechsler ME, Akuthota P, Jayne D, Khoury P, Klion A, Langford CA, et al. Mepolizumab or placebo for eosinophilic granulomatosis with polyangiitis. N Engl J Med. 2017;376:1921–1932. doi: 10.1056/NEJMoa1702079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kahn JE, Grandpeix-Guyodo C, Marroun I, Catherinot E, Mellot F, Roufosse F, et al. Sustained response to mepolizumab in refractory Churg-Strauss syndrome. J Allergy Clin Immunol. 2010;125:267–270. doi: 10.1016/j.jaci.2009.10.014. [DOI] [PubMed] [Google Scholar]
- 114.Soeda S, To M, Kono Y, Yamawaki S, Tsuzuki R, Katsube O, et al. Case series of allergic bronchopulmonary aspergillosis treated successfully and safely with long-term mepolizumab. Allergol Int. 2019;68:377–379. doi: 10.1016/j.alit.2018.12.008. [DOI] [PubMed] [Google Scholar]
- 115.Soeda S, Kono Y, Tsuzuki R, Yamawaki S, Katsube O, To M, et al. Allergic bronchopulmonary aspergillosis successfully treated with benralizumab. J Allergy Clin Immunol Pract. 2019;7:1633–1635. doi: 10.1016/j.jaip.2018.11.024. [DOI] [PubMed] [Google Scholar]
- 116.Ange N, Alley S, Fernando SL, Coyle L, Yun J. Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS) syndrome successfully treated with mepolizumab. J Allergy Clin Immunol Pract. 2018;6:1059–1060. doi: 10.1016/j.jaip.2017.10.020. [DOI] [PubMed] [Google Scholar]
- 117.Matucci A, Liotta F, Vivarelli E, Dies L, Annunziato F, Piccinni MP, et al. Efficacy and safety of mepolizumab (anti-interleukin-5) treatment in Gleich's syndrome. Front Immunol. 2018;9:1198. doi: 10.3389/fimmu.2018.01198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Taillé C, Pison C, Nocent C, Devouassoux G, Prud’homme A, Gruber A, et al. Patients in the IDEAL cohort: a snapshot of severe asthma in France. Rev Mal Respir. 2019;36:179–190. doi: 10.1016/j.rmr.2018.10.005. [DOI] [PubMed] [Google Scholar]
- 119.Jeffery MM, Shah ND, Karaca-Mandic P, Ross JS, Rank MA. Trends in Omalizumab Utilization for Asthma: Evidence of Suboptimal Patient Selection. J Allergy Clin Immunol Pract. 2018;6:1568–1577.e4. doi: 10.1016/j.jaip.2017.07.034. [DOI] [PubMed] [Google Scholar]
- 120.Llanos JP, Bell CF, Packnett E, Thiel E, Irwin DE, Hahn B, et al. Real-world characteristics and disease burden of patients with asthma prior to treatment initiation with mepolizumab or omalizumab: a retrospective cohort database study. J Asthma Allergy. 2019;12:43–58. doi: 10.2147/JAA.S189676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Inselman JW, Jeffery MM, Maddux JT, Shah ND, Rank MA. Trends and disparities in asthma biologic use in the United States. J Allergy Clin Immunol Pract. 2019 doi: 10.1016/j.jaip.2019.08.024. Forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Singh H, Peters JI, Kaur Y, Maselli DJ, Diaz JD. Long-term evaluation of response to omalizumab therapy in real life by a novel multimodular approach: the Real-life Effectiveness of Omalizumab Therapy (REALITY) study. Ann Allergy Asthma Immunol. 2019;123:476–482.e1. doi: 10.1016/j.anai.2019.07.026. [DOI] [PubMed] [Google Scholar]
- 123.Sullivan PW, Li Q, Bilir SP, Dang J, Kavati A, Yang M, et al. Cost-effectiveness of omalizumab for the treatment of moderate-to-severe uncontrolled allergic asthma in the United States. Curr Med Res Opin. 2019 doi: 10.1080/03007995.2019.1660539. Forthcoming. [DOI] [PubMed] [Google Scholar]
- 124.Busse W, Chupp G, Nagase H, Albers FC, Doyle S, Shen Q, et al. Anti-IL-5 treatments in patients with severe asthma by blood eosinophil thresholds: Indirect treatment comparison. J Allergy Clin Immunol. 2019;143:190–200.e20. doi: 10.1016/j.jaci.2018.08.031. [DOI] [PubMed] [Google Scholar]
- 125.Casale TB, Pacou M, Mesana L, Farge G, Sun SX, Castro M. Reslizumab compared with benralizumab in patients with eosinophilic asthma: a systematic literature review and network meta-analysis. J Allergy Clin Immunol Pract. 2019;7:122–130.e1. doi: 10.1016/j.jaip.2018.08.036. [DOI] [PubMed] [Google Scholar]
- 126.Menzella F, Biava M, Bagnasco D, Galeone C, Simonazzi A, Ruggiero P, et al. Efficacy and steroid-sparing effect of benralizumab: has it an advantage over its competitors? Drugs Context. 2019;8:212580. doi: 10.7573/dic.212580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Signorovitch JE, Sikirica V, Erder MH, Xie J, Lu M, Hodgkins PS, et al. Matching-adjusted indirect comparisons: a new tool for timely comparative effectiveness research. Value Health. 2012;15:940–947. doi: 10.1016/j.jval.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 128.Phillippo D, Ades T, Dias S, Palmer S, Abrams KR, Welton N. NICE DSU Technical Support Document 18: Methods for population-adjusted indirect comparisons in submissions to NICE. Sheffield: Decision Support Unit, ScHARR, University of Sheffield; 2016. [Google Scholar]
- 129.Bourdin A, Husereau D, Molinari N, Golam S, Siddiqui MK, Lindner L, et al. Matching-adjusted indirect comparison of benralizumab versus interleukin-5 inhibitors for the treatment of severe asthma: a systematic review. Eur Respir J. 2018;52:180. doi: 10.1183/13993003.01393-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]